Abstract
Background
Amphotericin B (Amp) and Betulinic acid (BA) as antileishmanial agents have negligible water solubility and high toxicity. To solve these problems, for the first time, chitosan nanoparticles and Anionic Linear Globular Dendrimer (D) were synthesized for the treatment of Leishmania major (L. major).
Method
Chitosan and dendrimer nanoparticles were synthesized, and Amp and BA were loaded into the nanoparticles. The particles were then characterized using various methods and their efficacy was evaluated in vitro and in vivo environments (parasite burden was confirmed using pathological studies and real-time PCR methods).
Result
The results of docking showed that Amp and BA can be loaded into chitosan and dendrimer nanoparticles. The results of physically drug loading efficiency for AK (Amphotericin B-chitosan), BK (Betulinic acid-chitosan), AD (Amphotericin B-Dendrimer) and BD (Betulinic acid- Dendrimer) were 90, 93, 84 and 96 percent, respectively. The characterization results indicated that the drugs were loaded into nanoparticles physically. Moreover, the increased solubility rate for AD=478, BD=790, AK=80 and BK=300 folds. Furthermore, the results of the drug delivery system showed the slow controlled drug release pattern with cellular uptake of more than 90%. The treatment results showed a 100 percent decrease of toxicity for the all nanodrugs was observed in vivo and in vitro environments. Moreover, AK10 and BK20 mg/kg reduced parasite burden by 83 percent (P<0.001), while AD50 and BD40 mg/kg reduced it to a lesser extent compared to glucantime.
Conclusion
All the synthesized nanodrugs were completely succeeded by 100% to recovery the L. major induced pathological effects in the infected footpad. Also, the results of present study were confirmed with real-time PCR and the results showed that AK and BK were succeeded in a large extent to the treatment of L. major infection (P<0.001), therefore AK and BK could be considered as proper alternatives of choices drugs.
Keywords: chitosan, L. major; anionic linear globular dendrimer; treatment
The leishmaniases as complex diseases in (sub) tropical regions throughout the world are produced by Leishmania species and diffused by sand flies. The World Health Organization (WHO) regards the disease as a noticeable cause of deaths among infectious diseases worldwide.1 The disease causes approximately 20,000 to 40,000 deaths each year worldwide.2 Based on clinical manifestations, there are two forms of leishmania including visceral leishmaniasis (VL) and tegumentary forms. The tegumentary forms of leishmaniasis categories into cutaneous leishmaniasis (CL), diffuse cutaneous leishmaniasis (DCL) and mucocutaneous (MCL) leishmaniasis.1 The annual incidence of the cutaneous form of the disease is about 0.7 to 1.2 million cases worldwide.2
Although systematic pentavalent antimony or glucantime is the first line and gold standard therapy for tegumentary forms, this therapeutic agent cannot be regarded as a satisfactory agent due to a number of reasons, such as: requirement of daily dosage for 20 to 30 days, prohibition of use in pregnant women and severe side effects including cardiotoxicity and renal failure.3 Also, this compound has a narrow therapeutic window that in some regions parasite resistance is observed.1 Paromomycin, Miltefosine, Pentamidine, Pentostan and Ambisome as other antileishmanial agents are used for treatment of leishmaniasis.1 Moreover, other problems related to the leishmaniasis treatment are high economic and social cost, permanent scarring (abnormal scar healing), infectiousness, long term of treatment period, development of secondary bacterial complications, septicaemia, risk of tetanus development, progress in drug resistance and painful injection.
In addition, Amphotericin B (Amp) as an antileishmanial agent is used for the treatment of leishmaniasis. However, severe nephrotoxicity and hematologic toxicity, hemolysis, liver damage, nausea, fever, significant insolubility and negligible excretion from the body limit its clinical use.4–7 The findings of studies showed that Amp functions through interaction with sterols in bilayer membrane, and pore development in this organelle, resulting in membrane destruction or prevention of its repair. Correspondingly, Amp produces pores in cholesterol-containing membranes, resulting in host cell toxicity.8
Betulinic acid (BA) as a natural material is a triterpenoid pentacyclic compound with some biological properties such as antileishamaial function. It is obtained from the numerous plants types such as P. andrieeuxii and betulin as a metabolic precursor.9 BA induces apoptosis through alterations in the expression levels of Bcl-2 protein family, disruption in mitochondrial function and by NF-κB activation.10 However, its clinical use is limited owing to poor solubility and comparatively short plasma half.11 The studies have shown that BA as a betulin heterocyclic derivatives has antiparasitic activity against different genus of leishamania.12–14 It has been shown that a BA derivative induces apoptosis through DNA topoisomerase I and II inhibition in L. donovani.14,15 Totally, Amp and BA are two antileismanial agents which have high toxicity, low solubility, and severe side effects. In this context, to solve these shortcomings, nano drug delivery systems have been used.
Drug delivery systems such as microspheres, nanospheres, and liposomes can increase the drugs concentrations and their slower distribution in organs such as spleen, liver, and kidneys.16 Furthermore, nanotechnology based drug delivery systems decrease the drug toxicity effects17-19 and enhance the solubility rate of drugs. In this regard, various carriers such as liposome, dendrimer, and modified chitosan nanoparticles have been used for delivery of antileishmanial drugs. In addition, immunomodulatory and antileishmanial effects of chitin and chitosan microparticles and chitosan nanoparticles have been approved.20–22
Chitosan possesses ideal properties for drug delivery for leishmaniasis treatment, including (a) antileishmanial activity;23 (b) specific binding to the receptors on the macrophages surface (targeted drug delivery);24 (c) stimulating macrophages and inducing nitric oxide production;5 inducing Th1 cytokines and adsorbing some plasma components such as immune protein and complement C protein, as a result rapidly recognized by reticuloendothelial system (RES);25 (d) decreasing the toxicity effects of drugs; (e) improving the process of wound healing; (f) reducing the drug side effects in non-target cells or tissues and as a drug carrier can target liver, spleen, lung, and colon;25 (g) enhancing the drug solubility; and (h) having a high cellular uptake due to positive surface charge. Positive charge plays important roles such as prolonging the drug retention time, prolonged drug release in vivo and improving drug bioavailability.25
Nanoparticles as solid colloidal particles have the size of 1 to 1000 nm. They have strong mobility in comparison to micron-grade particles due to their small size and easy penetration into cells, resulting in their accumulation in the lesion site. Therefore, they have a high rate of cellular uptake.25 Drugs loaded into chitosan can be released through degradation and corrosion, resulting in a pattern of sustained drug release.25
The methods for synthesis of chitosan nanoparticles are precipitation, ionic cross-linking, polymerization, self-assembly, covalent cross-linking and spray-drying.25 Phase separation is a subtype of precipitation method. In addition to abovementioned methods, ionic gelation, solvent evaporation, reverse micellar and sieving methods are used for chitosan nanoparticle synthesis.26
Drug-loaded chitosan nanoparticles degrade in vivo into chitosan and drug. Chitosan is chiefly decomposed by the catalytic activity of lysozyme and bacterial enzyme in the colon. The kidney is responsible for blood clearance of a high amount of blood absorbed chitosan, and the rest is excreted by feces.25
Furthermore, due to nature of polysaccharide, chitosan can specifically bind to those macrophage’s receptors which structurally are galactosamine like carbohydrate, and by which increase the uptake of loaded Amp.27 Due to ideal properties, such as immune adjuvanticity, low toxicity, and safety, chitosan is distinguished as one of the most appropriate polymer.5
Moreover, chitosan is an acid-resistive material, resulting in its resistance to immediate lysosomal digestion within macrophages for a short time, consequently, releases the loaded drug in a sustained manner at the target site (ie macrophages of RES organs as the host of leishmania parasite). In addition, the high molecular weight of chitosan nanoparticles causes them to be opsonized immediately after application.5 Eventually, studies have been shown that modified chitosan nanoparticles compared to other colloidal systems are more effective for drug delivery to macrophages, due to their preferential accumulation into macrophages rich organs such as liver and spleen.5
Also, dendrimers (Ds) are suitable nanoparticles characterized by onion-like structure.28 Ds are monodisperse synthetic macromolecules with several branching points and three-dimensional spherical shape. This structure considerably influences on the physicochemical properties of the nanoparticles. Ds are desirable for drug delivery purposes due to their spherical shape, cell wall penetration ability, small size and lipophilicity.29 The results of different studies showed that Ds are anti-infectious agents.30
In the present study, we aim to load Amp and BA into Anionic Linear Globular Dendrimer (ALGD) and K nanoparticles due to decreasing their toxicity and enhancing their solubility and efficacy. In this context, K and ALGD (D) were synthesized and Amp and BA were loaded into the nanoparticles. Thereafter, drug loading into carriers was proved using characterization methods. The toxicity effects were measured in vitro and in vivo environments using MTT assay, enzymatic evaluation, and histopathological studies. Moreover, the efficacy of the formulations to increase the drugs therapeutic and decrease their toxicity effects was measured in vitro and in vivo environments. In addition, real-time PCR and histopathological studies were performed to confirm the results of efficacy evaluation.
Materials and methods
Materials
Amp was supplied by Biobasic (Canada). Also, chitosan (20 KDa), tripolyphosphate (TPP), acetic acid, dimethylsulfoxide (DMSO), schneider culture medium, and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) were purchased from Sigma-Aldrich (USA). RPMI-1640, Fetal Bovine Serum (FBS) and Penicillin/Streptomycin antibiotics were obtained from Gibco Company (USA). Furthermore, the L. major strain of MRHO/IR/75/ER (an Iranian strain) was obtained from Pasteur Institute of Iran. All other chemicals were of analytical grade.
Methods
Molecular modeling
The 3D structure of the studied drugs and nanoparticles were generated using chemdraw Ultra 12.0 software. Geometry optimizations were performed in two steps by hyperchem 8.0.31 At first, the studied molecules were assigned to energy minimization using the “MM+” force field. Semi-emperical “PM3” method was then used to optimize the full geometry of the structures.
Docking calculations
Docking studies were carried out using Autodock program (using MGL tools 1.5.6).32 Input files required for docking study were made in Autodock tools. Autogrid 4 was used to generate the grid maps. A grid size of 120×120×120 was set for all calculations and a grid spacing of 0.375 Å was used for covering whole molecules as blind docking. Autodock4 was applied to calculate the binding energy for discovery the best binding mode. In all simulation, Lamarckian genetic algorithm (LGA) and fifty independent runs for conformational search with the maximum number of 2,500,000 energy evaluations per run were carried out. Default values were set for the other operations. The best conformation of the drug-nanostructure complexes was identified based on binding affinity score. In fact, the lower the binding energies, the more effective the binding mode.
Preparation of ALGD, chitosan, AD, BD, AK and BK nanoparticles
These nanoparticles were prepared according to the previous research works.33–36
Determine the drug loading efficiency in the nanodrugs (AD, BD, AK, and BK) and determine the size, size distribution and zeta potential
The drug loading efficiency for the four nanodrugs (AD, BD, AK, and BK), size, size distribution and zeta potential of the all particles were determined according to the previous studies.33–36
Nanoparticles characterization using SEM, TEM, AFM, FTIR and H-NMR methods
The nanoparticles were characterized using SEM, TEM, AFM, FTIR and H-NMR methods according to the previous study.34
Thin-layer chromatography (TLC)
Two µl of the samples of Amp, BA, D, K, AD, BD, AK and BK were poured on a silica plate and dried at room temperature. The plate was then incubated at 45 °C angle in a chamber containing 10% methanol in chloroform for 45 min. Next, the plate was withdrawn, left to dry and analyzed to detect the components using iodine vapours as visualizing agent and the related Rf were obtained.37 TLC was performed to indicate and confirm the purity of the formulations.
Evaluation the kinetic of drug release and cellular uptake of AD, BD, AK and BK formulations
The drug release studies and cellular uptake of the formulations were carried out using dialysis membrane method and flow cytometry methods, respectively according to a literature method.38–40
Biological activity of the formulations
The solvent design
The protocol for preparing the appropriate solvent and effective therapeutic dose has been mentioned in the previous works.33,35
The viability effects of the nanoformulations on peritoneal macrophages
The viability effects of the formulations on peritoneal macrophages were evaluated according to a literature method using MTT assay.41,42 All animal experiments were approved by the Animal Experimental Committee of Pasteur Institute of Iran (No# IR.PII.REC.1395.20) and all procedures were performed in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Killing effects of the nanoformulations on the promastigote and amastigote
The killing effects of the nanoformulations were measured against promastigotes of L. major according to the literature methods43,44 and intracellular form of the parasite in infected peritoneal macrophages based on.45,46
In vivo toxicity of the nanodrugs AD, BD, AK and BK formulations using blood factors
The in vivo toxicity was evaluated on female Balb/c mice, received these formulations based on previous study.34 After this time, the serum concentrations of Alanine Transaminase (ALT), Alkaline Phosphatase (ALP), Aspartate Transaminase (AST), creatinine and Blood Urea Nitrogen (BUN) were measured spectrophotometrically to determine the non-toxic dose.34
Nanoformulations efficacy on the lesion size
The lesion size of the infected footpad of Balb/c mice was measured using caliper according to the previous studies.47,48
Parasite burden measurement using limiting dilution assay (LDA) method
The parasite burden was measured using the popliteal lymph node of infected footpad according to the methods and ELIDA software described in the literature.48,49 Briefly, popliteal lymph node was divided into two equal sections, one section was used for determining the parasite burden and the other one was used for evaluation by Real-time PCR method.4,5,27,30
Real-time PCR
Firstly, to prepare a standard curve, DNA was extracted from 5.3×104 Leishmania promastigotes using a QIAamp® DNA Mini Kit (Qiagen) based on the manufacturer’s instructions and five times serial dilutions of the parasite DNA, consistent with 5.3×104 parasites to 5.3 parasites per reaction. The specific primers to amplify a 75 bp fragment of the SODB1 gene were designed by Beacon Designer software (ver. 8). SODB1 is placed on chromosome 32 of L. major. The primers sequences, polymerization conditions and the reactions used were based on the previous study.50 All assays were carried out in duplicates. The average cycle threshold (CT) of duplicates in each dilution was plotted against the number of parasites. Reproducibility of the assay was also performed using evaluation of inter-assay and intra-assay coefficients of the CT values variation. The average cycle threshold (CT) of duplicates for each dilution was plotted against the parasites number.
The number of parasites per 2×106 cells of half popliteal lymph node was calculated by interpolating cycle threshold (CT) of the samples in the standard curve (half of the popliteal lymph node was used for real-time PCR and another half was used for LDA assay).50,51
Statistical analysis
The results of the study were analyzed by one- and two-way ANOVA tests, as well as Prism software and the significance level, was set at P<0.05 considering appropriate post hoc tests.
Results
Molecular modeling and docking calculations
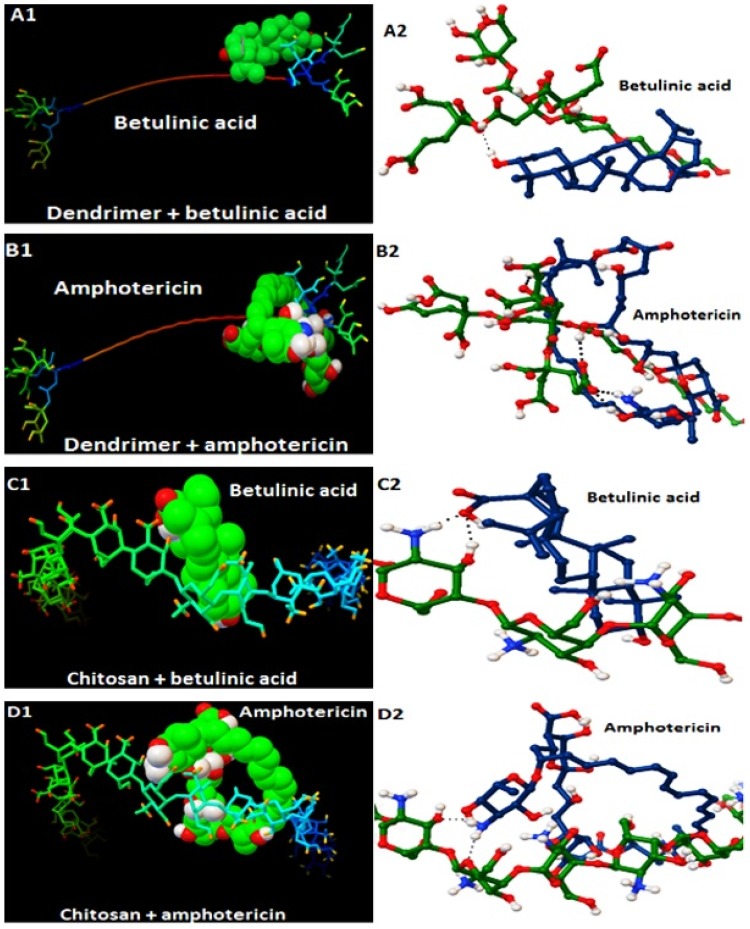
Docking results presented in Table 1 and Figure 1, have shown the binding energy of the best docking position. As the Table shows, this energy among all complexes is under zero indicating that Amp and BA were loaded into nanocarriers. Generally, the higher the negative charge, the higher drug loading efficiency is achieved. Figure 1 shows the predicted binding site and hydrogen bonds between drug molecules and the nanocarriers. According to the results (Figure 1 and Table 1), the proper bonds and binding energy values were provided between the drugs molecules and the related nanocarriers, therefore it was proved that the drugs were successfully loaded into the carriers.
Table 1.
Binding energy (Kcal.mol−1) in the four nanodrugs complexes at the optimized stable condition
| Complex | Binding energy |
|---|---|
| BD | −4.48 |
| AD | −5.27 |
| BK | −5.00 |
| AK | −5.08 |
Figure 1.
The results of molecular modeling and docking calculations for the four different nanodrugs.
Nanoparticles characterization
The results of the present study showed that the size of nanodrugs AK=112 nm, BK=124, AD=138 and BD=156 nm was achieved (Table 2). Also, the drug loading efficiency for nanodrugs AK=90%, BK=93%, AD=84% and BD=96% (Figures S1 and S2).33,35,52 The characterization results (DLS, SEM, AFM, FTIR, HNMR, and TEM) confirmed that Amp and BA were loaded into K and D physically (Figures S3–S5, Tables S1 and S2).33,35,52
Table 2.
The results of size, size distribution (PDI) and zeta potential of different nanoparticles
| Parameters Nanoparticle |
Size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| K | 102±10 | 0.2±0.1 | 14±2 |
| BK | 124±14 | 0.3±0.1 | 6.5±1 |
| AK | 112±15 | 0.45±0.15 | 8±1.5 |
| D | 90±13 | 0.25±0.17 | −5.8±0.9 |
| BD | 156±15 | 0.31±0.14 | −14.6±1.7 |
| AD | 138±11 | 0.11±0.13 | −18.8±2.5 |
TLC
According to the TLC results all compounds were found to be pure.
Drug release study and the cellular uptake of the nanodrugs
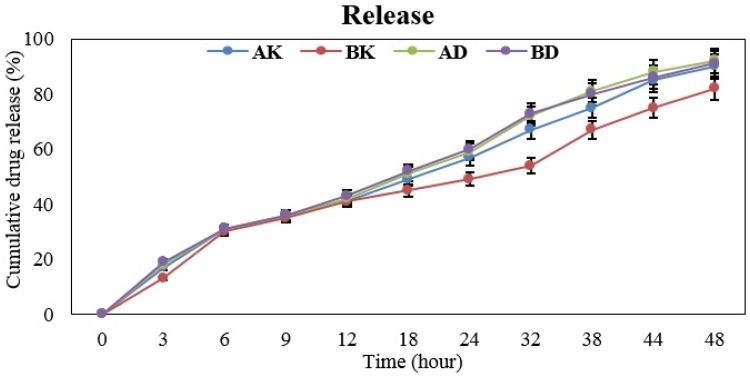
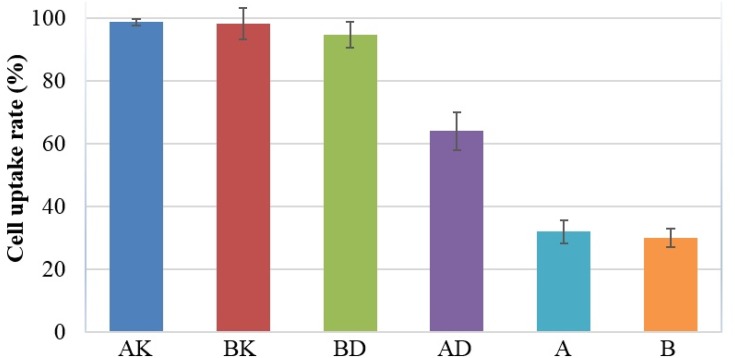
As the results showed the drugs were released from the four nanodrugs in a controlled and sustained manner (Figure 2). The study results indicated the cellular uptake of AK=98.6%, BK=98%, AD=64% and BD=94.6% (Figure 3).
Figure 2.
The mean cumulative release of Amp and BA from the four nanodrugs of AK, BK, AD and BD after 48 h (slow release).
Figure 3.
The flow cytometry results related to cellular uptake of nanodrug AK (nano amphotericin chitosan) =98.6%, BK=98%, AD=64% and BD=94.6. Also, the cellular uptake of A was 30% and B was equal to 28%.
Drug solubility in vivo environment
The results of solvent design mentioned in the previous studies,33,34 indicated that the solubility of Amp and BA in AK and BK was increased by 80 and 300 fold, respectively (Figure S6). Also, the enhanced solubility for AD=478 and BD=790. The details of solvent design procedure have been mentioned in the previous works.33,34
In vitro evaluation the viability rate of the nanoformulations
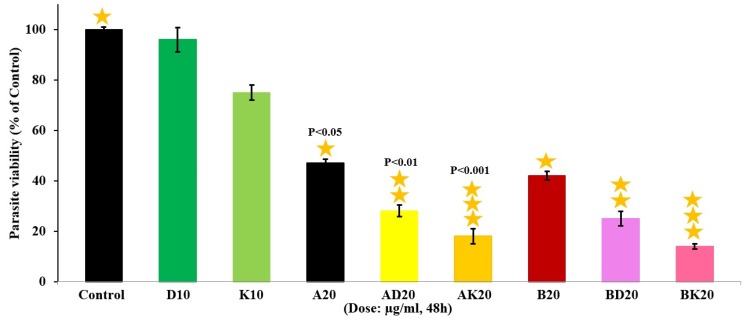
The viability rate of macrophages was firstly found to be 97% by trypan blue exclusion assay. The results showed AD20 µg/mL, AK20 µg/mL, BK20 µg/mL and BD20 µg/mL were completely non-toxic comparative to the negative and positive control groups (P˂0.001). Also, the results showed that D10 µg/mL and K10 µg/mL were perfectly non-toxic by the viability rate of 100% (Figure 4).
Figure 4.
The viability effects of the nanodrugs AK, BK, AD and BD at the same concentration of 20 µg/mL along with K(Chitosan) and D(dendrimer) at the concentration of 10 µg/mL compared to control group on the Balb/c mouse-derived peritoneal macrophages after 48 h incubation. The values are expressed as Mean ± SD from three independent experiments with P˂0.001.
The nanoformulations killing effects on the promastigotes
In this section, L. major promastigotes were negative, and promastigotes incubated with Amp20 µg/mL were positive control groups. The highest killing effects were obtained by AK20 µg/mL and BK20 µg/mL (86%) (P˂0.001) comparative to the negative control group and these killing effects were higher comparative to AD20 µg/mL and BD40 µg/mL (Figure 5).
Figure 5.
The killing effects of nanodrugs BK(nano amphotericin chitosan)20 µg/mL (with the killing effects of 88%), AK20 µg/mL, AD20 µg/mL, BD20 µg/mL, nano K(Chitosan)10 µg/mL, A20 µg/mL and B20 µg/mL on the viability of L. major promastigotes after 48 h incubation. The values are expressed as Mean ± SD from three independent experiments with P˂0.001.
The nanoformulations inhibition effects on the amastigotes
The results of present study showed that AK20 µg/mL and BK20 µg/mL had the highest killing effects (81%) compared to the negative control group (P˂0.001), while AD20 µg/mL, and BD20 µg/mL had lower killing effects comparative to AK20 µg/mL and BK20 µg/mL (Figure 6).
Figure 6.
The therapeutic effects of nanodrugs AK(nano amphotericin chitosan)20 µg/mL, AD20 µg/mL, BK20 µg/mL and BD20 µg/mL on the L. major-infected macrophages after 48 h incubation. The highest killing effects were related to BK20 µg/mL with the killing effects of 81%. The values are expressed as Mean ± SD from three independent experiments with P˂0.001.
The results of nanoformulations toxicity in vivo environment
The results of in vivo toxicity showed that AK10, BK20, AD50 and BD40 mg/kg were non-toxic in terms of measurement the blood concentrations of ALT, ALP, AST, creatinine and BUN. Therefore, while the drugs were toxic, the nanodrugs were effective to reduce the drug toxicity.
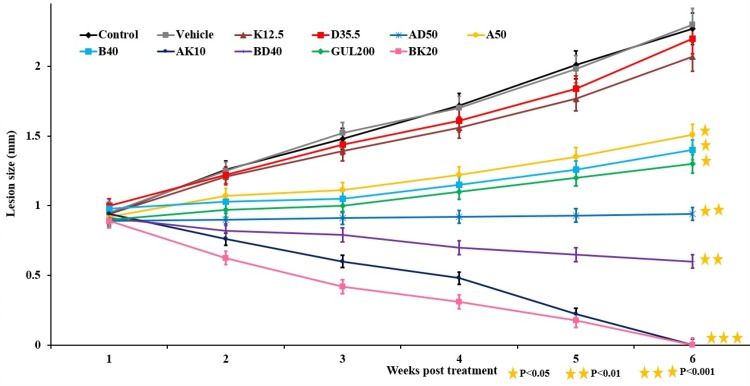
Nanoformulations efficacy on the lesion size
The results of lesion size measurement showed that AK10 and BK20 mg/kg were more effective to decrease the lesion size by decreasing the lesion size to zero compared to the AD50 and BD40 mg/kg (Figure 7).
Figure 7.
The lesion size in L. major-infected mice received various formulations including K12.5, D35.5, AD50, B(20 and 40), GUL200, AK10, BD40, A (10 and 50) and BK20 mg/kg. As the figure shows, the lesion size reached to zero in nanodrugs of AK10 mg/kg and BK20 mg/kg receivers (P˂0.001).
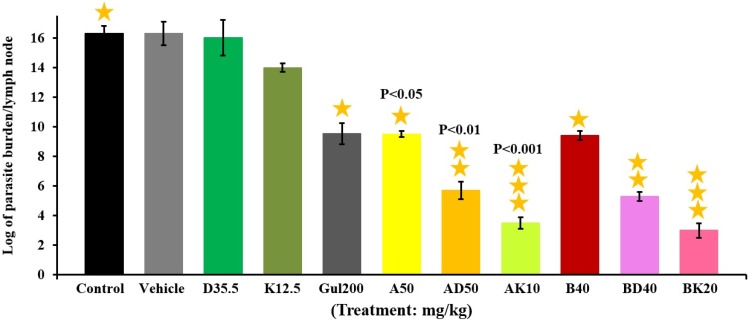
Parasite burden measurement using limiting dilution assay (LDA) method
The results of lesion size measurement showed that AK10 and BK20 mg/kg were more effective to decrease the parasite burden by 83% compared to the AD50 and BD40 mg/kg (Figure 8).
Figure 8.
The evaluation results of parasite burden in mice groups of BK20, AK10, BD40, AD50, A(10 and 50), B(20 and 40), GUL200, K12.5, D35.5 mg/kg and control group. As the results showed the lowest parasite burden was related to BK20=5.57×106±0.2 and AK10 mg/kg=8.22×106±0.3 groups. The results are expressed as Mean ± SD from three independent experiments.
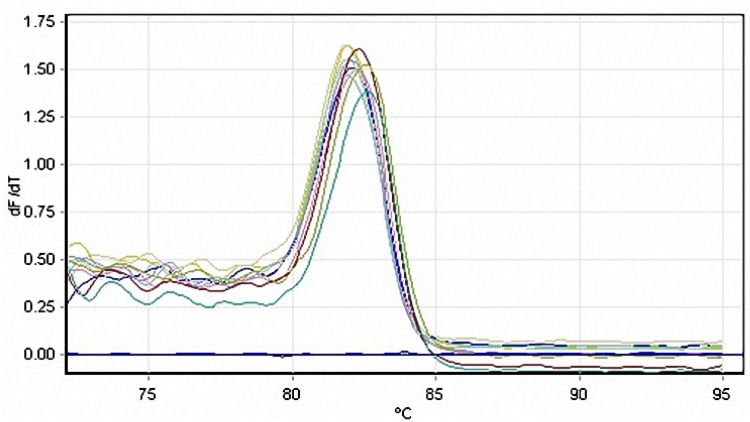
Real-time PCR assay
Melting curve analysis was carried out at the end of each run, to recognize the non-specific double-stranded reaction products (Figure S7) and the results of the analysis showed the purity of the products. Also, the standard curve plotted by 5-fold serial dilutions of L. major DNA was used to complete quantification of leishmania parasites and define the limit of detection (Figure 9). A linear standard curve with the correlation coefficient (R2) value of 0.99 and amplification efficiency of 0.92 was found over at least five serial dilutions of the parasitic DNA. Also, the test detection limit of 5.3 parasites per reaction was discerned. The plot indicates the mean CT values±SD from three replicates versus the parasite number. Inter-assay coefficients of variation of CT values for 5-fold serial dilutions of L. major DNA was correspond to 5.3×104 parasites.
Figure 9.
The standard curve obtained from the amplification of L. major SODB1 gene over 5-fold serial dilutions of the parasitic DNA, ranging from 5.3×104 parasites to 5.3 parasites per reaction.
The number of parasites per 2×106 cells of half of the popliteal lymph node was calculated using real-time PCR (popliteal lymph node was divided into two equal sections; one section was used for real-time PCR and another half was used for LDA assay). The parasite number per 2×106 cells of half of the popliteal lymph node was measured using real-time PCR (popliteal lymph node was divided into two equal sections; one section was used for real-time PCR and another half was used for LDA assay). The results of real-time PCR confirmed the results of LDA assay (Table 3), indicating that AK10 and BK20 mg/kg were more effective in parasite clearance.
Table 3.
Calculation of the parasite number of popliteal lymph nodes in different groups of L. major infected BALB/C mice at 8 weeks post infection using real-time PCR and LDA
| Groups | Number of parasites/half of the popliteal lymph node (LDA) | Mean CT ± SD | Number of parasites/2×106 cells of half of popliteal lymph node (real-time PCR) |
|---|---|---|---|
| Dendrimer | 12.24×106 | 26.10 | 10,000 |
| Chitosan nanoparticles | 11.35×106 | 26.49 | 8000 |
| Glucantime | 10.75×106 | 26.55 | 7600 |
| Betulinic acid | 10.47×106 | 26.54 | 7400 |
| Amphotericin B | 10.44×106 | 26.60 | 7200 |
| AD50 mg/kg | 10.36×106 | 26.70 | 6800 |
| BD40 mg/kg | 9.44×106 | 26.80 | 6400 |
| AK10 mg/kg | 8.22×106 | 27.30 | 5400 |
| BK20 mg/kg | 5.47×106 | 27.61 | 4600 |
Discussion
In the present study, we aimed to load Amp and BA into D and K nanoparticles in order to decrease their toxicity and enhance their solubility and as a result enhance their therapeutic efficacy. Initially, the results of docking calculations showed that Amp and BA can be loaded into D and K nanoparticles with high loading efficiency. The results of drug loading confirmed that the drug loading was completely occurred physically.
Also, the results current study indicated that the size of K=102, AK=112, BK=124, D=90, AD=138 and BD=156 nm. The results of drug loading efficiency for AK=90%, BK=93%, AD=84% and BD=96%. Moreover, the results of increased solubility rate for AD=478 and BD=790 folds. The novel solvent increased the solubility rate for AK=80 and BK=300 folds. Furthermore, the results of in vitro and in vivo toxicity showed that all the nanodrugs decreased the toxicity by 100%. Also, the results of parasite burden showed that AK and BK were effective to decrease the parasite burden by 83%, while AD and BD decreased the parasite burden to a lesser extent. The results of pathological studies showed that all the nanodrugs were succeeded by 100% to improve the L. major induced pathological lesions in the infected footpad. Totally, the results of the present study showed that AK and BK were succeeded in the treatment of L. major infectious by 100%, therefore they could be proper alternatives to current drugs for the treatment of L. major infectious whereas AD and BD were significantly effective in decreasing the toxicity and enhancing the solubility.
In the present study, firstly docking calculations proved that Amp and BA can be loaded into K and D nanoparticles properly. In Table 1, the binding energy for the best docking mode of the nanoparticle-drug complexes was shown. This energy in all complexes is under zero indicating loading of Amp and BA into nanoparticles. The charge of binding energy is inversely associated with the amount of drug loading into a nanocarrier. Moreover, the results of docking calculations showed that the proper hydrogen bonds between the drugs and nanocarriers have been occurred. Furthermore, in the previous studies, it was shown that the drugs were loaded into carriers by drug loading efficiency more than 90% (AK=90%, BK=93%, AD=84% and BD=96%), which were confirmed by characterization methods including DLS, SEM, TEM, AFM, FTIR, TLC, and HNMR.
Researchers in studies used release studies and cellular uptake measurements for evaluating the potency of nanocarriers for drug delivery.5,7,24,27,53–59
The results of the present study showed that nanodrugs AD, BD, AK, and BK had a slow drug release manner in which maximum release was occurred after 48 h that is more appropriate compared to the conventional release. The pattern of release for four nanoformulations was in accordance with the other studies.5,27,57 The results of studies showed that slow drug release from nanoparticles leads to more effective therapy with reduced drug toxicity. Controlled and slow drug release also leads to the more effective delivery of therapeutics to the target cells such as macrophages as the host of intracellular leishmania parasites.5 Also, the results of present study showed that four nanodrugs had the proper rate of cellular uptake (AK=98.6%, BK=98%, AD=64% and BD=94.6%). In general, the presence of chitosan with positive surface charge, and anionic globular dendrimer were effective in enhancing the cellular uptake of the drugs.5,59
After confirming the suitability of drug release and cellular uptake, researchers determine the non-toxic dose of a nanodrug in vitro environment using cell culture and MTT assay. In various studies, the viability effects of chitosan modified nanoparticles containing Amp (nanodrug AK) and AD (PAMAM) were evaluated using the MTT assay.5,7,24,27,57,60 In addition, it has been determined that nanochitosan is non-toxic and FDA approved material.61
In another study, nanodrug AK (P-127) at the dose of 20 μg/mL had the cell viability of 72%.27 Also, in a study, AD (PPI) decreased the Amp toxicity.58
In the current study, the results showed that nanodrugs AK, BK, AD, and BD increased the cell viability to more than 95% on peritoneal macrophages. Furthermore, in accordance to the previous studies, the results of the current study indicated that the non-toxicity effects observed in nanodrugs AD, BD, AK, and BK are resulted from slow drug release from the nanoparticles and the potency of ALGD and nanochitosan in decreasing the drug toxicity.
After determining and obtaining the non-toxic dose of a drug, researchers as the next step evaluate the drug efficacy on the promastigote and amastigote forms of a parasite. The results of a study showed that AK1 µg/mL (NQC) was not effective in killing of promastigote of L. amazonensis.57 Also, the results of a study indicated that BA had killing effects against promastigotes of L. infantum.62 In another study, the findings showed that AD (PAMAM) was effective in parasite killing to some extent.63
The results of present study showed that nanodrug AK20 µg/mL and BK20 µg/mL, prepared by phase separation and without any modification, increased the killing effects of the loaded drugs against promastigotes of L. major by 86%. Moreover, the results of the present study showed that AD20 µg/mL and BD20 µg/mL were effective to a lesser extent in killing the parasite which can be resulted from lesser cellular uptake. Also, the results of the present study showed that nanodrug AK20 µg/mL and BK20 µg/mL increased the killing effects of the drugs against amastigote of L. major more than 81%, while AD20 µg/mL and BD20 µg/mL had lower killing effects due to their lesser cellular uptake. This can be due to the antileishmanial effects of chitosan and the synergistic antileishmanial effects of Amp and BA with nanochitosan as well as slow drug release. It has been shown that the killing effects were increased by increasing the dose and drug loading efficiency.
Totally, the results of in vitro evaluation showed that nanocarriers of K12.5 and D35.5 mg/kg were effective in reducing the toxicity and increasing the solubility. Also, AK and BK were more effective in treatment compared to AD50 and BD40 mg/kg which could be results from the antileishmanial effects of nanochitosan.
After determining and obtain the therapeutic dose in vitro environment, researchers designed the in vivo therapeutic dose. In this regard, for increasing the therapeutic dose, various solvents have been designed. For in vivo evaluation the higher and more effective doses of drugs are needed. It has been known, Amp and BA as hydrophobic large molecules have negligible water solubility (˂0.05 mg/mL and 0.02 µg/mL, respectively).6 In various studies, AK has been used for the treatment of leishmaniasis at the dose of 1 mg7,27,60,64,65 using different solvents.
The results of the current study indicated that Amp at the dose of 50 and BA at the dose 40 were readily dissolved in ALGD (with increased solubility of 478 and 790 folds for Amp and BA, respectively).
The results of present study showed that the effective therapeutic dose for treatment of L. major was equal to 10 mg/kg of Amp in AK (AK10 mg/kg) and 20 mg/kg of BA in BK (BK20 mg/kg), whereas due to lower water solubility of Amp, BA and K, designing a proper solvent for increasing the solubility and preparing of effective therapeutic dose is needed. In this context, the results of present study showed that we could dissolve 10 mg of Amp and 20 mg of BA in chitosan formulation through the development of a novel solvent. By increasing the dose of the drug and viscosity, the administered dose was increased and as a result, treatment of leishmaniasis was performed more effectively. In addition, the prepared solvent was safe and nontoxic which increased the nanodrug AK solubility by more than 80 folds and nanodrug BK by 300 folds.
For in vivo evaluation of drug effects, researchers evaluate the drug toxicity by measuring the enzyme concentration. In various studies, the toxicity effects of nanoformulations were evaluated by measuring the concentrations of blood factors such as BUN, creatinine, AST, ALT, and ALP.
The results of the present study showed that the nanodrugs AK10 mg/kg, AD50 mg/kg, BD40 mg/kg and BK20 mg/kg were non-toxic in increasing the concentration of the blood factors (BUN, creatinine, AST, ALT, and ALP). These results were confirmed using histopathological studies of the liver, kidney, and spleen, where these four nanodrugs were non-toxic.
After determining the non-toxic therapeutic dose, researchers measure the therapeutic effects of this dose on the lesion size induced by leishmania species.4,5,24,27,60
The results of Ribeiro et al study showed that nanodrug AK (NQC)1 mg/kg slightly decreased the lesion size in mice compared to Amp1 mg/kg.4
The results of the current study indicated that the lesion size reached to zero in nanodrugs AK10 mg/kg and BK20 mg/kg receiver mice owing to increasing the effective dose, while AD50 and BD40 mg/kg were less effective to decreasing the lesion size. The measurement results of lesion size for the four nanodrugs were confirmed using histopathological studies. The results of histopathological studies showed that AK10 and BK20 mg/kg were completely succeeded in lesion recovery, while AD50 and BD40 were not succeeded as much as AK10 and BK20 mg/kg. To confirm the results of pathological effects, parasite burden was measured.
In this context, various investigators have measured the parasite burden to evaluate the drug effects and confirm the results of pathological effects. The results of previous studies showed that the synthesized nanodrugs were not succeeded to decrease the parasite burden due to lower therapeutic dose,5,27,60 (Table 4A and B), while in the present study, owing to increasing the effective dose, AK10 and BK20 mg/kg were more effective by 83% than AD50 and BD40 mg/kg in decreasing the parasite burden. In the present study, these results were confirmed using Real-time PCR method, where the Real-time PCR results confirmed that AK10 and BK20 mg/kg were more effective than AD50 and BD40 mg/kg in the parasite inhibition. The researchers have used real-time PCR to confirm the LDA results.50 In the current study, the results of real-time PCR were in agreement with the results of LDA.
Table 4(A).
Synthesis and characterization of various types of chitosan-based nanoparticles in vitro and in vivo environments, reported in different studies
| Reference Properties |
48 | 5 | 27 | 24 | 7 | Present study |
|---|---|---|---|---|---|---|
| Name | NQC-AmpB | HePC-AmB-CNLCs | Cs-PF-AmB-M | Chitosan-coated AmB-loaded SLNs | MTC AmB | AK |
| Nanoparticle size (nm) | 135 | – | – | 148 | – | 102 |
| Nanodrugs size (nm) | 155 | 150 | 156 | 166 | 362 | 112 |
| Synthesis method | Ionic gelation | Emulsification and homogenization | Ionic gelation | Emulsification and solvent evaporation | Ionic gelation | Phase separation |
| Drug loading efficiency (%) and methods used for confirmation | 81%; DLS, SEM, TEM | 80%; DLS, TEM | 63%; DLS | 82%; SEM, DLS | 73%; FTIR, SEM | 90%; DLS, SEM, TEM, AFM, HNMR, FTIR |
| Pattern of drug release | Controlled release | Controlled release | Controlled release | Biphasic | Controlled release | Controlled release |
| Cellular uptake (%) | 32% | 55% | 22% | 34% | 45% | 98.6% |
| In vitro viability (%) (MTT) | 50% at 9 µg/mL | 58% at 10 µg/mL | 72% at 20 µg/mL | 49% at 20 µg/mL | 34% at 57 µg/mL | 100% at 20 µg/mL |
| Promastigote killing (%) | 50% at 1 µg/mL | – | – | – | – | 82% at 20 µg/mL |
| Amastigote inhibition (%) | 61% | 68% | 50% | 50% | 61% | 78% |
| Leishmania species | Amazonensis | Donovani | Donovani | Donovani | Donovani | Major |
Table 4(B).
Synthesis and characterization of various types of dendrimer nanoparticles in vitro and in vivo environments, reported in different studies
| Reference properties |
7 | 6 | 30 | Present study | |
|---|---|---|---|---|---|
| Name | PADRE-PDD-LAmB | AmB-PPI dendrimer | MdPPIA | AD | BD |
| Size of dendrimer (nm) | - | 93 | 80 | 90 | 90 |
| Size of drug loaded dendrimer (nm) | 142 | 138 | 122 | 138 | 156 |
| Synthesis method | Divergent | Divergent | Divergent | Divergent | Divergent |
| Drug loading efficiency (%) and its confirmation method | 58%; TEM, DLS | 55%; flowcytometry and fluorescent microscopy | 76%; FTIR, DLS | 84%; AFM, DLS, FTIR, HNMR | 96%; AFM, DLS, FTIR, HNMR |
| Pattern of drug release | – | Controlled release | Biphasic | Controlled release | Controlled release |
| Cellular uptake (%) | – | 42% | 76% | 64% | 94.6% |
| In vitro viability (%) (MTT) | 75% at 1 µg/mL | 56% at 33 µg/mL | 55% at 1, 5, 10, 25 µg/mL | 100% at 20 µg/mL | 100% at 20 µg/mL |
| Promastigote killing (%) | – | – | – | 61% at 20 µg/mL | 73% at 20 µg/mL |
| Amastigote inhibition (%) | – | 23% | 32% | 52% | 61% |
| Leishmania species | Donovani | Donovani | Donovani | Major | Major |
Totally, the results of the present study showed that nanodrugs AK and BK were found with the highest drug loading efficiency and proper characterization results. We could increase the therapeutic dose in both nanodrugs using a novel solvent and synthesis method by 10-fold, resulting in the development of non-toxic AK and BK with 85% efficiency in parasite clearance, while in the previous studies due to using very lower therapeutic dose, the researchers were negligibly succeeded in the parasite clearance (Table 4A and B). Moreover, the results of the current study indicated that AD50 mg/kg and BD40 mg/kg were significantly efficient in decreasing the toxicity and increasing the solubility rate of the drugs. Therefore, AK10 and BK20 mg/kg can be considered as novel therapeutic candidates for the treatment of leishmaniasis.
Conclusion
The nanodrugs of AD, BD, AK, and BK were constructed and characterized. Overall, in vitro, in vivo, physicochemical, solvent design and histopathological studies showed very promising results for nanodrug AK10 mg/kg, BK20 mg/kg, AD50 mg/kg and BD40 mg/kg which were associated with the decrease of their toxicity and increase the solubility. Also, AK10 mg/kg and BK20 mg/kg were more effective to decrease the parasite burden and increased the therapeutic effects and relief indicators. It was concluded that nanodrugs AK10 mg/kg and BK20 mg/kg prepared with phase separation method were successful to completely reduce the parasite burden, lesion size, and parasite number. On the other hand, nanodrugs AK10 mg/kg and BK20 mg/kg were completely effective to improve the wound healing process due to the increased effective dose of these nanodrugs.
Suggestions
Due to the requirement of novel medications in L. major treatment and the world’s treatment approaches toward the development of novel medicines, it is suggested that AK10 and BK20 mg/kg can be regarded as appropriate alternative regimens for L. major treatment.
Acknowledgment
The authors would like to thank Pasteur Institute of Iran. Authors would like also to thanks Tehran University of Medical Sciences, and Shahid Beheshti University of Medical Sciences, Tehran, Iran for their invaluable help. Also, it should be noted that there was no financial support for doing the present study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ponte-Sucre A, Gamarro F, Dujardin J-C, et al. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis. 2017;11(12):e0006052. doi: 10.1371/journal.pntd.0006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvar J, Velez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galvão EL, Rabello A, Cota GF. Efficacy of azole therapy for tegumentary leishmaniasis: a systematic review and meta-analysis. PLoS One. 2017;12(10):e0186117. doi: 10.1371/journal.pone.0186117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro TG, Franca JR, Fuscaldi LL, et al. An optimized nanoparticle delivery system based on chitosan and chondroitin sulfate molecules reduces the toxicity of amphotericin B and is effective in treating tegumentary leishmaniasis. Int J Nanomedicine. 2014;9:5341–5353. doi: 10.2147/IJN.S68966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripathi P, Jaiswal AK, Dube A, Mishra PR. Hexadecylphosphocholine (Miltefosine) stabilized chitosan modified Ampholipospheres as prototype co-delivery vehicle for enhanced killing of L. donovani. Int J Biol Macromol. 2017;105:625–637. doi: 10.1016/j.ijbiomac.2017.07.076 [DOI] [PubMed] [Google Scholar]
- 6.Yu B, Okano T, Kataoka K, Kwon G. Polymeric micelles for drug delivery: solubilization and haemolytic activity of amphotericin B. J Controlled Release. 1998;53(1):131–136. [DOI] [PubMed] [Google Scholar]
- 7.Tripathi P, Dwivedi P, Khatik R, et al. Development of 4-sulfated N-acetyl galactosamine anchored chitosan nanoparticles: a dual strategy for effective management of Leishmaniasis. Colloids Surf B. 2015;136:150–159. doi: 10.1016/j.colsurfb.2015.08.037 [DOI] [PubMed] [Google Scholar]
- 8.Cole P, Bishop J, Beckstead J, Titus R, Ryan R. Effect of amphotericin B nanodisks on Leishmania major infected mice. Pharm Anal Acta. 2014;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meira CS, Barbosa-Filho JM, Lanfredi-Rangel A, Guimarães ET, Moreira DRM, Soares MBP. Antiparasitic evaluation of betulinic acid derivatives reveals effective and selective anti-Trypanosoma cruzi inhibitors. Exp Parasitol. 2016;166:108–115. doi: 10.1016/j.exppara.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 10.Haavikko R. Synthesis of Betulin Derivatives with New Bioactivities University of Helsinki, Finland: Helsingin yliopisto; 2015:45–49. [Google Scholar]
- 11.Saneja A, Kumar R, Singh A, et al. Development and evaluation of long-circulating nanoparticles loaded with betulinic acid for improved anti-tumor efficacy. Int J Pharm. 2017;531(1):153–166. doi: 10.1016/j.ijpharm.2017.08.076 [DOI] [PubMed] [Google Scholar]
- 12.Alakurtti S, Heiska T, Kiriazis A, Sacerdoti-Sierra N, Jaffe CL, Yli-Kauhaluoma J. Synthesis and anti-leishmanial activity of heterocyclic betulin derivatives. Bioorg Med Chem. 2010;18(4):1573–1582. doi: 10.1016/j.bmc.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 13.Domínguez-Carmona D, Escalante-Erosa F, García-Sosa K, et al. Antiprotozoal activity of betulinic acid derivatives. Phytomedicine. 2010;17(5):379–382. doi: 10.1016/j.phymed.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury S, Mukherjee T, Sengupta S, Chowdhury SR, Mukhopadhyay S, Majumder HK. Novel betulin derivatives as antileishmanial agents with mode of action targeting type IB DNA topoisomerase. Mol Pharmacol. 2015;80(4):694–703. doi: 10.1124/mol.111.072785 [DOI] [PubMed] [Google Scholar]
- 15.Chowdhury AR, Mandal S, Goswami A, et al. Dihydrobetulinic acid induces apoptosis in Leishmania donovani by targeting DNA topoisomerase I and II: implications in antileishmanial therapy. Mol Med. 2003;9(1–2):26–36. [PMC free article] [PubMed] [Google Scholar]
- 16.Pundir S, Badola A, Sharma D. Sustained release matrix technology and recent advance in matrix drug delivery system: a review. Int J Drug Res Technol. 2017;3(1):8. [Google Scholar]
- 17.Koohi Moftakhari Esfahani M, Alavi SE, Shahbazian S, Ebrahimi Shahmabadi H. Drug delivery of cisplatin to breast cancer by polybutylcyanoacrylate nanoparticles. Adv Polymer Technol. 2018;37(3):674–678. doi: 10.1002/adv.2018.37.issue-3 [DOI] [Google Scholar]
- 18.Doun SKB, Alavi SE, Esfahani MKM, Shahmabadi HE, Alavi F, Hamzei S. Efficacy of Cisplatin-loaded poly butyl cyanoacrylate nanoparticles on the ovarian cancer: an in vitro study. Tumor Biol. 2014;35(8):7491–7497. doi: 10.1007/s13277-014-1996-8 [DOI] [PubMed] [Google Scholar]
- 19.Shahabi J, Shahmabadi HE, Alavi SE, et al. Effect of gold nanoparticles on properties of nanoliposomal hydroxyurea: an in vitro study. Indian J Clin Biochem. 2014;29(3):315–320. doi: 10.1007/s12291-013-0355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehghani F, Hoseini MHM, Memarnejadian A, et al. Immunomodulatory activities of chitin microparticles on leishmania major-infected murine macrophages. Arch Med Res. 2011;42(7):572–576. doi: 10.1016/j.arcmed.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 21.Malik A, Gupta M, Mani R, Gogoi H, Bhatnagar R. Trimethyl chitosan nanoparticles encapsulated protective antigen protects the mice against anthrax. Front Immunol. 2018;9:562. doi: 10.3389/fimmu.2018.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik A, Gupta M, Gupta V, Gogoi H, Bhatnagar R. Novel application of trimethyl chitosan as an adjuvant in vaccine delivery. Int J Nanomedicine. 2018;13:7959. doi: 10.2147/IJN.S177627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salah R. Antileishmanial activities of chitin and chitosan prepared from shrimp shell waste. Int Conf Chem Mettalurgy Environ Eng. 2015;29:177–280. [Google Scholar]
- 24.Jain V, Gupta A, Pawar VK, et al. Chitosan-assisted immunotherapy for intervention of experimental leishmaniasis via amphotericin B-loaded solid lipid nanoparticles. Appl Biochem Biotechnol. 2014;174(4):1309–1330. doi: 10.1007/s12010-014-1084-y [DOI] [PubMed] [Google Scholar]
- 25.Wang JJ, Zeng ZW, Xiao RZ, et al. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine. 2011;6:765. doi: 10.2147/IJN.S25646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MubarakAli D, LewisOscar F, Gopinath V, Alharbi NS, Alharbi SA, Thajuddin N. An inhibitory action of chitosan nanoparticles against pathogenic bacteria and fungi and their potential applications as biocompatible antioxidants. Microb Pathog. 2018;114:323–327. doi: 10.1016/j.micpath.2017.11.043 [DOI] [PubMed] [Google Scholar]
- 27.Singh PK, Pawar VK, Jaiswal AK, et al. Chitosan coated PluronicF127 micelles for effective delivery of amphotericin B in experimental visceral leishmaniasis. Int J Biol Macromol. 2017;105:1220–1231. doi: 10.1016/j.ijbiomac.2017.07.161 [DOI] [PubMed] [Google Scholar]
- 28.Xu L, Zhang H, Wu Y. Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chem Neurosci. 2013;5(1):2–13. doi: 10.1021/cn400182z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalomiraki M, Thermos K, Chaniotakis NA. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int J Nanomedicine. 2016;11:1–12. doi: 10.2147/IJN.S93069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain K, Verma AK, Mishra PR, Jain NK. Characterization and evaluation of amphotericin B loaded MDP conjugated poly (propylene imine) dendrimers. Nanomed. 2015;11(3):705–713. doi: 10.1016/j.nano.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 31.Hypercube Incorporation. HyperChem (TM) Professional 8.0. Gainesville, FL: Hypercube, Inc.; 2007. [Google Scholar]
- 32.Molecular Graphics Laboratory, Department of Molecular Biology, The Scripps Research Institute, MB-510550 N. Torrey Pines Rd. La Jolla, CA 92037-1000U.S.A. Available from: http://autodock.scripps.edu/faqs-help/tutorial/using-autodock-4-with-autodocktools.
- 33.Zadeh Mehrizi T, Shafiee Ardestani M, Haji Molla Hoseini M, Khamesipour A, Mosaffa N, Ramezani A. Novel nano-sized chitosan amphotericin B formulation with considerable improvement against Leishmania major. Nanomedicine (Lond). 2018;13(24):3129–3147. doi: 10.2217/nnm-2018-0063 [DOI] [PubMed] [Google Scholar]
- 34.Zadeh Mehrizi T, Shafiee Ardestani M, Haji Molla Hoseini M, Khamesipour A, Mosaffa N, Ramezani A. Novel nanosized chitosan-betulinic acid against resistant Leishmania major and first clinical observation of such parasite in kidney. Sci Rep. 2018;8(1):11759. doi: 10.1038/s41598-018-30103-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zadeh Mehrizi T, Shafiee Ardestani M, Khamesipour A, et al. Reduction toxicity of amphotericin B through loading into a novel nanoformulation of anionic linear globular dendrimer for improve treatment of leishmania major. J Mate Sci. 2018;29(8):125. [DOI] [PubMed] [Google Scholar]
- 36.Zadeh Mehrizi T, Shafiee Ardestani M, Khamesipour A, et al. Reduction toxicity of Amphotericin B through loading into a novel nanoformulation of anionic linear globular dendrimer for improve treatment of leishmania major. Journal of Materials Science: Materials in Medicine 2018;29(8):125. [DOI] [PubMed] [Google Scholar]
- 37.Safari J, Azizi F, Sadeghi M. Chitosan nanoparticles as a green and renewable catalyst in the synthesis of 1, 4-dihydropyridine under solvent-free conditions. N J Chem. 2015;39(3):1905–1909. doi: 10.1039/C4NJ01730G [DOI] [Google Scholar]
- 38.Jain A, Thakur K, Sharma G, Kush P, Jain UK. Fabrication, characterization and cytotoxicity studies of ionically cross-linked docetaxel loaded chitosan nanoparticles. Carbohydr Polym. 2016;137:65–74. doi: 10.1016/j.carbpol.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 39.Mohammadi E, Amanlou M, Ebrahimi SES, et al. Cellular uptake, imaging and pathotoxicological studies of a novel Gd [III]–DO3A-butrol nano-formulation. RSC Adv. 2014;4(86):45984–45994. doi: 10.1039/C4RA05596A [DOI] [Google Scholar]
- 40.Ghoreishi SM, Bitarafan-Rajabi A, Azar AD, Ardestani MS, Novel AA. 99mTc-radiolabeled anionic linear globular PEG-based dendrimer-chlorambucil: non-invasive method for in-vivo biodistribution. Drug Res. 2017;67(03):149–155. doi: 10.1055/s-0042-118448 [DOI] [PubMed] [Google Scholar]
- 41.Morakul B, Suksiriworapong J, Chomnawang MT, Langguth P, Junyaprasert VB. Dissolution enhancement and in vitro performance of clarithromycin nanocrystals produced by precipitation–lyophilization–homogenization method. Eur J Pharm Biopharm. 2014;88(3):886–896. doi: 10.1016/j.ejpb.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 42.Koch S, Kessler M, Mandel K, Dembski S, Heuze K, Hackenberg S. Polycarboxylate ethers: the key towards non-toxic TiO 2 nanoparticle stabilisation in physiological solutions. Colloids Surf B. 2016;143:7–14. doi: 10.1016/j.colsurfb.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 43.Mahmoudvand H, Kheirandish F, Mirbadie SR, et al. The potential use of methotrexate in the treatment of cutaneous Leishmaniasis: in vitro assays against sensitive and meglumine antimoniate-resistant strains of Leishmania tropica. Iran J Parasitol. 2017;12(3):339. [PMC free article] [PubMed] [Google Scholar]
- 44.Abamor ES. Antileishmanial activities of caffeic acid phenethyl ester loaded PLGA nanoparticles against Leishmania infantum promastigotes and amastigotes. In Vitro. Asian Pacific J Trop Med. 2017;10(1):25–34. doi: 10.1016/j.apjtm.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 45.Vellozo NS, Pereira-Marques ST, Cabral-Piccin MP, et al. All-trans retinoic acid promotes an M1-to M2-phenotype shift and inhibits macrophage-mediated immunity to Leishmania major. Front Immunol. 2017;8:1560–1571. doi: 10.3389/fimmu.2017.01560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marinho FA, Sangenito LS, Oliveira SS, et al. The potent cell permeable calpain inhibitor MDL28170 affects the interaction of Leishmania amazonensis with macrophages and shows anti-amastigote activity. Parasitol Int. 2017;66(5):579–583. doi: 10.1016/j.parint.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 47.Davoudi N, Khamesipour A, Mahboudi F, McMaster WR. A dual drug sensitive L. major induces protection without lesion in C57BL/6 mice. PLoS Negl Trop Dis. 2014;8(5):e2785. doi: 10.1371/journal.pntd.0002785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kheirandish F, Mahmoudvand H, Khamesipour A, et al. The therapeutic effects of olive leaf extract on Leishmania major infection in BALB/c mice. Marmara Pharm J. 2017;21(4):837–842. doi: 10.12991/mpj.2017.6 [DOI] [Google Scholar]
- 49.Hoseini MHM, Moradi M, Alimohammadian MH, Shahgoli VK, Darabi H, Rostami A. Immunotherapeutic effects of chitin in comparison with chitosan against Leishmania major infection. Parasitol Int. 2016;65(2):99–104. doi: 10.1016/j.parint.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 50.Ghotloo S, Mollahoseini MH, Najafi A, Yeganeh F. Comparison of parasite burden using real-time polymerase chain reaction assay and limiting dilution assay in leishmania major infected mouse. Iran J Parasitol. 2015;10(4):571. [PMC free article] [PubMed] [Google Scholar]
- 51.Hajjaran H, Kazemi‐Rad E, Mohebali M, et al. Expression analysis of activated protein kinase C gene (LACK 1) in antimony sensitive and resistant Leishmania tropica clinical isolates using real‐time RT‐PCR. Int J Dermatol. 2016;55(9):1020–1026. doi: 10.1111/ijd.13321 [DOI] [PubMed] [Google Scholar]
- 52.Zadeh Mehrizi T, Mosaffa N, Haji MollaHoseini M, Shafiee Ardestani M, Khamesipour A, Ramezani A. In vivo therapeutic effects of four synthesized antileishmanial nanodrugs in the treatment of Leishmaniasis. Arch Clin Infect Dis. 2018;13(5):e80314. [Google Scholar]
- 53.Huynh CT, Lee DS. Controlled release. Encycl Polym Nanomate. 2015;4(Springer):439–449. [Google Scholar]
- 54.Tamizharasi S, Dubey A, Rathi V, Rathi J. Development and characterization of niosomal drug delivery of gliclazide. J Young Pharm. 2009;1(3):205. doi: 10.4103/0975-1483.57065 [DOI] [Google Scholar]
- 55.Irby D, Du C, Li F. Lipid–drug conjugate for enhancing drug delivery. Mol Pharm. 2017;14(5):1325–1338. doi: 10.1021/acs.molpharmaceut.6b01027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barzegar-Jalali M, Adibkia K, Valizadeh H, et al. Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci. 2008;11(1):167–177. [DOI] [PubMed] [Google Scholar]
- 57.Ribeiro TG, Fumagalli MAC, Valadares DG, et al.Novel targeting using nanoparticles: an approach to the development of an effective anti-leishmanial drug-delivery system. Int J Nanomedicine, 2014;9:877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain K, Verma AK, Mishra PR, Jain NK. Surface-engineered dendrimeric nanoconjugates for macrophage-targeted delivery of amphotericin B: formulation development and in vitro and in vivo evaluation. Antimicrob Agents Chemother. 2015;59(5):2479–2487. doi: 10.1128/AAC.04213-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaminskas LM, Boyd BJ, Porter CJ. Dendrimer pharmacokinetics: the effect of size, structure and surface characteristics on ADME properties. Nanomedicine. 2011;6(6):1063–1084. doi: 10.2217/nnm.11.67 [DOI] [PubMed] [Google Scholar]
- 60.Gupta PK, Jaiswal AK, Asthana S, et al. Self assembled ionically sodium alginate cross-linked amphotericin B encapsulated glycol chitosan stearate nanoparticles: applicability in better chemotherapy and non-toxic delivery in visceral leishmaniasis. Pharm Res. 2015;32(5):1727–1740. doi: 10.1007/s11095-014-1571-4 [DOI] [PubMed] [Google Scholar]
- 61.Dash M, Chiellini F, Ottenbrite R, Chiellini E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. 2011;36(8):981–1014. doi: 10.1016/j.progpolymsci.2011.02.001 [DOI] [Google Scholar]
- 62.Sousa MC, Varandas R, Santos RC, Santos-Rosa M, Alves V, Salvador JA. Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: synergistic effects with miltefosine. PLoS One. 2014;9(3):e89939. doi: 10.1371/journal.pone.0089939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daftarian PM, Stone GW, Kovalski L, et al. A targeted and adjuvanted nanocarrier lowers the effective dose of liposomal amphotericin B and enhances adaptive immunity in murine cutaneous leishmaniasis. J Infect Dis. 2013;208(11):1914–1922. doi: 10.1093/infdis/jit378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asthana S, Jaiswal AK, Gupta PK, Pawar VK, Dube A, Chourasia MK. Immunoadjuvant chemotherapy of visceral leishmaniasis in hamsters using amphotericin B-encapsulated nanoemulsion template-based chitosan nanocapsules. Antimicrob Agents Chemother. 2013;57(4):1714–1722. doi: 10.1128/AAC.01984-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shahnaz G, Edagwa BJ, McMillan J, et al. Development of mannose-anchored thiolated amphotericin B nanocarriers for treatment of visceral leishmaniasis. Nanomedicine. 2017;12(2):99–115. doi: 10.2217/nnm-2016-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Molecular Graphics Laboratory, Department of Molecular Biology, The Scripps Research Institute, MB-510550 N. Torrey Pines Rd. La Jolla, CA 92037-1000U.S.A. Available from: http://autodock.scripps.edu/faqs-help/tutorial/using-autodock-4-with-autodocktools.