Abstract
Background:
Drug-induced colitis is a known complication of therapies that alter the immune balance, damage the intestinal barrier or disturb intestinal microbiota. Immune checkpoint inhibitors (ICI) directed against cancer cells may result in activated T lymphocyte-induced immune-related adverse events (AEs), including immune-related colitis and hepatitis. The aim of this review article is to summarize the incidence of gastrointestinal (GI) and hepatic AEs related to ICI therapy. We have also looked at the pathogenesis of immune-mediated AEs and propose management strategies based on current available evidence.
Methods:
A literature search using PubMed and Medline databases was undertaken using relevant search terms pertaining to names of individual drugs, mechanism of action, related AEs and their management.
Results:
ICI-related GI AEs are common, and colitis appears to be the most common side effect, with some studies reporting incidence as high as 30%. The incidence of both all-grade colitis and hepatitis were highest with combination therapy with anti-CTLA-4/PD-1; severity of colitis was dose-dependent (anti-CTLA-4). Early intervention is associated with better outcomes.
Conclusion:
ICI-related GI and hepatic AEs are common and clinicians need to be aware. Patients with GI AEs benefit from early diagnosis using endoscopy and computed tomography. Early intervention with oral steroids is effective in the majority of patients, and in steroid-refractory colitis infliximab and vedolizumab have been reported to be useful; mycophenolate has been used for steroid-refractory hepatitis.
Keywords: anti-CTLA-4, anti-PD1, anti-PDL1, immune checkpoint inhibitors, immune-related hepatitis, immune-related colitis, management
Introduction
Cancer immunotherapy has progressed dramatically after the introduction of immune checkpoint inhibitors (ICI), which include antagonistic antibodies that block key co-inhibitory molecules, including cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein (PD-1) and programmed death-ligand 1 (PD-L1), all of which are overexpressed in certain tumour microenvironments. Targeting CTLA-4, PD-1 and PD-L1 reactivates cytotoxic T lymphocytes, allowing the immune system to destroy cancer cells.
The ICI currently in use are ipilimumab (anti-CTLA-4), nivolumab, pembrolizumab (anti-PD-1), and atezolizumab, avelumab and durvalumab (anti-PD-L1), against a number of cancers including melanoma, renal cell carcinoma, bladder cancer, head and neck cancers and lung cancers. While ICIs reactivate cytotoxic T lymphocytes, allowing them to destroy cancer cells, it may also result in immune-related adverse events (IrAEs) in a proportion of patients as the reactivated T cells attack other tissues. The intestine, especially the colon, appears to be one of the most common target organs for acute IrAEs,1 with lymphocytic and neutrophilic inflammation and in some cases granuloma and crypt abscesses.2 However, other AEs, including endocrine dysfunction, skin and mucosal manifestations, polyarthritis, pneumonitis and haematologic disorders, also occur.
Most studies that have described and reported AEs related to ICI therapies include data from therapy with ipilimumab, pembrolizumab, nivolumab and atezolizumab. The spectrum of IrAEs induced by these drugs can be disabling and may lead to discontinuation of cancer immunotherapy. This narrative review focuses on immune-related colitis and hepatitis and their management, though involvement of other organs in IrAE may influence management strategies. Due to a lack of randomized studies on the topic, we have not conducted a systematic review.
Search strategy
A literature search using PubMed and Medline databases was undertaken first using the search terms ‘immune check point inhibitors’ or ‘check point inhibitors’; searches also included individual drugs ‘ipilimumab’ or ‘pembrolizumab’ or ‘nivolumab’ or ‘atezolizumab’ or ‘avelumab’ and mechanism, that is ‘anti-CTLA-4’, ‘anti-PD-1’ or ‘anti-PD-L1’. These terms were then combined with keywords ‘colitis’ or ‘immune mediated colitis’ or ‘complications’, ‘side effects’ or ‘adverse events’ or ‘gastrointestinal and hepatic adverse events’ and ‘management of adverse events’.
Gastrointestinal and hepatic AEs in checkpoint inhibitor therapy
Incidence of GI and hepatic AEs
The incidence of GI AEs appears to be dependent on various factors. A systematic review and meta-analysis by Wang and colleagues, published in 2017, looked at 34 studies with a total of 8863 patients, and reported differences in incidence rates of colitis related to ICI therapy based on the type of therapy (single, combination), tumour type and the dosage of therapies used.3 The overall incidence of all grades of colitis in their review was noted to be 2.4% (95% CI, 1.6–3.6%) and 1.7% (95% CI, 1.1–2.5%) for grade 3–4 colitis.3 The grading system used is as per the common terminologies used in clinical trials as per guidance from the National Cancer Institute (see Table 1).4
Table 1.
The National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 4.
| Adverse effect | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Diarrhoea | Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline | Increase of 4–6 stools per day over baseline; moderate increase in ostomy output compared to baseline | Increase of 7 or more stools per day over baseline; incontinence; hospitalization indicated; severe increase in ostomy output compared to baseline; limiting self-care ADL | Life-threatening consequences; urgent intervention indicated | Death |
| Colitis | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Abdominal pain; mucus or blood in stool | Severe abdominal pain; change in bowel habits; medical intervention indicated; peritoneal signs | Life-threatening consequences; urgent intervention indicated | Death |
| Hepatitis | AST or ALT 1–2.5× ULN and/or T-BIL 1–1.5× ULN | AST or ALT 2.5–5× ULN and/or T-BIL 1.5–3× ULN | AST or ALT >5× ULN and/or T-BIL >3× ULN | AST or ALT >8× ULN | Death |
The current version in use is CTCAE version 5 but all studies included in this study reported adverse events using CTCAE version 4.
ADL, activities of daily living; T-BIL, total bilirubin; ULN, upper limit of normal.
A systematic review of IrAEs related to anti-PD1 and anti-PD-L1 therapies showed a lower proportion of colitis compared to patients on anti-CTLA-4 therapy.5 Another comprehensive systematic review by Wang and colleagues, which focused on fatal toxic effects of all ICI therapies using the World Health Organization database, reported 613 fatal events from 2009 to January 2018.6 Among these, 193 deaths were related to anti-CTLA-4 therapy, most commonly from colitis [135 (70%)], whereas pneumonitis and hepatitis (22%) were more often the causes of death with anti-PD-1/PD-L1-related fatalities. With combination PD-1/CTLA-4 therapy, death was more frequently from colitis (37%) and myocarditis (25%).6 Although the colon is most commonly affected, there have been reports of upper GI involvement. A case report of lymphocytic gastritis secondary to pembrolizumab therapy has recently been published.7
A detailed breakdown of incidence rates of GI and hepatic adverse events related to ICI therapy is provided in Table 2.
Table 2.
Incidence of gastrointestinal and hepatic adverse events.
| Overall incidence (all-grade colitis) |
Grade 3–4 colitis | Grade 3–4 diarrhoea | Hepatitis all grades |
Hepatitis Grade 3–4 |
|
|---|---|---|---|---|---|
| Single-agent therapy | |||||
| Anti-CTLA-4 | 9.1% (6.6–12.5%)3 | 6.8% (5.3–8.6%) | 7.9% (5.5–11.4%) | 1.9% (0.9–3.9%)8 | |
| Anti-PD-1 | 1.4% (1.1–1.8%)3 | 0.9% (0.7–1.3%) | 1.3% (1.0–1.7%) | 1.2% (0.7–1.8%)9,10 | 1.1% (0.5–1.7%) |
| Anti-PD-L1 | 1.0% (0.4–2.2%)3,5 | 0.6% (0.2–1.6%) | 0.3% (0.1–1.1%) | 1.5% (0.9–2.5%)9,10 | 0.8% (0.6–1.0%) |
| Combination therapy | |||||
| Anti-CTLA-4/PD-1 | 13.6% (7.7–22.9%)3 | 9.4% (4.8–17.4%) | 9.2% (6.8–12.3%) | 17.6%6 | 8.3% |
| Tumour type | |||||
| Melanoma | 1.8%3 | 1.2% (0.8–1.7%) | 1.4% | 3.8% | 1.3% |
| Renal cell carcinoma | 0.4%3 | 0.4% (0.1–1.8%) | 1.0% | ||
| Non-small cell lung cancer | 0.8% | 0.5% (0.3–1.0%) | 1.2% | ||
| Dosage of therapies | |||||
| Ipilimumab (3 mg/kg) | 9.6% (7.6–12.0%)8 | 7.1% (5.3–9.4%) | 5.2% (3.3–8.2%) | ||
| Ipilimumab (10 mg/kg) | 6.6% (2.4–16.75%)8 | 5.1% (2.5–9.9%) | 11.5% (8.5–15.5%) | ||
In some studies, approximately 30% of patients treated with anti-CTLA-4 antibodies were seen to develop diarrhoea, with about 10% having symptoms severe enough to consider interruption of therapy. Immune-related colitis is diagnosed in approximately 5% of patients.8,11 Diarrhoea is less common with inhibition of PD-1 and PD-L1 (about 12% of patients develop diarrhoea and 2% severe diarrhoea), with immune-related colitis reported in 1–2% of cases.12 In one meta-analysis looking at risk of IrAEs of anti-PD-1 and anti-PD-L1 therapy, the authors concluded that anti-PD-1 therapy may result in a higher risk of all-grade immune-related colitis when compared with chemotherapy.13 Also, pembrolizumab was noted to carry a higher risk of all-grade colitis compared to chemotherapy. This increased risk was more commonly seen in patients with non-small cell lung compared to malignant melanoma.
They also noted that nivolumab and atezolizumab probably did not increase the risk of immune-related colitis when compared with chemotherapy. The reduced risk of all-grade colitis seen with nivolumab is unusual, considering that pembrolizumab and nivolumab have a similar mechanism of action. This was also investigated by Fessas and colleagues, who reported on the molecular and preclinical comparisons between nivolumab and pembrolizumab.14 In this comprehensive study, they found significant molecular similarities between the two drugs and concluded that the differences in AEs seen in clinical trials may be due to drug-independent factors, such as differences in the patient populations in the trials.
An analysis of the AE profiles spontaneously reported to the US Food and Drug Administration Adverse Event Reporting System database showed that nivolumab and pembrolizumab have very similar safety profiles, but the signal strength of AEs increased when combined with ipilimumab.15 In addition, the quality of IrAE data-reporting in clinical trials may be suboptimal,16 and this may also account for subtle differences in reporting of different agents. Safety profiles of immunotherapies are also not similar for all tumour types.
The incidence of liver dysfunction (mainly hepatitis) caused by ICI therapy is much lower compared to diarrhoea, and is reported in about 1–6% of patients, mostly at grades 1 and 2.8,9 In studies reporting hepatic dysfunction, deranged transaminases is the most common form of abnormality. The median time to onset of liver dysfunction varied greatly with therapy and type of cancer. The details of differences in incidence of hepatitis among therapies10 is given in Table 2.
Pathogenesis
The cellular and molecular basis of IrAEs upon ICI therapy and the reason for predominance of GI toxicity is still not completely understood, but it might be because tumour neoantigens and normal tissue antigens of the GI tract are cross-reactive,17 and microbial epitopes important for host protection to GI infection may overlap with tumour neoantigens.18
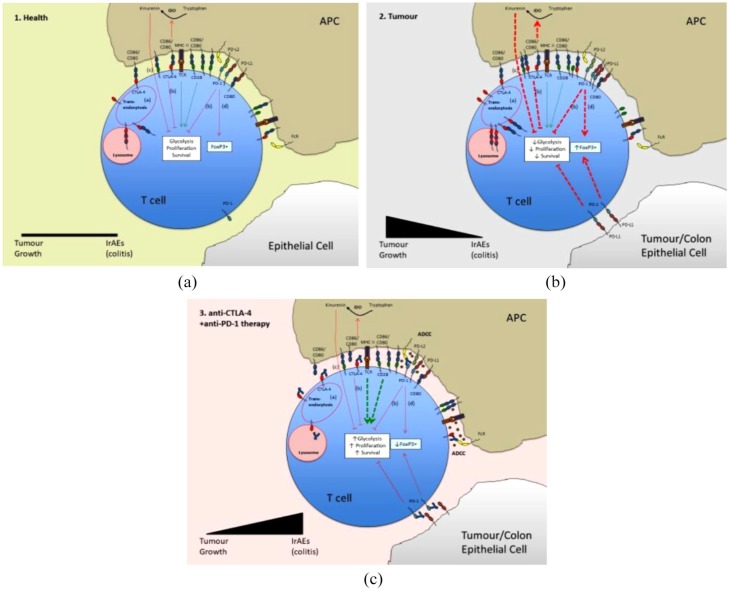
Under these conditions in the presence of ICI that block co-inhibitory pathways, activation and expansion of effector CD4+ and CD8+ T cells is favoured as co-stimulation signals that drive T cell glycolysis, proliferation and survival are sustained. The mechanism might also involve depletion of Treg numbers caused by antibody dependent cellular cytotoxicity (ADCC) or phagocytosis of antibody-marked Tregs by FcR-expressing tissue macrophages.19,20 In the case of anti-PD-1/PD-L1 therapy, iTreg depletion might also be due to the role of PD-1 signalling in enhancing FoxP3 expression and the maintenance of iTreg.21 The co-inhibitory pathways controlled by CTLA-4 and PD-1, and the mechanisms by which their blockade with antagonistic antibodies can lead to IrAEs and GI toxicity, are summarized in Figure 1 and described comprehensively in the respective legends.
Figure 1.
Pathology of checkpoint-inhibitor-induced gastrointestinal toxicities. In healthy tissues (A) low levels of self- or non-self-antigen-TCR and CD28 co-stimulation signals that lead to increased glycolysis, T cell proliferation and survival are balanced by inhibitory signals through co-inhibitory receptors CTLA-4 and PD-1 that are constitutively expressed on patrolling Treg and induced on stimulated effector T cells. The inhibitory pathways that extend from these receptors include (a) competitive binding of CTLA-4 and CD28 for their shared ligands CD80 and CD86 – this is enhanced by CD86/80 transendocytosis in which CTLA-4 recruits its ligands into vesicles that deliver them to the lysosome for degradation; (b) dephosphorylation of activatory phosphate groups on signalling proteins assembled downstream of CD28 and antigen-TCR; (c) production of kinurenins that inhibit T cell proliferation from tryptophan by indoleamine dioxygenase (IDO), which is activated downstream of CD86/80 engagement of CTLA-4; and (d) induction of FoxP3 downstream of PD-1.
In tumour (B), CTLA-4, PD-1 and their ligands are elevated. Inhibitory signals (a–d) are therefore increased relative to stimulatory signals through tumour neoantigen-TCR and CD28. This reduces the activation and expansion of effector T cells, enabling the tumour to grow.
Anti-CTLA-4 and anti-PD-1/PD-L1 therapies (C) block inhibitory pathways (a–d) and Treg with constitutively high expression of CTLA-4 and PD-1 are destroyed by tissue macrophages through antibody dependent cellular cytotoxicity (ADCC) and antibody-mediated phagocytosis. Altogether this results in T cell activation, proliferation and survival and differentiation into inflammatory effector classes that mediate destruction of the tumour but promote IrAEs in peripheral tissues, especially the colon, where self-antigens or microbial antigens might overlap with tumour neoantigens.
Key: Green lines represent co-stimulation pathways and red lines co-inhibitory pathways. Arrowheads indicate induction and wedges inhibition of the response. Line thickness indicates the strength of the pathway.
The generally greater incidence and severity of IrAEs under anti-CTLA-4 therapy compared to ani-PD-1/PD-L1 therapies probably reflects the more dominant role of CTLA-4 as an inhibitor of T cell activation compared to PD-1, as it is the principal counter regulator of CD28, which delivers the primary co-stimulation signal. CTLA-4 is also induced more rapidly on activated T cells and constitutively expressed by a greater proportion of Tregs than PD-1.22 Greater toxicity of anti-CTLA-4 therapy might also relate to the relative presence of ligands at areas of the body where maintenance of tolerance is most critical and is the consequence of losing inhibitory back signals in antigen-presenting cells (APCs).22,23
Overall, it appears that an inevitable consequence of targeting CTLA-4, PD-1 and PD-L1 is to reactivate cytotoxic T cells to drive destruction of tumour cells in the tumour micro-environment where these immunosuppressive molecules are significantly overexpressed. Such a disturbance of the homeostasis of the immune system favours the development of IrAEs, especially in the colon. Tumour neoantigens, microbial epitopes and host susceptibility to GI toxicity all have a role to play. Diarrhoea is more common than colitis and it is likely that the former represents a milder, more microscopic involvement of the colon than the latter, which includes ulcerative changes, though this requires further study. Colitis shares some of the features of Crohn’s disease and the small bowel may be involved (with lymphocytic and neutrophilic inflammation and in some cases granuloma and crypt abscesses).24 Recently, attention has focused on delayed immune-related events (DIREs) after discontinuation of immunotherapy, but these appear uncommon for GI toxicity and more common for endocrine, dermatologic or neurologic toxicities.25
Diagnosis
Diagnosis of GI-related AEs
There is considerable variation in time to onset of colitis following anti-CTLA-4 therapy. Weber and colleagues reported that GI IrAEs were observed after a median of 8 weeks into treatment in a phase III trial.26 In another study, patients developed immune-related enterocolitis after a median of 11 days (range, 0–59 days) from last dose of ipilimumab.27 In a pooled analysis, the median time to onset of GI IrAEs was 7 weeks for nivolumab and 18 weeks for pembrolizumab (for any grade of colitis).12,28 Any new onset of symptoms soon after commencing ICI therapy must prompt clinicians to consider and maintain a high index of suspicion of IrAEs until proven otherwise.
Investigations
In addition to routine blood tests, investigation of patients with ICI-related diarrhoea should first include stool tests to exclude enteric infections. Once ruled out, other tests should follow swiftly. The role of faecal calprotectin as a diagnostic marker is still unclear. One study demonstrated that an elevated level compared to baseline was not specific to patients reporting grade 2 or higher GI IrAEs.29
Endoscopy and its role
Endoscopy is an important tool for diagnosis, and clinicians should have a low threshold to use it once ICI-related GI symptoms are reported by patients. A full colonoscopy is recommended as index procedure wherever possible. In one study, the authors noted that nearly 10% of patients had involvement only in the right colon or terminal ileum.30 They also noted that timing of endoscopy was an important factor affecting outcomes. The duration of steroid therapy, recurrence of symptoms, admission to intensive care and the need to use infliximab were all lower in patients who underwent endoscopy within 30 days of onset of GI symptoms.30
Endoscopic features can vary widely, from a macroscopically normal-looking mucosa to various degrees of mucosal ulcerations and erythema. Patients who have a normal endoscopy are likely to require a shorter course of steroids and less likely to go on infliximab.30 In a case series of 39 patients with anti-CTLA-4-related enterocolitis, all patients underwent at least a sigmoidoscopy and 25 had a complete ileo-colonoscopy. Among these patients, nearly all had ulceration involving the rectum and sigmoid colon. It was noted that 66% had extensive colitis and 20% had ileal involvement.31
Histological features of ICI-related colitis
There is some variation in histological features that have been observed. Although the features are generally similar in colitis resulting from all ICI therapies, there are some minor differences with each.
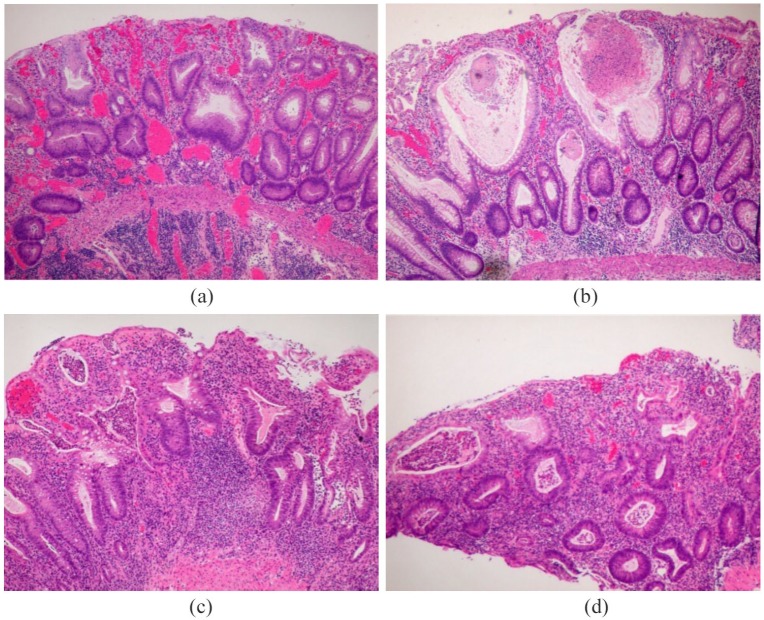
In patients treated with anti-CTLA-4 therapy, most commonly seen features appear to be dense lymphocytic infiltration in the lamina propria with frequent presence of plasma cells and eosinophils. Karamchandani and colleagues describe that in such patients features such as neutrophilic infiltration, neutrophilic cryptitis or crypt microabscesses and increased crypt epithelial apoptosis are common (Figure 2).32
Figure 2.
(a) A case of ipilimumab-related colitis. A patient with melanoma was treated with ipilimumab (anti- CTLA-4). Severe active colitis, with expansion of lamina propria lymphoplasma cells, cryptitis, crypt destruction/dropout and crypt architecture alteration. (b) Additionally, many crypts show significant distention (‘ballooning’) due to intraluminal inflammatory exudate accumulation. (c) A male patient was given ipilimumab, nivolumab and IL2 to treat metastatic prostatic carcinoma, then developed bloody diarrhoea. Severe active chronic colitis mimics ulcerative colitis. (d) Severe active colitis, with expansion of lamina propria lymphoplasma cells, cryptitis, crypt abscesses, crypt destruction/dropout, crypt architecture alteration and basal lymphoplasmacytosis.
In another study, out of 90 colonic biopsies taken from patients with ICI-related colitis, the most common feature was an increase in lamina propria cellularity (83%) and the second most common feature was neutrophilic infiltration (79%).33 Other notable features are increased intraepithelial lymphocytes and absence of granulomas. It is worth mentioning that features of chronicity, for example basal plasmacytosis and architectural distortion, are usually absent (Figure 2).32
Although the histological features due to anti-PD1 therapy tend to be similar, there have been some reports of granulomas being seen in biopsies and features of chronicity could be seen in recurrent colitis related to the drug.32,34
Overall, for most cases of ICI-related colitis, histologic features resemble that of severe active ulcerative colitis (UC), but no specific histologic pattern or feature has been identified. As compared to true UC, however, the crypt destruction and ballooning distention are more prominent, and features of chronicity are less impressive, particularly with only minimal crypt architecture distortion. In addition, some cases also show marked apoptotic activity in cryptal epithelium, which is not a common feature of inflammatory bowel disease (IBD). It is worth noting, however, that IBD itself is a group of disorders with considerable heterogeneity, and although there are some features common to both IBD and ICI-related colitis, there is considerable variation noted in this condition.
Imaging
Cross-sectional imaging primarily in the form of computed tomography (CT) or magnetic resonance imaging is helpful to determine the extent of inflammation and exclude complications such as perforation. A CT scan should be considered when patients present with alarm symptoms like sudden-onset abdominal pain or features of sepsis. In a retrospective study of patients who developed ipilimumab-associated colitis, three predominant radiological patterns were observed on CT or positron emission tomography/CT studies. Isolated recto-sigmoid colitis without diverticulosis, diffuse colitis and segmental colitis associated with diverticulosis were observed in 50%, 33% and 17% of patients, respectively.35 In a study published in 2017, CT findings were found to be highly predictive of colitis on biopsy, with a positive predictive value of 96%.36 In symptomatic patients who had CT evaluation, CT was highly predictive of the need for steroids to reach resolution of symptoms, with a positive predictive value of 92%.36
Other CT findings, such as mesenteric vessel engorgement, bowel wall thickening and colonic distention could be seen (extensive or segmental); segmental findings could lead to a reported differential diagnosis of diverticular sigmoiditis.37,38
Diagnosis of hepatic dysfunction
As previously mentioned, the most common form of hepatotoxicity related to ICI therapy is hepatitis, defined by elevations in serum aminotransferases (ALT and AST) with or without change in bilirubin levels. Based on current available data, it is noted in about 5–10% of patients treated with a single ICI agent, but is more commonly seen (nearly 20%) with combination therapies.39,40
The rise in liver enzyme levels is asymptomatic unless accompanied by a significant rise in bilirubin causing jaundice, which is rare. The condition is usually picked up on routine blood tests. The rise in enzyme levels appears to be significantly high, starting at about 6 weeks after initiation of therapy, and the risk appears to be sustained up to 14 weeks after the drug is given.
Patients who have documented high transaminases should be monitored closely. They should have investigations in keeping with the general principles of testing for liver disease. A full noninvasive liver screen including for viral hepatitis [hepatitis A, B and C viruses, Epstein–Barr virus (EBV), cytomegalovirus (CMV) and varicella zoster virus], an autoimmune screen which includes antinuclear antibodies, anti-cytoplasmic antibodies, anti-mitochondrial antibodies and anti-smooth muscle antibodies should be completed. An ultrasound of the liver is mandatory, and getting a portal vein Doppler to rule out a thrombus would be prudent in view of the increased thrombosis risk due to malignancy. Any positive tests from the above panel should prompt appropriate further investigations. If negative, patients can be treated as per guidance for ICI-related hepatotoxicity, which is discussed in more detail in a separate section.
In rare cases, a liver biopsy may be indicated, but this has to be carefully considered in select patients, given the significant risk and relatively low benefit. Histologically, the ICI-related hepatotoxicity is commonly hepatitis and is characterized by predominantly lobular inflammation with milder portal inflammation. The infiltrating inflammatory cells are largely CD3+/CD8+ T cells. Bile duct injury is rare and very mild if present.41 Anti-CTLA-4 related hepatitis is also often associated with nonnecrotizing granulomas.42
Management
Management of GI AEs
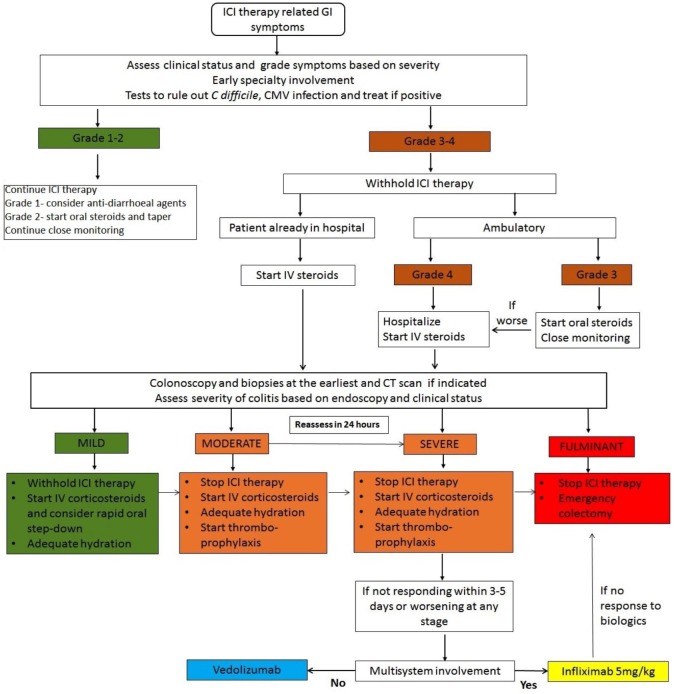
There are a number of suggested management algorithms based on the severity of diarrhoea. There is one recent proposed treatment algorithm that suggests managing the ICI-related AE based on the type of immune infiltrate noted in the affected organ and drawing from experience in other autoimmune conditions.43 Although this is a very reasonable approach, in clinical practice the distinction in infiltrates may not always be clear enough to guide treatment decisions. We propose a simple and effective algorithm that is guided by clinical symptoms and supported by endoscopic appearance and imaging (Figure 3).
Figure 3.
Proposed algorithm for management of checkpoint inhibitor-associated diarrhoea or colitis.
CMV, cytomegalovirus; CT, computed tomography; ICI, immune checkpoint inhibitors; IV, intravenous.
Management of mild diarrhoea (bowels open <4 times per day) is generally supportive. Budesonide can be considered as an oral corticosteroid. For moderate colitis (bowels open 4–6 times per day) treatment with prednisolone 0.5–1.0 mg/kg/day is recommended. Patients with severe diarrhoea (bowels opened >6 times per day) should ideally be commenced on intravenous corticosteroids (methylprednisolone 1–2 mg/kg/day), and in steroid-responsive patients this should be followed by a tapering course of oral prednisolone over 6–8 weeks. There are currently no predictive markers to determine nonresponse to corticosteroid therapy.
Steroid-refractory patients (nonresponse to intravenous corticosteroids after 72 h) require treatment with anti-TNF therapy. Infliximab has been used successfully in this setting with a dosing regimen similar to that in IBD, starting with an initial dose of 5 mg/kg.44 A further dose may be required after 2 weeks. Following response to infliximab, prolonged therapy with a tapering course of oral prednisolone may be necessary and a subgroup of patients may develop steroid-dependent disease. Johnson and colleagues evaluated the effect of early use of infliximab for ICI-related colitis.45 The authors noted that patients who received infliximab and steroids combined had significantly shorter times to resolution of diarrhoea (median 3 days versus 9 days; p < 0.001) and steroid titration (median 4 days versus 13 days; p < 0.001) compared to patients who were given corticosteroids alone. Among patients receiving combined infliximab and steroids, the majority (86%) had documented grade 3/4 colitis. It was also noted that there was no increased risk of failure of the ICI therapy in patients exposed to infliximab and the overall survival was greater in the group receiving combined infliximab and steroids after a median follow up of 26 months.45
There is now emerging evidence that the anti-integrin antibody, vedolizumab, is also an effective option. A case report first showed the efficacy of vedolizumab in the setting of steroid-dependent ipilimumab-associated colitis.46 Another study recently published in 2018 reported the outcomes of vedolizumab use in patients with ICI-related colitis.47 A retrospective review of 28 patients who received vedolizumab for steroid-refractory disease showed that 24 achieved clinical remission, with mean duration of follow up of 15 months (Table 3). It is worth mentioning that in this cohort, although patients who had not received infliximab prior to vedolizumab therapy had a higher success rate (95%), the success rate among patients exposed to infliximab remained as high as 67%. Figure 3 is a proposed algorithm to manage patients with or without multisystem involvement.
Table 3.
Summary of studies of ICI-related GI and liver complications refractory to steroids.
| Therapy for ICI-related colitis | Study | Dosages used | Results |
|---|---|---|---|
| Infliximab | Johnson et al.45
Compared infliximab versus corticosteroids (75 patients, 48% received infliximab) |
1–3 infusions of infliximab | • Infliximab + steroids superior to steroids
alone • Resolution of diarrhoea 3 days versus 9 days (median) • Duration of steroid use shorter at 35 days versus 51 days (median) |
| Pagès et al.44
(case report of one patient) |
Infliximab (5 mg/kg) single dose | • Symptom resolution in 2 days • Mucosal healing on endoscopy noted on day 7 |
|
| Vedolizumab | Hsieh et al.46
(case report of one patient) |
Standard induction dose (300 mg at 0, 2 and 6 weeks) | • Resolution of symptoms in 6 weeks • Successfully weaned off steroids • Mucosal healing on endoscopy by 6 weeks |
| Abu-Sbeih et al.47
(28 patients) |
3 infusions of vedolizumab | • Duration for improvement in symptoms after vedolizumab was
5 days (median) • Sustained clinical remission in 84% of patients |
|
| Faecal microbial transplant | Wang et al.48
(case report of two patients) |
FMT delivered via colonoscopy (50 g/250 ml) of liquid donor stool | • Clinical improvement with one patient but patient died
after 3 months due to primary malignancy • Sustained remission after 7 months of treatment with second patient |
| Therapy for ICI-related hepatic complications | |||
| Mycophenolate mofetil | Tanaka et al.49
(case report of one patient) |
2 g/day in addition to steroids (2 g for about 6 weeks and then tapered down and stopped in 2 weeks) | • Improvement in both AST and ALT with no recurrence after stopping MMF therapy |
| Anti-thymocyte globulin | Chmiel et al.50
(case report of one patient) |
1.5 mg/kg over 2 consecutive days followed by 2 doses over next 2 weeks | • Reduction in transaminases within 24 h and was sustained |
| Toclizumab | Stroud et al.51
(39 patients received toclizumab but included other indications) |
4 mg/kg infusion over 1 h Multiple infusions as per clinician decision |
• Clinical improvement reported in 79% of patients but no specific data on hepatitis only |
ICI, immune checkpoint inhibitors; GI, gastrointestinal; MMF, Mycophenolate mofetil.
There is a theoretical concern that immunosuppressive therapy may compromise the anti-tumour response to checkpoint inhibitor therapy. Systemic corticosteroids do not appear to affect the response rates to ipilimumab or nivolumab; however, the effect of other immunosuppressive therapy such as infliximab remains unclear.52,53
ICI therapy should be interrupted on development of colitis and permanent discontinuation is recommended for episodes that progress to severe or life-threatening stages, classified as grade 3 or 4 according to the National Cancer Institute’s Common Terminology (NCI CTCAE) criteria.4 In other cases, reintroduction can be cautiously considered on a case-by-case basis. A retrospective study suggested that anti-PD-1 therapy can be administered safely in patients who previously experienced grade 3 or 4 colitis with ipilimumab.54 At present there are no data supporting prophylactic corticosteroid therapy for IrAEs.55
Faecal microbiota transplant (FMT) has recently been used for ICI-related colitis and early reports from a case series of two patients treated with FMT appear encouraging.48 FMT resulted in complete resolution of symptoms followed by improvement in endoscopic appearances (Table 3). This is very early work and more data and evidence is required in this area before this can be recommended.
A summary of therapies currently used, relevant studies regarding the same and the dose regimes used in these studies are listed in Table 3.
Management of hepatic dysfunction
The standard management of grade 1–2 hepatic dysfunction is generally in the form of closer monitoring to ensure that worsening liver tests towards a grade 3–4 AE are picked up early on.9 The management of grades 3–4 liver toxicity requires high-dose intravenous glucocorticoids for 24–48 h, followed by an oral steroid taper with prednisolone at 1–2 mg/kg over at least a period of 30 days.9 It is safer to withhold immunotherapy until the liver function tests return to at least grade 1.10 Any derangement beyond eight times the upper limit of normal should be measured three times per week until an improving trend is noticed.
During the course of treatment, if there is no improvement or there is a worsening trend of liver function tests within 48 h of systemic steroids, mycophenolate mofetil 500 mg every 12 h should be considered.49 In refractory cases, anti-thymocyte globulin can be tried at a dose of 1.5 g/kg for 48 h, which has been reported to be successful in a patient who developed ipilimumab-related liver toxicity.50 There is one study where anti IL-6 antibody toclizumab was used to manage all IrAEs, including hepatitis.51 There was clinical improvement in nearly 80% of patients (Table 4).
Table 4.
Management of hepatitis related to ICI therapy.
| Grade 1–2 hepatitis | Grade 3 hepatitis | Grade 4 hepatitis | |
|---|---|---|---|
| Criteria | AST or ALT 1–2.5 × ULN and/or total bilirubin 1–1.5 × ULN | AST or ALT >5 × ULN and/or total bilirubin >3 × ULN | AST or ALT >8 × ULN |
| ICI therapy | Continue but with close monitoring | Discontinue | Discontinue |
| Steroids | Consider oral steroids | High-dose IV steroids for 48 h and taper with oral steroids | High-dose IV steroids |
| Other drugs to consider | None at this stage | • Mycophenolate mofetil 500 mg BD if no improvement after 48 h of steroids | • Longer duration of high-dose IV
steroids • Anti-thymocyte globulin • Toclizumab (anti IL-6 antibody) |
BD, twice daily; IV, intravenous; ULN- upper limit of normal.
ICI-related hepatitis may take up to 3 months to resolve completely after withdrawal of the offending drug.
Predicting GI and hepatic AEs
It has been suggested that intrinsic patient factors may be responsible for IrAEs and this could possibly be identified by identification of genetic, epigenetic or other predictive markers.43
There are patient factors – for example, female sex, baseline sarcopenia, concurrent medications – that have been identified as affecting outcomes and severity of ICI-related toxicity; some biomarkers, such as T cell population, increased eosinophil counts, increased IL-17 levels, reduced circulating IL-6 levels and gut microbiome, have been shown to predict ICI-related AEs.56–58 In one study reported by Chaput and colleagues, it was noted that patients with melanoma who had baseline microbiota enriched with the Faecalibaterium genus and other Firmicutes had higher incidence of ICI-related colitis when exposed to ipilimumab; on the other hand, it was also noted that patients who had Bacteroidetes remained free of ICI-related colitis.59 These findings may be useful in predicting IrAEs in the future, but more studies are needed before this is used routinely.
In another study published by Friedlander and colleagues in 2018, the group retrospectively looked at gene signatures from patients on tremelimumab in a clinical trial to see if there are any predictive markers of ICI-related IrAEs in patients with melanoma.60 Peripheral blood gene expression signatures were checked pre- and post-treatment for patients who had documented GI toxicity. In the pre-treatment data, no gene significantly predicted development of grade 2 or higher colitis, but they identified a 16-gene signature that probably could distinguish onset of severe versus mild or no diarrhoea. The gene signature dataset was validated in another tremelimumab clinical trial at a later date. Out of the 16-gene signature, six were found to be predictive – CCL3, CCR3, IL5, IL8, PTGS2, GADD45A – and were seen to be upregulated in patients with toxicity.60
Conclusion
ICI therapy has led to a paradigm shift in oncology. The IrAEs due to ICI are common and with their increasing use it is imperative that clinicians recognize these early and initiate prompt treatments. Immune-related colitis and hepatitis are likely to be encountered more frequently by gastroenterologists, who will need to be aware of these AEs in order to manage patients safely and effectively. Early recognition and treatment are critical as the majority of patients who are managed appropriately show good clinical response, go into remission and have fewer serious complications. Based on current evidence, early aggressive management of colitis with steroids and biologics like infliximab or vedolizumab appears to be beneficial, with good success rates. In refractory colitis, FMT is an emerging option although more studies are required to establish its efficacy and safety. Immune-mediated hepatitis requires close monitoring and sometimes temporary withdrawal of ICI in severe cases, but overall the response to steroids appears to be good.
Footnotes
Author contributions: UNS, literature search, evidence procurement, writing and editing the manuscript, revision, approval and submission; LJ, writing and editing the manuscript, images and approval; XG, histology images and legends, sections of the manuscript, revision and final approval; CLSS, revision of the manuscript and approval; OFA, literature search, writing and editing sections of the manuscript, revision and approval; AA, revision, critical review of the manuscript and approval; MI, revision, critical review of the manuscript and approval; SG, plan of the review, critical review of the manuscript, revision, overall supervision and final approval.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: UNS, SG and MI are funded by the NIHR Birmingham Biomedical Research Centre.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Uday N Shivaji  https://orcid.org/0000-0002-6800-584X
https://orcid.org/0000-0002-6800-584X
Contributor Information
Uday N. Shivaji, National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, UK Institute of Immunology and Immunotherapy, University of Birmingham, UK.
Louisa Jeffery, National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, UK; Institute of Immunology and Immunotherapy, University of Birmingham, UK.
Xianyong Gui, Department of Pathology, University of Washington, Seattle, WA, USA.
Samuel C. L. Smith, Institute of Immunology and Immunotherapy, University of Birmingham, UK Institute of Translational Medicine, Birmingham, UK.
Omer F. Ahmad, Department of Gastroenterology, University College London Hospital, London, UK
Ayesha Akbar, St Mark’s Hospital, IBD Unit, London, UK.
Subrata Ghosh, National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, UK; Institute of Immunology and Immunotherapy, University of Birmingham, UK; Institute of Translational Medicine, University of Birmingham, Edgbaston, Birmingham B15 2TH, UK.
Marietta Iacucci, National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, UK; Institute of Immunology and Immunotherapy, University of Birmingham, UK; Institute of Translational Medicine, Birmingham, UK.
References
- 1. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016; 54: 139–148. [DOI] [PubMed] [Google Scholar]
- 2. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006; 24: 2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang DY, Ye F, Zhao S, et al. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: a systematic review and meta-analysis. Oncoimmunology 2017; 10: e1344805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Institute of Health. Common Terminology Criteria for Adverse Events (CTCAE). NIH Publ. 2010;2009:0–71. [Google Scholar]
- 5. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ (Online) 2018;360:k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yip RH, Lee LH, Schaeffer DF, et al. Lymphocytic gastritis induced by pembrolizumab in a patient with metastatic melanoma. Melanoma Res 2018;28:645–647. [DOI] [PubMed] [Google Scholar]
- 8. Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010;11:155–164. [DOI] [PubMed] [Google Scholar]
- 9. Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 2016;45:7–18. [DOI] [PubMed] [Google Scholar]
- 10. Hahn AW, Gill DM, Agarwal N, et al. PD-1 checkpoint inhibition: toxicities and management. Urol Oncol 2017;35:701–707. [DOI] [PubMed] [Google Scholar]
- 11. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 2016;45:7–18. [DOI] [PubMed] [Google Scholar]
- 13. Su Q, Zhang X, Shen X, et al. Risk of immune-related colitis with PD-1/PD-L1 inhibitors vs chemotherapy in solid tumors: systems assessment. J Cancer 2018;9:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fessas P, Lee H, Ikemizu S, et al. A molecular and preclinical comparison of the PD-1–targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol 2017;44:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ji HH, Tang XW, Dong Z, et al. Adverse event profiles of anti-CTLA-4 and anti-PD-1 monoclonal antibodies alone or in combination: analysis of spontaneous reports submitted to FAERS. Clin Drug Investig 2019;39:319–330. [DOI] [PubMed] [Google Scholar]
- 16. Chen WW, Razak AR, Bedard PL, et al. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann Oncol 2015;26:1824–1829. [DOI] [PubMed] [Google Scholar]
- 17. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Granier C, De Guillebon E, Blanc C, et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017;2:e000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar P, Bhattacharya P, Prabhakar BS. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun 2018;95:77–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206:3015–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith SC, Zardo D, Cannatelli R, et al. Endoscopic findings of checkpoint inhibitor-induced ileitis with use of the latest advanced endoscopic optical diagnosis: near-focus narrow-band imaging. VideoGIE 2019;4:133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer 2019;7:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013;119:1675–1682. [DOI] [PubMed] [Google Scholar]
- 27. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006;24:2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2016;34:785–792. [DOI] [PubMed] [Google Scholar]
- 29. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun 2010;10:11. [PMC free article] [PubMed] [Google Scholar]
- 30. Abu-Sbeih H, Ali FS, Luo W, et al. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer 2018;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marthey L, Mateus C, Mussini C, et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis 2016;10:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karamchandani DM, Chetty R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists’ perspective. J Clin Pathol 2018;71:665–671. [DOI] [PubMed] [Google Scholar]
- 33. Geukes Foppen MH, Rozeman EA, van Wilpe S, et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open 2018;3:e000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen JH, Pezhouh MK, Lauwers GY, et al. Histopathologic features of colitis due to immunotherapy with anti-PD-1 antibodies. Am J Surg Pathol 2017;41:643–654. [DOI] [PubMed] [Google Scholar]
- 35. Barina AR, Bashir MR, Howard BA, et al. Isolated recto-sigmoid colitis: a new imaging pattern of ipilimumab-associated colitis. Abdom Radiol 2016;41:207–214. [DOI] [PubMed] [Google Scholar]
- 36. Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic comparison of CT scans and colonoscopy for immune-related colitis in ipilimumab-treated advanced melanoma patients. Cancer Immunol Res 2017;5:286–291. [DOI] [PubMed] [Google Scholar]
- 37. Kim KW, Ramaiya NH, Krajewski KM, et al. Ipilimumab-associated colitis: CT findings. Am J Roentgenol 2013;200:W468–W474. [DOI] [PubMed] [Google Scholar]
- 38. Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut 2018;67:2056–2067. [DOI] [PubMed] [Google Scholar]
- 39. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reddy HG, Schneider BJ, Tai AW. Immune checkpoint inhibitor-associated colitis and hepatitis. Clin Transl Gastroenterol 2018;9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol 2018;31:965–973. [DOI] [PubMed] [Google Scholar]
- 42. De Martin E, Michot JM, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181–1190. [DOI] [PubMed] [Google Scholar]
- 43. Martins F, Sykiotis GP, Maillard M, et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol 2019;20:e54–e64. [DOI] [PubMed] [Google Scholar]
- 44. Pagès C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res 2013;23:227–230. [DOI] [PubMed] [Google Scholar]
- 45. Johnson DH, Zobniw CM, Trinh VA, et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer 2018;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsieh A, Ferman M, Brown M, et al. Vedolizumab: a novel treatment for ipilimumab-induced colitis. BMJ Case Rep 2016:bcr2016216641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abu-Sbeih H, Ali FS, Alsaadi D, et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J Immunother Cancer 2018;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med 2018;24:1804–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tanaka R, Fujisawa Y, Sae I, et al. Severe hepatitis arising from ipilimumab administration, following melanoma treatment with nivolumab. Jpn J Clin Oncol 2017;47:175–178. [DOI] [PubMed] [Google Scholar]
- 50. Chmiel KD, Suan D, Liddle C, et al. Resolution of severe ipilimumab-induced hepatitis after antithymocyte globulin therapy. J Clin Oncol 2011;29:e237–e240. [DOI] [PubMed] [Google Scholar]
- 51. Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 2017;25:551–557. [DOI] [PubMed] [Google Scholar]
- 52. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2016;35:785–792. [DOI] [PubMed] [Google Scholar]
- 54. Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–376. [DOI] [PubMed] [Google Scholar]
- 55. Maraveyas A, Weber J, Thompson JA, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009;15:5591–5598. [DOI] [PubMed] [Google Scholar]
- 56. Hopkins AM, Rowland A, Kichenadasse G, et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer 2017;117:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nakamura Y. Biomarkers for immune checkpoint inhibitor-mediated tumor response and adverse events. Front Med 2019;6:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28:1368–1379. [DOI] [PubMed] [Google Scholar]
- 60. Friedlander P, Wood K, Wassmann K, et al. A whole-blood RNA transcript-based gene signature is associated with the development of CTLA-4 blockade-related diarrhea in patients with advanced melanoma treated with the checkpoint inhibitor tremelimumab. J Immunother Cancer 2018;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]