Short abstract
Background
Serum neurofilament light chain concentration is a proposed biomarker of axonal injury in multiple sclerosis. Mesenchymal stem cells have anti-inflammatory and repair-promoting activities, making them of interest for potential multiple sclerosis treatment.
Objectives
The purpose of this study was to assess correlation of serum neurofilament light chain concentration and measures of multiple sclerosis disease activity/severity, longitudinal stability of serum neurofilament light chain concentration, and treatment effect of mesenchymal stem cell transplantation on serum neurofilament light chain concentration.
Methods
Twenty-four multiple sclerosis patients underwent intravenous infusion of autologous mesenchymal stem cells. Clinical assessments, serum collection, and brain magnetic resonance imaging were performed at months –1, 0 (transplant), 1, 3, and 6. Matched control serum was collected once (n = 10). Serum neurofilament light chain concentration was measured by single-molecule array. Serum neurofilament light chain concentration correlations with disease measures were analyzed by Spearman correlations and linear mixed effect models. Pre-post transplant serum neurofilament light chain concentration was compared by Wilcoxon signed rank testing.
Results
There were significant (p<0.01) correlations between serum neurofilament light chain concentration and gadolinium-enhancing lesion number (rho = 0.55) and volume (rho = 0.65), and new/enlarging T2 lesions (rho = 0.65). Patients without disease activity had lower fluctuation in serum neurofilament light chain concentration (p = 0.01). Mean pre- versus post-treatment serum neurofilament light chain concentration values were not significantly different.
Conclusions
Serum neurofilament light chain concentration correlated with magnetic resonance imaging measures of disease activity cross sectionally and longitudinally, and was stable in patients without disease activity. There was no clear treatment effect of mesenchymal stem cell transplantation on serum neurofilament light chain concentration.
Keywords: Multiple sclerosis, biomarkers, neurofilament light chain, mesenchymal stem cells
Introduction
Neurofilaments are protein components of the cytoskeleton of myelinated axons. Neurofilament-light chain concentration (NfL-c) has generated substantial interest as a putative biomarker of axonal injury and is being investigated in several neurological diseases, including multiple sclerosis (MS).1–11 However, it has yet to be fully validated for clinical use in these settings, as the significance and variability at the individual patient level is not yet known. Development of a biomarker for disease activity and potentially prognosis in MS is of significant interest and would be of great utility in clinical trials, observational studies, and patient care.12
In MS, prior studies demonstrated elevated NfL-c concentrations in cerebrospinal fluid (CSF) correlate with relapse, gadolinium-enhancing lesions on magnetic resonance imaging (MRI), and disease severity.1,11,13,14 Initiation of disease modifying therapy in MS is associated with decreased NfL-c levels in the CSF, possibly reflecting reduced axonal damage.15–17 Single-molecule array (Simoa) technology allows NfL-c to be detected in serum,18 and there is a strong correlation between CSF and serum NfL-c.1,14 Given the less invasive nature of a blood draw compared to lumbar puncture, evaluation of serum rather than CSF NfL-c is of substantial interest as a biomarker for MS disease activity and treatment response.
Mesenchymal stem cells (MSCs) are pluripotent, non-hematopoietic precursor cells that can be isolated from various tissues, including bone marrow and adipose, and culture-expanded to purity. MSCs are postulated to have a range of anti-inflammatory and repair-promoting activities, making them of interest in several neuroinflammatory and tissue injury conditions, including MS.19–21 Previously published studies regarding MSC transplantation in MS have been mostly phase I trials to examine safety and tolerability, and to date, the safety of MSC transplantation has been largely supported.20,22–26
In the MSC transplantation study performed at our institution, there was suggestion of benefit on the Expanded Disability Status Scale (EDSS), as 17 of 24 patients had improved EDSS at three months post-treatment versus baseline.22 Additionally, there was suggestion of benefit in two MRI measures, whole brain magnetization transfer ratio and magnetization transfer ratio peak height, which trended upwards over post-treatment months 1–3 compared to baseline. NfL-c has not been examined in previous studies of MSC transplantation.
In this study, we sought to compare serum NfL-c (sNfL-c) between MS patients and healthy controls, evaluate the correlation of sNfL-c and various imaging and clinical markers of MS disease activity and severity, evaluate the short-term longitudinal stability of sNfL-c, and examine the treatment effect of MSC transplantation on sNfL-c in a previously completed phase 1/2 trial of MSC transplantation in MS.
Materials and methods
Study design
A phase 1/2, single-center, single-arm clinical trial of autologous culture-expanded MSC transplantation enrolled patients between March 2011–April 2013 at the Cleveland Clinic Mellen Center. Methods have been previously described.22,27 The study involved laboratory, clinical, and imaging evaluations over a seven-month period, with MSC transplantation (considered month 0 (M0)) occurring two months following screening (month –2 (M–2)). Each patient had five banked serum samples, collected at month (M) –1, 0 (baseline), 1, 3, and 6, with concomitant clinical assessments and brain MRI. Cleveland Clinic Institutional Review Board approval was obtained, and informed consent was obtained from all patients prior to any study procedures (Study #10-726, #08-964).
Patient population
Eligible patients had relapsing–remitting MS (RRMS) or secondary progressive MS (SPMS), based on the 2010 McDonald criteria,28 were between the ages of 18–55 years, had an EDSS score of 3.0–6.5, and had evidence of disease activity or disability worsening in the two prior years. Patients were permitted to continue interferon-beta or glatiramer acetate during the study. Complete inclusion/exclusion criteria have been previously reported.22 Age- and sex-matched healthy controls provided a single serum sample, 10 of which were available for the current study.
Clinical assessment
At each study visit, patients underwent clinical assessment, including evaluation for relapse, EDSS, and Multiple Sclerosis Functional Composite (paced auditory serial addition test (PASAT), 9-hole peg test (9HPT), timed 25-foot walk (T25FW), and high- and low-contrast (2.5% and 1.25%) letter acuity (LCLA) using Sloan charts).29–31 PASAT score was reported as the three-second total correct. For 9HPT, patients underwent two trials per hand, and the average of the two trials (in seconds) in the worse hand was used for this analysis. For T25FW, patients underwent two trials and the average time (in seconds) of the trials was used for analysis. For LCLA, 2.5% visual acuity was reported as the number correct in the worse eye.
Imaging assessment
Brain MRIs were obtained on a 3T Siemens Trio MRI scanner (Erlangen, Germany) according to a standardized protocol at each study visit.22 Images were analyzed by the MRI Analysis Center in the Biomedical Engineering Department of Lerner Research Institute (Cleveland Clinic, Cleveland, Ohio, USA). MRI measurements included in the original study were gadolinium-enhancing lesion number and volume, new/enlarging T2-hyperintense lesion number, T2-hyperintense lesion volume (T2LV), T1-hypointense lesion volume (T1LV), normalized whole brain volume (brain parenchymal fraction (BPF)), gray matter fraction, whole brain diffusion tensor imaging (DTI, mean diffusivity (MD), fractional anisotropy (FA)), and whole brain magnetization transfer ratio (MTR). Additional MRI metrics analyzed post-hoc included cortical thickness and further DTI parameters (FA, MD, transverse diffusivity (TD), and axial diffusivity (AD)) in lesional tissue, corticospinal tracts (reported as average of left and right), and the transcallosal motor tract.32
Optical coherence tomography (OCT) was performed at each study visit using a Cirrus spectral domain machine (Carl Zeiss Meditec, Dublin, California, USA). Patients underwent two OCT scans at each visit. OCT parameters of interest include average and temporal retinal nerve fiber layer thickness (RNFL), both of which are reported as the mean of two measurements in the better eye at each visit, in microns.
Visual evoked potentials
Patients underwent visual evoked potentials (VEPs) at each visit, using an Eclipse Neurological Workstation (Axon Systems, Hauppauge, New York, USA) at a single center (Cleveland Clinic EEG Laboratory, Cleveland, Ohio, USA). Mean P100 latency for each eye was determined from two recordings.
Serum NfL-c measurement
Patient and control serum samples were run in duplicate on the Quanterix (Cambridge, Massachusetts, USA) Simoa assay (NF-light Advantage Kit) to quantify sNfL-c.
Statistical analysis
Demographics of MS patients and controls were compared by Student’s t-tests for continuous and Chi-squared testing for categorical variables. Given the non-normality of sNfL-c distributions, analyses employed non-parametric methods. Matched MS and control sNfL-c were compared at baseline by Wilcoxon rank sum testing for paired samples. sNfL-c was compared between patients with RRMS and SPMS via Kruskal-Wallis testing. Cross-sectional associations between sNfL-c and markers of MS disease activity and severity at baseline were evaluated via Spearman rank correlation coefficients. Metrics with a significant cross-sectional correlation were further investigated longitudinally using linear mixed effect models, using patient as a random effect.
To assess short-term longitudinal stability of sNfL-c, the mean absolute difference in sNfL-c compared to the first measurement (M–1) was calculated for each patient (sNfL-c fluctuation). Patients were then characterized as having inflammatory disease activity if they had any relapse, new/enlarging T2 hyperintense lesion, or gadolinium-enhancing lesion over the course of the study. Patients were further delineated into each component of inflammatory activity, and these groups (presence versus absence of relapse, new/enlarging T2 hyperintense lesion, or gadolinium-enhancing lesion) were compared as well. The mean sNfL-c fluctuation was then compared between the active versus non-active disease groups via an unpaired t-test.
To investigate a potential MSC treatment effect, pre- and post-treatment average sNfL-c values were compared by Wilcoxon signed rank testing for paired samples. The correlations between change in sNfL-c and change in selected clinical and MRI measures of interest in the pre- versus post-treatment periods (difference calculated between means for the pre- and post-treatment values) were evaluated using Spearman rank correlation coefficients. Given the exploratory nature of this study, we did not adjust for multiple comparisons. Statistical analyses were conducted in R (version 3.5.3), utilizing the packages spearmanCI (version 1.0), nlme (version 3.1–140), and tidyverse (version 1.2.1).
Results
Patient characteristics
Twenty-four patients with MS, 10 with relapsing–remitting and 14 with secondary progressive disease underwent MSC transplantation between 2011 and 2013.22 Twenty-two patients consented to accessory studies and had banked samples available. Sixteen of these 22 patients were female, and the patient age range was 18–55 years (mean 46.4 ± 5.2 years) (Table 1). Serum samples were available from 10 age- and sex-matched controls.
Table 1.
Patient characteristics.
| MS(n=22) | Controls(n=10) | |
|---|---|---|
| Age, years, mean (SD) | 46.4 (5.3) | 47.1 (6.2) |
| Gender, n (%) female | 15 (68.2) | 6 (60.0) |
| Race, n (%) | ||
| Caucasian | 21 (95.5) | 10 (100.0) |
| African-American | 1 (4.5) | 0 (0) |
| MS phenotype, n (%) | ||
| Relapsing–remitting (RR) | 9 (40.9) | N/A |
| Secondary progressive (SP) | 13 (59.1) | |
| MS disease duration, years, mean (SD) | 12.4 (9.4) | N/A |
| Expanded Disability Status Scale, median (IQR) | 6.0 (4.1–6.5) | N/A |
| sNfL-c, pg/ml, median (IQR) | 14.7 (11.1–16.3) | 6.9 (4.3–7.8) |
IQR: interquartile range; MS: multiple sclerosis; SD: standard deviation; sNfL-c: serum neurofilament light chain concentration.
sNfL-c in MS patients versus controls
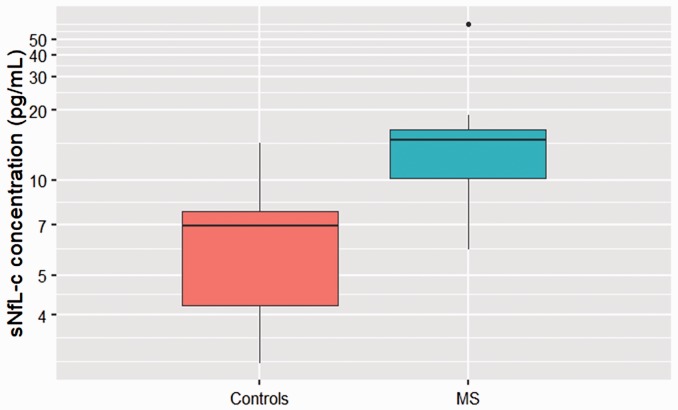
sNfL-c was higher in MS patients at baseline versus matched controls (n = 10, p = 0.008) (Figure 1). The median (interquartile range) sNfL-c in MS patients with a matched control was 14.60 (6.02) pg/ml (versus 6.92 (3.41) pg/ml in controls), and without a matched control was 14.71 (5.85) pg/ml. The median sNfL-c difference between paired MS patients and controls was 5.95 pg/ml (95% confidence interval (CI) 1.60–30.66). There was no difference in sNfL-c between patients with RRMS and SPMS (p = 0.393).
Figure 1.
Serum neurofilament light chain concentration (sNfL-c) in multiple sclerosis (MS) patients versus controls.
A boxplot compares sNfL-c in MS patients and matched controls, with y-axis on log scale. sNfL-c was higher in MS patients at baseline (median 14.60 pg/ml) versus matched controls (median 6.92 pg/ml) (n=10, p=0.008).
Clinical disability and sNfL-c
No significant correlations were observed between sNfL-c and clinical disability measures (EDSS, PASAT, 9HPT, T25FW, and LCLA) at baseline (n = 22) (Table 2). There were no relapses documented at the baseline visit, so the correlation between relapse and sNfL-c was instead investigated via longitudinal analysis, described below.
Table 2.
Correlations of serum neurofilament light chain concentration (sNfL-c) and disease activity measures.
| Spearman’s Rho(95% CI) | |
|---|---|
| Clinical | |
| Relapse | N/A |
| Expanded Disability Status Scale | 0.03 (–0.51–0.57) |
| Paced Auditory Serial Addition Test | –0.27 (–0.76–0.22) |
| 9-Hole Peg Test (worse hand) | 0.31 (–0.18–0.80) |
| Timed 25-Foot Walk | –0.08 (–0.56–0.41) |
| Low contrast visual acuity (2.5%, worse eye) | 0.23 (–0.35–0.80) |
| Anterior visual system | |
| Average RNFL (better eye) | –0.31 (–0.76–0.14) |
| Temporal RNFL (better eye) | –0.08 (–0.55–0.38) |
| P100 latency (better eye) | 0.39 (–0.10–0.86) |
| Brain MRI | |
| Gadolinium-enhancing lesions | |
| Number | 0.55 (0.27–0.83) a |
| Volume | 0.65 (0.35–0.94) |
| New/enlarging T2 lesion number | 0.65 (0.29–0.99) |
| T2 hyperintense lesion volume | 0.49 (0.02–0.95) |
| T1 hypointense lesion volume | 0.51 (0.12–0.90) |
| Brain parenchymal fraction | –0.16 (–0.73–0.41) |
| Gray matter fraction | –0.10 (–0.67–0.46) |
CI: confidence interval; MRI: magnetic resonance imaging; RNFL: retinal nerve fiber layer.
aBold indicates statistical significance (p<0.05)
MRI measures and sNfL-c
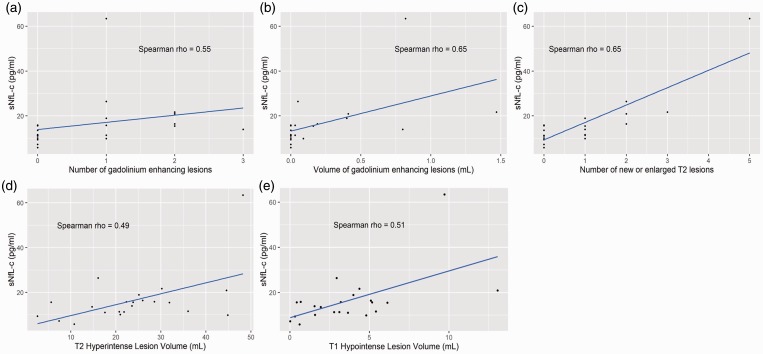
There were significant (p<0.05) correlations at M0 (baseline) between sNfL-c and gadolinium-enhancing lesion number (Spearman’s rho = 0.55, 95% CI 0.27–0.83) and volume (rho = 0.59, 95% CI 0.35–0.94), new/enlarging T2 lesions (rho = 0.65, 95% CI 0.29–0.99), T2LV (rho = 0.49, 95% CI 0.02–0.95) and T1-hypointense lesion volume (rho = 0.51, 95% CI 0.12–0.90) (n = 21) (Table 2, Figure 2). However, there was no correlation between sNfL-c and brain parenchymal fraction, gray matter fraction, or DTI-derived metrics (Table 2, Supplemental Material Table 1).
Figure 2.
Serum neurofilament light chain concentration (sNfL-c) significantly correlated with measures of magnetic resonance imaging (MRI) disease activity and severity.
Scatterplots demonstrate that at baseline, sNfL-c significantly correlated with (a) number and (b) volume of gadolinium-enhancing lesions, (c) number of new/enlarging T2 hyperintense lesions, (d) T2 hyperintense lesion volume, and (e) T1 hypointense lesion volume. The blue line in each figure represents a simple linear regression line.
Anterior visual system measures and sNfL-c
No significant correlations were observed between sNfL-c and visual pathway measures at baseline, including RNFL thickness and P100 latency (n = 22) (Table 2).
Longitudinal analyses
We explored clinical and MRI measures of new disease activity and/or burden longitudinally using linear mixed effects modeling with a subject random effect (Table 3). For each gadolinium-enhancing lesion, there was a 2.62 (95% CI 0.79–4.45) pg/ml significant increase in sNfL-c. An additional 1 ml of gadolinium-enhancing lesion volume was associated with a 14.61 (95% CI 6.67–22.56) pg/ml significant increase in sNfL-c. Each additional new/enlarging T2 hyperintense lesion was associated with a 2.41 (95% CI 1.06–3.76) pg/ml significant increase in sNfL-c. An additional mL of T2-hyperintense lesion volume was associated with a 0.47 (95% CI 0.15–0.79) pg/ml significant increase in sNfL-c. An additional 1 ml of T1-hypointense lesion volume was associated with a 2.06 (95% CI 0.89–3.22) pg/ml significant increase in sNfL-c. A 1 mm decrease in cortical thickness was associated with a 19.28 (95% CI 1.91–36.64) pg/ml significant increase in sNfL-c. A 1.0% decrease in BPF was associated with a 1.33 (95% CI 0.33–2.33) pg/ml significant increase in sNfL-c. A relapse was associated with an 8.37 (95% CI –2.56–19.29) pg/ml non-significant increase in sNfL-c.
Table 3.
Longitudinal analyses via linear mixed effects models.
| Clinical or MRI metric | Beta (95% CI) | p-Value |
|---|---|---|
| Gadolinium-enhancing lesion number | 2.62 (0.79–4.45) | 0.006 |
| Gadolinium-enhancing lesion volume (ml) | 14.61 (6.67–22.56) | 0.0004 |
| New/enlarging T2 lesions (number) | 2.41 (1.06–3.76) | 0.0006 |
| T2 hyperintense lesion volume (ml) | 0.47 (0.15–0.79) | 0.0046 |
| T1 hypointense lesion volume (ml) | 2.06 (0.89–3.22) | 0.0007 |
| Brain parenchymal fraction (%) | –1.33 (–2.33––0.33) | 0.0099 |
| Cortical thickness (mm) | –19.28 (–36.7––1.9) | 0.03 |
| Relapse | 8.37 (–2.56–19.29) | 0.1315 |
CI: confidence interval; MRI: magnetic resonance imaging.
Longitudinal stability of sNfL-c
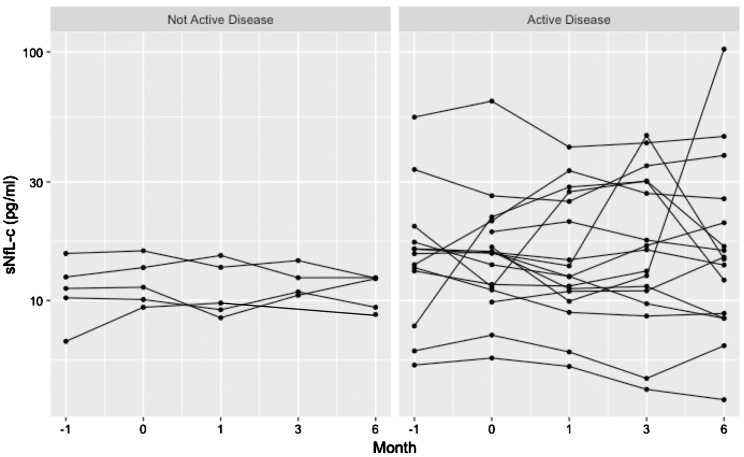
Overall, the mean ( ± standard deviation) fluctuation in sNfL-c over the course of the study was 5.67 ± 7.2 pg/ml. In patients with inflammatory disease activity (n = 17), fluctuation in sNfL-c was 6.93 ± 7.8 pg/ml, which was significantly higher than those without inflammatory disease activity (n = 5), 1.38 ± 0.7 pg/ml (p = 0.01) (Figure 3). Two patients with active disease (one with new/enlarging T2 hyperintense lesions at M–1 and M6, and the other with a clinical relapse and new/enlarging T2 hyperintense lesions at M1) had overall low sNfL-c, and no apparent changes in sNfL-c associated with observed disease activity.
Figure 3.
Longitudinal stability of serum neurofilament light chain concentration (sNfL-c) in non-active versus active disease.
sNfL-c fluctuation in patients with (n=17) and without (n=5) disease activity (defined by clinical relapses, new/enlarging T2 lesions on brain magnetic resonance imaging (MRI), or gadolinium-enhancing lesions on brain MRI) is demonstrated in a faceted spaghetti plot. On the left, patients without active disease had lower sNfL-c fluctuation (1.38±0.7 pg/ml). On the right, patients with active disease had higher degrees of sNfL-c fluctuation (6.93±7.8 pg/ml, p-value for comparison=0.01).
Investigation of sNfL-c fluctuation by each component of inflammatory disease activity demonstrated that patients with new/enlarging T2 lesions (n = 16) had significantly higher fluctuation versus those without (7.08 ± 8.1 vs 1.89 ± 1.4 pg/ml, respectively, p = 0.02) (Supplemental Material Figure 1). Similarly, patients with gadolinium-enhancing lesions (n = 13) had significantly higher sNfL-c fluctuation versus those without (8.57 ± 8.3 vs 1.48 ± 1.2 pg/ml, respectively, p = 0.01) (Supplemental Material Figure 2). However, patients with and without clinical relapses were not significantly different in terms of sNfL-c fluctuation (6.30 ± 8.0 vs 3.52 ± 3.4 pg/ml, respectively, p = 0.28) (Supplemental Material Figure 3).
Treatment effect of MSCs
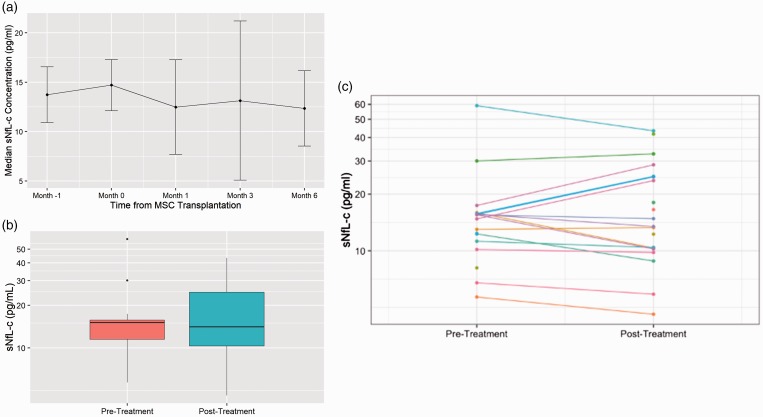
Post-transplant, sNfL-c decreased at M1 (median difference –0.91 pg/ml), M3 (–0.11 pg/ml), and M6 (–0.75 pg/ml) compared to baseline, but differences did not reach statistical significance (Figure 4(a)). Mean pre- (M–1, M0) versus post-treatment (M1–6) sNfL-c values were not significantly different (p = 0.82, Figure 4(b)). At the individual patient level, changes in sNfL-c were heterogeneous: some patients had decreased, increased, or stable sNfL-c comparing pre- and post-treatment (Figure 4(c)).
Figure 4.
Pre- vs post-treatment serum neurofilament light chain concentration (sNfL-c).
(a) Overall, median sNfL-c decreased post-transplant at M1 (median difference –0.91 pg/ml), M3 (–0.11 pg/ml), and M6 (–0.75 pg/ml) compared to baseline, but differences did not reach statistical significance. (b) Median and mean pre- versus post-treatment sNfL-c were not significantly different (p=0.82). (c) Patients had heterogeneous pre- versus post- treatment trajectories of sNfL-c concentrations. The spaghetti plot demonstrates that some patients had decreased, increased, or stable sNfL-c comparing pre- and post-treatment. MSC: mesenchymal stem cell.
We also investigated the correlation of pre- versus post-treatment change in sNfL-c and in selected other measures. There were significant correlations between pre-post treatment differences in sNfL-c and T2LV (rho = 0.41, 95% CI 0.01–0.80) and gadolinium-enhancing lesion number (rho = 0.51, 95% CI 0.04–0.97). Given the lack of change observed pre-post treatment in EDSS, whole brain MTR, whole brain MTR peak height, new/enlarging T2 lesions, gadolinium-enhancing lesion volume, cortical thickness, brain parenchymal fraction,22,32 and relapse, we did not observe significant correlations between pre-post change in these measures and change in sNfL-c.
Discussion
sNfL-c was higher in MS patients compared to controls and correlated with markers of disease severity and activity on MRI, both cross-sectionally and longitudinally. There were significant correlations between change in pre- versus post-treatment sNfL-c and corresponding change in T2LV and gadolinium-enhancing lesion number. Additionally, sNfL-c appeared stable over a relatively short study period (six months) in the absence of disease activity. These results are consistent with the growing body of evidence supporting use of sNfL-c as a biomarker in MS.1,11,13,14 Additionally, this study is the first to investigate sNfL-c in the context of MSC transplantation.
Our results differ in some ways from the published literature. The study did not demonstrate association between relapses and sNfL-c elevation, possibly due to the low incidence of relapse in this cohort (n = 5) and the overall small sample size. As sNfL-c is thought to be a marker of acute axonal injury, our findings that sNfL-c was cross-sectionally associated with T1LV and T2LV were somewhat surprising, as they are considered indicators of a more chronic disease burden. However, the cross-sectional association of CSF NfL-c with T1LV in patients with progressive MS33 and longitudinal association with T2LV in patients with relapsing-onset MS34 have been reported in recent studies. Further studies are needed to delineate the relative utility and correlates of sNfL-c in relapsing and progressive forms of MS to better inform clinical trial design in the future.
The longitudinal variability of sNfL-c at the individual patient level is not clearly established, but our results demonstrated overall stability over a six-month period in patients without disease activity. The presence of gadolinium-enhancing lesions was the most substantial contributor to increased fluctuation in sNfL-c and most reliably delineated patients with higher versus lower sNfL-c (Supplemental Material Figure 2). Though new/enlarging T2-hyperintense lesions and clinical relapses are indicative of inflammatory disease activity, there were two patients in particular who had such events without concomitant increase in sNfL-c. These findings may be attributed to the timing of clinical assessments or brain MRI in relation to disease activity, though we would expect to detect a meaningful change in sNfL-c with such close clinical and radiographic surveillance in this study. Further studies are needed to better quantify the sensitivity of sNfL-c increase in response to clinical and imaging disease biomarkers of interest, particularly as sNfL-c is becoming incorporated as a biomarker into larger clinical trials. The degree of fluctuation of sNfL-c from baseline could also be investigated as a potential predictor of disease activity in future studies, as interpretation of an sNfL-c value in isolation may be misleading.
There was no clear treatment effect of MSC transplantation on sNfL-c. Though the original phase 1/2 clinical trial reported potential improvement in a few exploratory outcomes, namely EDSS, whole brain MTR, and MTR peak height, change in sNfL-c did not correlate with these potential indicators of treatment response, either cross-sectionally or longitudinally. This overall stability in sNfL-c also coincided with lack of clear clinical improvement observed in the trial, in contrast to the treatment response in sNfL-c seen with existing MS disease-modifying therapies. The small sample size limited power to detect such changes, as did variability of sNfL-c change in the study population (Figure 4(c)). However, we did observe correlations in pre-post treatment changes of sNfL-c and T2LV and gadolinium-enhancing lesion number in expected directions; this finding does not necessarily reflect a treatment effect of MSCs.
The main limitation of this study is its small sample size. As NfL-c is primarily a biomarker of axonal damage, it is thought to primarily reflect acute inflammatory activity, including new/enlarging MRI lesions and clinical relapses. The drivers of sNfL-c in patients with relapsing and progressive forms of MS may therefore be different, which may have limited our ability to observe consistent effects in this potentially heterogeneous population.
Given the proposed repair mechanism of action for MSCs, future trials should consider incorporation of sNfL-c as an outcome measure given its overall consistent correlations with various markers of disease severity and ease of collection compared to CSF NfL-c. However, patients with progressive MS are less likely to have inflammatory disease activity that is more readily captured by sNfL-c, so using sNfL-c as an indirect marker of repair requires understanding of the biomarker in the patient population of interest when planning a clinical trial. Additionally, future work is needed to determine longitudinal stability and the clinical relevance of sNfL-c changes in an individual patient.
Supplemental Material
Supplemental material, MSO887198 Supplemetal Material for Serum neurofilament light chain concentration in a phase 1/2 trial of autologous mesenchymal stem cell transplantation by Laura E Baldassari, Sarah M Planchon, Robert A Bermel, Kunio Nakamura, Elizabeth Fisher, Jenny Feng, Ken E Sakaie, Daniel Ontaneda and Jeffrey A Cohen in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Conflicts of interests
The author(s) declared the following potential conflicts ofinterest with respect to the research, authorship, and/orpublication of this article: Laura E Baldassari received funding via a Sylvia Lawry Physician Fellowship Grant through the National Multiple Sclerosis Society (#FP-1606-24540), and has received personal fees for serving on a scientific advisory board for Teva. Sarah M Planchon has received research support from the Guthy Jackson Charitable Foundation. Robert A Bermel has served as a consultant for Biogen, Genzyme/Sanofi, Genentech/Roche, and Novartis. He receives research support from Biogen and Genentech. Kunio Nakamura has received personal fees for consulting from NeuroRx Research, speaking from Sanofi Genzyme, and license from Biogen Idec. He has received research support from NIH NINDS, NMSS, DOD, Biogen, Sanofi Genzyme, and Novartis. Elizabeth Fisher is an employee and stockholder of Biogen, but her contribution to this study was during her employment at the Cleveland Clinic. Jenny Feng receives funding via a Sylvia Lawry Physician Fellowship Grant through the National Multiple Sclerosis Society (#FP-1707-28768), and has received personal fees for speaking for Sanofi/Genzyme and served on an advisory board for Sanofi/Genzyme. Ken Sakaie has received salary support from Novartis and Genzyme. Daniel Ontaneda has received research support from National Multiple Sclerosis Society, National Institutes of Health, Patient Centered Outcomes Research Institute, Race to Erase MS Foundation, Genentech, and Genzyme. He has also received consulting fees from Biogen, Genentech/Roche, Genzyme, Novartis, and Merck. Jeffrey A Cohen has received personal compensation for consulting for Convelo, Population Council; speaking for Mylan; and serving as an Editor of Multiple Sclerosis Journal.
Funding
The author(s) disclosed receipt of the following financialsupport for the research, authorship, and/or publicationof this article: This study was funded by Cleveland Clinic Lerner Research Institute Research Program Committee Award (RPC 298 S1). The previously completed MSC trial was funded by Department of Defense, National Institutes of Health, National Multiple Sclerosis Society, and Cleveland Clinic RPC. Anonymized data will be shared with qualified investigators by request from the corresponding author for purposes of replicating procedures and results.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017; 89: 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahim P, Tegner Y, Gustafsson B, et al. Neurochemical aftermath of repetitive mild traumatic brain injury. JAMA Neurol 2016; 73: 1308–1315. [DOI] [PubMed] [Google Scholar]
- 3.Shahim P, Gren M, Liman V, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep 2016; 6: 36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahim P, Zetterberg H, Tegner Y, et al. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017; 88: 1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohrer JD, Woollacott IO, Dick KM, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 2016; 87: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gisslen M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: A cross-sectional study. EBioMedicine 2016; 3: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lista S, Toschi N, Baldacci F, et al. Diagnostic accuracy of CSF neurofilament light chain protein in the biomarker-guided classification system for Alzheimer's disease. Neurochem Int 2017; 108: 355–360. [DOI] [PubMed] [Google Scholar]
- 8.Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res 2003; 987: 25–31. [DOI] [PubMed] [Google Scholar]
- 9.Rojas JC, Bang J, Lobach IV, et al. CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology 2018; 90: e273–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weston PSJ, Poole T, Ryan NS, et al. Serum neurofilament light in familial Alzheimer disease: A marker of early neurodegeneration. Neurology 2017; 89: 2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comabella M, Montalban X. Body fluid biomarkers in multiple sclerosis. Lancet Neurol 2014; 13: 113–126. [DOI] [PubMed] [Google Scholar]
- 13.Novakova L, Axelsson M, Khademi M, et al. Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing–remitting multiple sclerosis. J Neurochem 2017; 141: 296–304. [DOI] [PubMed] [Google Scholar]
- 14.Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing–remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler 2016; 22: 1550–1559. [DOI] [PubMed] [Google Scholar]
- 15.Gunnarsson M, Malmestrom C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol 2011; 69: 83–89. [DOI] [PubMed] [Google Scholar]
- 16.Axelsson M, Malmestrom C, Gunnarsson M, et al. Immunosuppressive therapy reduces axonal damage in progressive multiple sclerosis. Mult Scler 2014; 20: 43–50. [DOI] [PubMed] [Google Scholar]
- 17.Novakova L, Axelsson M, Khademi M, et al. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult Scler 2017; 23: 62–71. [DOI] [PubMed] [Google Scholar]
- 18.Rissin DM, Kan CW, Campbell TG, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 2010; 28: 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. J Neurol Sci 2013; 333: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scolding NJ, Pasquini M, Reingold SC, et al. Cell-based therapeutic strategies for multiple sclerosis. Brain 2017; 140: 2776–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korbling M, Estrov Z. Adult stem cells for tissue repair – a new therapeutic concept? N Engl J Med 2003; 349: 570–582. [DOI] [PubMed] [Google Scholar]
- 22.Cohen JA, Imrey PB, Planchon SM, et al. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult Scler 2018; 24(4): 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol 2012; 11: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010; 67: 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llufriu S, Sepulveda M, Blanco Y, et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PloS One 2014; 9: e113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamout B, Hourani R, Salti H, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J Neuroimmunol 2010; 227: 185–189. [DOI] [PubMed] [Google Scholar]
- 27.Planchon SM, Lingas KT, Reese Koc J, et al. Feasibility of mesenchymal stem cell culture expansion for a phase I clinical trial in multiple sclerosis. Mult Scler J Exp Transl Clin 2018; 4: 2055217318765288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer JS, Rudick RA, Cutter GR, et al. The Multiple Sclerosis Functional Composite Measure (MSFC): An integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 1999; 5: 244–250. [DOI] [PubMed] [Google Scholar]
- 30.Rudick RA, Cutter G, Reingold S. The multiple sclerosis functional composite: A new clinical outcome measure for multiple sclerosis trials. Mult Scler 2002; 8: 359–365. [DOI] [PubMed] [Google Scholar]
- 31.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 32.Feng J, Offerman E, Lin J, et al. Exploratory MRI measures after intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult Scler J Exp Transl Clin. Epub before print 14 June 2019. DOI: 10.1177/2055217319856035. [DOI] [PMC free article] [PubMed]
- 33.Damasceno A, Dias-Carneiro R, Moraes A, et al. Clinical and MRI correlates of CSF neurofilament light chain levels in relapsing and progressive MS. Mult Scler Relat Disord 2019; 30: 149–153. [DOI] [PubMed] [Google Scholar]
- 34.Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. Epub before print 12 August 2019. DOI: 10.1001/jamaneurol.2019.2137. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSO887198 Supplemetal Material for Serum neurofilament light chain concentration in a phase 1/2 trial of autologous mesenchymal stem cell transplantation by Laura E Baldassari, Sarah M Planchon, Robert A Bermel, Kunio Nakamura, Elizabeth Fisher, Jenny Feng, Ken E Sakaie, Daniel Ontaneda and Jeffrey A Cohen in Multiple Sclerosis Journal – Experimental, Translational and Clinical