Abstract
Stage III non-small cell lung cancer (NSCLC) has a dismal prognosis, with only 15–20% of patients alive at 5 years after concomitant chemo–radiotherapy, which represents the standard treatment. Targeting immune-checkpoint inhibitors represents a standard option for advanced NSCLC. Improvements in understanding of the immune profile of NSCLC has led to the development of immunotherapeutic strategies, including inhibitory molecules responsible for abrogating an anticancer immune response such as programmed cell-death 1 and programmed cell-death ligand 1. A recently published phase III trial (PACIFIC) showed for the first time an improved overall survival in stage III NSCLC patients with consolidative durvalumab.
The aim of this review is to summarize and discuss the clinical evidence for the use of durvalumab in stage III NSCLC, with a brief overview on future perspectives in this setting.
Keywords: chemo–radiation, consolidation, durvalumab, NSCLC, stage III
Introduction
Lung cancer represents the leading cause of cancer death worldwide.1 Despite advances in diagnostic techniques over recent years, one third of patients are still diagnosed with locally advanced disease,2 for which platinum-based doublet concurrent chemo–radiotherapy (CRT) has become the first-choice treatment over the past decade.3–5 However, prognosis is still dismal, as median overall survival (OS) ranges between 17 and 28.7 months3,6 and only about 15–20% of patients are alive at 5 years.3,4 These survival data remain approximately the same with the addition of surgery.4
In recently published phase III trials with modern radiotherapy (RT) and positron emission tomography–computed tomography (PET-CT) for staging, the local tumour progression rates were about 30–40% and distant progression was detected in half of the patients.6,7
New approaches that preferentially tackle both local and distant disease sites are needed to improve long-term survival and cure rates.
Attempts to improve the long-term survival include RT dose escalation,6 different chemotherapy combinations8 and adding biological agents to standard regimens.6 At present, none of these have demonstrated solid improved outcomes.
Targeting immune-checkpoint inhibitors (ICIs) represents a standard option for advanced non-small cell lung cancer (NSCLC).9,10
Improved understanding of the immune profile of NSCLC has led to immunotherapeutic strategies, including inhibitory molecules responsible for abrogating an anticancer immune response such as programmed cell-death 1 (PD-1) and programmed cell-death ligand 1 (PD-L1).
To date, nivolumab, pembrolizumab and atezolizumab have been approved by the US Food and Drug Administration (FDA)11 and European Medicines Agency (EMA)12 as a treatment option for pretreated patients with advanced NSCLC. Pembrolizumab is also licensed as first-line treatment for treatment-naïve metastatic NSCLC patients with PD-L1 expression ⩾ 50% and for metastatic NSCLC with ⩾1% PDL1 expression after progression following first-line platinum-based doublet chemotherapy.9 In addition and more recently, pembrolizumab has received the authorization by both agencies, in combination with platinum-based chemotherapy, in previously untreated advanced NSCLC regardless of the PD-L1 status, becoming the standard of care in this setting.10
These results might address the current poor prognosis of stage III NSCLC by providing newer treatment paradigms that incorporate immunotherapy.
Durvalumab (MEDI4736), a novel ICI, particularly as an anti-PD-L1 antibody, inhibits binding of PD-L1 to PD-1 and B7-1, thus promoting the ability of T cells to recognize and eliminate tumour cells. Antonia and colleagues reported a longer progression-free survival (PFS) with durvalumab as consolidation therapy compared with placebo in stage III NSCLC patients who did not have disease progression after two or more cycles of platinum-based CRT.13
On the basis of these data, durvalumab received FDA approval as consolidation chemotherapy in stage III disease after CRT in February 2018, the first such case in the use of ICIs.11 In September 2018, the EMA approved durvalumab as consolidation therapy in stage III disease after CRT, only if PD-L1 is expressed in ⩾1% of tumour cells.12
The aim of this review is to summarize and discuss the clinical evidence for the use of durvalumab in stage III NSCLC, with a brief overview on future perspectives in this setting.
Mechanism of action
The PD-1 receptor is an immune-checkpoint protein expressed on activated T cells (after antigen exposure) with two corresponding ligands, PD-L1 and PD-L2. PD-L1 (also named B7-H1 and CD274) is a transmembrane protein, expressed in haematopoietic cells, tumour cells and tumour stroma.13 PD-L1 can bind to either the PD-1 receptor or to CD80 (also named B7-1, expressed on activated T cells and on antigen-presenting cells (APCs). Interactions between PD-L1 and PD-1/CD-80 can lead to inhibition of cytotoxic T-cell activation.14,15
The expression of PD-L2 is less common in cancer, and regulates the priming and the polarization of T cells.16 To date, there is no evidence indicating that antibodies against PD-1, which block binding to both PD-L1 and PD-L2, are more effective when compared with antibodies against PD-L1 only.14
Durvalumab (MEDI4736) is fully human high-affinity immunoglobulin G1-kappa (IgG1κ) monoclonal antibody that blocks PD-L1 by binding to PD-1 (IC50 0.1 nmol/l) and CD-80 (IC50 0.04 nmol/l).15 Durvalumab binds with a high affinity to PD-L1 but not to PD-L2, the latter playing a role in controlling inflammation in normal tissue; this mechanism of action potentially decreases the immune-related toxicity associated with the PD-L2 interaction.
Efficacy
Durvalumab in the metastatic disease
Durvalumab was first evaluated in a large phase I/II study on patients with an advanced solid tumour, including refractory advanced NSCLC patients [ClinicalTrials.gov identifier: NCT01693562].16 Patients received durvalumab intravenously (IV) from 0.1 to 10 mg/kg every 2 weeks (Q2W) or 15 mg/kg every 3 weeks for up to 12 months or until unacceptable toxicity or disease progression. A total of 304 patients received durvalumab 10 mg/kg Q2W,16 with an overall response rate (ORR) of 17.5%. Higher response rates were reported in treatment-naïve patients (ORR of 27.1% versus 13.0% in heavily pretreated patients) and when PD-L1 expression was high (⩾25% PD-L), with ORR of 25.3% versus 6.1% in PD-L1 < 25% (low).
A subsequent study from Antonia and colleagues17 reported results from 59 treatment-naïve NSCLC patients, showing an ORR of 29% in patients with high PD-L1 expression versus 11% in low PD-L1 patients.
Similar results were shown in the ATLANTIC trial [ClinicalTrials.gov identifier: NCT02087423],18 where the patients with higher PD-L1 expression had better OS. The 1-year OS rate was 50.8% for very high PD-L1 (⩾90% PD-L1), 47.7% for high PD-L1 and 34.5% in low PD-L1 populations.
However, AstraZeneca has reported an update of the large randomized phase III MYSTIC study on 16 November 2018, where durvalumab as monotherapy and in combination with tremelimumab (an ICI anti-CTL-4) did not meet the primary endpoints of improving OS and PFS compared with chemotherapy in patients with ⩾25% PD-L1 (determined by Ventana assay, SP263). Few phase III trials, such as the PEARL, POSEIDON and NEPTUNE studies [ClinicalTrials.gov identifiers: NCT03003962, NCT03164616 and NCT02542293, respectively], evaluating the role of durvalumab in monotherapy or in combination with tremelimumab as first-line treatment in advanced NSCLC patients, are currently ongoing.
Durvalumab in stage III NSCLC
Durvalumab and radiotherapy: preclinical evidence
Preclinical data consistently show a clear beneficial and possibly synergistic effect when radiotherapy is combined with anti-PD-1.17,19,20
During the development of cancer, the relationship between the tumour and the host immune system evolves from one in which the tumour cells are recognized and destroyed by the immune system (immune elimination) to immune equilibrium, where tumour cells and immune system coexist, and finally to immune escape.21 The immune-escape stage is characterized by upregulated inhibitory ligands and cytokines and reduced major histocompatibility complex (MHC) class I expression, which ultimately causes poor antigen presentation and masks the tumour from immune surveillance and elimination.21 Radiation may ‘unmask’ the tumour and make it more visible to both the innate and adaptive immune systems through the activation of downstream immune responses and priming of T cells,22 and the upregulation of the expression of MHC-I on the tumour surface to enable better presentation of tumour-specific peptides (which enhances the visibility of the tumour to cytotoxic T cells).23
By inducing the antigen recognition, radiation might also induce the T-cell-mediated inhibition of untreated distant tumours (known as the abscopal effect).24 Moreover, radiation-induced deoxyribonucleic acid damage may generate neoantigen and trigger the immune surveillance.25
Since different types of ICIs target different pathways, the timing of the ICI–RT combination should be designed to maximize the potential synergistic effect.
Unfortunately, the paucity of data does not allow drawing of firm conclusions.
The secondary analysis of the KEYNOTE-001 trial [ClinicalTrials.gov identifier: NCT01295827] showed the NSCLC patients who received radiotherapy before pembrolizumab had better OS and PFS rates compared with the patients who did not receive radiotherapy, suggesting radiation may enhance the efficacy of immunotherapy.26
Qian and colleagues showed that, in a cohort of 75 melanoma patients with 566 brain metastases treated with stereotactic radiosurgery (SRS) and ICI (anti-CTL-4 and anti-PD-1/PDL-1), the concomitant use of ICI and SRS resulted in a higher median volume reduction 45 days (63.1% versus −43.2%, p < 0.0001), 3 months (−83.0% versus −52.8%, p < 0.0001), and 6 months (−94.9% versus −66.2%, p < 0.0001) when compared with nonconcurrent therapy.27
A recent retrospective analysis of 758 patients treated with ICI (anti-CTLA4 with or without anti-PD1/anti-PDL1) and RT suggested that OS was better for patients who received concurrent ICI and RT, especially when ICIs were started within 30 days before RT [median OS: 20 months (<30 days) versus 11 months (>30 days)].28
Moreover, the post hoc analysis of the PACIFIC trial suggests that starting the durvalumab within 14 days after CRT (rather than ⩾14 days) is associated with a higher benefit to OS and PFS.29
Clinical evidence: durvalumab efficacy
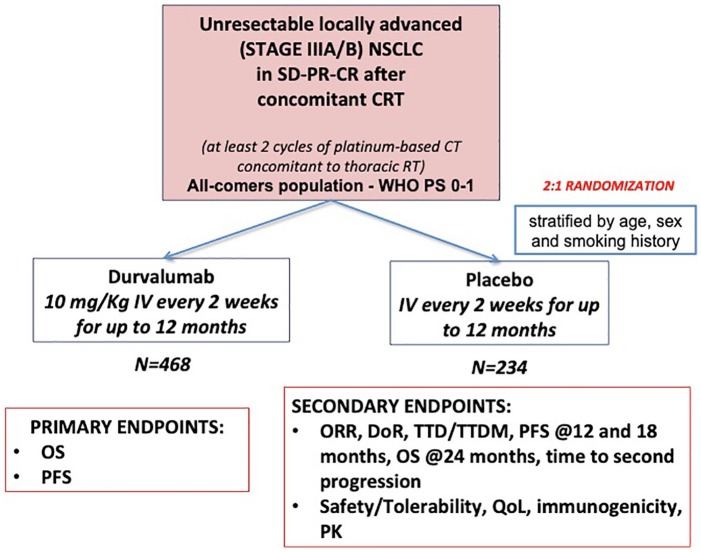
This preclinical evidence and the abovementioned trials on durvalumab in advanced NSCLC form the core of the hypothesis behind the multicentre, randomized double-blind phase III PACIFIC trial [ClinicalTrials.gov identifier: NCT02125461] (Figure 1), which compared durvalumab as consolidation therapy with placebo in patients with stage III, locally advanced, unresectable NSCLC that had not progressed after platinum-based CRT.10
Figure 1.
PACIFIC phase III trial scheme.
CT, chemotherapy; DoR, duration of response; IV, intravenous; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PK, pharmacokinetics, PR, partial response, PS, performance status; QoL, quality of life; CR, complete response; RT, radiotherapy; SD, stable disease; TTD, time to death; TTDM, time to distant metastases; WHO, World Health Organization.
Eligible patients had received two or more cycles of platinum-based chemotherapy concurrently with definitive RT (54–66 Gy), had no disease progression after this treatment and had completed the last radiation dose within 1–14 days before randomization. However, due to a protocol amendment, the delay between the end of RT and the randomization was augmented to 1–42 days.
A total of 713 patients were randomized in a 2:1 ratio, 1–42 days after CRT to receive durvalumab IV at a dose of 10 mg/kg of body weight (n = 473) or placebo (n = 236) Q2W for up to 12 months. Randomization was stratified according to patient’s age (<65 years versus ⩾65 years), sex and smoking history (current or former smoker versus never smoked). OS and PFS were the coprimary endpoints, assessed by blinded independent central review. Secondary endpoints were PFS at 12 and 18 months, ORR, duration of response, the time to death/distant metastasis, OS at 24 months, time to second progression, safety/tolerability, quality of life, immunogenicity and pharmacokinetics. PFS was defined from randomization (which occurred up to 6 weeks after CRT).
Patients provided archived tumour tissue samples, obtained before chemoradiotherapy, for PD-L1 testing (determined by Ventana assay, SP263). However, PD-L1 expression status was not mandatory for the inclusion.
The main results of the PACIFIC trial are summarized in Table 1.
Table 1.
Overview of the results (clinical efficacy) of the PACIFIC study.
| Study | Study design | Treatment scheme | Population | ORR | PFS, median (95% CI) | 12- month OS rate (%) |
24- month OS rate (%) | Median OS (months)# |
|---|---|---|---|---|---|---|---|---|
| PACIFIC–interim analysis on PFS* and OS analysis# | Randomized phase III |
Durvalumab (dose of 10 mg/kg Q2W) for up to 1 year |
n = 476
Stage III after CRT |
28.4%# | 16.8 months (95% CI 13.0–18.1)* | 83.1% (95% CI 79.4–86.2)# | 66.3% (95% CI 61.7–70.4)# | NR (95% CI 34.7 to NR)# |
| Post hoc analysis PD-L1 ⩾ 1% | NR** | 17.8 months (95% CI 16.9 to NR)** | NR** | NR** | NR (95% CI NR to NR)** | |||
| Post hoc analysis PD-L1 < 1% | NR** | 10.7 months (95% CI 7.3 to NR)** | NR** | NR** | NR (95% CI 20.8 to NR)** | |||
| Placebo |
n = 237 Stage III after CRT |
16.0%# | 5.6 months (95% CI 4.6–7.8)* | 75.3% (95% CI 69.2–80.4)# | 55.6% (95% CI 48.9–61.8)# | 28.7 months (95% CI 22.9 to NR)# | ||
| Post hoc analysis PD-L1 ⩾ 1% | NR** | 5.6 months (95% CI 3.6–11)** | NR** | NR** | 29.1 (95% CI 17.7 to NR)** | |||
| Post hoc analysis PD-L1 < 1% | NR** | 5.6 months (95% CI 3.7–10.6)** | NR** | NR** | NR (95% CI 27.3 to NR)** |
The first planned interim analysis showed that median PFS was 16.8 months [95% confidence interval (CI) 13.0–18.1] with durvalumab versus 5.6 months (95% CI 4.6–7.8) with placebo [hazard ratio (HR) 0.52; 95% CI 0.42–0.65; p < 0.001].13 The ORR was 28.4% with durvalumab versus 16% with placebo (p < 0.001). In this primary analysis, the time to second progression or death was longer in the durvalumab group (median 28.3 months versus 17.1 months).
The recently published analysis for the second primary endpoint of OS30 showed a 12-month OS rate of 83.1% (95% CI 79.4–86.2) in the durvalumab group, as compared with 75.3% (95% CI 69.2–80.4) in the placebo group. The 24-month OS rate was 66.3% (95% CI 61.7–70.4) in the durvalumab group, as compared with 55.6% (95% CI 48.9–61.8) in the placebo group (two-sided p = 0.005). Durvalumab significantly prolonged median OS, as compared with placebo (stratified HR 0.68; 99.73% CI 0.47–0.997; p = 0.0025). Median OS was not reached [95% CI 34.7 to not reached (NR)] with durvalumab versus 5.6 months (95% CI 22.9 to NR) with placebo. The updated frequency of new lesions was 22.5% in the durvalumab group and 33.8% in the placebo group, with a lower incidence of new brain metastases in the durvalumab group than in the placebo group (6.3% versus 11.8%). No differences in OS were detected, on the basis of the PD-L1 expression.
On the basis of those results, the FDA (on 16 February 2018) and the EMA (24 September 2018) approved durvalumab for the treatment of unresectable stage III NSCLC without progression after treatment with concurrent CRT.
A post hoc (not preplanned) exploratory analysis based on PD-L1 expression and components of the concurrent CRT was presented at the European Society of Medical Oncology Congress 2018.29 In the group PD-L1 ⩾ 1%, a median PFS of 17.8 months (95% CI 16.9 to NR) was observed with durvalumab versus 5.6 months (95% CI 3.6–11) with placebo (HR 0.46; 95% CI 0.33–0.64). The analysis in the group PD-L1⩽1% showed that median PFS was 10.7 months (95% CI 7.3 to NR) with durvalumab versus 5.6 months (95% CI 3.7–10.6) with placebo (HR 0.73; 95% CI 0.48–1.1).
With regard to OS, the group of PD-L1 ⩾ 1% showed that median OS was NR (95% CI NR to NR) versus 29.1 (95% CI 17.7 to NR) with placebo (HR 0.53; 95% CI 0.36–0.77). This analysis of the group PD-L1 < 1% showed that median OS was NR (95% CI 20.8 to NR) with durvalumab versus NR (95% CI 27.3 to NR) with placebo (HR 1.36; 95% CI 0.79–2.34).
Globally, this post hoc analysis showed a survival advantage in the group PD-L1 ⩾ 1% (consistently with the PFS). However, in the group PD-L1 < 1%, the placebo arm behaved differently (number of events/number of patients (%): 19/58 (32.8%) when compared with the placebo arm in the PD-L1 ⩾ 1%: 45/91 (49.5%). The difference in restricted mean survival time was not significant (−0.6 months; 95% CI −3.4 to 2.3).
The subgroup analyses confirmed the improved PFS and OS with durvalumab regardless of type of chemotherapy, radiation dose used or time from radiation to randomization.
The 3-year OS rates were presented at the American Society of Clinical Oncology (ASCO) meeting 2019.31 Updated OS remained consistent with that previously reported, with the median OS NR (95% CI 38.4 months to NR) with durvalumab versus 29.1 months (95% CI 22.1–35.1) with placebo. The 12-, 24- and 36-month OS rates with durvalumab and placebo were 83.1% versus 74.6%, 66.3% versus 55.3%, and 57.0% versus 43.5%, respectively.
Caution must be used when drawing definitive conclusions on outcomes by PD-L1 status on the basis of post hoc exploratory subgroup analyses. Moreover, the PACIFIC trial was designed to evaluate durvalumab in the intention-to-treat (all comers) population, and PD-L1 testing was not mandatory and status was unknown for 37% of patients. Nevertheless, the EMA decided to approve durvalumab as consolidation treatment exclusively in the positive PD-L1 population.
Clinical evidence: durvalumab tolerability
In the first phase I/II study on durvalumab in metastatic disease, Antonia and colleagues showed that 57% of patients experienced treatment-related adverse events, mostly fatigue (17%), hyporexya (9%) and diarrhoea (9%), with a grade ⩾3 rate of 10% patients that led to discontinuation in 5% of patients.16 Similar results were reported in the ATLANTIC study.17
As expected, the phase III PACIFIC study confirmed the favourable tolerability profile of the durvalumab, even in association with the CRT (Table 2). The authors reported a maximum G3–4 adverse event (AE) rate of 30.5% of the patients in the durvalumab group and 26.1% in the placebo group. The most frequent AEs leading to the discontinuation of the regimen were pneumonitis (in 4.8% of the patients in the durvalumab group and in 2.6% of those in the placebo group), radiation pneumonitis (1.3% in both groups), and pneumonia (in 1.1% and 1.3%, respectively).30
Table 2.
Overview of the results (tolerability) of the PACIFIC study.
| Study | Treatment scheme | All AEs (%) | Most common all grades AEs (%) | G3/4 AEs (%) | Most common G3/4 AEs (%) | Ir-AEs (%) | Discontinuation (%) | Death due to AEs |
|---|---|---|---|---|---|---|---|---|
| PACIFIC OS analysis# | Durvalumab | 96.8% | Cough (35.4%) Pneumonitis or RP* (33.9%) Fatigue (23.8%) Dyspnoea (22.3) |
29.9% | Pneumonia (4.4%) Pneumonitis or RP*(3.4%) Anaemia (2.9%) |
24.2% | 15.4% | 4.4% |
| Placebo | 94.9% | Cough (25.2%) Pneumonitis or RP* (24.8%) Fatigue (20.5%) Dyspnoea (23.9%) |
26.1% | Pneumonia (3.8%) Pneumonitis or RP*(2.6%) Anaemia (3.4%) |
8.1% | 9.8% | 5.6% |
Pneumonitis or radiation pneumonitis was assessed by investigators with subsequent review and adjudication by the study sponsor. In addition, pneumonitis is a grouped term that includes acute interstitial pneumonitis, interstitial lung disease, pneumonitis, and pulmonary fibrosis.
Antonia et al.30
AE, adverse event; Ir, Immune-related; OS, overall survival; RP, radiation pneumonitis.
Ongoing trials and future prospects
Durvalumab has been the first ICI approved in unresectable stage III NSCLC patients as consolidation therapy, but other ICIs are currently being investigated in this patient population.
Durm and colleagues reported at the ASCO 2018 meeting the preliminary data of a phase II, single-arm study of pembrolizumab (PD-1 inhibitor) as a consolidation treatment after concurrent CRT in patients with unresectable stage III NSCLC (Table 3).32 The primary endpoint was time to metastatic disease or death (TMDD), and the secondary endpoints included PFS, OS and toxicity. A total of 93 patients were included. Median TMDD was NR (95% CI 18.7 to NR). The estimated 1-year and 2-year OS rates were 80.5% and 68.7%, respectively. The median PFS was 15.4 months (95% CI 10.4 to NR), which compares favourably with the PACIFIC trial results. Only 5.4% of patients developed a G3–4 pneumonitis, with one pneumonitis-related death.
Table 3.
Overview of the published studies of consolidation immune-checkpoint inhibitors in stage III non-small-cell lung cancer.
| Study | Study design | Treatment scheme | Population | ORR# | PFS, median (95% CI)* | 12-month OS rate (%)# | 24-month OS rate (%)# | Median OS (months)# |
|---|---|---|---|---|---|---|---|---|
| PACIFIC OS analysis# | Randomized phase III |
Durvalumab (dose: 10 mg/kg Q2W) for up to 1 year |
n = 476
Stage III NSCLC after CRT |
28.4% | 16.8 months (95% CI 13.0–18.1) | 83.1% (95% CI 79.4–86.2) | 66.3% (95% CI 61.7–70.4) | NR (95% CI 34.7 to NR) |
| Placebo |
n = 237
Stage III NSCLC after CRT |
16.0% | 5.6 months (95% CI 4.6–7.8) | 75.3% (95% CI 69.2–80.4) | 55.6% (95% CI 48.9–61.8) | 28.7 months (95% CI 22.9 to NR) | ||
| LUN 14-17925 | Phase II | Pembrolizumab (dose: 200 mg IV Q3W) for up to 1 year |
n = 93
Stage III NSCLC after CRT |
NS | 15.4 months (95% CI 10.4 to NR) | 80.5% (95% CI NS) | 68.7% (95% CI NS) | NS |
CI, confidence interval; CRT, chemo–radiotherapy; NR, not reached; NS, not specified; NSCLC, non-small-cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival, Q2W, once every 2 weeks; Q3W, once every 3 weeks.
The ongoing phase II NICOLAS trial [ClinicalTrials.gov identifier: NCT02434081] is currently evaluating the combination of concurrent CRT and nivolumab (PD-1 inhibitor), followed by consolidative nivolumab (12 months or until disease progression), with results expected for 2020.
The impressive results of the PACIFIC clinical trial have led to the design of several clinical trials combining RT with ICI, including a PACIFIC-2 study, where a concomitant CRT plus durvalumab arm will be studied [ClinicalTrials.gov identifier: NCT03519971] (Table 4).
Table 4.
Overview of the ongoing studies of consolidation immune-checkpoint inhibitors in stage III non-small cell lung cancer.
| Study and ClinicalTrials.gov identifier | Study design | Population | Treatment scheme | Primary endpoints | Status |
|---|---|---|---|---|---|
| PACIFIC-2 [NCT03519971] | Randomized phase III |
n = 300 Unresectable stage III NSCLC after CRT |
Concomitant durvalumab with platinum-based CRT | PFS ORR |
Active, recruiting (estimated study completion date: August 2022) |
| SoC (platinum-based CRT + consolidative durvalumab in responding patients) | |||||
| BTCRC-LUN16-081 [NCT03285321] | Phase II |
n = 108 Unresectable stage III NSCLC after CRT |
Consolidation ipilimumab and nivolumab following CRT | PFS | Active, recruiting (estimated study completion date: September 2022) |
| Consolidation nivolumab following CRT | |||||
| NCT02768558 | Phase III | NA Unresectable stage III NSCLC after CRT |
Consolidation nivolumab following CRT (cisplatin and etoposide based) | PFS OS |
Active, not recruiting (estimated study completion date: October 2024) |
| CRT (cisplatin and etoposide based) | |||||
| NICOLAS [NCT02434081] | Phase II |
n = 78 Unresectable stage III NSCLC after CRT |
Consolidation nivolumab following CRT | Grade ⩾ 3 pneumonitis | Active, not recruiting (estimated study completion date: August 2020) |
Hopefully, these ongoing studies will contribute to elucidating the role of the ICI–RT timing (concurrent versus sequential).
CRT, chemo–radiotherapy; ICI, immune-checkpoint inhibitor; NSCLC, non-small-cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; SoC, standard of care.
Conclusion
Durvalumab as consolidation is currently the standard of care after CRT in unresectable stage III NSCLC patients. The impressive results from the PACIFIC trial have demonstrated for the first time that ICI benefit is not limited to advanced disease and are the first practice-changing results in this setting in recent decades. Moreover, the safety profile and tolerability after CRT are favourable.
Long-term data and larger cohorts, together with mirror studies with other ICIs, are eagerly awaited, to confirm the integration of ICI in treatment strategies for unresectable stage III NSCLC patients.
Acknowledgments
The authors thank Sarah MacKenzie, PhD, for English editing.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: AB: nothing to declare.
LM: Consulting, advisory role: Roche Diagnostics. Lectures and educational activities: Bristol-Myers Squibb, Tecnofarma, Roche, AstraZeneca. Travel, accommodations, expenses: Chugai.
DP: Consulting, advisory role or lectures: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche. Honoraria: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche. Clinical trials research: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Pfizer, Roche, Medimmun, Sanofi-Aventis, Taiho Pharma, Novocure, Daiichi Sankyo. Travel, accommodations, expenses: AstraZeneca, Roche, Novartis, prIME Oncology, Pfizer.
ORCID iD: Angela Botticella  https://orcid.org/0000-0002-9652-8744
https://orcid.org/0000-0002-9652-8744
Contributor Information
Angela Botticella, Department of Radiation Oncology, Gustave Roussy, Villejuif, France.
Laura Mezquita, Medical Oncology Department, Gustave Roussy, Villejuif, France.
Cecile Le Pechoux, Department of Radiation Oncology, Gustave Roussy, Villejuif, France.
David Planchard, Head of Thoracic Oncology Group, Medical Oncology Department, Gustave Roussy, 114 Rue Edouard Vaillant, Villejuif 94805, France.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Morgensztern D, Ng SH, Gao F, et al. Trends in stage distribution for patients with non-small. J Thorac Oncol 2010; 5: 29–33. [DOI] [PubMed] [Google Scholar]
- 3. Curran WJ, Paulus R, Langer CJ, et al. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer : randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103: 1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albain KS, Swann RS, Rusch VR, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small cell lung cancer. Lancet 2015; 374: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aupérin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2018; 28. [DOI] [PubMed] [Google Scholar]
- 6. Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small- cell lung cancer (RTOG 0617): a randomised, two-by-two factorial. Lancet Oncol 2016; 95: 222–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Senan S, Brade A, Wang L, et al. PROCLAIM: Randomized phase III trial of pemetrexed- cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2016; 34: 953–962. [DOI] [PubMed] [Google Scholar]
- 8. Santana-davila R, Devisetty K, Szabo A, et al. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small- cell lung cancer: an analysis of veterans health administration data. J Clin Oncol 2015; 33: 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 10. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol 2018; 29(Suppl. 4): iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 11. US Food and Drug Adminstration. FDA expands approval of Imfinzi to reduce the risk of non-small cell lung cancer progressing, https://www.fda.gov/news-events/press-announcements/fda-expands-approval-imfinzi-reduce-risk-non-small-cell-lung-cancer-progressing (2018).
- 12. European Medical Agency. Imfinzi: summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/imfizi-epar-product-information_en.pdf. (2018).
- 13. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 14. Yearley JH, Gibson C, Yu N, et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res 2017; 23: 19–21. [DOI] [PubMed] [Google Scholar]
- 15. Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res 2015; 3: 1052–1062. [DOI] [PubMed] [Google Scholar]
- 16. Antonia SJ, Brahmer JR, Khleif S, et al. Phase 1/2 study of the safety and clinical activity of durvalumab in patients with non-small cell lung cancer (NSCLC). Ann Oncol 2016; 27(Suppl. 6): vi416–vi454. [Google Scholar]
- 17. Antonia SJ, Kim SW, Spira AI, et al. Safety and clinical activity of durvalumab (MEDI4736), an anti-PD-L1 antibody, in treatment-naïve patients with advanced non‒small-cell lung cancer. J Clin Oncol 2016; 34: 9029. [Google Scholar]
- 18. Garassino MC, Cho B, Kim J, et al. Articles durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018; 19: 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124: 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014; 74: 5458–5468. [DOI] [PubMed] [Google Scholar]
- 21. Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013; 123: 2756–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Golden EB, Pellicciotta I, Demaria S, et al. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol 2012; 2: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203: 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58: 862–870. [DOI] [PubMed] [Google Scholar]
- 25. Germano G, Lamba S, Rospo G, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 2017; 552: 116–120. [DOI] [PubMed] [Google Scholar]
- 26. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017; 18: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qian JM, Yu JB, Kluger HM, et al. Timing and type of immune checkpoint therapy affects early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2017; 122: 3051–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samstein R, Rimner A, Barker CA, et al. Combined immune checkpoint blockade and radiation therapy: timing and dose fractionation associated with greatest survival duration among over 750 treated patients. Int J Radiat Oncol Biol Phys 2018; 99: S129–S130. [Google Scholar]
- 29. Faivre-Finn C, Spigel DR, Senan S, et al. Efficacy and safety evaluation based on time from completion of radiotherapy to randomization with durvalumab or placebo in pts from PACIFIC. Ann Oncol 2018; 29(Suppl. 8): viii488–viii492. [Google Scholar]
- 30. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379: 2342–2350. [DOI] [PubMed] [Google Scholar]
- 31. Gray JE, Villegas A, Daniel D, et al. Three-year overall survival update from the PACIFIC trial. J Clin Oncol 2019; 37: (Suppl. 15): abstract 8526. [Google Scholar]
- 32. Durm GA, Althouse SK, Sadiq AA, et al. Phase II trial of concurrent chemoradiation with consolidation pembrolizumab in patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. J Clin Oncol 2018; 20; 36(Suppl. 15): abstract 8500. [Google Scholar]