Highlights
-
•
Compared with control participants, participants with bipolar disorder showed blunted NAcc activity during monetary gain anticipation.
-
•
Blunted NAcc activity during gain anticipation is related to individual differences in the tendency to respond impulsively to positive emotional states (Positive Urgency).
-
•
Individual differences in Positive Urgency could statistically account for the association of blunted NAcc activity with bipolar diagnosis.
Keywords: Bipolar, Imaging, Reward, Anticipation, Accumbens, Monetary, Human
Abstract
Although behavioral sensitivity to reward predicts the onset and course of mania in bipolar disorder, the evidence for neural abnormalities in reward processing in bipolar disorder is mixed. To probe neural responsiveness to anticipated and received rewards in the context of bipolar disorder, we scanned individuals with remitted bipolar I disorder (n = 24) and well-matched controls (n = 24; matched for age and gender) using Functional Magnetic Resonance Imaging (FMRI) during a Monetary Incentive Delay (MID) task. Relative to controls, the bipolar group showed reduced NAcc activity during anticipation of gains. Across groups, this blunting correlated with individual differences in impulsive responses to positive emotions (Positive Urgency), which statistically accounted for the association of blunted NAcc activity with bipolar diagnosis. These results suggest that blunted NAcc responses during gain anticipation in the context of bipolar disorder may reflect individual differences in Positive Urgency. These findings may help resolve discrepancies in the literature on neural responses to reward in bipolar disorder, and clarify the relationship between brain activity and the propensity to experience manic episodes.
Bipolar disorder ranks among the most severe and costly of psychiatric illnesses, and is accordingly associated with high rates of suicide and general mortality (Angst et al., 2002). Heritability estimates for bipolar disorder exceed 80% (Kieseppä, 2004; McGuffin et al., 2003), which implies underlying physiological mechanisms that might include neural responses to reward (Depue and Iacono, 1989; Fowles, 1988). Indeed, early researchers proposed that manic symptoms (i.e., elevated mood, excessive energy, racing thoughts, and enhanced engagement in goal-directed activity) might reflect excessive activity in neural circuits implicated in reward processing (Depue et al., 1989). Consistent with this idea, irregularities in dopamine function have been invoked in models of bipolar disorder across a broad range of human and comparative research (Ashok et al., 2017; Cousins et al., 2009). Nonetheless, the precise association of activity in neural circuits implicated in reward processing with bipolar disorder remains unclear.
Substantial behavioral research indicates that people with remitted bipolar disorder show elevated behavioral sensitivity to rewards compared to controls, as reflected in both self-report measures (e.g., Behavioral Activation Scales (Carver and White, 1994)) and behavioral tasks (Harmon-Jones et al., 2008; Hayden et al., 2008; Johnson et al., 2012b). Increased behavioral responsiveness to rewards does not merely index manic symptoms, since self-report (Applegate et al., 2009; Carvalho and Nobre, 2011; Fulford et al., 2008; Johnson et al., 2009; Johnson and Carver, 2006; Kundakç and Emre, 2004; Mason et al., 2012; Meyer et al., 1999) and physiological measures of reward sensitivity (Harmon-Jones et al., 2002; Mason et al., 2012; Sutton SK, 2002) remain elevated even after remission from manic symptoms (Meyer et al., 2001). Increased behavioral responsiveness to reward is also apparent in individuals with a history of subsyndromal symptoms who have not yet experienced a full-blown manic episode. Longitudinally, behavioral measures of reward sensitivity predict: the onset of bipolar disorder spectrum diagnoses among vulnerable students (Alloy et al., 2009a); conversion to more severe forms of bipolar disorder among diagnosed individuals (Alloy et al., 2012); and increases in manic symptoms among diagnosed individuals (e.g., over six months) (Bhugra et al., 2010; Meyer et al., 2001).
Because individuals at risk for bipolar disorder show increased behavioral responsiveness to reward, theorists have extrapolated that these individuals might also show greater neural sensitivity to reward (Johnson et al., 2012b). Over the past two decades, researchers have used Functional Magnetic Resonance Imaging (FMRI) in healthy humans to develop tasks capable of consistently eliciting neural responses during both anticipation and receipt of basic as well as abstract rewards (Knutson and Cooper, 2005). Meta-analyses of these findings consistently implicate activity in brain regions that receive mesolimbic dopamine projections in reward anticipation and receipt (Bartra et al., 2013; Knutson and Greer, 2008). Some of this evidence suggests that ventral striatal regions (including the Nucleus Accumbens or NAcc) respond more prominently to reward anticipation, whereas a region of the Medial PreFrontal Cortex (MPFC) responds more prominently to reward outcomes (Knutson et al., 2003; Knutson and Greer, 2008). Many of these neuroimaging tasks use monetary incentives, which allow researchers to directly contrast anticipation and receipt of monetary gains versus losses, as well as to control for potential confounds related to arousal, salience, attention, and motor demands. Some neural responses during these tasks also show temporal stability (e.g., over > 2 years), suggesting that they might serve as reliable “neurophenotypic” markers of psychiatric symptom profiles (Wu et al., 2014). More recent translational work further implies that neural responses to reward cues may be related to phasic dopamine release, since optogenetic stimulation of midbrain dopamine neurons in awake rats generates a pattern of increased striatal FMRI activity that matches neural activity observed in humans as they anticipate monetary rewards (Ferenczi et al., 2016).
Several neuroimaging studies have used these incentive tasks to probe neural responses to reward in the context of bipolar disorder. Consistent with behavioral findings, reviews of this growing literature have concluded that individuals with bipolar disorder are “…characterized by elevated activation in a fronto-striatal reward neural circuit…” (Nusslock et al., 2014) (see also Whitton et al., 2015). Scrutiny of these collected findings, however, suggests that such an intuitive conclusion may be premature. Results from several FMRI studies utilizing monetary incentive tasks in the context of bipolar disorder, for instance, paint a more variable picture (Table 1; k = 15 studies; omitting studies using other scanning techniques such as electroencephalography or positron emission tomography, focusing on youth (Bebko et al., 2014; Urošević et al., 2016), or including previously-published data (Dutra et al., 2017)). Specifically, focusing on the most commonly studied condition of reward anticipation, qualitative comparison of these findings suggests that only a few studies found evidence for increased NAcc (or ventral striatal) activity (k = 3 studies), whereas others reported decreased NAcc activity (k = 2 studies), and the rest reported no significant differences (k = 10 studies) in NAcc activity of bipolar versus control participants.
Table 1.
Characteristics of FMRI studies investigating neural responses to incentives in adults diagnosed with bipolar disorder versus healthy control participants.
| Authors | Year | Phase | BD n (%M) / HC | Task | Gain anticipation | Gain outcome | Loss anticipation | Loss outcome |
|---|---|---|---|---|---|---|---|---|
| Abler et al | 2007 | Manic | 12 BD I (58.3) / 12 HC (58.3) | MID | none | ↓ VS | N/A | N/A |
| Bermpohl et al | 2009 | Manic | 15 BP I (53.3) / 26 HC (57.7) | MID | ↑ l LOFC | none | ↓ l lateral OFC | none |
| O'Sullivan et al | 2011 | Hypomanic | 12 manic / 12 HC (not reported) | Probabilistic reinforcement learning | ↓ VS, ↑ DS | ↓ insula, DS | N/A | N/A |
| Nusslock et al | 2012 | Euthymic | 21 BD I (42.9) / 20 HC (40) | Card-guessing | ↑ r VS, r OFC, ↑ l LOFC | none | none | none |
| Linke et al | 2012 | Euthymic | 19 BD I (42.1) / 19 HC (42.1) | Reversal Learning | N/A | ↑ medial OFC | N/A | ↑ r amygdala |
| Caseras et al | 2013 | Euthymic | 17 BD I (36) / 15 BD II (40) / 20 HC (35) | Card-guessing | ↑ VS, l VLPFC, insula in BP I, ↑ DS, l DLPFC in BP-II | ↑ l VS in BP I (trend) | N/A | N/A |
| Chase et al | 2013 | Bipolar depression | 23 BD I (17.4) / 37 HC (32.4) | Card-guessing | ↓ ACC, ↑ l VLPFC | none | N/A | N/A |
| Trost et al | 2014 | Euthymic | 16 BD I (37.5) / 16 HC (43.8) | Desire-reason dilemma | N/A | ↓ VS, ↓ correlated VLPFC and VS | N/A | N/A |
| Mason et al | 2014 | Euthymic | 20 BD (18 BD I, 2 BD II) (50) / 20 HC (45) | Roulette | ↑ l VS (trend) | ↑ VS, ↓ DLPFC | ↑ VS trend in BD | none |
| Dutra et al | 2015 | Remitted | 24 BD I (37.5) / 25 HC (40) | MID and SID | ↓ OFC, IFG, r occipital ctx | ↑ VS, DS | none | none |
| Yip et al | 2015 | Euthymic | 20 BD II / NOS (60) / 20 HC (50) | MID | ↓ r DS | none | ↓ VS, DS | none |
| Satterthwaite et al | 2015 | Bipolar depression | 23 BD (21 BD I, 2 BD II) (37) / 32 HC (51) | Monetary reward | N/A | ↓ VS, ↑ correlated reward nodes | none | none |
| Redlich et al | 2015 | Bipolar depression | 33 BD I (51.5) / 34 HC (52.9) | Card-guessing | N/A | ↓ VS, caudate, thalamus, putamen, insula, | none | none |
| Schreiter et al | 2016 | Euthymic | 20 BD (40) / 20 HC (40) | MID | ↓ VS, ↓ l VS and APFC correlated activity | none | none | none |
| Berghost et al | 2016 | Euthymic or mildly depressed | 13 BD (38) / 15 HC (33) |
MID | ↑ amygdala no stress, ↓ amygdala during stress | ↑ DS during stress | N/A | N/A |
| Kollman et al | 2017 | Euthymic | 16 BD (37.5) / 19 HC (50) |
MID | ↑ ACC | N/A | none | none |
These findings might vary for a number of reasons. Study design might play a role, since studies reporting increased NAcc activity during reward in individuals with bipolar disorder tended to use card guessing or roulette tasks (Caseras et al., 2013; Mason et al., 2014; Nusslock et al., 2014), whereas studies reporting decreased NAcc activity tended to use Monetary Incentive Delay (or MID) tasks (O'Sullivan et al., 2011; Schreiter et al., 2016). Other methodological reasons for variation might include smaller sample sizes (e.g., fewer than 20 per group (Abler et al., 2008; Berghorst et al., 2016; Bermpohl et al., 2010; Kollmann et al., 2017; Linke et al., 2012; Mason et al., 2014; Nusslock et al., 2012; O'Sullivan et al., 2011; Schreiter et al., 2016; Singh et al., 2013; Trost et al., 2014; Urošević et al., 2016; Yip et al., 2015)), insufficiently large incentives to increase NAcc activity in healthy controls (Nusslock et al., 2012), or the lack of neutral or loss conditions for comparison (Nusslock et al., 2012). Thus, while some FMRI evidence implies abnormalities in the neural processing of incentives in bipolar disorder, the direction and strength of these effects remains unclear.
Differences in psychological states might also account for variability in diagnostic group differences across studies. Variation across samples in mood state, comorbid diagnoses, and medication profiles might make complicate comparisons. For instance, reward-related activity was reported to normalize in one small bipolar sample after remission (Bermpohl et al., 2010), and depressive symptoms might blunt NAcc activity during reward anticipation in nonbipolar (Hägele et al., 2015) as well as bipolar samples (Satterthwaite et al., 2015; Sharma et al., 2017; Urošević et al., 2016), while hypomanic symptoms might intensify responses to reward outcomes (Urošević et al., 2016). Further, some evidence suggests that antipsychotic medications (which are sometimes prescribed to treat bipolar disorder) might generally blunt neural responses to reward anticipation and outcomes (Caseras et al., 2013; Chase et al., 2013).
Further, individual differences in psychological traits might also increase the variability of these findings. In this study, we specifically focus on a dimensional trait which has gained considerable support as an important correlate of bipolar disorder –– the Positive Urgency Measure (PUM), which assesses a trait-like tendency to respond impulsively to positive emotional states. Several studies have reported increased PUM scores in individuals who are diagnosed with remitted bipolar I disorder as well as in those with mild subsyndromal manic symptoms (Giovanelli et al., 2013; Johnson et al., 2013). Moreover, after accounting for clinical, treatment, and personality characteristics in individuals diagnosed with bipolar I disorder, individual differences in PUM were robustly associated with a host of difficulties including aggression (Johnson and Carver, 2016), self-harm, suicidal ideation and behavior (Johnson et al., 2017), functional impairment, and poor quality of life (Muhtadie et al., 2014; Victor et al., 2011). PUM scores were also significantly more elevated in individuals with remitted bipolar disorder than other measures of impulsivity (Muhtadie et al., 2014). Because PUM indexes impulsive responses to positive emotions, this construct may prove particularly relevant for understanding neural responses to reward. To our knowledge, no study has yet examined neural correlates of PUM in individuals with bipolar disorder (but see Cyders et al. (2014) and Tervo-Clemmens et al. (2017) for neuroimaging studies of PUM in alcoholism with nonincentivized tasks, as well as Chase et al. (2017) for a neuroimaging study of a mixed clinical group with an incentivized gambling task).
Given mixed FMRI evidence for abnormal neural responses to reward in bipolar disorder, we sought to directly compare neural activity during anticipation of monetary incentives and in response to their outcomes in a group of individuals diagnosed with bipolar (I) disorder versus an age- and gender-matched non-mood disordered control group. We also examined whether individual differences in Positive Urgency (PUM) might help to explain further variability within the bipolar disordered group. Our analyses could account for potentially confounding effects of antipsychotic medications (Bermpohl et al., 2010), illness severity (Herbenick et al., 2010), comorbid conditions including substance-related disorders (Alloy et al., 2009b), anxiety disorders (Morgan et al., 2009), and subsyndromal depressive and manic symptoms). To control for these potential confounds, we stratified recruitment based on lifetime anxiety and substance abuse disorders and oversampled individuals who were free of medications. We also excluded individuals who were taking first-generation antipsychotic medications, which might blunt NAcc activity during reward anticipation (Juckel et al., 2006). To probe neural responses to monetary incentives, individuals diagnosed with bipolar disorder and healthy control participants completed a MID Task while undergoing scanning with FMRI. We specifically tested whether participants diagnosed with bipolar disorder would show altered NAcc activity during gain anticipation, and also whether individual differences in PUM could account for group differences in neural responses to reward. Since previous studies of bipolar disorder have reported neural responses to gain and loss in other regions implicated in incentive processing, we additionally considered activity in those regions (i.e., the MPFC as well as the Anterior Insula or AIns).
1. Method
Participants were recruited through community and internet advertisements, as well as local clinics. Individuals who contacted the study team were pre-screened by telephone for demographic medical exclusion criteria and likely diagnosis. Potential participants were scheduled for individual in-person appointments. Written informed consent was obtained from participants upon arriving at the university. Potential participants completed a comprehensive interview to assess diagnostic status and inclusion/exclusion criteria, and completed other measures not relevant for the current report (Johnson et al., 2012a; Victor et al., 2011). FMRI scans were conducted during separate testing sessions to limit participant fatigue. Medication assessments were conducted prior to FMRI scans. The study was approved by the Institutional Review Board of the Stanford University Medical School, and data collection occurred between April of 2007 and July of 2009.
1.1. Participants
The current study included 24 participants with bipolar I disorder (the “bipolar group”; 14 men, mean age = 36.22, 11 minorities) and 24 participants with no lifetime history of mood disorder (the “control group”; 13 men, mean age = 33.92, 9 minorities; Table 2), such that a total of 48 individuals were included in the final analysis. To be eligible for the study, individuals had to be between the ages of 18 and 60 years, be fluent English speakers, and meet criteria for Bipolar I Disorder (BD I) or for no lifetime mood disorder using the Structured Clinical Interview for the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (SCID-IV) (First et al., 1995). Exclusion criteria included a history of alcohol or substance abuse or dependence in the past year, primary psychotic disorders, history of brain injury, hemorrhage, or tumor, stroke, medical conditions influencing the central nervous system, developmental disabilities, electroconvulsive treatment within the past 18 months, weight greater than 300 pounds, pain medication during the 24 h before the scan, diabetes, epilepsy, cardiovascular disorder, loss of consciousness for more than 5 min in the past year or more than 1 h during lifetime, and history of amnesia. To ensure adequate ability to complete the computerized cognitive tasks, individuals were excluded if they were color blind or dyslexic. To ensure safety and comfort, individuals were excluded if they reported claustrophobia or any occupational or medical history entailing a risk of a metallic object in their body that could be dislodged by scanning (e.g., work as a metal worker, eye injury involving a metallic object, nonremovable body piercings, metal implants or ear tubes, certain tattoos). Women with late menstrual periods or possible pregnancies were also excluded, as were individuals taking first-generation antipsychotic medications.
Table 2.
Demographic, clinical, and behavioral responses by group.
| Bipolar (n = 24) | Control (n = 24) | |
|---|---|---|
| Mean (SD) or% | Mean (SD) or% | |
| Age | 37.04 (9.866) | 33.92 (12.151) |
| Gender (female) | 50.0% | 45.8% |
| Employed | 70.8% | 54.2% |
| Race | ||
| Asian | 29.2% | 16.7% |
| Pacific Islander or Native Hawaiian | 4.2% | 0.0% |
| African American | 0.0% | 8.3% |
| Caucasian | 66.7% | 75.0% |
| Ethnicity | ||
| Hispanic | 8.3% | 12.5% |
| Alcohol or Drug Abuse or Dependence | 37.0% | 25.0% |
| Anxiety Disorder Diagnosis | 37.0% | 25.0% |
| Depressive Episodes | 10.96 (11.710) | 0 |
| Manic Episodes | 8.92 (10.946) | 0 |
| Lithium | 37.5% (9/24) | |
| Valproate | 16.7% (4/24) | |
| Atypical Antipsychotic | 25.0% (6/24) | |
| Benzodiazapene | 12.5% (3/24) | |
| Lamictal | 45.8% (11/24) | |
| Antidepressant | 8.3% (2/24) | |
| HRSD* | 4.03 (5.434) | 0.90 (1.586) |
| BRMS* | 1.71 (1.989) | 0.70 (1.418) |
| PUM⁎⁎⁎ | 33.35 (10.15) | 18.26 (5.42) |
Note. BRMS = Bech Rafaelsen Mania Rating Scale; HRSD = Modified Hamilton Rating Scale for Depression; PUM = Positive Urgency Measure; *p < .05, uncorrected, ⁎⁎⁎p < .001, uncorrected.
To increase generalizability, individuals who were symptomatic at the time of the diagnostic interview were not excluded from participation. Rather, we conducted monthly telephone symptom severity assessments and proceeded with the fMRI session after they had achieved remission criteria based on symptom scale scores (described below). To verify sustained remission, participants completed symptom severity measures 24 h before the FMRI session. Prior to analysis, we excluded 10 participants (5 control; 5 bipolar) with excessive motion (i.e., > 2 mm in any dimension from one scan to the next), leading to analysis of data from 48 of the 58 individuals who completed the entire protocol.
Bipolar and control groups were matched with respect to age, gender, employment status, race, ethnicity, handedness, lifetime diagnoses of substance-related abuse and dependence, and lifetime diagnoses of anxiety disorders. Power analysis indicated that samples of at least 23 participants per group would be required to detect large group differences in NAcc activity during gain versus nongain anticipation (d = 0.75) at a power of 0.80 and an error probability (directional) of 0.05 (using G*Power software, version 3.1).
1.2. Measures
Diagnostic criteria for bipolar disorder and comorbid conditions were assessed using the Structured Clinical Interview for DSM-IV (SCID-IV) for Axis I disorders. Before administering SCID-IV interviews, interviewers completed extensive didactic and role-play training and achieved inter-rater reliability of 1.0 for both depression and mania diagnoses for 10 randomly selected interviews, as assessed using intraclass correlation coefficient.
As an interviewer-rated measure of current manic symptoms (including elevated mood, self-esteem, motor activity, verbal activity, sleep, hostility, and sexual interest), we used the Bech Rafaelsen Mania Scale (BRMS); 11-items). Our version also included suggested probes as well as anchor points. This scale has been sensitive to changes with treatment, and correlates with diagnosis as well as other measures of mania symptom severity (Bech et al., 1979). We used standardized probes to achieve high inter-rater reliability (intraclass correlation = 0.93 in a sample of 14 randomly selected audiotapes) and internal consistency (alpha = 0.94).
As an interview-based measure of depressive symptom severity, we used the Modified Hamilton Rating Scale for Depression (MHRSD; 17 items; Miller et al., 1985). While scores on the modified scale correlate robustly with the original Hamilton Rating Scale for Depression (HAM-D), the scale was revised to include standardized probes and behavioral anchors for rating each item. The MHRSD scale shows strong psychometric qualities, including inter-rater reliability, concordance with other depression measures, and sensitivity to change with treatment (Keitner et al., 1991). Our coders achieved robust inter-rater reliability for a set of 14 randomly-selected audiotaped interviews (alpha = 0.92). Participants were scheduled for FMRI sessions if their BRMS and MHRSD scores were both less than or equal to a score of seven, indicating remission.
The Positive Urgency Measure (PUM) was designed to capture tendencies to act impulsively in response to positive emotion (e.g. “Others would say I make bad choices when I am extremely happy about something”) (Cyders et al., 2007). The scale includes 14 items that are self-rated on a scale ranging from 1 (agree strongly) to 4 (disagree strongly). The PUM has shown factor analytic support, strong internal consistency, and correspondence with interviewer and family ratings (Cyders et al., 2010; Cyders and Smith, 2008). High scores have been associated with risky behavior, externalizing syndromes (Cyders and Smith, 2007), depression diagnoses (Carver et al., 2013), and bipolar diagnosis (Muhtadie et al., 2014). High scores have also been associated with poor function and quality of life (Muhtadie et al., 2014; Victor et al., 2011) and with aggression and suicidality (Johnson et al., 2017; Johnson and Carver, 2016) in individuals diagnosed with bipolar disorder. In the current sample, the scale showed high internal consistency (α=0.97).
Pharmacotherapy was assessed by interview. While more than one patient was taking lithium, valproic acid, lamotrigine, benzodiazepines, antidepressants or second-generation antipsychotics, no patient was taking carbamazepine or gabapentin. Because dose distributions were positively skewed, measures of drugs taken were converted from dosages to presence versus absence indices for analysis. Exploratory analyses revealed that of the reported medications, only treatment with second-generation antipsychotics was related to the primary outcome variable (i.e., the linear coefficient of gain anticipation activity in the NAcc; Wilcoxon rank sum W = −21, n = 6, m = 18, p=.024). Therefore, parallel analyses excluding participants on atypical antipsychotic medications were conducted to verify robustness of the critically predicted findings.
1.3. Task
Participants completed a version of the Monetary Incentive Delay (MID) Task designed to elicit neural and behavioral responses to monetary incentives and their outcomes during FMRI scan acquisition (Knutson et al., 2001). Each trial (8000 ms total) began with presentation of a visual cue (cue period; 2000 ms). Cue shapes indicated the valence (gain: circle, or loss: square) and horizontal lines across the cues indicated magnitude ($0.00: no lines, $0.20: one line, $1.00: two lines, or $5.00: three lines) of incentives that participants could try to gain or avoid losing by responding to an upcoming target. In addition to gain and loss trials, triangle cues indicated nonresponse trials, in which participants were instructed to not respond to upcoming targets. This version of the MID task therefore included 9 total conditions (4 gain, 4 loss, and 1 nonresponse). After viewing the cue, participants were shown a fixation cross for a variable interval (anticipation period; 2000 – 2500 ms), followed by a target that briefly appeared (150 – 470 ms). Participants were instructed to try to press a button before the disappearance of each target to either gain or avoid losing the previously cued amount of money. After a second variable delay (1030 – 2350 ms), participants received feedback informing them of the amount they had gained or lost on each trial (outcome period; 2000 ms). Participants completed two blocks including 90 trials each (20 trials per condition; 180 trials total). Trials were presented in a pseudo-random sequence within each block. An adaptive timing algorithm applied to the targets maintained an approximately constant hit rate within each condition (i.e., if an individual's hit rate for a condition did not approximate an average of 66%, the duration of the next target was shortened or lengthened).
Before scanning, participants were taught how to play the task (including the meaning of each cue), and then played a training version of the task until they understood the incentives and task demands (typically lasting less than 10 min). Participants were then allowed to ask questions, shown the cash they could win, and explicitly quizzed about the meaning of a subset of the cues they had seen. After completing the task, participants rated levels of arousal and valence they experienced in response to the presentation of each cue during the MID task. Ratings were made on 7-point Likert scales for each cue in which the arousal scale ranged from not aroused to very aroused and the valence scale ranged from negative through neutral to positive. These scores were then mean-deviated within subject and rotated 45° through two-dimensional space to derive independent measures of positive arousal and negative arousal for each cue prior to analyses (Knutson et al., 2005). These rotated “anticipatory affective” dimensions of positive arousal and negative arousal align most closely with NAcc and AIns activity, respectively, in previous research (Knutson et al., 2014). Participants received cash payments for their task earnings after rating the cues.
1.4. FMRI acquisition and analysis
Participants were scanned with a General Electric 1.5 T Signa scanner using a standard birdcage quadrature head coil. Stimuli were presented using E-Prime 1.1 software and projected onto a mirror mounted on the coil. Participants were fitted with a bite bar and padding to minimize head motion. Functional images covered the whole brain and consisted of 24 contiguous 4-mm thick axial slices (TR: 2000 ms, TE: 40 ms, flip: 90°, 3.44 × 3.44 mm in-plane resolution, 64 × 64 matrix), collected using a T2*-sensitive spiral in/out pulse sequence that minimizes dropout in ventral frontal and medial temporal regions of interest (Glover and Law, 2001; Preston et al., 2004).
We first examined behavior (hit rate, hit reaction time) and affect ratings (positive arousal and negative arousal ratings in response to incentive cues), to verify that diagnostic groups did not differ significantly. To do so, we conducted four parallel, mixed-model Analyses Of Variance (ANOVAs) to assess the effects of diagnostic group (bipolar vs. control) as a between-subjects factor, valence (gain vs. loss) and amount of money ($0.00, $0.20, $1.00, $5.00) as within-subjects factors, and the interaction of these two factors.
Analyses of brain activity then included three stages: (1) whole brain localization to confirm significant responses in predicted regions; (2) targeted group comparisons to test for the predicted differences in Volumes Of Interest (VOIs); and (3) regressions to examine whether individual differences correlated with activity in VOIs. These analyses were conducted using Analysis of Functional NeuroImaging (AFNI version AFNI_18.0.25, Cox, 1996). For preprocessing, each voxel's time series was concatenated across runs and corrected for differences in the timing of slice acquisition using sync-interpolation. Head motion was estimated and corrected in three dimensions and three rotations. We visually examined motion estimates to ensure that no participant's head movement exceeded 2.0 mm in any direction from one volume to the next. Data was then spatially smoothed using a 4 mm full-width-at-half-maximum Gaussian blurring kernel (Sacchet and Knutson, 2012), high-pass filtered, and normalized to percent signal change using each voxel's average activation across the entire task as a baseline.
For whole-brain analysis, we used a multiple regression model that included four orthogonal parametric regressors of interest: (1) gain anticipation (linearly weighted as ++0.00 / −3, ++0.20 / −1, ++1.00 / 1, ++5.00 / 3); (2) loss anticipation (linearly weighted as −$0.00 / −3, −$0.20 / −1, −$1.00 / 1, −$5.00 / 3); (3) gain versus nongain outcomes (hit vs. miss weighted as ++1.00 / ±1, ++5.00 / ±3); (4) and non-loss versus loss outcomes (hit vs. miss weighted as −$1.00 / ±1, −$5.00 / ±3). Covariates of noninterest included two regressors weighting each modeled trial period (anticipation period and outcome period), six regressors indexing motion (3 dimensions of linear and rotated displacement), and two regressors indexing white-matter and cerebrospinal-fluid intensity (Chang and Glover, 2009). All regressors of interest were convolved with a gamma-variate function that modeled a canonical hemodynamic response function (Cohen et al., 1997). The model output maps of t-statistics were transformed into maps of Z-scores, resampled at 3.75 mm3 (to approximate the originally-acquired voxel size and resolution), and coregistered with the structural scan. Whole brain statistical maps were estimated using a grey matter mask and cluster-thresholded (cluster size ≥ 3 contiguous 3.75 mm cubic voxels) to yield corrected maps (p < .05 corrected, p < .0001 uncorrected, derived with 10,000 Monte Carlo iterations using 3dClustSim in AFNI version 18.0.25, which estimates spatial autocorrelation by default to minimize false positive results).
Neural activity in predicted volumes of interest (VOIs) during incentive anticipation was then compared between groups. To extract raw activity time courses for targeted analyses, spherical VOIs (8 mm diameter) were centered on bilateral foci for the NAcc (Talairach coordinates: ±10, 12, –2), AIns (±34, 24, –4), and MPFC (±4, 45, 0), based on coordinates identified in previous meta-analytic reviews of neuroimaging research on incentive processing (Knutson and Greer, 2008). To directly test for group differences in NAcc activity during gain anticipation, we analyzed peak activity (i.e., at a 4 s lag) extracted from bilateral NAcc volumes of interest (foci: ±10, 10, –2) during incentive anticipation. To test the regional specificity of effects observed in the NAcc, parallel analyses were conducted on bilateral AIns and MPFC activity during anticipation of incentives. Activity from these VOIs was submitted to mixed model group (control versus bipolar; between) x valence (gain, loss; within) x magnitude ($0.00, $0.20, $1.00, $5.00; within) ANOVAs for anticipatory activity. Post-hoc group comparisons involved t-tests thresholded by a Bonferroni correction for multiple tests (i.e., p<.05 / 8 conditions = 0.00625).
To verify that activity in predicted VOIs could classify groups even in a model-free context, we applied classification models (i.e., Support Vector Machine with Recursive Feature Elimination or SVM-RFE) to whole brain coefficient maps of the linear gain anticipation contrast (De Martino et al., 2008) using scikit-learn (Pedregosa et al., 2011). We initially applied the SVM classifier using Leave-One-Subject-Out Cross-Validation (LOSO CV) to determine which hyperparameter (i.e., C-value) could best classify bipolar versus control participants. Next, we ran the SVM classifier using the optimal hyperparameter value and Leave-One-Subject-Out CrossValidation (LOSO CV) for several iterations, recursively eliminating the lowest 5% of features at each step until the top 0.1% of discriminating features remained. Finally, we back-projected the remaining features into normalized brain space (i.e., Talairach space) to visualize the location of features that most robustly discriminated bipolar versus control participants.
To construct models of individual differences, we first conducted bivariate correlation analyses to explore whether relevant activity in the predefined volume of interest (i.e., NAcc activity during gain anticipation) was associated with potentially confounding effects of demographic, clinical, or medication variables. Next, we conducted logistic regression analyses to examine the ability of individual differences in Positive Urgency (PUM), anticipatory brain activity (e.g., linear NAcc gain anticipation coefficient; linear AIns loss anticipation coefficient; linear MPFC gain outcome coefficient), and their combination to distinguish individuals with bipolar disorder from controls. Bootstrapped accelerated mediation models (n = 1000 draws) then tested whether individual differences in PUM could account for associations of NAcc activity during gain anticipation with bipolar versus control diagnostic status using the “lavaan” package in R (Rosseel, 2012). Finally, to verify the importance of NAcc activity during gain anticipation, whole brain linear gain anticipation coefficients were regressed against individual differences in PUM across both bipolar and control participants.
2. Results
2.1. Participant characteristics
Although the bipolar group reported significantly higher mania (BRMS) and depression (MHRSD) symptoms than the control group, both groups’ scores fell within the remitted range. Participants diagnosed with bipolar disorder had an average of approximately 9 previous manic episodes and approximately 11 previous depressed episodes (Table 2).
2.2. Behavior and affect
Groups did not significantly differ in hit rates (overall range: 0.61–.71), consistent with the hit rate targeted by the adaptive timing algorithm (i.e., 66%). For hit rate, a mixed-model ANOVA of diagnostic group, valence, magnitude, and the interaction of these effects yielded no main effect of incentive valence and a main effect of magnitude (F(3,138)=5.25, p=.002), but groups did not significantly differ. Similarly, there were main effects of incentive valence (F(1,46)=7.01, p=.011) and magnitude (F(3,138) = 20.27, p<.001) on hit reaction time; but no group differences. Thus, diagnostic groups did not significantly differ in their behavioral performance on the MID task, and the effects of task conditions on behavior replicated patterns reported in previous research (Knutson and Greer, 2008).
With respect to cue-induced affect ratings, two mixed-model ANOVAs assessed effects of diagnostic group, valence, and magnitude on positive arousal and negative arousal ratings. Two participants (1 bipolar and 1 control) did not provide affective ratings and so were omitted from this analysis. For positive arousal, the analysis yielded the predicted main effects of valence (F(1,43)=90.09, p<.001, ηp2 = 0.682) and magnitude (F(3,129)=51.95, p<.001, ηp2 = 0.553), which were qualified by an interaction of valence and magnitude (F(3,129)=28.92, p<.001; ηp2 = 0.408; see Table 3). There was no significant main effect of diagnostic group or interaction on positive arousal ratings. For negative arousal, a similar analysis yielded the predicted main effects of valence (F(1,43)=42.26, p<.001, ηp2 = 0.507) and magnitude (F(3,129)=40.18, p<.001, ηp2 = 0.489), which were qualified by an interaction of valence and magnitude (F(3,129)=25.72, p<.001, ηp2 = 0.380). As with positive arousal ratings, there was no significant main effect of diagnostic group or interaction on negative arousal ratings. Thus, while MID task cues induced self-reported affect, diagnostic groups did not significantly differ in their responses (Table 3).
Table 3.
Cue-induced affect and behavioral performance by group and condition.
| Bipolar (n = 24) | Control (n = 24) | T-statistic / | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Levene's F | |
| Positive Arousal | |||
| +5.00 | 2.42 (0.957) | 2.68 (0.825) | 0.954 / 1.17 |
| +1.00 | 1.26 (1.155) | 1.63 (1.006) | 1.152 / 0.99 |
| +0.20 | 0.07 (1.087) | 0.83 (0.866) | 2.648 / 0.35 |
| +0.00 | −0.83 (1.064) | −0.52 (0.981) | 1.015 / 0.22 |
| −0.00 | −0.89 (0.775) | −1.17 (1.044) | −1.024 / 2.87 |
| −0.20 | −0.80 (0.859) | −0.95 (0.677) | −0.679 / 0.87 |
| −1.00 | −0.24 (0.894) | −0.61 (0.733) | −1.535 / 0.07 |
| −5.00 | 0.37 (1.178) | −0.49 (0.897) | −2.791 / 1.32 |
| Negative Arousal | |||
| +5.00 | 0.01 (0.924) | −0.25 (0.780) | −0.999 / 0.28 |
| +1.00 | −0.45 (0.629) | −0.43 (0.567) | 0.103 / 1.14 |
| +0.20 | −0.48 (0.755) | −0.43 (0.797) | 0.214 / 0.08 |
| +0.00 | −0.76 (0.881) | −0.98 (0.948) | −0.844 / 0.62 |
| −0.00 | −0.57 (0.758) | −0.71 (0.874) | −0.562 / 0.23 |
| −0.20 | 0.20 (0.952) | 0.55 (0.875) | 1.322 / 0.06 |
| −1.00 | 1.18 (1.145) | 1.63 (1.352) | 1.214 / 0.90 |
| −5.00 | 2.10 (1.552) | 2.43 (1.317) | 0.767 / 0.28 |
| Hit percent | |||
| +5.00 | 0.71 (0.096) | 0.67 (0.084) | −1.686 / 0.03 |
| +1.00 | 0.69 (0.073) | 0.68 (0.075) | −0.541 / 0.01 |
| +0.20 | 0.65 (0.086) | 0.61 (0.062) | −1.490 / 1.91 |
| +0.00 | 0.65 (0.094) | 0.63 (0.103) | −0.651 / 0.49 |
| −0.00 | 0.63 (0.098) | 0.65 (0.070) | 0.943 / 2.82 |
| −0.20 | 0.68 (0.098) | 0.68 (0.072) | 0.093 / 4.34* |
| −1.00 | 0.64 (0.092) | 0.64 (0.104) | 0.000 / 1.01 |
| −5.00 | 0.63 (0.108) | 0.65 (0.089) | 0.945 / 1.14 |
| Hit reaction time (ms) | |||
| +5.00 | 185.3 (30.36) | 175.8 (19.94) | −1.276 / 1.76 |
| +1.00 | 184.6 (32.98) | 176.5 (23.78) | −0.976 / 1.91 |
| +0.20 | 189.3 (35.52) | 179.4 (16.43) | −1.233 / 2.91 |
| +0.00 | 193.6 (33.68) | 189.4 (20.13) | −0.530 / 1.81 |
| −0.00 | 197.3 (35.65) | 187.6 (18.13) | −1.196 / 5.06* |
| −0.20 | 192.7 (32.43) | 186.8 (17.92) | −0.777 / 3.03 |
| −1.00 | 189.0 (31.68) | 178.5 (22.39) | −1.334 / 0.51 |
| −5.00 | 183.9 (32.49) | 174.4 (20.66) | −1.215 / 1.29 |
| Motion (mm) | |||
| Displacement (avg x,y,z) | 0.194 (0.09) | 0.199 (0.10) | 0.181 / 1.54 |
Note. *p < .05, uncorrected.
2.3. Brain activity
2.3.1. Whole brain analyses
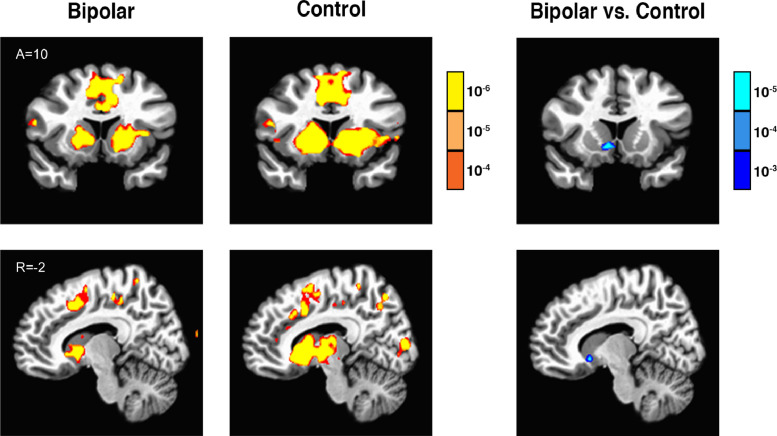
Across both groups, gain anticipation and loss anticipation contrasts significantly correlated with increased activity in dopamine mesolimbic target projection regions including the striatum (specifically, the NAcc), the AIns, and other regions, while the gain outcome contrast correlated with activity in MFPC regions, consistent with previous findings (Tables S1 and S2). Direct group comparisons of these contrasts of interest revealed that the bipolar group showed less activity for the gain anticipation contrast specifically in the ventral striatum (including the right NAcc and right globus pallidus), as well as less activity for the gain outcome contrast in the left occipital gyrus, relative to the control group. Groups did not significantly differ, however, in their neural responses either to loss anticipation or to loss outcomes (Fig. 1; Table 4).
Fig. 1.
Bipolar, control, and bipolar versus control contrast for gain anticipation linear contrast (A=Anterior, R=Right; p<.001 uncorrected for display, with each color gradation representing an order of magnitude increase in significance).
Table 4.
Comparison of bipolar (n = 24) versus control groups (n = 24; p<.0001 uncorrected, p<.05 corrected, cluster ≥ 3 × 3.75 mm3 voxels; positive Z-score indicates bipolar > control; x=right, y=anterior; z=superior).
| Region | x | y | z | Peak Z | Voxels | |
|---|---|---|---|---|---|---|
| Gain anticipation (linear) | R N Accumbens | 8 | 12 | −2 | −4.27 | 5 |
| R Globus Pallidus | 18 | −7 | 2 | −4.26 | 3 | |
| Loss anticipation (linear) | N/A | |||||
| Gain outcomes (linear) | L M Occipital Gyrus | −30 | −86 | 8 | −4.33 | 5 |
| Nonloss outcomes (linear) | N/A |
2.3.2. Volume of interest (VOI) analyses
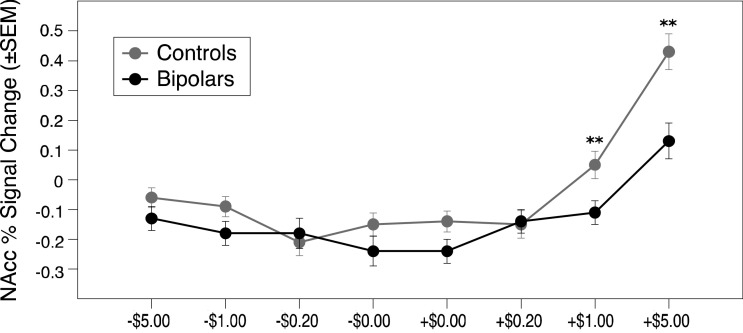
For NAcc VOI activity during incentive anticipation, a mixed-model ANOVA of diagnostic group, valence, and magnitude yielded the predicted main effects of magnitude (F(3,138) = 43.12, p < .001, ηp2 = 0.537) and valence (F(1,46) = 66.29, p < .001, ηp2 = 0.313), which were qualified by a predicted interaction of magnitude and valence (F(3,138) = 19.96, p < .001, ηp2 = 0.303). This analysis also yielded a significant main effect of group (F(1, 46) = 6.110, p = .017, ηp2 = 0.198), which was qualified by a significant interaction of magnitude by group (F(3,138) = 4.779, p = .003, ηp2 = 0.114). Posthoc comparisons revealed that bipolar versus control groups significantly differed with respect to NAcc activity during anticipation of high gains (t(46)=−3.18, p=.003; d = 0.92) and medium gains (t(46)=−3.24, p=.002; d = 0.93), but not in any other condition (p<.05 Bonferroni corrected for 8 comparisons at 0.006; see Fig. 2).
Fig. 2.
Nucleus Accumbens volume of interest peak activity by condition for bipolar versus control groups. (**p < .005 uncorrected, p < .05 corrected for 6 comparisons).
This pattern of significance persisted in an identical analysis after removing the 6 participants on atypical antipsychotics. Specifically, for NAcc activity during incentive anticipation, a mixed-model ANOVA of diagnostic group, valence, and magnitude yielded the predicted main effects of magnitude (F(3,120) = 48.37, p < .001, ηp2 = 0.585) and valence (F(1,40) = 61.99, p < .001, ηp2 = 0.330), which were qualified by a predicted interaction of magnitude and valence (F(3,120) = 20.62, p < .001, ηp2 = 0.340). This analysis also yielded a significant main effect of group (F(1, 40) = 6.84, p = .012, ηp2 = 0.165), which was qualified by a significant interaction of magnitude by group (F(3,120) = 3.87, p = .011, ηp2 = 0.101).
By comparison, analysis of bilateral AIns activity during anticipation of incentives yielded a significant main effect only of magnitude (F(3,138) = 23.94, p<.001; ηp2 = 0.428), which was qualified by unpredicted interactions of valence and magnitude (F(3,138) = 7.07, p<.001; ηp2 = 0.133) and valence and group (F(1,46) = 6.62 p=.013; ηp2 = 0.057). Posthoc comparisons, however, indicated significant differences between diagnostic groups with respect to AIns activity only during anticipation of high gains (t(46) = −2.93, p=.005; d = 0.81), but not in any other condition (p<.05 Bonferroni corrected for 8 comparisons at 0.006). Analysis of MPFC activity during incentive anticipation yielded significant main effects of valence (F(3,46) = 13.60, p<.001; ηp2 = 0.083) and magnitude (F(3,138) = 23.32, p<.001; ηp2 = 0.444), which were qualified by a significant interaction of valence and magnitude (F(3,138) = 4.17, p=.007; ηp2 = 0.083), but no main effects or interactions with group. Posthoc comparisons also indicated no significant differences between diagnostic groups in any condition with respect to MPFC activity.
To validate the focus on the diagnostic properties of NAcc activity, a classifier (SVM-RFE) was applied to whole brain data for the coefficients of the gain anticipation contrast to distinguish groups. After recursive elimination of 99.9% of the features, leave-one-subject-out cross-validated accuracy at a c-value of 10 yielded a classification rate of 60%. Projecting this remaining 0.1% of the selected features back into standardized (i.e., Talairach) brain space revealed negative features in the ventral striatum, which overlapped with the predefined NAcc VOI (see Figure S1).
2.4. Associations of neural activity with individual differences
To explore whether predicted brain activity was associated with potential demographic and clinical confounds, we examined the association of the NAcc gain anticipation contrast with age, gender, current manic symptoms (BRMS), current depressive symptoms (MHRSD), number of lifetime hospitalizations for depression and mania, lifetime diagnoses of anxiety disorders, lifetime diagnoses of problems with substance or alcohol use, and presence of concurrent medications (separately examined for lithium, valproate, atypical antipsychotics, antidepressants, benzodiazepenes, and lamictal). Only the presence versus absence of atypical antipsychotic medications was associated with decreased NAcc gain anticipation activity in bipolar participants (t(22)= –2.30, p=.031).
Next, we conducted logistic regression analyses to examine whether individual differences in the Positive Urgency Measure (PUM), NAcc gain anticipation activity, and their combination could distinguish the bipolar group from the control group. Leave-one-subject-out classifiers indicated that PUM classified diagnosis at 83%, NAcc gain anticipation coefficients classified diagnosis at 77%, and their combination classified diagnosis at 80%. Thus, PUM symptoms alone best differentiated participants with bipolar disorder from controls. Similar results were obtained after removing the 6 individuals on atypical antipsychotics from analysis. Specifically, leave-one-subject-out classifiers indicated that PUM classified diagnosis at 80%, NAcc gain anticipation coefficients classified diagnosis at 67%, and their combination classified diagnosis at 80%.
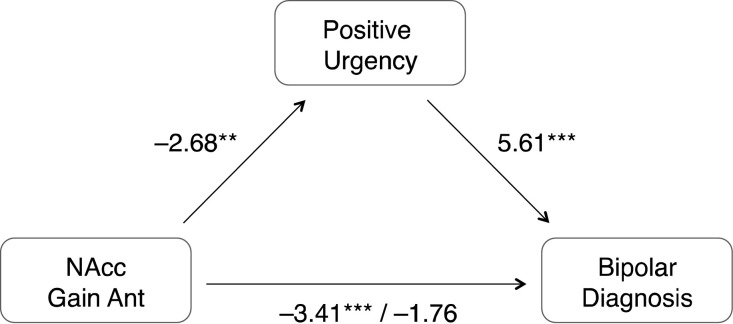
Finally, we conducted bootstrapped accelerated mediation analyses to test whether individual differences in PUM could statistically account for the association of NAcc gain anticipation activity with bipolar diagnosis. The direct path of NAcc gain anticipation to bipolar diagnosis was significant (Z=−3.41; p=.000), as were the indirect paths from NAcc gain anticipation to PUM (Z=−2.68; p=.007) and from PUM to bipolar diagnosis (Z = 5.61; p=.000). Including these indirect paths statistically mediated the influence of the direct path (Z=−1.76, p=.079), suggesting that individual differences in PUM could account for the association of NAcc gain anticipation with bipolar diagnosis. An alternative specification of this mediation model in which NAcc gain anticipation and PUM were switched did not fit the data as well (i.e., total model fit of Z = 3.50, p=.000 versus Z = 6.43, p=.000).
Identical mediation analyses that substituted manic (BRMS) or depressive (HRSD) symptoms (which also differed by group) for positive urgency (PUM) were not significant. Thus, the best-fitting model suggested that individual differences in PUM could statistically account for the observed association of NAcc gain anticipation activity with bipolar diagnosis (Fig. 3). Identical mediation analyses further indicated that individual differences in PUM could statistically account for the association of NAcc gain anticipation activity with bipolar diagnosis, even after excluding participants on atypical antipsychotics. The direct path of NAcc gain anticipation to bipolar diagnosis was significant (Z=−2.31; p=.021), as were the indirect paths from NAcc gain anticipation to PUM (Z=−1.84; p=.066) and from PUM to bipolar diagnosis (Z = 5.72; p=.000). Including the indirect path statistically mediated the influence of the direct path (Z=−1.39, p=.163), again suggesting that individual differences in PUM could account for the association of NAcc gain anticipation with bipolar diagnosis, even after excluding individuals on atypical antipsychotics.
Fig. 3.
Statistical mediation of association of Nucleus Accumbens activity with bipolar versus control group status by individual differences in positive urgency (Z-scores; n = 48; **p<.01, ***p<.001).
Finally, supplementary whole-brain analyses confirmed this study's focus on NAcc activity during gain anticipation, since an exploratory regression of the gain anticipation contrast against individual differences in PUM across both groups revealed peak associations in the NAcc (Table S3).
3. Discussion
The goal of this study was to examine neural responses to monetary incentives in individuals diagnosed with Bipolar Disorder. Although extensive behavioral research has implicated altered reward processing in Bipolar Disorder, neuroimaging findings have proven less consistent. For instance, investigators have reported increases, decreases, and no significant difference in ventral striatal activity of bipolar versus control participants during reward anticipation (see Table 1). In the present study – somewhat surprisingly – participants with bipolar disorder showed reduced rather than increased activity in the ventral striatum (including the NAcc) during anticipation of monetary gains relative to controls. Bipolar and control participants did not significantly differ in their neural responses during anticipation of losses or in response to incentive outcomes. Blunted NAcc activity was also more pronounced in individuals who reported more impulsive responses to positive emotion (PUM) – which could statistically account for the association of blunted NAcc activity during reward anticipation with bipolar diagnosis. Analyses of other conditions revealed few other diagnostic group differences, suggesting a relatively specific distinction between diagnostic groups with respect to blunted ventral striatal activity during gain anticipation.
This study is novel in its integration of bipolar diagnostic status with individual differences in Positive Urgency (PUM). The findings associate for the first time a behavioral measure of PUM, which indexes a tendency to engage in regrettable behavior during positive emotion states, with neural anomalies in reward processing. Beyond a diagnostic association with remitted bipolar disorder, PUM scores have been associated with lower behavioral functioning, decreased quality of life, and more severe outcomes (such as suicidality and aggression). The current findings imply that in individuals with bipolar disorder, individual differences in PUM may also help account for blunted NAcc activity during reward anticipation. Thus, these findings might help to elucidate a physiological basis for the behavioral correlates of PUM. The emphasis on individual differences in symptom profiles is also consistent with recent efforts (e.g., by the National Institute of Mental Health Research Domain Criteria or RDoC initiative) to characterize biopsychological dimensions that underlie psychiatric disorders (Cuthbert, 2015). A future goal in might involve extending these probes to other disorders involving blunted neural responses to conventional rewards and impulsive behavior, such as addictions (Riley et al., 2016; Stojek and Fischer, 2013).
Beyond positive urgency, other individual difference variables might also help to explain inconsistent findings across studies of bipolar disorder. Although individuals taking first-generation antipsychotics were excluded from this study, those taking second-generation antipsychotics showed modestly diminished neural responses during gain anticipation. Thus, medications that influence dopamine function may modulate neuroimaging signals related to reward (Ferenczi et al., 2016). These medication effects are not likely to explain all of the reward-related abnormalities observed in this study, however, since other researchers have documented blunted dorsal striatal activity during gain anticipation even in medication-naïve bipolar patients (Yip et al., 2015). Further, even after excluding individuals taking second-generation antipsychotics in supplemental analyses in the current study, blunted neural responses during gain anticipation were still evident in the bipolar group.
The current study features some strengths relative to previous studies of neural responses to gain anticipation in bipolar disorder, including an adequately-powered sample size, stratification based on anxiety and substance abuse diagnoses, a control group that was matched on anxiety and substance abuse disorders, exclusion of individuals who were taking first-generation neuroleptic medications, longitudinal assessment to ensure remission of bipolar symptoms, and specific analyses of individual differences in neural responsiveness to incentives. Groups were also well-matched in their ability to learn and perform the Monetary Incentive Delay task, which featured parametric manipulation of the magnitude of both gains and losses. Although different subgroups within a clinical sample might show distinct behavioral and neural profiles even while performing the same task (Misaki et al., 2016), statistical comparisons revealed no evidence of increased variability in the behavioral or neural responses of the bipolar versus control groups in this study (Table 2).
The study also has some limitations. First, while adequately powered to detect group differences in neural responses to incentives, this study was not designed to detect small effects or interactions in the variables of interest. Second, some selection criteria (e.g., absence of claustrophobia or cardiovascular conditions) might covary with a bipolar diagnosis. For example, as many as half of participants with a bipolar I disorder diagnosis typically meet criteria for simple phobias (such as claustrophobia; Merikangas et al., 2007), and bipolar disorder has been related to a two-fold increase in risk of cardiovascular disease relative to the general population (Weiner et al., 2011). Finally, the sample recruited for this study was not structured to assess whether neural responses to incentives might fluctuate with episode status, which could provide additional information for understanding dynamic processes underlying bipolar disorder (Linke et al., 2012; Nusslock et al., 2012; O'Sullivan et al., 2011; Urošević et al., 2016).
Despite these limitations, analyses revealed a clear directional bias in neural responses to anticipated monetary gain in bipolar disorder. Consistent with some, but not all, previous findings (O'Sullivan et al., 2011; Schreiter et al., 2016; Yip et al., 2015), both volume of interest and whole-brain analyses indicated that relative to controls, individuals with bipolar disorder showed blunted NAcc activity during gain anticipation. These results might seem surprising, given that reviews of the literature have reached opposite conclusions (Nusslock et al., 2012; Whitton et al., 2015). Nonetheless, our initial qualitative review of existing findings suggests that striatal blunting was consistent with more recent findings using the MID task (Schreiter et al., 2016), and which included studies of unmedicated individuals diagnosed with milder forms of bipolar disorder (Yip et al., 2015). Blunted ventral striatal responses during gain anticipation have been observed in other psychiatric conditions characterized by abnormal reward processing including externalizing syndromes in adolescents (Gatzke-Kopp et al., 2009), attention deficit hyperactivity disorder (Scheres et al., 2007; Ströhle et al., 2008), stimulant use (Schouw et al., 2013), and alcohol abuse (Beck et al., 2009). In the present study, the effect sizes of blunted NAcc activity during reward anticipation in the bipolar versus control group (d's∼.90; Fig. 2) were comparable to effect sizes identified in meta-analyses of similar neuroimaging studies of schizophrenia (d's∼.70; Radua et al., 2015), attention deficit hyperactivity disorder (d's∼.50; Plichta and Scheres, 2014), and substance use disorder (d's∼.20; Balodis et al., 2016; Luijten et al., 2017). Blunted NAcc activity during reward anticipation might also play a role in anhedonic symptoms related to unipolar depression (Hägele et al., 2015; Knutson and Heinz, 2015), but it is not clear whether this blunted activity occurs during reward anticipation or in response to reward outcomes (Knutson et al., 2008; Pizzagalli et al., 2009) and sufficient research has not yet accumulated to support quantitative meta-analysis. Blunted neural anticipation of reward might provoke attempts to compensate with increased reward-seeking behavior (Robbins and Everitt, 1999), which could manifest as individual differences in the tendency to respond impulsively to positive emotional states (as indexed by the PUM), and may foreshadow future problems involving impulsive behavior (e.g., problematic drug use in adolescents, (Büchel et al., 2017)).
Together, these findings suggest that individuals with remitted bipolar disorder show blunted ventral striatal responses during anticipation of conventional rewards (e.g., money). In future research, methodological details related to task type and timing (e.g., incentive anticipation versus outcome) may moderate the sensitivity of neural probes. For instance, the existing literature suggests that in individuals with bipolar disorder, ventral striatal activity is blunted in studies that use the MID task, but possibly potentiated in studies using card-guessing tasks (though fewer of these studies exist; see Table 1). Thus, distinguishing and comparing neural responses to gain anticipation and outcomes might enhance the consistency and generality of findings. Future clinical applications might also involve correlating neural responses to incentives with externally valid behaviors and symptom profiles. By better delineating conceptual extensions and methodological boundaries, neuroimaging findings may yield the most informative and reliable markers of psychiatric symptoms that underlie bipolar disorder.
Archive
Scripts and data relevant to this manuscript can be found at osf.io, and whole brain unthresholded statistical maps are available at neurovault.org.
Financial disclosures
The authors have no conflicts of interest to declare.
Acknowledgment
This study was supported by NIMH Grant RO1 076021 to Sheri L. Johnson. We thank Sarah Victor, Meggy Wang, and Daniel J. Yoo for their assistance in conducting this study, as well as Ben Rosenberg, Tara Srirangarajan, and two anonymous reviewers for feedback on previous drafts.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102018.
Appendix. Supplementary materials
References
- Abler B., Greenhouse I., Ongur D., Walter H., Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy L.B., Abramson L.Y., Walshaw P.D., Gerstein R.K., Keyser J.D., Whitehouse W.G., Urosevic S., Nusslock R., Hogan M.E., Harmon-Jones E. Behavioral approach system (BAS)-relevant cognitive styles and bipolar spectrum disorders: concurrent and prospective associations. J. Abnorm. Psychol. 2009;118:459–471. doi: 10.1037/a0016604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy L.B., Bender R.E., Wagner C.A., Whitehouse W.G., Abramson L.Y., Hogan M.E., Sylvia L.G., Harmon-Jones E. Bipolar spectrum-substance use co-occurrence: behavioral approach system (BAS) sensitivity and impulsiveness as shared personality vulnerabilities. J. Pers. Soc. Psychol. 2009;97:549–565. doi: 10.1037/a0016061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy L.B., Urošević S., Abramson L.Y., Jager-Hyman S., Nusslock R., Whitehouse W.G., Hogan M. Progression along the bipolar spectrum: a longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. J. Abnorm. Psychol. 2012;121:16–27. doi: 10.1037/a0023973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst F., Stassen H.H., Clayton P.J., Angst J. Mortality of patients with mood disorders: follow-up over 34-38 years. J. Affect. Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- Applegate E., El-Deredy W., Bentall R.P. Reward responsiveness in psychosis-prone groups: hypomania and negative schizotypy. Pers. Individ. Dif. 2009;47:452–456. [Google Scholar]
- Ashok A.H., Marques T.R., Jauhar S., Nour M.M., Goodwin G.M., Young A.H., Howes O.D. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol. Psychiatry. 2017;22:666–679. doi: 10.1038/mp.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis I.M., Kober H., Worhunsky P.D., Stevens M.C., Pearlson G.D., Carroll K.M., Potenza M.N. Neurofunctional reward processing changes in cocaine dependence during recovery. Neuropsychopharmacology. 2016;71:743–757. doi: 10.1038/npp.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebko G., Bertocci M.A., Fournier J.C., Hinze A.K., Bonar L., Almeida J.R.C., Perlman S.B., Versace A., Schirda C., Travis M., Gill M.K., Demeter C., Diwadkar V.A., Ciuffetelli G., Rodriguez E., Olino T., Forbes E., Sunshine J.L., Holland S.K., Kowatch R.A., Birmaher B., Axelson D., Horwitz S.M., Eugene Arnold L., Fristad M.A., Youngstrom E.A., Findling R.L., Phillips M.L. Parsing dimensional vs diagnostic category-related patterns of reward circuitry function in behaviorally and emotionally dysregulated youth in the longitudinal assessment of manic symptoms study. JAMA Psychiatry. 2014;71:71–80. doi: 10.1001/jamapsychiatry.2013.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech P., Bolwig T.G., Kramp P., Rafaelsen O.J. The Bech-Rafaelsen Mania Scale and the Hamilton Depression Scale. Acta Psychiatr. Scand. 1979;59:420–430. doi: 10.1111/j.1600-0447.1979.tb04484.x. [DOI] [PubMed] [Google Scholar]
- Beck A., Schlagenhauf F., Wüstenberg T., Hein J., Kienast T., Kahnt T., Schmack K., Hägele C., Knutson B., Heinz A., Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Berghorst L.H., Kumar P., Greve D.N., Deckersbach T., Ongur D., Dutra S.J., Pizzagalli D.A. Stress and reward processing in bipolar disorder: a functional magnetic resonance imaging study. Bipolar Disord. 2016;18:602–611. doi: 10.1111/bdi.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl F., Kahnt T., Dalanay U., Hägele C., Sajonz B., Wegner T., Stoy M., Adli M., Krüger S., Wrase J., Ströhle A., Bauer M., Heinz A. Altered representation of expected value in the orbitofrontal cortex in Mania. Hum. Brain Mapp. 2010;31:958–969. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhugra D., Popelyuk D., McMullen I. Paraphilias across cultures: contexts and controversies. J. Sex. Res. 2010;47:242–256. doi: 10.1080/00224491003699833. [DOI] [PubMed] [Google Scholar]
- Büchel C., Peters J., Banaschewski T., Bokde A.L.W., Bromberg U., Conrod P.J., Flor H., Papadopoulos D., Garavan H., Gowland P., Heinz A., Walter H., Ittermann B., Mann K., Martinot J.L., Paillère-Martinot M.L., Nees F., Paus T., Pausova Z., Poustka L., Rietschel M., Robbins T.W., Smolka M.N., Gallinat J., Schumann G., Knutson B. Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nat. Commun. 2017;8:14140. doi: 10.1038/ncomms14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho J., Nobre P. Biopsychosocial determinants of men's sexual desire: testing an integrative model. J. Sex. Med. 2011;8:754–763. doi: 10.1111/j.1743-6109.2010.02156.x. [DOI] [PubMed] [Google Scholar]
- Carver C., White T. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment. J. Pers. Soc. Psychol. 1994;67:319–333. [Google Scholar]
- Carver C.S., Johnson S.L., Joormann J. Major depressive disorder and impulsive reactivity to emotion: toward a dual-process view of depression. Br. J. Clin. Psychol. 2013;52:285–299. doi: 10.1111/bjc.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X., Lawrence N.S., Murphy K., Wise R.G., Phillips M.L. Ventral striatum activity in response to reward: differences between bipolar i and ii disorders. Am. J. Psychiatry. 2013;170:533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Glover G.H. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H.W., Fournier J.C., Bertocci M.A., Greenberg T., Aslam H., Stiffler R., Lockovich J., Graur S., Bebko G., Forbes E.E., Phillips M.L. A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Transl. Psychiatry. 2017;7:e1096. doi: 10.1038/tp.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H.W., Nusslock R., Almeida J.R., Forbes E.E., Labarbara E.J., Phillips M.L. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15:839–854. doi: 10.1111/bdi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.D., Perlstein W.M., Braver T.S., Nystrom L.E., Noll D.C., Jonides J., Smith E.E. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Cousins D., Butts K., Young A. The role of dopamine in bipolar disorder. Bipolar Disord. 2009;11:787–806. doi: 10.1111/j.1399-5618.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N. Research domain criteria: toward future psychiatric nosologies. Dialog. Clin. Neurosci. 2015;17:89–97. doi: 10.31887/DCNS.2015.17.1/bcuthbert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders M.A., Dzemidzic M., Eiler W.J., Coskunpinar A., Karyadi K., Kareken D.A. Negative urgency and ventromedial prefrontal cortex responses to alcohol cues: FMRI evidence of emotion-based impulsivity. Alcohol. Clin. Exp. Res. 2014, 409-417;38 doi: 10.1111/acer.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders M.A., Smith G.T. Emotion-Based dispositions to rash action: positive and negative urgency. Psychol. Bull. 2008;134:807–828. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders M.A., Smith G.T. Mood-based rash action and its components: positive and negative urgency. Pers. Individ. Dif. 2007;43:839–850. [Google Scholar]
- Cyders M.A., Smith G.T., Spillane N.S., Fischer S., Annus A.M., Peterson C. Integration of impulsivity and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychol. Assess. 2007;19:107–118. doi: 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- Cyders M.A., Zapolski T.C.B., Combs J.L., Settles R.F., Fillmore M.T., Smith G.T. Experimental effect of positive urgency on negative outcomes from risk taking and on increased alcohol consumption. Psychol. Addict. Behav. 2010;24:367–375. doi: 10.1037/a0019494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino F., Valente G., Staeren N., Ashburner J., Goebel R., Formisano E. Combining multivariate voxel selection and support vector machines for mapping and classification of fMRI spatial patterns. Neuroimage. 2008;43:44–58. doi: 10.1016/j.neuroimage.2008.06.037. [DOI] [PubMed] [Google Scholar]
- Depue R.A., Iacono W.G. Neurobehavioral aspects of affective disorders. Annu. Rev. Psychol. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Depue R.A., Arbisi P., Spoont M.R., Leon A. Seasonal Affective Disorders and Phototherapy. 1989. Dopamine functioning in the behavioral facilitation system and seasonal variation in behavior: normal population and clinical studies; pp. 230–259. [Google Scholar]
- Dutra S.J., Man V., Kober H., Cunningham W.A., Gruber J. Disrupted cortico-limbic connectivity during reward processing in remitted bipolar I disorder. Bipolar Disord. 2017;19:661–675. doi: 10.1111/bdi.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi E.A., Zalocusky K.A., Liston C., Grosenick L., Warden M.R., Amatya D., Katovich K., Mehta H., Patenaude B., Ramakrishnan C., Kalanithi P., Etkin A., Knutson B., Glover G.H., Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science (80-. ) 2016:351. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M.B., Spitzer, M.B., Gibbon, M., Williams, J.B.W., 1995. Structured Clinical Interview for DSM-IV Axis I Disorders Patient Edition (SCID I/P, Version 2.0), Biometrics Research.
- Fowles D.C. Psychophysiology and psychopathology: a motivational approach. Psychophysiology. 1988;25:373–391. doi: 10.1111/j.1469-8986.1988.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Fulford D., Johnson S.L., Carver C.S. Commonalities and differences in characteristics of persons at risk for narcissism and mania. J. Res. Pers. 2008;42:1427–1438. doi: 10.1016/j.jrp.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp L.M., Beauchaine T.P., Shannon K.E., Chipman J., Fleming A.P., Crowell S.E., Liang O., Johnson L.C., Aylward E. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. J. Abnorm. Psychol. 2009;118:203–213. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli A., Hoerger M., Johnson S.L., Gruber J. Impulsive responses to positive mood and reward are related to mania risk. Cogn. Emot. 2013;27:1091–1104. doi: 10.1080/02699931.2013.772048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover G.H., Law C.S. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn. Reson. Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Hägele C., Schlagenhauf F., Rapp M., Sterzer P., Beck A., Bermpohl F., Stoy M., Ströhle A., Wittchen H.U., Dolan R.J., Heinz A. Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology (Berl) 2015;232:331–341. doi: 10.1007/s00213-014-3662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E., Abramson L.Y., Nusslock R., Sigelman J.D., Urosevic S., Turonie L.D., Alloy L.B., Fearn M. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biol. Psychiatry. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Abramson L.Y., Sigelman J., Bohlig A., Hogan M.E., Harmon-Jones C. Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. J. Pers. Soc. Psychol. 2002;82:610–618. [PubMed] [Google Scholar]
- Hayden E.P., Bodkins M., Brenner C., Shekhar A., Nurnberger J.I., O'Donnell B.F., Hetrick W.P. A multimethod investigation of the behavioral activation system in bipolar disorder. J. Abnorm. Psychol. 2008;117:164–170. doi: 10.1037/0021-843X.117.1.164. [DOI] [PubMed] [Google Scholar]
- Herbenick D., Reece M., Schick V., Sanders S.A., Dodge B., Fortenberry J.D. Sexual behavior in the United States: results from a national probability sample of men and women ages 14-94. J. Sex. Med. 2010;7:255–265. doi: 10.1111/j.1743-6109.2010.02012.x. [DOI] [PubMed] [Google Scholar]
- Johnson S.L., Carver C.S. Emotion-relevant impulsivity predicts sustained anger and aggression after remission in bipolar I disorder. J. Affect. Disord. 2016;189:169–175. doi: 10.1016/j.jad.2015.07.050. [DOI] [PubMed] [Google Scholar]
- Johnson S.L., Carver C.S. Extreme goal setting and vulnerability to mania among undiagnosed young adults. Cognit. Ther. Res. 2006;30:377–395. doi: 10.1007/s10608-006-9044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.L., Carver C.S., Gotlib I.H. Elevated ambitions for fame among persons diagnosed with bipolar I disorder. J. Abnorm. Psychol. 2012;121:602–609. doi: 10.1037/a0026370. [DOI] [PubMed] [Google Scholar]
- Johnson S.L., Carver C.S., Mulé S., Joormann J. Impulsivity and risk for mania: towards greater specificity. Psychol. Psychother. Theory, Res. Pract. 2013;86:401–412. doi: 10.1111/j.2044-8341.2012.02078.x. [DOI] [PubMed] [Google Scholar]
- Johnson S.L., Carver C.S., Tharp J.A. Suicidality in bipolar disorder: the role of emotion-triggered impulsivity. Suicide Life-Threaten. Behav. 2017;47:177–192. doi: 10.1111/sltb.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S.L., Edge, M.D., Holmes, M.K., Carver, C.S., 2012b. The behavioral activation system and mania. Ann. Rev. Clin. Psychol., 8, 2012, 243-267, ssrn. doi: 10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed]
- Johnson S.L., Eisner L.R., Carver C.S. Elevated expectancies among persons diagnosed with bipolar disorder. Br. J. Clin. Psychol. 2009;48:217–222. doi: 10.1348/014466509X414655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G., Schlagenhauf F., Koslowski M., Filonov D., Wüstenberg T., Villringer A., Knutson B., Kienast T., Gallinat J., Wrase J., Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006;29:409–416. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Keitner G.I., Ryan C.E., Miller I.W., Kohn R., Epstein N.B. 12-month outcome of patients with major depression and comorbid psychiatric or medical illness (Compound depression) Am. J. Psychiatry. 1991;148:345–350. doi: 10.1176/ajp.148.3.345. [DOI] [PubMed] [Google Scholar]
- Kieseppä T. High concordance of bipolar I disorder in a nationwide sample of twins. Am. J. Psychiatry. 2004;161:1814. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. 20015472 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Bhanji J.P., Cooney R.E., Atlas L.Y., Gotlib I.H. Neural responses to monetary incentives in major depression. Biol. Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Cooper J. Functional magnetic resonance imaging of reward prediction. Curr. Opin. Neurol. 2005;10:59–70. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Bennett S.M., Adams C.M., Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B., Greer S.M. Anticipatory affect: neural correlates and consequences for choice. Philos. Trans. R. Soc. B Biol. Sci. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Heinz A. Probing psychiatric symptoms with the monetary incentive delay task. Biol. Psychiatry. 2015;77:419–420. doi: 10.1016/j.biopsych.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Knutson B., Katovich K., Suri G. Inferring affect from fMRI data. Trends Cogn. Sci. 2014;18:422–428. doi: 10.1016/j.tics.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Knutson B., Taylor J., Kaufman M., Peterson R., Glover G. Distributed neural representation of expected value. J. Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann B., Scholz V., Linke J., Kirsch P., Wessa M. Reward anticipation revisited- evidence from an fMRI study in euthymic bipolar I patients and healthy first-degree relatives. J. Affect. Disord. 2017;219:178–186. doi: 10.1016/j.jad.2017.04.044. [DOI] [PubMed] [Google Scholar]
- Kundakç, T., Emre, K., 2004. Comorbidity in patients with conversion disorder 2271–2276. [DOI] [PubMed]
- Linke, J., King, A.V., Rietschel, M., Strohmaier, J., Hennerici, M., Gass, A., Meyer-Lindenberg, A., Ph, D.. Increased medial orbitofrontal and amygdala activation: evidence for a systems-level endophenotype of bipolar I disorder Am. J. Psychiatr. 169, 2012, 316–325. [DOI] [PubMed]
- Luijten M., Schellekens A.F., Kühn S., MacHielse M.W.J., Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74:387–398. doi: 10.1001/jamapsychiatry.2016.3084. [DOI] [PubMed] [Google Scholar]
- Mason L., O'Sullivan N., Bentall R.P., El-Deredy W. Better than I thought: positive evaluation bias in hypomania. PLoS ONE. 2012;7:e47754. doi: 10.1371/journal.pone.0047754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L., O'sullivan N., Montaldi D., Bentall R.P., El-Deredy W. Decision-making and trait impulsivity in bipolar disorder are associated with reduced prefrontal regulation of striatal reward valuation. Brain. 2014;137:2346–2355. doi: 10.1093/brain/awu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P., Rijsdijk F., Andrew M., Sham P., Katz R., Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch. Gen. Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Merikangas K.R., Akiskal H.S., Angst J., Greenberg P.E., Hirschfeld R.M.A., Petukhova M., Kessler R.C. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Johnson S.L., Carver C.S. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. Pychopathol. J. Psychopathol. Behav. Assess. 1999;21:275–292. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Johnson S.L., Winters R. Responsiveness to threat and incentive in bipolar disorder: relations of the BIS/BAS scales with symptoms. J. Psychopathol. Behav. Assess. 2001;23:133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I.W., Bishop S., Norman W.H., Maddever H. The modified Hamilton rating scale for depression: reliability and validity. Psychiatry Res. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Misaki M., Suzuki H., Savitz J., Drevets W.C., Bodurka J. Individual variations in nucleus accumbens responses associated with major depressive disorder symptoms. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B.E., Van Honk J., Hermans E.J., Scholten M.R.M., Stein D.J., Kahn R.S. Gray's BIS/BAS dimensions in non-comorbid, non-medicated social anxiety disorder. World J. Biol. Psychiatry. 2009;10:925–928. doi: 10.1080/15622970802571695. [DOI] [PubMed] [Google Scholar]
- Muhtadie L., Johnson S.L., Carver C.S., Gotlib I.H., Ketter T.A. A profile approach to impulsivity in bipolar disorder: the key role of strong emotions. Acta Psychiatr. Scand. 2014;129:100–108. doi: 10.1111/acps.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Almeida J.R.C., Forbes E.E., Versace A., Frank E., Labarbara E.J., Klein C.R., Phillips M.L. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Young C.B., Damme K.S.F. Elevated reward-related neural activation as a unique biological marker of bipolar disorder: assessment and treatment implications. Behav. Res. Ther. 2014;62:74–87. doi: 10.1016/j.brat.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan N., Szczepanowski R., El-Deredy W., Mason L., Bentall R.P. FMRI evidence of a relationship between hypomania and both increased goal-sensitivity and positive outcome-expectancy bias. Neuropsychologia. 2011;49:2825–2835. doi: 10.1016/j.neuropsychologia.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Pedregosa F. Michel V., Grisel O., Blondel M., Prettenhofer P., Weiss R., Vanderplas J., Cournapeau D., Pedregosa F., Varoquaux G., Gramfort A., Thirion B., Grisel O., Dubourg V., Passos A., Brucher M., Perrot M., Duchesnay É. Scikit-learn: machine learning. J. Mach. Learn. Res. 2011;12:2825–2830. [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., Goetz E.L., Birk J.L., Bogdan R., Dougherty D.D., Iosifescu D.V., Rauch S.L., Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta M.M., Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston A.R., Thomason M.E., Ochsner K.N., Cooper J.C., Glover G.H. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. Neuroimage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J., Schmidt A., Borgwardt S., Heinz A., Schlagenhauf F., McGuire P., Fusar-Poli P. Ventral striatal activation during reward processing in psychosis a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72:1243–1251. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- Riley E.N., Rukavina M., Smith G.T. The reciprocal predictive relationship between high-risk personality and drinking: an 8-wave longitudinal study in early adolescents. J. Abnorm. Psychol. 2016;125:798–804. doi: 10.1037/abn0000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W., Everitt B.J. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. lavaan: an R package for structural equation modeling and more. J. Stat. Softw. 2012;48:1–36. [Google Scholar]
- Sacchet M.D., Knutson B. Spatial smoothing systematically biases the localization of reward-related brain activity. Neuroimage. 2012;66:270–277. doi: 10.1016/j.neuroimage.2012.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Kable J.W., Vandekar L., Katchmar N., Bassett D.S., Baldassano C.F., Ruparel K., Elliott M.A., Sheline Y.I., Gur R.C., Gur R.E., Davatzikos C., Leibenluft E., Thase M.E., Wolf D.H. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015;40:2258–2268. doi: 10.1038/npp.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A., Milham M.P., Knutson B., Castellanos F.X. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Schouw M.L.J., De Ruiter M.B., Kaag A.M., van den Brink W., Lindauer R.J.L., Reneman L. Dopaminergic dysfunction in abstinent dexamphetamine users: results from a pharmacological fMRI study using a reward anticipation task and a methylphenidate challenge. Drug Alcohol Depend. 2013;130:52–60. doi: 10.1016/j.drugalcdep.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Schreiter S., Spengler S., Willert A., Mohnke S., Herold D., Erk S., Romanczuk-Seiferth N., Quinlivan E., Hindi-Attar C., Banzhaf C., Wackerhagen C., Romund L., Garbusow M., Stamm T., Heinz A., Walter H., Bermpohl F. Neural alterations of fronto-striatal circuitry during reward anticipation in euthymic bipolar disorder. Psychol. Med. 2016;46:3187–3198. doi: 10.1017/S0033291716001963. [DOI] [PubMed] [Google Scholar]
- Sharma A., Wolf D.H., Ciric R., Kable J.W., Moore T.M., Vandekar S.N., Katchmar N., Daldal A., Ruparel K., Davatzikos C., Elliott M.A., Calkins M.E., Shinohara R.T., Bassett D.S., Satterthwaite T.D. Common dimensional reward deficits across mood and psychotic disorders: a connectome-wide association study. Am. J. Psychiatry. 2017;174:657–666. doi: 10.1176/appi.ajp.2016.16070774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.K., Chang K.D., Kelley R.G., Cui X., Sherdell L., Howe M.E., Gotlib I.H., Reiss A.L. Reward processing in adolescents with bipolar 1 disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:68–83. doi: 10.1016/j.jaac.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojek M., Fischer S. Impulsivity and motivations to consume alcohol: a prospective study on risk of dependence in young adult women. Alcohol. Clin. Exp. Res. 2013;37:292–299. doi: 10.1111/j.1530-0277.2012.01875.x. [DOI] [PubMed] [Google Scholar]
- Ströhle A., Stoy M., Wrase J., Schwarzer S., Schlagenhauf F., Huss M., Hein J., Nedderhut A., Neumann B., Gregor A., Juckel G., Knutson B., Lehmkuhl U., Bauer M., Heinz A. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39:966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Sutton S.K. Hypomanic tendencies predict lower startle magnitudes during pleasant pictures. Psychophysiology. 2002;39(suppl.):S80. [Google Scholar]
- Tervo-Clemmens B., Quach A., Luna B., Foran W., Chung T., De Bellis M.D., Clark D.B. Neural correlates of rewarded response inhibition in youth at risk for problematic alcohol use. Front. Behav. Neurosci. 2017;11:205. doi: 10.3389/fnbeh.2017.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost S., Diekhof E.K., Zvonik K., Lewandowski M., Usher J., Keil M., Zilles D., Falkai P., Dechent P., Gruber O. Disturbed anterior prefrontal control of the mesolimbic reward system and increased impulsivity in bipolar disorder. Neuropsychopharmacology. 2014;39:1914–1923. doi: 10.1038/npp.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S., Luciana M., Jensen J.B., Youngstrom E.A., Thomas K.M. Age associations with neural processing of reward anticipation in adolescents with bipolar disorders. NeuroImage Clin. 2016;11:476–485. doi: 10.1016/j.nicl.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor S.E., Johnson S.L., Gotlib I.H. Quality of life and impulsivity in bipolar disorder. Bipolar Disord. 2011;13:303–309. doi: 10.1111/j.1399-5618.2011.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M., Warren L., Fiedorowicz J.G. Cardiovascular morbidity and mortality in bipolar disorder. Ann. Clin. Psychiatry. 2011;23:40–47. [PMC free article] [PubMed] [Google Scholar]
- Whitton A.E., Treadway M.T., Pizzagalli D.A. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.C., Samanez-Larkin G.R., Katovich K., Knutson B. Affective traits link to reliable neural markers of incentive anticipation. Neuroimage. 2014;84:279–289. doi: 10.1016/j.neuroimage.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S.W., Worhunsky P.D., Rogers R.D., Goodwin G.M. Hypoactivation of the ventral and dorsal striatum during reward and loss anticipation in antipsychotic and mood stabilizer-naive bipolar disorder. Neuropsychopharmacology. 2015;40:658–666. doi: 10.1038/npp.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.