Short abstract
Background
Brain iron accumulation is associated with multiple sclerosis (MS). Hepcidin is the master regulator of iron homeostasis and distribution. Dysregulation of hepcidin is a feature of different chronic inflammatory diseases but has not been investigated in MS so far.
Objective
The aim of this study was to determine serum hepcidin levels of MS patients and healthy volunteers serving as controls and to investigate possible relations between hepcidin levels, disease activity and disease course.
Methods
In a cross-sectional design, we measured serum hepcidin levels in 71 MS patients and 16 healthy controls (HC). MS patients were sub-grouped in active relapsing–remitting MS (aRRMS), inactive (i)RRMS, active progressive MS (aPMS) and inactive (i)PMS. Blood parameters were measured by standard laboratory methods.
Results
Median hepcidin levels were 26.9 ng/ml (confidence interval (CI) 22.8; 30.9) in MS and 17.3 ng/ml (CI 12.8; 23.4) in HC with significant age and sex effects. Hepcidin correlates were in line with hepcidin as an indicator of iron stores. After correction for age and sex, hepcidin was neither associated with MS subgroups nor degree of disability and occurrence of relapses.
Conclusions
Serum hepcidin levels are not associated with disease activity and disease course in MS.
Keywords: Multiple sclerosis, hepcidin, iron, progressive, active, biomarker
Introduction
Metabolism and deposition of iron is crucial for a normal function of the brain. A dysregulation of iron homeostasis is known to be associated with normal aging but also neurodegenerative disorders of the central nervous system (CNS), including multiple sclerosis (MS).1–3
In MS, iron accumulation occurs within both grey and white brain matter.4,5 However, the link between observed iron deposition and the underlying pathophysiology within the brain is not well understood.
Several studies investigated a correlation between iron accumulation in the brain and parameters of iron metabolism within serum and cerebrospinal fluid (CSF). While serum and CSF levels of iron were found to be normal, surveys of indices of iron metabolism such as ferritin, transferrin receptor (TfR) and soluble transferrin receptor (sTfR) have yielded conflicting results.6–10
The liver-derived peptide hormone hepcidin is the master regulator of iron homeostasis and tissue distribution in mammals. Hepcidin controls the transport of iron from the intestinal lumen and cells such as macrophages into the plasma.11–14 Mechanistically, hepcidin binds extracellular domains of the sole cellular iron exporter, ferroportin (FPN) causing its internalization and subsequent degradation.15 Hepcidin expression is induced mainly in hepatocytes by iron loading, inflammatory signals and endoplasmic reticulum stress, whereas iron deficiency, hypoxia, anaemia and several hormones reduce hepcidin formation.3 Hepcidin lowers systemic iron levels and shifts iron from plasma to cellular iron stores via reducing duodenal iron absorption and iron release from hepatocytes and macrophages. Dysregulation of hepcidin is associated with different diseases, such as anaemia of chronic disease due to permanent inflammation and overexpression of hepcidin or genetic iron overload, haemochromatosis, caused by a lack of or a resistance to hepcidin.11,14,16
Despite the crucial roles of iron in MS and hepcidin in regulating iron homeostasis, hepcidin has not been investigated in MS so far.
Therefore, the aim of this study was to determine serum hepcidin levels of MS patients, to compare them with laboratory data of healthy volunteers serving as controls and to investigate possible relations between hepcidin levels and disease activity and disability progression.
Methods
In a cross-sectional design, we recruited 71 MS patients (diagnosed according to McDonald criteria 2010) aged between 18 and 65 years from the MS Clinic of the Department of Neurology at the Medical University Innsbruck and 16 healthy controls.17 Patients with an acute infection defined as fever within 14 days before the study visit or C-reactive protein (CRP) levels >1 mg/dl were excluded. Other exclusion criteria were disease-modifying treatment (DMT) with interferon beta, malignant disease, rheumatic disease, a glomerular filtration rate <60 ml/min/1.73 m2, diabetes mellitus defined as HbA1c > 6.5mg/dl and anaemia defined as haemoglobin <120 g/l in women and <130 g/l in men.
At the clinical study visit, we obtained demographic data, neurological and treatment history including DMT, occurrence and date of relapses, and expanded disability status scale (EDSS).18 A relapse was defined as patient-reported symptoms or objectively confirmed neurological signs typical of an acute CNS inflammatory demyelinating event with a duration of at least 24 hours in the absence of fever or infection and separated from the last relapse by at least 30 days.17 Disability progression was defined as a confirmed EDSS increase of ≥1.0 point in patients with a previous score of ≤5.5, or an increase of ≥0.5 points in patients with a previous score of >5.5 sustained for at least six months.
MS patients were then divided into four subgroups according to disease course: (a) active relapsing–remitting MS (aRRMS), (b) inactive (i)RRMS, (c) active progressive MS (aPMS) and (d) inactive (i)PMS. Activity was defined as the occurrence of one or more relapses within three months prior to the study visit or disability progression within six months prior to the study visit.19
Hepcidin measurement
Serum was collected at the date of the study visit in our MS outpatient clinic. Serum hepcidin concentration was determined by a commercial ELISA (Intrinsic Lifesciences, La Jolla, CA) according to manufacturer’s protocol in samples obtained from healthy and MS individuals.
The investigators performing the hepcidin testing were blinded to clinical parameters and the investigators assessing clinical parameters were blinded to hepcidin results.
Ethics
Confidentiality and data protection are ensured in keeping with the recommendations of the declaration of Helsinki. The study was approved by the ethics committee of the Medical University Innsbruck (ethical approval number: AM3743-281/4.3) and all participants gave written informed consent before inclusion into the study. Participants’ data were stored and analysed in an anonymized form which precludes linking clinical information with personal data.
Statistics
Statistical analysis was performed using SPSS 25.0 (SPSS Inc, Chicago, IL, USA). Categorical variables were expressed in absolute numbers and percentages. Continuous variables were tested for normal-distribution by the Kolmogorow–Smirnow test and displayed as mean and 95% confidence intervals (CI) or median and inter-quartile range (IQR) as appropriate.
Univariate comparisons were done by Chi-square-test, Kruskal–Wallis test, independent t-test (with Welch’s correction in case of unequal standard deviations between the groups) or ANOVA as appropriate.
We performed linear regression models (fixed effect: study group, sex and age) with hepcidin and parameters of iron metabolism (iron, ferritin, transferrin, transferrin saturation (TFS), sTfR, hepcidin–ferritin ratio, hepcidin–TFS ratio and hepcidin–TfR ratio) as the dependent variable.
Results
Characteristics of MS patients and healthy controls (HC) are given in Table 1.
Table 1.
Characteristics of multiple sclerosis (MS) patients and healthy controls (HC).
| MS relapsing active (n = 9) | MS relapsing inactive (n = 32) | MS progressive active (n = 14) | MS progressive inactive (n = 16) | HC (n = 16) | p-value | |
|---|---|---|---|---|---|---|
| Femalea | 7 (77.8) | 25 (78.1) | 10 (71.4) | 10 (62.5) | 8 (50) | 0.328d |
| Age (years)b | 41.5 (9.1) | 41.1 (8.2) | 51.6 (7.0) | 54.3 (7.5) | 31.8 (11.6) | <0.001e |
| Disease duration (years)b | 11.6 (5.8) | 12.7 (6.8) | 19.0 (8.7) | 22.8 (8.5) | NA | 0.003e |
| Relapse within last three monthsa | 9 (100) | 0 (0) | 3 (21.4) | 0 (0) | NA | <0.001d |
| EDSSc | 2.0 (0–6.5) | 2.0 (0–6.5) | 4.5 (3–7.5) | 4.5 (3–8.0) | NA | 0.027f |
| EDSS progression withinlast six monthsa | 6 (66.6) | 0 (0) | 14 (100) | 0 (0) | <0.001d | |
| DMT receiveda | 6 (66.6) | 18 (56.3) | 6 (42.9) | 6 (37.5) | NA | 0.681d |
| Current DMT at baselinea | NA | NA | ||||
| Glatirameracetate | 1 (11.1) | 4 (11.4) | 0 (0) | 0 (0) | NA | |
| Dimethylfumarate | 2 (22.2) | 5 (14.3) | 0 (0) | 0 (0) | NA | |
| Fingolimod | 1 (11.1) | 1 (2.9) | 0 (0) | 0 (0) | NA | |
| Natalizumab | 2 (22.2) | 8 (22.9) | 0 (0) | 6 (33.3) | NA | |
| Rituximab | 0 (0) | 0 (0) | 6 (42.9) | 0 (0) | NA |
an (percentage); bmean (SD); cmedian (range); dChi-square test; eANOVA; fKruskal–Wallis test.
DMT: disease-modifying treatment; EDSS: expanded disability status scale.
Hepcidin levels correlated significantly with age (r = 0.258, p = 0.017), but not with disease duration (Supplementary Figure 1). Women had significantly lower mean hepcidin levels than men both in the MS (23.6 ng/ml (CI 19.2–28.1) v. 35.4 (CI 27.1–23.8), p = 0.008) and the HC group (13.2 (CI 6.1–20.3) v. 21.3 (CI 13.0–29.7), p = 0.010).
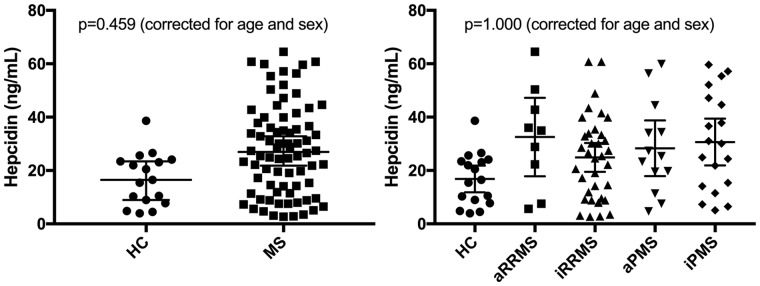
After correcting for age and sex, hepcidin levels neither significantly differed between MS patients and HC nor between MS subgroups (Figure 1(a) and 1(b), Table 2).
Figure 1.
Hepcidin levels in multiple sclerosis (MS) patients and healthy controls (HC). aRRMS: active relapsing-remitting MS; iRRMS: inactive RRMS; aPMS: active progressive MS; iPMS: inactive PMS.
p-values calculated by linear-regression models (fixed effect: study group, sex and age). Error bars indicate 95% confidence intervals.
Table 2.
Parameters of iron metabolism in multiple sclerosis (MS) patients and healthy controls.
| MS v. HC |
MS subgroups |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MS overall (n = 71) | HC (n = 16) | Unadjusted p-valueb | Adjusted p-valuec | aRRMS (n = 9) | iRRMS (n = 32) | aPMS (n = 14) | iPMS (n = 16) | Unadjusted p-valued | Adjusted p-valuee | |
| Hepcidin (ng/ml)a | 26.9 (16.9) |
17.3 (9.9) |
0.261 (0.029) [–9.8; –18.6–1.0] |
0.459 (0.051) [–9.4; –18.9–0.3] |
32.5 (19.1) |
22.8 (14.8) |
28.3 (17.3) |
30.4 (18.7) |
1.000 (0.313) |
1.000 (0.627) |
| Iron (µmol/l)a | 15.3 (5.8) |
19.1 (10.0) |
0.828 (0.092) [3.2; –2.2–8.6)] |
1.000 (0.592) [1.1; –3.0–5.3] |
16.0 (7.5) |
15.7 (6.2) |
13.9 (6.4) |
15.3 (3.4) |
1.000 (0.793) |
1.000 (0.951) |
| Ferritin (µg/l)a | 105.8 (113.9) |
68.2 (54.6) |
1.000 (0.212) [–38.2; –98.7–22.3] |
0.459 (0.051) [–56.5; –113.3–0.2] |
139.8 (105.1) |
90.3 (101.6) |
112.6 (171.5) |
111.8 (81.2) |
1.000 (0.694) |
0.936 (0.104) |
| Transferrin (mg/l)a | 263.8 (45.6) |
273.4 (48.3) |
1.000 (0.429) [–10.2; –35.6–15.2] |
1.000 (0.905) [–1.6; –28.9–25.6] |
263.7 (48.1) |
273.5 (45.6) |
264.5 (51.6) |
243.9 (35.2) |
1.000 (0.213) |
1.000 (0.982) |
| TFS (%)a | 23.8 (9.4) |
29.0 (14.5) |
1.000 (0.305) [4.0; –4.0–12.1] |
1.000 (0.591) [1.8; –4.7–8.3] |
25.4 (12.9) |
23.7 (9.8) |
21.2 (9.6) |
25.3 (5.9) |
1.000 (0.635) |
1.000 (0.903) |
| sTfR (mg/l)a | 2.7 (0.84) |
3.3 (1.01) |
0.387 (0.043) [0.6; 0.0–1.1] |
0.099 (0.011) [0.7; 0.2–1.3] |
2.5 (1.05) |
2.6 (0.91) |
3.0 (0.47) |
2.8 (0.85) |
1.000 (0.541) |
1.000 (0.182) |
| Hepcidin–Ferritin ratioa | 0.36 (0.20) |
0.38 (0.20) |
1.000 (0.698) [0.02; –0.09–0.14] |
1.000 (0.206) [0.08; –0.04–0.19] |
0.29 (0.12) |
0.37 (0.21) |
0.44 (0.25) |
0.32 (0.15) |
1.000 (0.244) |
1.000 (0.262) |
| Hepcidin–TFS ratioa | 1.16 (0.69) |
0.73 (0.42) |
0.126 (0.014) [–0.46; –0.82– –0.09] |
1.000 (0.129) [–0.31; –0.71–0.09] |
1.26 (0.56) |
0.99 (0.60) |
1.47 (0.60) |
1.18 (0.75) |
1.000 (0.191) |
1.000 (0.564) |
| Hepcidin–TfR ratioa | 11.5 (9.09) |
6.1 (3.54) |
0.144 (0.016) [–5.8; –10.4– –1.1] |
0.090 (0.010) [–6.6; –11.6– –1.6] |
17.5 (16.2) |
10.1 (7.4) |
9.8 (6.0) |
12.4 (8.3) |
1.000 (0.154) |
1.000 (0.130) |
aMean (SD).
bp-values calculated by t-test, corrected for multiple testing (Bonferroni) with uncorrected p-values in brackets and estimated mean differences between MS and HC with 95% confidence intervals in square brackets.
cp-values calculated by linear-regression models (fixed effects: study group, sex and age) corrected for multiple testing (Bonferroni) with uncorrected p-values in brackets and estimated mean differences between MS and HC with 95% confidence intervals in square brackets.
dp-values calculated by ANOVA corrected for multiple testing (Bonferroni) with uncorrected p-values in brackets.
ep-values calculated by linear-regression models (fixed effects: study group, sex and age) corrected for multiple testing (Bonferroni) with uncorrected p-values in brackets.
aRRMS: active relapsing–remitting MS; iRRMS: inactive RRMS; aPMS: active progressive MS; iPMS: inactive PMS; sTfR: soluble transferrin receptor; TFS: transferrin saturation.
We did not detect any significant differences in other parameters of iron metabolism (iron, ferritin, transferrin, TFS, sTfR, hepcidin–ferritin ratio, hepcidin–TFS ratio and hepcidin–TfR ratio) between MS patients and HC nor between MS subgroups (Table 2).
Neither hepcidin levels nor other parameters of iron metabolism correlated with EDSS or occurrence of relapses in the year before sampling (Supplementary Table 1). Also, there were no significant differences in hepcidin and other parameters of iron metabolism between MS patients with and without DMT.
Hepcidin significantly correlated with serum iron (r = 0.250, p = 0.038), ferritin (r = 0.625, p < 0.001), sTfR (r = –0.358, p = 0.003), transferrin (r = –0.652, p < 0.001), TFS (r = 0.484, p < 0.001), hepcidin–ferritin ratio (r = 0.729, p < 0.001), hepcidin–TFS ratio (r = 0.763, p < 0.001) and hepcidin–TfR ratio (r = 0.872, p < 0.001).
Discussion
The main finding of our study is that neither serum hepcidin levels nor other serum parameters of iron metabolism (iron, ferritin, transferrin, TFS, sTfR) significantly differ in MS patients compared to HC and are not associated with disease activity and disease course in MS.
Brain iron accumulation particularly in basal ganglia structures is a well-established feature of MS which has been associated with progressing disease.20–22 Although there is evidence that iron accumulation does not precede the development of MS pointing towards iron deposition as an epiphenomenon of MS pathology, the underlying pathophysiology is not well understood and the ‘chicken and egg question’ remains.20–22 Different hypotheses, for example dysfunction of the blood–brain barrier (BBB) and limited iron clearance and transport have been postulated.5
Hepcidin is the master regulator in controlling iron homeostasis and distribution.11–14 Dysregulation of hepcidin is a feature of different chronic inflammatory diseases.11,14,16 Elevated hepcidin levels were found in experimental autoimmune encephalomyelitis, an animal model of MS, indicating that iron accumulation may be due to hepcidin-mediated disruption of iron elimination from the brain.23–25 Similar to prior studies, we found hepcidin levels to be higher in men and positively correlated with age.26–28 Since we were primarily interested in the potential effect of MS diagnosis and disease activity on hepcidin levels, we corrected all analyses for sex and age and also excluded known confounding factors for hepcidin such as acute or chronic infection, malignant or rheumatic disease, diabetes mellitus, renal dysfunction, chronic inflammation and anaemia. Even in this confined and optimized setting, we detected neither significantly different serum hepcidin levels in MS patients compared to HC nor between MS subgroups, that is between relapsing and progressive MS or between active and inactive MS. There are two potential explanations for this finding: (a) hepcidin and consequently iron homeostasis is not involved in MS pathology at all or (b) the relevant pathophysiological processes involving iron metabolism are taking place in compartments behind the BBB and thus, cannot be captured by measurement in serum.
Various parameters of iron metabolism have been studied in both serum and CSF in MS. While iron levels in serum and CSF were consistently normal, data concerning ferritin, TfR and sTfR have been conflicting. Ferritin, the iron storage protein, was demonstrated to be increased in progressive MS in one study, while others did find increased ferritin only in CSF but not in serum.6–10 Transferrin, which is considered the main iron transporter in the body, in the brain and across the BBB interacting with TfR, was reported normal both in serum and CSF in some studies, while a more recent study showed significantly lower CSF transferrin levels within clinical isolated syndrome (CIS) and early MS.1,29–32 sTfR levels were postulated to be increased in active MS, both in progressive and relapsing–remitting forms.1 However, the one study reporting increased ferritin and sTfR in serum did not account for the confounding influence of sex, age, inflammatory status and specifically of interferon beta treatment, which is known to increase ferritin levels, and was limited by small sample size.6,33 Thus, while pathophysiological processes involving hepcidin and iron homeostasis might be involved in MS pathology, these are likely located behind the BBB and not reflected by alterations of iron metabolism parameters in serum.
In accordance with prior studies, hepcidin correlates were in line with hepcidin as an indicator of iron stores with positive correlations with serum iron, ferritin, hepcidin–ferritin ratio and negative correlations with sTfR and transferrin.11,12
Strengths of our study include that its participants and study groups are well characterized, that confounding factors were very rigorously excluded and that correction for multiple testing was performed. As a limitation, we did not have MRI parameters of iron deposition available in this study. Another weakness is the cross-sectional design which does not enable the assessment of potential temporal relationships between hepcidin and MS disease activity or disease course. Also, the HC group was significantly younger and included less women than the MS group. However, all our analyses were corrected for age and sex, thus limiting the potential bias resulting from the imbalance.
In conclusion, in serum neither hepcidin nor other parameters of systemic iron metabolism are useful as biomarkers of disease activity or disease course in MS. Further research on hepcidin and other parameters of iron metabolism may focus on CSF in order to elucidate the role of iron metabolism in MS.
Supplemental Material
Supplemental material, MSO885984 Supplemetal Material for Serum hepcidin levels in multiple sclerosis by Gabriel Bsteh Piotr Tymoszuk Klaus Berek Verena Petzer Florian Deisenhammer Guenter Weiss Thomas Berger in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Contributor Information
Gabriel Bsteh, Department of Neurology, Medical University of Vienna, Austria;; Department of Neurology, Medical University of Innsbruck, Austria
Piotr Tymoszuk, Department of Internal Medicine II, Medical University of Innsbruck, Austria.
Klaus Berek, Department of Neurology, Medical University of Innsbruck, Austria.
Verena Petzer, Department of Internal Medicine II, Medical University of Innsbruck, Austria.
Florian Deisenhammer, Department of Neurology, Medical University of Innsbruck, Austria.
Guenter Weiss, Department of Internal Medicine II, Medical University of Innsbruck, Austria.
Thomas Berger, Department of Neurology, Medical University of Vienna, Austria.
Conflicts of Interests
The author(s) declared the following potential conflicts ofinterest with respect to the research, authorship, and/orpublication of this article: Gabriel Bsteh has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene, Merck, Novartis, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Roche and Teva.
David Haschka declares no conflict of interest.
Piotr Tymoszuk declares no conflict of interest.
Klaus Berek declares no conflicts of interest.
Verena Petzer declares no conflicts of interest.
Harald Hegen has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Merck, Novartis, Sanofi-Genzyme and Teva, and received honoraria for consulting Teva.
Sebastian Wurth has participated in meetings sponsored by, received honoraria or travel funding from Allergan, Biogen, Ipsen Pharma, Merck, Novartis, Sanofi-Genzyme, Roche and Teva.
Michael Auer received speaker honoraria and/or travel grants from Biogen, Merck and Novartis.
Anne Zinganell has participated in meetings sponsored by, received speaking honoraria or travel funding from Biogen, Merck, Sanofi-Genzyme and Teva.
Franziska Di Pauli has received speaking honoraria from Biogen and Sanofi-Genzyme.
Florian Deisenhammer has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Bayer, Biogen, Merck, Novartis, Roche and Sanofi-Genzyme.
Guenter Weiss declares no conflict of interest.
Thomas Berger has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, Celgene, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Teva. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, Merck, Novartis, Sanofi Aventis, Teva) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Teva.
Funding
The author(s) received no financial support for theresearch, authorship, and/or publication of this article.
References
- 1.Sfagos C, Makis AC, Chaidos A, et al. Serum ferritin, transferrin and soluble transferrin receptor levels in multiple sclerosis patients. Mult Scler 2005; 11(3): 272–275. [DOI] [PubMed] [Google Scholar]
- 2.Khalil M, Teunissen C, Langkammer C. Iron and neurodegeneration in multiple sclerosis. Mult Scler Int 2011; 2011(2): 606807–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hentze MW, Muckenthaler MU, Galy B, et al. Two to tango: regulation of Mammalian iron metabolism. Cell 2010; 142(1): 24–38. [DOI] [PubMed] [Google Scholar]
- 4.Ropele S, de Graaf W, Khalil M, et al. MRI assessment of iron deposition in multiple sclerosis. J Magn Reson Imaging 2011; 34(1): 13–21. [DOI] [PubMed] [Google Scholar]
- 5.Stankiewicz JM, Neema M, Ceccarelli A. Iron and multiple sclerosis. Neurobiol Aging 2014; 35(S2): S51–58. [DOI] [PubMed] [Google Scholar]
- 6.Sfagos C, Makis AC, Chaidos A, et al. Serum ferritin, transferrin and soluble transferrin receptor levels in multiple sclerosis patients. Mult Scler 2005; 11(3): 272–275. [DOI] [PubMed] [Google Scholar]
- 7.LeVine SM, Lynch SG, Ou CN, et al. Ferritin, transferrin and iron concentrations in the cerebrospinal fluid of multiple sclerosis patients. Brain Res 1999; 821(2): 511–515. [DOI] [PubMed] [Google Scholar]
- 8.Petzold A, Eikelenboom MJ, Gveric D, et al. Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain 2002; 125(7): 1462–1473. [DOI] [PubMed] [Google Scholar]
- 9.Worthington V, Killestein J, Eikelenboom MJ, et al. Normal CSF ferritin levels in MS suggest against etiologic role of chronic venous insufficiency. Neurology 2010; 75(18): 1617–1622. [DOI] [PubMed] [Google Scholar]
- 10.Khalil M, Riedlbauer B, Langkammer C, et al. Cerebrospinal fluid transferrin levels are reduced in patients with early multiple sclerosis. Mult Scler 2014; 20(12): 1569–1577. [DOI] [PubMed] [Google Scholar]
- 11.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med 2011; 62(1): 347–360. [DOI] [PubMed] [Google Scholar]
- 12.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta Gen Sub 2009; 1790(7): 682–693. [DOI] [PubMed] [Google Scholar]
- 13.Brasse-Lagnel C, Karim Z, Letteron P, et al. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology 2011; 140(4): 1261–1271. [DOI] [PubMed] [Google Scholar]
- 14.Sebastiani G, Pantopoulos K. Disorders associated with systemic or local iron overload: from pathophysiology to clinical practice. Metallomics 2011; 3(10): 971–916. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004; 306(5704): 2090–2093. [DOI] [PubMed] [Google Scholar]
- 16.Pantopoulos K, Porwal SK, Tartakoff A, et al. Mechanisms of mammalian iron homeostasis. Biochemistry 2012; 51(29): 5705–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33(11): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 19.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83(3): 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ropele S, de Graaf W, Khalil M, et al. MRI assessment of iron deposition in multiple sclerosis. J Magn Reson Imaging 2011; 34(1): 13–21. [DOI] [PubMed] [Google Scholar]
- 21.Khalil M, Enzinger C, Langkammer C, et al. Quantitative assessment of brain iron by R 2* relaxometry in patients with clinically isolated syndrome and relapsing–remitting multiple sclerosis. Mult Scler 2009; 15(9): 1048–1054. [DOI] [PubMed] [Google Scholar]
- 22.Khalil M, Langkammer C, Ropele S, et al. Determinants of brain iron in multiple sclerosis: a quantitative 3T MRI study. Neurology 2011; 77(18): 1691–1697. [DOI] [PubMed] [Google Scholar]
- 23.Zarruk JG, Berard JL, Santos Dos RP, et al. Expression of iron homeostasis proteins in the spinal cord in experimental autoimmune encephalomyelitis and their implications for iron accumulation. Neurobiol Dis 2015; 81: 1–15. [DOI] [PubMed] [Google Scholar]
- 24.Ćurko-Cofek B, Kezele TG, Barac-Latas V. Hepcidin and metallothioneins as molecular base for sex-dependent differences in clinical course of experimental autoimmune encephalomyelitis in chronic iron overload. Med Hypotheses 2017; 107: 1–18. [DOI] [PubMed] [Google Scholar]
- 25.Varga E, Pandur E, Abrahám H, et al. Cuprizone administration alters the iron metabolism in the mouse model of multiple sclerosis. Cell Mol Neurobiol 2018; 38: 1081–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theurl I, Aigner E, Theurl M, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood 2009; 113(21): 5277–5286. [DOI] [PubMed] [Google Scholar]
- 27.Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood 2011; 117(25): e218–225. [DOI] [PubMed] [Google Scholar]
- 28.Pechlaner R, Kiechl S, Mayr M, et al. Correlates of serum hepcidin levels and its association with cardiovascular disease in an elderly general population. Clin Chem Lab Med 2016; 54(1): 5705–5711. [DOI] [PubMed] [Google Scholar]
- 29.Moos T, Nielsen TR, Skjørringe T, et al. Iron trafficking inside the brain. J Neurochem 2007; 103(5): 1730–1740. [DOI] [PubMed] [Google Scholar]
- 30.Rouault TA. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci 2013; 14(8): 551–564. [DOI] [PubMed] [Google Scholar]
- 31.LeVine SM, Lynch SG, Ou CN, et al. Ferritin, transferrin and iron concentrations in the cerebrospinal fluid of multiple sclerosis patients. Brain Res 1999; 821(2): 511–515. [DOI] [PubMed] [Google Scholar]
- 32.Khalil M, Riedlbauer B, Langkammer C, et al. Cerebrospinal fluid transferrin levels are reduced in patients with early multiple sclerosis. Mult Scler 2014; 20(12): 1569–1577. [DOI] [PubMed] [Google Scholar]
- 33.Sena A, Pedrosa R, Ferret-Sena V, et al. Interferon therapy increases serum ferritin levels in patients with relapsing-remitting multiple sclerosis. Mult Scler 2008; 14(6): 857–859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSO885984 Supplemetal Material for Serum hepcidin levels in multiple sclerosis by Gabriel Bsteh Piotr Tymoszuk Klaus Berek Verena Petzer Florian Deisenhammer Guenter Weiss Thomas Berger in Multiple Sclerosis Journal—Experimental, Translational and Clinical