Abstract
Background:
Infection remains a major cause of morbidity and mortality in patients with systemic lupus erythematosus (SLE). This study aimed to establish a clinical prediction model for the 3-month all-cause mortality of invasive infection events in patients with SLE in the emergency department.
Methods:
SLE patients complicated with invasive infection admitted into the emergency department were included in this study. Patient’s demographic, clinical, and laboratory characteristics on admission were retrospectively collected as baseline data and compared between the deceased and the survivors. Independent predictors were identified by multivariable logistic regression analysis. A prediction model for all-cause mortality was established and evaluated by receiver operating characteristic (ROC) curve analysis.
Results:
A total of 130 eligible patients were collected with a cumulative 38.5% 3-month mortality. Lymphocyte count <800/ul, urea >7.6mmol/l, maximum prednisone dose in the past ⩾60 mg/d, quick Sequential Organ Failure Assessment (qSOFA) score, and age at baseline were independent predictors for all-cause mortality (LUPHAS). In contrast, a history of hydroxychloroquine use was protective. In a combined, odds ratio-weighted LUPHAS scoring system (score 3–22), patients were categorized to three groups: low-risk (score 3–9), medium-risk (score 10–15), and high-risk (score 16–22), with mortalities of 4.9% (2/41), 45.9% (28/61), and 78.3% (18/23) respectively. ROC curve analysis indicated that a LUPHAS score could effectively predict all-cause mortality [area under the curve (AUC) = 0.86, CI 95% 0.79–0.92]. In addition, LUPHAS score performed better than the qSOFA score alone (AUC = 0.69, CI 95% 0.59–0.78), or CURB-65 score (AUC = 0.69, CI 95% 0.59–0.80) in the subgroup of lung infections (n = 108).
Conclusions:
Based on a large emergency cohort of lupus patients complicated with invasive infection, the LUPHAS score was established to predict the short-term all-cause mortality, which could be a promising applicable tool for risk stratification in clinical practice.
Keywords: emergency department, infection, mortality, prediction, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous systemic autoimmune disease with protean clinical manifestations.1 The overall survival of SLE has appreciably improved over the past decades due to better disease control benefiting, at least partially, from immunosuppressive agents. However, invasive infection remains an important and major cause of morbidity and mortality in lupus patients in the context of the immune disturbed or immunocompromised status.2–5
Several risk factors including age, active disease, renal involvement, previous exposure of high-dose glucocorticoid, and immunosuppressants (e.g. cyclophosphamide, rituximab) have been reported to be associated with incidence of infection in SLE.6–11 In contrast, it has been accepted that antimalarial drugs have a protective role against infection.9,12–14
It is common that these acutely ill patients with severe infection presented to the emergency room seeking medical attention. Although existing predictive tools including Quick Sequential Organ Failure Assessment (qSOFA) and CURB-65 have been widely used in the general population with infection for risk assessment,15,16 there is a lack of robust data on lupus patients who are admitted to the emergency department with invasive infections. However, no applicable mortality prediction model, to the best of our knowledge, is available for this specific subpopulation.
As a large tertiary referral center with a powerful rheumatology team, many severe lupus patients are rereferred or transferred to the emergency department of our hospital. We have built up a so called ‘Emergency-Rheum’ based on a multidisciplinary approach with rheumatologists and emergency physicians working closely together to manage these patients. In this unique setting, we have the advantage of being able to investigate our cohort of SLE patients, complicated with invasive infections, in the emergency department. In this study, we aimed to identify the independent risk factors and to establish a clinical prediction model for the 3-month all-cause mortality for patients with this life-threatening condition.
Methods
Study cohort
We conducted a retrospective observational study in a prospective ‘Emergency-Rheum’ cohort. The ‘Emergency-Rheum’ cohort was established in the south campus of Renji Hospital in 2015. On the basis of multidisciplinary collaboration, when patients with rheumatology diseases including lupus present at the emergency department of our center, rheumatologists give consultation and opinion upon disease evaluation and specific treatment as soon as possible. The patients would also be followed up by the rheumatologist until they are admitted to a ward if necessary.
Written informed consent was obtained from the patients included in our ‘Emergency-Rheum’ cohort. Then data including demographic, clinical, and laboratory characteristics on admission covering the disease evaluation of both critical condition and rheumatology diseases were collected. Once included in the database, every patient was continuously followed up in the inpatient and outpatient departments of our center using medical records or occasionally by phone.
The current study was conducted by retrospectively analyzing the subcohort of lupus patients with invasive infection. The study protocol was approved by the ethics committees of Renji Hospital.
Eligible patients for this study fulfilled the following criteria: diagnosis of SLE according to the 1997 American College of Rheumatology classification criteria,17 complications with an invasive infection when admitted into the emergency department between May 2015 and June 2018.
Invasive infection was defined as a deep infection with definite microbiological evidence, or was judged to be by the treating physician combining the symptoms, laboratory, and imaging tests.8,10,18 For example, a patient was diagnosed with pneumonia by combining respiratory symptoms and signs, positive tests for sputum or bronchoalveolar lavage fluid culture, positive chest X-ray or CT findings, elevated microorganism-associated serum markers including procalcitonin, (1-3)-β-D-glucan or virus DNA. When it was difficult to differentiate between infection and lupus activity in patients with negative culture tests, treatment response to antimicrobial therapy was considered by the treating physician to confirm the diagnosis of infection.16
For every patient suspected of viral infection, possible organ-specific manifestations were comprehensively evaluated, including pneumonia (interstitial lung disease), hepatitis (elevated bilirubin, liver enzyme levels or both, and absence of any other documented cause), retinitis (confirmed by an ophthalmologist). In patients clinically suspected of viral infection, cytomegalovirus (CMV) DNA and Epstein–Barr virus (EBV) DNA were then tested in serum samples by quantitative PCR-based techniques. Positive CMV or EBV DNA tests combined with objective findings of at least one infected organ were required to establish the diagnosis of viral infection.19,20
All patients were followed up for at least 3 months or until death. We chose 3 months as the end-point for follow-up because most death events occur within a short period due to uncontrolled, severe infection or accompanying lupus activity in this subpopulation.
Data collection
Patient’s demographic, clinical, and laboratory characteristics on admission and medication history were retrospectively collected as baseline data. The detailed information about invasive infection (sites and pathogens) were also collected. The outcome was defined as all-cause death within 3 months since baseline. According to the outcome data, patients were divided into two groups, deceased and survivors.
The disease activity at baseline was evaluated by SLE Disease Activity Index 2000 (SLEDAI-2K).21 Existing tools that have been used to evaluate the severity of infection including qSOFA and CURB-65 were also assessed as indicated at baseline.15,16
Statistical analysis
Baseline characteristics were described and compared between the deceased and survivors by univariable analyses followed by Bonferroni correction. The independent sample Student’s t test, Mann–Whitney U test, Chi-square (χ2) test, and Fisher’s exact test were conducted, as appropriate.
The independent predictors for all-cause mortality within 3 months were determined by multivariable logistic regression analysis. Candidate predictors for the multivariable regression were selected by expert opinion based on clinical significance, previous studies, and feasibility.
Independent predictors were then weighted by odds ratio (OR) values and combined to establish a prediction model for all-cause mortality. Predictive and discriminatory performance of the new prediction model was examined by applying Kaplan–Meier survival plot and ROC curve analysis and then compared with qSOFA and CURB-65.
All above-mentioned statistical analyses were performed using SPSS V.23 (Armonk, NY, USA) or Graphpad 5.0 (San Diego, CA, USA) software. Significance was defined as p < 0.05.
Results
Study population
A total of 130 SLE patients complicated with invasive infection who met the inclusion criteria were analyzed in our cohort. Of those, a total of 50 (38.5%) patients died within the 3-month follow-up. The mean follow-up period in the deceased group was 3.34 ± 2.54 weeks. The numbers of deceased patients in the 1st, 2nd and 3rd months were 38, 9 and 3, respectively. Patients were predominantly female (91%) with a mean age of 43.0 years on admission. The median disease duration for SLE on admission was 4.0 years (IQR 0.5–10.0), and the median disease duration for infection (time between initial symptom attributed to infection and admission in our center) was 10.0 days (IQR 3.8–15.0). The detailed demographic, clinical, laboratory characteristics, and medication history at baseline are provided in Table 1.
Table 1.
Patient’s baseline characteristics and univariable comparisons in SLE patients complicated with invasive infection in the emergency department.
| Characteristics | Whole cohort (n = 130) | Survivors (n = 80) | Deceased (n = 50) | p value |
|---|---|---|---|---|
| Demographic | ||||
| Age on admission (year) | 42.6 ± 14.2 | 40.9 ± 13.3 | 45.3 ± 15.3 | 0.099 |
| Male sex | 12 (9.2) | 8 (10.0) | 4 (8.0) | 0.703 |
| Disease duration of SLE (year) | 6.6 ± 6.8 | 6.5 ± 7.2 | 6.7 ± 6.4 | 0.538 |
| Disease duration of infection (day) | 15.0 ± 21.7 | 14.8 ± 17.3 | 15.3 ± 27.5 | 0.284 |
| SLE activity | ||||
| SLEDAI score | 9.0 ± 5.9 | 8.4 ± 5.5 | 9.9 ± 6.4 | 0.194 |
| Lupus nephritis | 78 (60.0) | 44 (55.0) | 34 (68.0) | 0.141 |
| Neuropsychiatric lupus | 27 (20.8) | 12 (15.0) | 15 (30.0) | 0.040 |
| Pulmonary hypertension* | 27 (20.8) | 17 (21.3) | 10 (20.0) | 0.864 |
| Infection site | ||||
| Lung infection | 108 (83.1) | 65 (81.3) | 43 (86.0) | 0.482 |
| Blood stream infection | 23 (17.7) | 9 (11.3) | 14 (28.0) | 0.015 |
| Laboratory tests | ||||
| ESR >20 mm/1 h | 98/129 (76.0) | 65 (81.3) | 33/49 (67.3) | 0.073 |
| CRP elevation | 103 (79.2) | 58 (72.5) | 45 (90.0) | 0.017 |
| Lymphocyte count <800/μl | 92 (70.8) | 50 (62.5) | 42 (84.0) | 0.009 |
| Platelet count <105/μl | 55 (42.3) | 28 (35.0) | 27 (54.0) | 0.033 |
| Hypoalbuminemia (<25 g/l) | 56 (43.1) | 25 (31.3) | 31 (62.0) | 0.001 |
| Hypoglobulinemia (<20 g/l) | 12/124 (9.7) | 4/77 (5.2) | 8/47 (17.0) | 0.056 |
| Urea >7.6 mmol/l | 72/129 (55.8) | 35 (43.8) | 37/49 (75.5) | <0.001 |
| Procalcitonin >0.5 μg/l | 58/126 (46.0) | 28/77 (36.4) | 30/49 (61.2) | 0.006 |
| (1-3)-β-D-glucan >100 pg/ml | 38/121 (31.4) | 16/73 (21.9) | 22/48 (45.8) | 0.006 |
| Medication history | ||||
| Maximum prednisone-equivalent dose in the past ⩾60 mg/d | 86/126 (68.3) | 45/77 (58.4) | 41/49 (83.7) | 0.003 |
| History of immunosuppressant use in the past 6 months$ | 78 (60.0) | 43 (53.8) | 35 (70.0) | 0.066 |
| History of hydroxychloroquine use | 74 (56.9) | 50 (62.5) | 24 (48.0) | 0.104 |
| Comorbidity | ||||
| Diabetes | 21 (16.2) | 10 (12.5) | 11 (22.0) | 0.152 |
| Chronic renal insufficiency | 43 (33.1) | 23 (28.7) | 20 (40.0) | 0.185 |
| qSOFA score ⩾2‡ | 9 (6.9) | 0 (0.0) | 9 (18.0) | <0.001 |
Data are presented as mean ± SD for continuous variables and number (frequency) (%) for categorical variables.
p values of univariable comparisons of baseline characteristics between survivors and deceased are shown (Chi-squared tests or Fisher’s exact tests were used for categorical variables and independent sample t tests were used for continuous variables, as appropriate).
Pulmonary hypertension was globally judged on echocardiography by the treating physician.
Immunosuppressant use was defined as treatment with any of methotrexate, azathioprine, cyclophosphamide, mycophenolate mofetil, cyclosporine, and rituximab.
The quick Sequential Organ Failure Assessment (qSOFA) score ranges 0–3 points, with 1 point each for systolic hypotension (⩽100 mm Hg), tachypnea (⩾22/min), or altered mentation. Patients with a score ⩾2 are associated with a greater risk of death or prolonged intensive care unit stay.
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; qSOFA, quick Sequential Organ Failure Assessment; SD, standard deviation; SLE, Systemic Lupus Erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
Data on invasive infection
A total of 102 (78.5%) patients had evidence of pathogens. A total of 28 (21.5%) patients were clinically diagnosed as having an invasive infection by the treating physician, all of which were pneumonia.
Among the patients with positive microbiology, we found that 58 (56.9%) patients had bacterial infections, 49 (48.0%) patients had fungal infections, 13 (12.7%) patients had viral infections, 7 (6.9%) patients had mycobacterium infections, and 25 (24.5%) patients had mixed infections (more than 1 species).
The most frequent bacteria species were Staphylococcus (15.7%), Klebsiella pneumoniae (10.8%), Escherichia coli (9.8%) and Pseudomonas aeruginosa (4.9%). The dominant fungal species were Candida (27.5%), Aspergillus (9.8%), Cryptococcus neoformans (4.9%), and Pneumocystis jeroveci (2.9%). The remaining pathogens included CMV (10.8%), Mycobacterium tuberculosis (6.9%), EBV (3.9%), and Nocardia (2.9%).
A total of 108 (83.1%) patients had lung infections, 23 (17.7%) patients had blood stream infections, and 9 (6.9%) patients had a central nervous system infection. These were the three most frequent infection sites in our cohort, followed by gastrointestinal (4.6%), urinary tract (4.6%), and joint (3.8%) infections, respectively.
Univariable analysis
Results of univariable comparison of baseline parameters between survivors and deceased are summarized in Table 1. Deceased patients had a higher incidence of neuropsychiatric lupus, blood stream infections, qSOFA score ⩾2, and maximum prednisone dose in the past ⩾60 mg/d. C-reactive protein elevation, lymphocyte count < 800/μl, platelet count <105/μl, hypoalbuminemia (<25 g/l), urea >7.6 mmol/l, procalcitonin >0.5 μl/l, and (1-3)-β-D-glucan >100 pg/ml were also observed more frequently in deceased patients. By using the Bonferroni correction, the modified critical p value (α) was determined as 0.002. Significantly higher incidence of qSOFA score ⩾2, hypoalbuminemia (<25 g/l), and urea >7.6 mmol/l were found in the deceased than survivors (p ⩽ 0.001).
Multivariable analysis
In the final multivariable logistic regression model, which included 10 clinically meaningful candidate predictors, lymphocyte count <800/μl (OR = 3.52, CI 95% 1.13–11.03), urea >7.6 mmol/l (OR = 3.75, CI 95% 1.25–11.22), maximum prednisone dose in the past ⩾60 mg/d (OR = 4.52, CI 95% 1.39–14.65), qSOFA score (OR = 5.40, CI 95% 2.20–13.27), and age on admission (OR = 1.05, CI 95% 1.01–1.09) were independently predictive for 3-month all-cause mortality in emergency lupus patients complicated with invasive infection. However, the history of hydroxychloroquine use (OR = 0.30, CI 95% 0.11–0.84) was protective (Table 2).
Table 2.
Multivariable logistic regression model for 3-month all-cause death in SLE patients complicated with invasive infection in the emergency department.
| Predictors | p value | OR | CI 95% |
|---|---|---|---|
| Age | 0.029 | 1.05 | 1.01–1.09 |
| SLEDAI score | 0.495 | 1.03 | 0.95–1.12 |
| Lymphocyte count <800/μl | 0.031 | 3.52 | 1.13–11.03 |
| Hypoalbuminemia (<25 g/l) | 0.352 | 1.62 | 0.59–4.46 |
| Urea >7.6 mmol/l | 0.018 | 3.75 | 1.25–11.22 |
| Blood stream infection | 0.112 | 3.01 | 0.77–11.67 |
| Maximum prednisone dose in the past ⩾60 mg/d | 0.012 | 4.52 | 1.39–14.65 |
| History of immunosuppressant use in the past 6 months | 0.063 | 2.85 | 0.95–8.56 |
| History of hydroxychloroquine use | 0.022 | 0.30 | 0.11–0.84 |
| qSOFA score | <0.001 | 5.40 | 2.20–13.27 |
Predictors highlighted in bold are significantly associated with all-cause mortality.
OR, odds ratio; qSOFA, quick Sequential Organ Failure Assessment; SLE, systemic Lupus Erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
Establishment of risk prediction model
We combined the six independent predictors to make a risk prediction model for all-cause mortality and denominated this new scoring model as LUPHAS by combining the initials of the predictors. All predictors were weighted by OR values, giving a LUPHAS score ranging from 3 to 22 (Table 3).
Table 3.
Establishment of the LUPHAS scoring system.
| Predictors | Points | |
|---|---|---|
| L | Lymphocyte count | |
| ⩾800/μl | 1 | |
| <800/μl | 4 | |
| U | Urea | |
| ⩽7.6 mmol/l | 1 | |
| >7.6 mmol/l | 4 | |
| P | Maximum Prednisone dose in the past | |
| <60 mg/d | 1 | |
| ⩾60 mg/d | 5 | |
| H | History of Hydroxychloroquine use | |
| Yes | −3 | |
| No | 0 | |
| A | Age, year | |
| ⩽20 | 1 | |
| 21–40 | 2 | |
| 41–60 | 3 | |
| >60 | 4 | |
| S | qSOFA score | |
| 0 | 0 | |
| 1 | 3 | |
| ⩾2 | 6 |
LUPHAS score was established by combining independent predictors, weighted by odds ratio values.
qSOFA, quick Sequential Organ Failure Assessment.
On the basis of the LUPHAS scoring system, apart from five patients without valid data for dose of prednisone, all patients could be categorized into three groups: low-risk (score 3–9), medium-risk (score 10–15), and high-risk (score 16–22). The mortalities were 4.9% (2/41), 45.9% (28/61), and 78.3% (18/23) in low-risk, medium-risk and high-risk patients, respectively, compared with the overall mortality of 38.4% (48/125).
Evaluation of LUPHAS scoring system
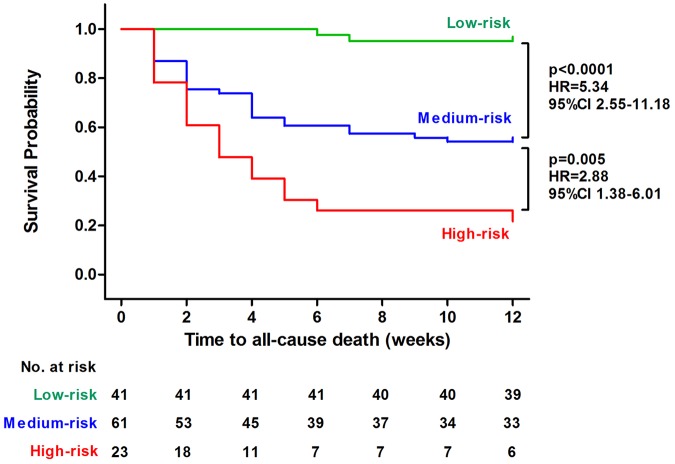
The subsequent Kaplan–Meier survival analysis indicated that the probability of all-cause death was significantly higher for medium-risk patients than low-risk patients (p < 0.0001).In addition, the high-risk patients had an even higher probability of all-cause death than medium-risk patients (p = 0.005), according to the LUPHAS score (Figure 1).
Figure 1.
Kaplan–Meier survival plot for time to all-cause death during follow-up depending on the LUPHAS risk categories.
ROC curve analysis indicated that the LUPHAS score could effectively predict all-cause mortality in this population (area under the curve [AUC] = 0.86, CI 95% 0.79–0.92), with a sensitivity of 79.2% and a specificity of 80.5%.
In addition, the LUPHAS score performed better than the qSOFA score [area under the curve (AUC) = 0.69, CI 95% 0.59–0.78] (sensitivity 64.0%, specificity 67.5%) when predicting the short-term mortality of emergency lupus patients with invasive infection in our cohort.
Similarly, in the subgroup of patients with lung infection (n = 108), the discriminatory performance of the LUPHAS score was also superior than the CURB-65 score (AUC = 0.69, CI 95% 0.59–0.80) (sensitivity 39.5%, specificity 87.7%) (Table 4).
Table 4.
Discriminatory performance of LUPHAS score compared with qSOFA score and CURB-65 score by receiver operating characteristic (ROC) curve analysis.
| Model | AUROC (CI 95%) | Sensitivity | Specificity |
|---|---|---|---|
| Total population (n = 130) | |||
| LUPHAS | 0.86 (0.79–0.92) | 79.2% | 80.5% |
| qSOFA | 0.69 (0.59–0.78) | 64.0% | 67.5% |
| Subgroup of lung infection (n = 108) | |||
| LUPHAS | 0.84 (0.76–0.92) | 78.0% | 79.4% |
| CURB-65 | 0.69 (0.59–0.80) | 39.5% | 87.7% |
The quick Sequential Organ Failure Assessment (qSOFA) score ranges 0–3 points, with 1 point each for systolic hypotension (⩽100 mm Hg), tachypnea (⩾22/min), or altered mentation. Patients with a score ⩾2 are associated with a greater risk of death or prolonged intensive care unit stay.
CURB-65 is a validated clinical assessment tool for predicting mortality in patients with community-acquired pneumonia, including confusion, urea >7 mmol/l, respiratory rate >30/min, low blood pressure (systolic <90 mm Hg, or diastolic <60 mm Hg, or both) and age ⩾65. Patients with a score ⩾2 are associated with a higher mortality and hospitalization needs to be considered.
AUROC, area under receiver operating characteristic curve; qSOFA, the quick Sequential Organ Failure Assessment.
Discussion
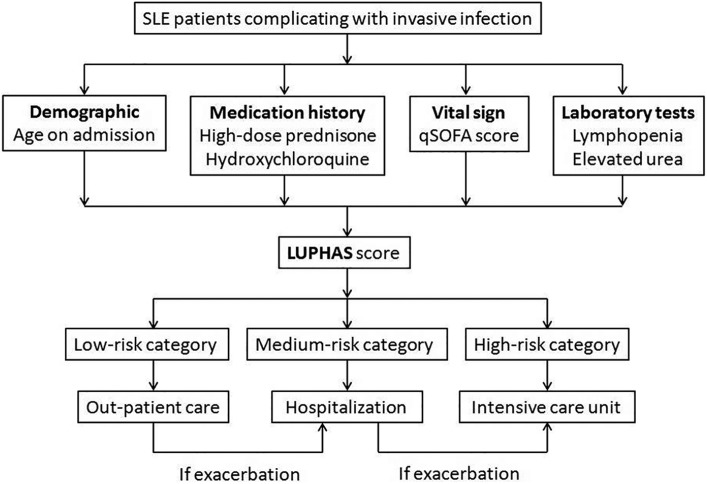
In this study, based on a large emergency cohort of SLE complicated with invasive infection, we reported an extremely high short-term all-cause mortality rate (38.5%), highlighting that special attention should be paid to these immunocompromised patients. Several independent predictors were successfully identified and a convenient mortality risk prediction model LUPHAS score was established. To the best of our knowledge, the LUPHAS score is the first available mortality risk prediction model in emergency patients with SLE and invasive infection. The LUPHAS model integrates several clinical and laboratory parameters including age, vital sign, medication history, and routine blood tests. All of these can be easily obtained in an appropriate manner when a lupus patient presents in the emergency department. Therefore, this applicable tool could assist the emergency physicians and rheumatologists to identify those patients at higher risk and to provide efficient triage for them, as well as appropriate management. We recommend the patients at high-risk according to their LUPHAS score should be referred to an intensive care unit as soon as possible due to the extremely high mortality (Figure 2).
Figure 2.
Recommended triage flow chart for SLE patients complicated with invasive infection admitted into the emergency department.
Among the predictors reported in our cohort, older age and previous exposure of high-dose glucocorticoids are the well-known risk factors for serious infection in SLE. In addition, previous use of antimalarial drugs has proved to be protective for infection in multiple studies. Of interest, the decreased peripheral lymphocyte count and elevated urea concentration were first identified as predictive for all-cause mortality in this population. The former parameters, easily accessed via complete blood count tests, could be a crude surrogate marker for the cellular immune function. The latter could be a composite indicator for renal function and catabolism, which is frequently elevated in critical patients. As expected, the qSOFA score, a common evaluation tool widely used in the field of emergency and critical care, was determined to be an independent predictor for mortality in our cohort. To the best of our knowledge, such composite parameters reflecting the vital signs have never been assessed in previous similar studies of SLE patients. In addition, we weighted the hazards of various risk factors so that a quantified scoring system could be established. This novel risk-staging system would be an applicable tool for fast evaluation and stratification of patients.
Using ROC curve analysis, the LUPHAS score was shown to be capable of predicting short-term all-cause mortality and performed better than the qSOFA score alone. Because the lungs are the most frequent site of infection in lupus patients, the LUPHAS score provided a superior evaluation tool to the CURB-65 score in this specific subpopulation. Our data reinforced that invasive infection in SLE is a complicated, heterogeneous clinical condition that requires a multidimensional prediction tool.
Our ‘Emergency-Rheum’ multidisciplinary approach attempted to integrate the first-aid skill of emergency physicians and the specialized knowledge of rheumatologists. This system could help to identify severe clinical conditions in patients with rheumatic diseases, therefore, the appropriate interventions could be implemented more appropriately and efficiently, as we have shown previously when managing patients with SLE-associated pulmonary hypertension in the emergency setting.22,23 Further prospective studies are required to address the question whether this approach, combined with a LUPHAS-guided triage protocol could eventually improve the short-term prognosis for patients with SLE invasive infections.
There are several limitations in our study. First, due to the retrospective study design, there is missing data issues that frequently presented in the real-life cohort. The parameters with >10% missing values (e.g. lymphocyte subset count by flow cytometer, and levels) could rarely be considered for multivariable analysis. Therefore, we could have missed some potential predictors. Second, as a result of the observational design, we did not evaluate the effect of antimicrobial treatment on the outcomes. However, antimicrobial therapy always depends on the individual disease severity and specific microorganism and it is, therefore, difficult to accurately exclude the influence of treatment in an unselected heterogeneous cohort. In addition, there is a meaningful treatment-by-indication error in observational studies, making interpretation of the results difficult. Third, we have already verified the new prediction model in our prospective validation cohort. However, external validation with qualified data from other centers is required to confirm our findings. Finally, it is worth highlighting that the prediction model in our study was derived from a relatively heterogeneous cohort in the emergency condition, with mixed pathogen-based and clinical diagnoses, and a combination of various infection types. The results should not be over-interpreted and extrapolated to the overall lupus population.
Conclusion
In this large emergency cohort of lupus patients complicated with invasive infection, an impressively high short-term all-cause mortality was recorded, highlighting that special attention should be paid to these patients. Several independent predictors for all-cause mortality were successfully identified in our study. The real-world evidence-based on the LUPHAS score could be a promising tool for the fast evaluation and risk stratification of this population in clinical practice.
Footnotes
Funding: Shuang Ye has received funding from Shanghai Shenkang promoting project for clinical skills of major diseases (16CR1013A). All other authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that they have no conflicts of interest.
Ethical approval: The retrospective study protocol was approved by the ethics committees of Renji Hospital.
ORCID iDs: Wanlong Wu  https://orcid.org/0000-0003-2727-7950
https://orcid.org/0000-0003-2727-7950
Contributor Information
Wanlong Wu, Department of Rheumatology, South Campus, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Jun Ma, Department of Emergency Medicine, South Campus, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Yuhong Zhou, Department of Emergency Medicine, South Campus, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Chao Tang, Department of Emergency Medicine, South Campus, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Feng Zhao, Department of Emergency Medicine, South Campus, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Fangfang Sun, Department of Rheumatology, South Campus, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Wenwen Xu, Department of Rheumatology, South Campus, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Jie Chen, Department of Rheumatology, South Campus, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Shuang Ye, Department of Rheumatology, South Campus, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Yi Chen, Department of Emergency Medicine, South Campus, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, No. 2000, Jiangyue Road, Minhang District, Shanghai 201112, China.
References
- 1. Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun 2019; 96: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ocampo-Piraquive V, Nieto-Aristizabal I, Canas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol 2018; 14: 1043–1053. [DOI] [PubMed] [Google Scholar]
- 3. Mu L, Hao Y, Fan Y, et al. Mortality and prognostic factors in Chinese patients with systemic lupus erythematosus. Lupus 2018; 27: 1742–1752. [DOI] [PubMed] [Google Scholar]
- 4. Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003; 82: 299–308. [DOI] [PubMed] [Google Scholar]
- 5. Wu XY, Yang M, Xie YS, et al. Causes of death in hospitalized patients with systemic lupus erythematosus: a 10-year multicenter nationwide Chinese cohort. Clin Rheumatol 2019; 38: 107–115. [DOI] [PubMed] [Google Scholar]
- 6. Jung JY, Yoon D, Choi Y, et al. Associated clinical factors for serious infections in patients with systemic lupus erythematosus. Sci Rep 2019; 9: 9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu P, Tan HZ, Li H, et al. Infections in hospitalized lupus nephritis patients: characteristics, risk factors, and outcomes. Lupus 2018; 27: 1150–1158. [DOI] [PubMed] [Google Scholar]
- 8. Zhan Z, Lao M, Su F, et al. Hospital-acquired infection in patients with systemic lupus erythematosus: a case-control study in a southern Chinese population. Clin Rheumatol 2018; 37: 709–717. [DOI] [PubMed] [Google Scholar]
- 9. Rua-Figueroa I, Lopez-Longo J, Galindo-Izquierdo M, et al. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum 2017; 47: 38–45. [DOI] [PubMed] [Google Scholar]
- 10. Chen D, Xie J, Chen H, et al. Infection in Southern Chinese patients with systemic lupus erythematosus: spectrum, drug resistance, outcomes, and risk factors. J Rheumatol 2016; 43: 1650–1656. [DOI] [PubMed] [Google Scholar]
- 11. Chen GL, Chen Y, Zhu CQ, et al. Invasive fungal infection in Chinese patients with systemic lupus erythematosus. Clin Rheumatol 2012; 31: 1087–1091. [DOI] [PubMed] [Google Scholar]
- 12. Teh CL, Wan SA, Ling GR. Severe infections in systemic lupus erythematosus: disease pattern and predictors of infection-related mortality. Clin Rheumatol 2018; 37: 2081–2086. [DOI] [PubMed] [Google Scholar]
- 13. Feldman CH, Hiraki LT, Winkelmayer WC, et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol 2015; 67: 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus 2013; 22: 1286–1294. [DOI] [PubMed] [Google Scholar]
- 15. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009; 64(Suppl. 3): iii1–iii55. [DOI] [PubMed] [Google Scholar]
- 17. Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- 18. Lu Z, Li J, Ji J, et al. Mortality prediction in systemic lupus erythematosus patients with pulmonary infection. Int J Rheum Dis 2019; 22: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 19. Cohen JI. Epstein-Barr virus infection. N Engl J Med 2000; 343: 481–492. [DOI] [PubMed] [Google Scholar]
- 20. Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097. [DOI] [PubMed] [Google Scholar]
- 21. Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29: 288–291. [PubMed] [Google Scholar]
- 22. Chen Y, Guo L, Li Y, et al. Severe pulmonary arterial hypertension secondary to lupus in the emergency department: proactive intense care associated with a better short-term survival. Int J Rheum Dis 2015; 18: 331–335. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y, Chen GL, Zhu CQ, et al. Severe systemic lupus erythematosus in emergency department: a retrospective single-center study from China. Clin Rheumatol 2011; 30: 1463–1469. [DOI] [PubMed] [Google Scholar]