Visual Abstract
Keywords: cardiovascular disease, chronic kidney disease, clinical epidemiology, end-stage renal disease, mortality, vascular calcification, humans, glomerular filtration rate, confidence intervals, risk factors, prospective studies, follow-up studies, peripheral arterial disease, chronic renal insufficiency, chronic kidney failure, atherosclerosis, myocardial infarction, heart failure, stroke
Abstract
Background and objectives
Patients with CKD are at high risk for cardiovascular disease, ESKD, and mortality. Vascular calcification is one pathway through which cardiovascular disease risks are increased. We hypothesized that a novel measure of serum calcification propensity is associated with cardiovascular disease events, ESKD, and all-cause mortality among patients with CKD stages 2–4.
Design, setting, participants, & measurements
Among 3404 participants from the prospective, longitudinal Chronic Renal Insufficiency Cohort Study, we quantified calcification propensity as the transformation time (T50) from primary to secondary calciprotein particles, with lower T50 corresponding to higher calcification propensity. We used multivariable-adjusted Cox proportional hazards regression models to assess the associations of T50 with risks of adjudicated atherosclerotic cardiovascular disease events (myocardial infarction, stroke, and peripheral artery disease), adjudicated heart failure, ESKD, and mortality.
Results
The mean T50 was 313 (SD 79) minutes. Over an average 7.1 (SD 3.1) years of follow-up, we observed 571 atherosclerotic cardiovascular disease events, 633 heart failure events, 887 ESKD events, and 924 deaths. With adjustment for traditional cardiovascular disease risk factors, lower T50 was significantly associated with higher risk of atherosclerotic cardiovascular disease (hazard ratio [HR] per SD lower T50, 1.14; 95% confidence interval [95% CI], 1.05 to 1.25), ESKD within 3 years from baseline (HR per SD lower T50, 1.68; 95% CI, 1.52 to 1.86), and all-cause mortality (HR per SD lower T50, 1.16; 95% CI, 1.09 to 1.24), but not heart failure (HR per SD lower T50, 1.06; 95% CI, 0.97 to 1.15). After adjustment for eGFR and 24-hour urinary protein, T50 was not associated with risks of atherosclerotic cardiovascular disease, ESKD, and mortality.
Conclusions
Among patients with CKD stages 2–4, higher serum calcification propensity is associated with atherosclerotic cardiovascular disease events, ESKD, and all-cause mortality, but this association was not independent of kidney function.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2019_10_28_CJN04710419.mp3
Introduction
CKD affects more than 10% of the population globally and is strongly associated with adverse health outcomes, including cardiovascular disease, ESKD, and death (1–4). In 2017, CKD accounted for 1.23 million deaths, representing a 33.7% increase from 2007 (5). Vascular calcification is prevalent in patients with CKD and ESKD, and is one mechanism by which risks for cardiovascular disease and death are increased (6,7).
The serum calcification propensity test (T50) is a novel, in vitro assay that quantifies the transformation time from primary to secondary calciprotein particles in serum when challenged with exogenous calcium and phosphate (8). The T50 test incorporates summary information about vascular calcification promoters (e.g., calcium and phosphate) and inhibitors (e.g., albumin, fetuin-A, magnesium, and pyrophosphate), and may signify the status of the humoral calcification-regulating system. T50 was associated with cardiovascular and all-cause mortality among patients with advanced CKD in several small studies (9–12). However, its associations with clinical events, including cardiovascular disease, ESKD, and mortality, among larger patient populations with earlier stages of CKD are unknown. T50 can be measured inexpensively and noninvasively, and quantifying its associations with these outcomes could provide insights into the effect of promoter-inhibitor imbalance on prognosis and aid in the early identification of high-risk patients.
The Chronic Renal Insufficiency Cohort (CRIC) Study provides the opportunity to quantify the associations of the T50 test with clinical events among a large, diverse sample of adults with CKD stages 2–4. We hypothesized that lower T50 (i.e., higher calcification propensity) is associated with higher risks of atherosclerotic cardiovascular disease, heart failure, ESKD, and mortality.
Materials and Methods
Study Design and Participants
The CRIC study is a prospective, longitudinal cohort study of participants with mild-to-moderate CKD on the basis of an eGFR entry criteria of 20–70 ml/min per 1.73 m2. A racially and ethnically diverse group of 3939 men and women aged 21–74 years was enrolled from seven clinical centers in the United States (Ann Arbor, MI; Baltimore, MD; Chicago, IL; Cleveland, OH; New Orleans, LA; Philadelphia, PA; and San Francisco, CA) in phase 1, between May 2003 and August 2008 (13). Patients with severe heart failure, cirrhosis, HIV infection, polycystic kidney disease, renal cell carcinoma, those receiving maintenance dialysis or an organ transplant, and those taking immunosuppressive medications were excluded from the CRIC study. The study was approved by the institutional review board at each institution and all participants provided written informed consent.
Exposure Assessment
T50 was measured among 3404 participants with stored samples from the first annual follow-up visit (i.e., baseline for the current analysis) and 96% of participants reported fasting. We quantified calcification propensity as the transformation time (T50), in minutes, from primary to secondary calciprotein particles in vitro, with lower T50 corresponding to higher calcification propensity (8). The T50 test was performed using a Nephelostar nephelometer at the Calciscon Laboratory in Switzerland, using serum samples stored at −80°C and shipped with sufficient dry ice. The mean intraassay and interassay coefficients of variation are 2.2% and 3.4%, respectively. T50 values have been reported in other populations, including 184 patients with CKD stages 3–4 (mean, 329 [SD 95] minutes) (14) and 2785 patients undergoing hemodialysis (median, 212 [10th–90th percentiles, 109–328] minutes) (11).
Outcome Assessment
The outcomes for this analysis are time to (1) a composite of atherosclerotic cardiovascular disease events (myocardial infarction, stroke, or peripheral artery disease events) (15), (2) heart failure, (3) all-cause and cause-specific mortality, and (4) ESKD. Cardiovascular disease events are reported every 6 months and confirmed by medical record adjudication as possible, probable, or definite events (15). All deaths were confirmed by death certificate. We used a superlearning algorithm to determine if a death was cardiovascular-related on the basis of causes of death listed on the death certificate, using the 233 adjudicated cardiovascular-related deaths as a gold standard. ESKD was defined as the receipt of dialysis or kidney transplantation ascertained every 6 months and confirmed by medical record review, supplemented by information from the US Renal Data System. Follow-up time was censored at the earliest occurrence of death, loss to follow-up (n=176; 5%), or end of the follow-up period through September 2015, for a maximum duration of 11.2 years.
Covariate Assessment
Sociodemographic characteristics, medical history, and medication use were obtained via self-reported questionnaire. Body weight, height, and BP were measured using standard protocols (13). We defined diabetes as fasting glucose ≥126 mg/dl, nonfasting glucose ≥200 mg/dl, and/or the use of antidiabetic medications. We defined history of cardiovascular disease as self-reported prior coronary artery disease, heart failure, stroke, or peripheral artery disease. Glucose, cholesterol, phosphate, calcium, bicarbonate, magnesium, and serum albumin were measured using standard laboratory methods. Twenty-four hour urinary protein was measured using the turbidimetric method, with benzethonium chloride. Total parathyroid hormone (PTH) was measured using the total PTH assay, which includes the 1–84 PTH molecule and 7–84 fragments assay (Scantibodies, Santee, CA). Fibroblast growth factor-23 (FGF23) was measured by a second-generation C-terminal assay (Immutopics). High-sensitivity C-reactive protein (hsCRP) and IL-6 were measured at the original baseline examination using the particle enhanced immunonephelometry method. Fetuin-A concentration was measured at the original baseline examination using the quantitative sandwich enzyme immunoassay technique. We calculated eGFR using the equation derived in the CRIC cohort (16). We obtained covariate data from the same study visit as T50 or, if missing, the original baseline visit 1 year prior (<5% missing for all covariates except 24-hour urinary protein [10%]).
Statistical Analyses
We summarized the baseline characteristics of the study participants, stratified by quartiles of T50, as the mean±SD or median (25th–75th percentile) for continuous variables or the number (percentage) for categorical variables. We transformed variables with skewed distributions by taking the natural log of their raw values. We visually assessed departures from linearity in the associations of T50 with events using plots of Martingale residuals and, given no apparent departures, retained a linear functional form for T50. Kaplan–Meier plots were used to characterize the unadjusted associations of T50 with events.
Cox proportional hazards regression models, stratified by study site, were used to estimate the multivariable-adjusted associations of T50 with time to events. Covariates included in regression models were selected on the basis of prior clinical knowledge. In addition to unadjusted analyses, we used three sequentially adjusted models: (1) adjusted for age, sex, and race/ethnicity; (2) adjusted for variables in model 1 plus history of cardiovascular disease and traditional cardiovascular disease risk factors (history of diabetes, systolic BP, use of antihypertensive medications, total cholesterol, HDL cholesterol, and current smoking) (17); and (3) adjusted for variables in model 2 plus eGFR and 24-hour urinary protein. We tested for effect modification by baseline age, sex, race/ethnicity, diabetes, and eGFR, by including interaction terms with T50 in the models. Additionally, we used linear regression to assess the strength of multivariable-adjusted associations of individual variables in model 3 with T50.
We tested the proportional hazards assumption visually using plots of Schoenfeld residuals and statistically using time-by-variable interaction terms in the models. The proportional hazards assumption was met for all variables in analyses of atherosclerotic cardiovascular disease, heart failure, and mortality. However, we detected violations of the assumption in analyses of ESKD for T50 and eGFR. Thus, as recommended by the CRIC study (18), we included a time interaction in the Cox models, reporting associations of T50 with early- (0 to <3 years) and late-onset (≥3 years) ESKD, time periods in which hazards were proportional across quartiles of T50.
We additionally conducted two supplementary analyses: the first excluding those with self-reported history of cardiovascular disease from analyses of atherosclerotic cardiovascular disease and heart failure, and the second assessing the effect of additional adjustment for variables potentially affecting T50 (calcium, phosphate, bicarbonate, magnesium, serum albumin, fetuin-A, FGF23, PTH, and use of medications, including warfarin, active vitamin D, phosphate binders, and calciferols) and inflammatory markers (hsCRP and IL-6). Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.4.2 (R Project for Statistical Computing). Two-sided P values <0.05 were considered statistically significant for all analyses.
Results
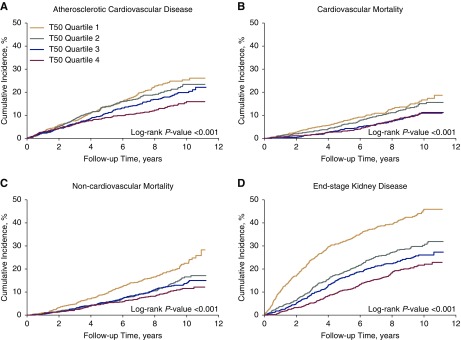
Among 3404 participants included in the analyses, the mean age was 59 (SD 11) years, 45% were women, 49% had diabetes, 36% had a history of cardiovascular disease, and the mean eGFR was 42 (SD 17) ml/min per 1.73 m2. The mean T50 was 313 (SD 79) minutes. Participants with lower T50 were more likely to be women, non-Hispanic black, and current smokers; have a history of cardiovascular disease and diabetes; and be taking antihypertensive, statin, active vitamin D, and phosphate binder medications (Table 1). On average, participants with lower T50 had higher systolic BP, 24-hour urinary protein, phosphate, FGF23, PTH, hsCRP, and IL-6; and lower eGFR, bicarbonate, calcium, magnesium, serum albumin, and fetuin-A. T50 was significantly correlated with eGFR (rho=0.26; Supplemental Figure 1). After multivariable adjustment, eGFR was the variable most strongly associated with T50 (Supplemental Table 1). In unadjusted Kaplan–Meier analyses, lower T50 was significantly associated with higher risk of all events, particularly atherosclerotic cardiovascular disease, cardiovascular mortality, noncardiovascular mortality, and ESKD (Figure 1).
Table 1.
Baseline characteristics of 3404 CRIC participants by quartiles of T50
| Variables | Quartiles of T50, min | |||
|---|---|---|---|---|
| Quartile 4 (n=850), T50=364–600 min | Quartile 3 (n=849), T50=318–363 min | Quartile 2 (n=854), T50=264–317 min | Quartile 1 (n=851), T50=72–263 min | |
| Age, yr | 59±11 | 59±11 | 59±11 | 59±11 |
| Women | 351 (41) | 371 (44) | 411 (48) | 397 (47) |
| Race/ethnicity | ||||
| Non-Hispanic White | 466 (55) | 382 (45) | 350 (41) | 265 (31) |
| Non-Hispanic Black | 274 (32) | 342 (40) | 368 (43) | 424 (50) |
| Hispanic | 62 (7) | 93 (11) | 97 (11) | 144 (17) |
| Other | 48 (6) | 32 (4) | 39 (5) | 18 (2) |
| Body mass index, kg/m2 | 31±7 | 32±8 | 33±8 | 32±8 |
| Current cigarette smoking | 84 (10) | 90 (11) | 103 (12) | 134 (16) |
| History of cardiovascular disease | 266 (31) | 305 (36) | 289 (34) | 359 (42) |
| Diabetes mellitus | 349 (41) | 388 (46) | 436 (51) | 511 (60) |
| Systolic BP, mm Hg | 124±20 | 126±21 | 127±22 | 130±23 |
| Antihypertensive medication use | 761 (90) | 787 (93) | 782 (92) | 804 (94) |
| Total cholesterol, mg/dl | 186±41 | 180±43 | 183±46 | 179±46 |
| HDL cholesterol, mg/dl | 49±15 | 48±15 | 49±17 | 48±16 |
| LDL cholesterol, mg/dl | 104±35 | 98±33 | 99±35 | 98±36 |
| Statin medication use | 466 (55) | 505 (59) | 511 (60) | 531 (62) |
| Warfarin medication use | 47 (6) | 54 (6) | 56 (7) | 45 (5) |
| eGFR, ml/min per 1.73 m2 | 48±16 | 43±16 | 42±17 | 36±17 |
| Urinary protein, g/24 h | 0.1 [0.1–0.5] | 0.2 [0.1–0.8] | 0.2 [0.1–1.0] | 0.3 [0.1–1.7] |
| Bicarbonate, mmol/L | 26±3 | 25±3 | 24±3 | 23±4 |
| Active vitamin D medication use | 30 (4) | 53 (6) | 57 (7) | 90 (11) |
| Phosphate binder medication use | 50 (6) | 65 (8) | 67 (8) | 94 (11) |
| Calciferol medication use | 113 (13) | 96 (11) | 104 (12) | 103 (12) |
| Calcium, mg/dl | 9.4±0.5 | 9.3±0.5 | 9.3±0.5 | 9.2±0.6 |
| Phosphate, mg/dl | 3.6±1.0 | 3.8±1.1 | 3.8±0.8 | 4.2±1.1 |
| Magnesium, mg/dl | 2.0±0.3 | 2.0±0.3 | 1.9±0.3 | 1.9±0.3 |
| Serum albumin, g/dl | 4.2±0.4 | 4.1±0.4 | 4.0±0.4 | 3.9±0.5 |
| Fetuin-A, mg/L | 579±110 | 538±105 | 519±102 | 487±108 |
| Fibroblast growth factor-23, RU/ml | 124 [82–208] | 146 [93–264] | 150 [97–283] | 197 [113–392] |
| Parathyroid hormone, pg/ml | 55 [39–85] | 62 [39–94] | 61 [40–95] | 74 [44–135] |
| IL-6, pg/ml | 1.5 [0.9–2.6] | 1.8 [1.1–2.9] | 1.9 [1.3–3.0] | 2.2 [1.3–3.7] |
| High-sensitivity C-reactive protein, mg/L | 2.0 [0.9–5.0] | 2.4 [0.9–5.9] | 2.8 [1.2–6.7] | 2.7 [1.1–7.7] |
Values are presented as mean±SD, median [interquartile range], or n (%).
Figure 1.
Lower T50 is associated with higher cumulative incidence of atherosclerotic cardiovascular disease, cardiovascular mortality, noncardiovascular mortality, and ESKD. (A) Cumulative incidence of atherosclerotic cardiovascular disease. (B) Cumulative incidence of cardiovascular mortality. (C) Cumulative incidence of noncardiovascular mortality. (D) Cumulative incidence of ESKD.
Table 2 shows the numbers of events and hazard ratios (HRs) of atherosclerotic cardiovascular disease and heart failure associated with T50 quartiles and per one SD lower T50. During 24,022 person-years of follow-up for cardiovascular disease events, we documented 571 atherosclerotic cardiovascular disease (312 myocardial infarction, 120 stroke, and 139 peripheral artery disease) events (23.8 per 1000 person-years) and 633 heart failure events (26.6 per 1000 person-years). T50 was significantly associated with both outcomes after adjustment for age, sex, and race/ethnicity. After additional adjustment for traditional cardiovascular disease risk factors, T50 remained associated with higher risk of atherosclerotic cardiovascular disease (HR per SD lower T50, 1.14; 95% confidence interval [95% CI], 1.05 to 1.25), but not heart failure (HR per SD lower T50, 1.06; 95% CI, 0.97 to 1.15). Further adjustment for eGFR and proteinuria attenuated the association between T50 and atherosclerotic cardiovascular disease, and the association was no longer significant (HR per SD lower T50, 1.07; 95% CI, 0.98 to 1.17). In a supplemental analysis excluding those with a self-reported history of cardiovascular disease, point estimates were similar to the main analyses, although 95% CIs were wider (Supplemental Table 2).
Table 2.
Associations of T50 with atherosclerotic cardiovascular disease and heart failure events
| Cardiovascular Disease Outcomes | Quartiles of T50, min | Per 1-SD Lower T50 (79 min) | P Value | |||
|---|---|---|---|---|---|---|
| Quartile 4 (≥364 min) | Quartile 3 (318–363 min) | Quartile 2 (264–317 min) | Quartile 1 (≤263 min) | |||
| Atherosclerotic cardiovascular disease (n=3397) | ||||||
| Events/total number | 107/847 | 142/847 | 155/852 | 167/851 | ||
| Hazard ratio (95% CI) | ||||||
| Unadjusted | Reference | 1.34 (1.05 to 1.73) | 1.56 (1.22 to 2.00) | 1.76 (1.38 to 2.24) | 1.25 (1.15 to 1.36) | <0.001 |
| Model 1a | Reference | 1.30 (1.01 to 1.68) | 1.53 (1.19 to 1.96) | 1.65 (1.28 to 2.11) | 1.23 (1.13 to 1.35) | <0.001 |
| Model 2b | Reference | 1.28 (0.99 to 1.65) | 1.42 (1.10 to 1.82) | 1.34 (1.04 to 1.73) | 1.14 (1.05 to 1.25) | 0.002 |
| Model 3c | Reference | 1.22 (0.94 to 1.57) | 1.30 (1.01 to 1.68) | 1.14 (0.88 to 1.48) | 1.07 (0.98 to 1.17) | 0.14 |
| Congestive heart failure (n=3396) | ||||||
| Events/total number | 132/ 847 | 142/ 847 | 166/ 852 | 193/ 850 | ||
| Hazard ratio (95% CI) | ||||||
| Unadjusted | Reference | 1.07 (0.84 to 1.35) | 1.32 (1.05 to 1.65) | 1.66 (1.33 to 2.07) | 1.21 (1.12 to 1.31) | <0.001 |
| Model 1a | Reference | 0.97 (0.76 to 1.23) | 1.17 (0.93 to 1.47) | 1.39 (1.10 to 1.74) | 1.14 (1.05 to 1.24) | 0.002 |
| Model 2b | Reference | 0.91 (0.71 to 1.15) | 1.06 (0.84 to 1.34) | 1.12 (0.89 to 1.41) | 1.06 (0.97 to 1.15) | 0.18 |
| Model 3c | Reference | 0.83 (0.65 to 1.06) | 0.95 (0.75 to 1.20) | 0.85 (0.67 to 1.07) | 0.95 (0.88 to 1.03) | 0.25 |
95% CI, 95% confidence interval.
Model 1: adjusted for age, sex, and race/ethnicity.
Model 2: adjusted for variables in model 1 plus history of cardiovascular disease, history of diabetes, systolic BP, use of antihypertensive medications, total cholesterol, HDL cholesterol, and current smoking.
Model 3: adjusted for variables in model 2 plus eGFR and 24-hour urinary protein.
Table 3 shows the numbers of events and HRs of all-cause, cardiovascular, and noncardiovascular mortality associated with T50. During 27,188 person-years of follow-up for mortality, we documented 924 all-cause deaths (34.0 per 1000 person-years), 349 of which were from cardiovascular causes and 433 from noncardiovascular causes; cause of death could not be determined in 142 cases. T50 was significantly associated with higher risk of all-cause mortality after adjustment for traditional cardiovascular disease risk factors (HR per SD lower T50, 1.16; 95% CI, 1.09 to 1.24), but this association was no longer significant after adjustment for eGFR and proteinuria (HR per SD lower T50, 1.05; 95% CI, 0.98 to 1.12). Only the association between T50 and noncardiovascular mortality remained statistically significant after adjustment for eGFR and proteinuria (HR per SD lower T50, 1.12; 95% CI, 1.01 to 1.24).
Table 3.
Associations of T50 with all-cause, cardiovascular, and noncardiovascular mortality
| Mortality Outcomes | Quartiles of T50, min | Per 1-SD Lower T50 (79 min) | P Value | |||
|---|---|---|---|---|---|---|
| Quartile 4 (≥364 min) | Quartile 3 (318–363 min) | Quartile 2 (264–317 min) | Quartile 1 (≤263 min) | |||
| All-cause mortality (n=3404) | ||||||
| Events/total number | 185/850 | 204/849 | 240/854 | 295/851 | ||
| Hazard ratio (95% CI) | ||||||
| Unadjusted | Reference | 1.13 (0.93 to 1.38) | 1.38 (1.14 to 1.67) | 1.83 (1.52 to 2.20) | 1.27 (1.19 to 1.36) | <0.001 |
| Model 1a | Reference | 1.07 (0.87 to 1.30) | 1.30 (1.07 to 1.58) | 1.63 (1.34 to 1.97) | 1.23 (1.15 to 1.32) | <0.001 |
| Model 2b | Reference | 1.04 (0.85 to 1.27) | 1.22 (1.00 to 1.48) | 1.40 (1.15 to 1.69) | 1.16 (1.09 to 1.24) | <0.001 |
| Model 3c | Reference | 0.96 (0.79 to 1.18) | 1.13 (0.93 to 1.37) | 1.09 (0.89 to 1.32) | 1.05 (0.98 to 1.12) | 0.17 |
| Cardiovascular mortality (n=3262) | ||||||
| Events/total number | 72/818 | 71/815 | 100/818 | 106/811 | ||
| Hazard ratio (95% CI) | ||||||
| Unadjusted | Reference | 1.01 (0.73 to 1.40) | 1.48 (1.09 to 2.00) | 1.70 (1.26 to 2.29) | 1.24 (1.12 to 1.38) | <0.001 |
| Model 1a | Reference | 0.98 (0.70 to 1.36) | 1.46 (1.08 to 1.99) | 1.59 (1.16 to 2.17) | 1.22 (1.09 to 1.36) | <0.001 |
| Model 2b | Reference | 0.94 (0.67 to 1.31) | 1.34 (0.98 to 1.83) | 1.30 (0.95 to 1.78) | 1.13 (1.01 to 1.26) | 0.03 |
| Model 3c | Reference | 0.85 (0.61 to 1.18) | 1.22 (0.89 to 1.67) | 0.99 (0.72 to 1.36) | 1.01 (0.91 to 1.13) | 0.81 |
| Noncardiovascular mortality (n=3262) | ||||||
| Events/total number | 81/818 | 99/815 | 104/818 | 149/811 | ||
| Hazard ratio (95% CI) | ||||||
| Unadjusted | Reference | 1.25 (0.93 to 1.68) | 1.37 (1.02 to 1.83) | 2.12 (1.62 to 2.78) | 1.33 (1.21 to 1.46) | <0.001 |
| Model 1a | Reference | 1.15 (0.86 to 1.54) | 1.26 (0.94 to 1.69) | 1.89 (1.43 to 2.50) | 1.30 (1.17 to 1.43) | <0.001 |
| Model 2b | Reference | 1.15 (0.85 to 1.54) | 1.19 (0.88 to 1.60) | 1.67 (1.26 to 2.21) | 1.23 (1.12 to 1.36) | <0.001 |
| Model 3c | Reference | 1.04 (0.77 to 1.41) | 1.09 (0.81 to 1.47) | 1.30 (0.97 to 1.74) | 1.12 (1.01 to 1.24) | 0.03 |
95% CI, 95% confidence interval.
Model 1: adjusted for age, sex, and race/ethnicity.
Model 2: adjusted for variables in model 1 plus history of cardiovascular disease, history of diabetes, systolic BP, use of antihypertensive medications, total cholesterol, HDL cholesterol, and current smoking.
Model 3: adjusted for variables in model 2 plus eGFR and 24-hour urinary protein.
Table 4 shows the numbers of events and HRs of ESKD associated with T50. During 23,146 person-years of follow-up for ESKD, we documented 887 ESKD events (38.3 per 1000 person-years), 405 of which occurred during the first 3 years of follow-up. After adjustment for traditional cardiovascular disease risk factors, T50 was significantly associated with ESKD, although more strongly in the early time period (HR per SD lower T50, 1.68; 95% CI, 1.52 to 1.86) versus the late time period (HR per SD lower T50, 1.14; 95% CI, 1.04 to 1.26). The associations between T50 and ESKD were attenuated and no longer significant after adjustment for eGFR and proteinuria.
Table 4.
Association of T50 with ESKD by follow-up time
| ESKD Outcomes | Quartiles of T50, min | Per 1-SD Lower T50 (79 min) | P Value | |||
|---|---|---|---|---|---|---|
| Quartile 4 (≥364 min) | Quartile 3 (318–363 min) | Quartile 2 (264–317 min) | Quartile 1 (≤263 min) | |||
| All follow-up (n=3345) | ||||||
| Events/total number | 157/843 | 190/837 | 224/840 | 316/825 | ||
| Years 0 to <3, hazard ratio (95% CI) | ||||||
| Unadjusted | Reference | 1.80 (1.24 to 2.62) | 2.25 (1.56 to 3.22) | 5.28 (3.79 to 7.34) | 1.89 (1.71 to 2.08) | <0.001 |
| Model 1a | Reference | 1.61 (1.10 to 2.34) | 1.97 (1.37 to 2.83) | 4.30 (3.07 to 6.02) | 1.76 (1.59 to 1.95) | <0.001 |
| Model 2b | Reference | 1.54 (1.06 to 2.24) | 1.78 (1.24 to 2.57) | 3.72 (2.65 to 5.21) | 1.68 (1.52 to 1.86) | <0.001 |
| Model 3c | Reference | 1.08 (0.74 to 1.58) | 0.90 (0.62 to 1.31) | 1.12 (0.78 to 1.60) | 1.05 (0.94 to 1.17) | 0.43 |
| Years 3–12, hazard ratio (95% CI) | ||||||
| Unadjusted | Reference | 1.08 (0.84 to 1.41) | 1.31 (1.02 to 1.69) | 1.63 (1.26 to 2.10) | 1.25 (1.13 to 1.37) | <0.001 |
| Model 1a | Reference | 1.00 (0.77 to 1.30) | 1.17 (0.91 to 1.51) | 1.36 (1.05 to 1.77) | 1.17 (1.06 to 1.29) | 0.001 |
| Model 2b | Reference | 1.00 (0.77 to 1.30) | 1.09 (0.84 to 1.40) | 1.29 (0.99 to 1.68) | 1.14 (1.04 to 1.26) | 0.008 |
| Model 3c | Reference | 0.88 (0.67 to 1.14) | 0.79 (0.60 to 1.02) | 0.78 (0.60 to 1.03) | 0.93 (0.85 to 1.03) | 0.17 |
95% CI, 95% confidence interval.
Model 1: adjusted for age, sex, and race/ethnicity.
Model 2: adjusted for variables in model 1 plus history of cardiovascular disease, history of diabetes, systolic BP, use of antihypertensive medications, total cholesterol, HDL cholesterol, and current smoking.
Model 3: adjusted for variables in model 2 plus eGFR and 24-hour urinary protein.
We did not detect effect modification of T50 by age, sex, race/ethnicity, diabetes, or baseline eGFR for any of the outcomes. In secondary analyses with additional adjustment for variables potentially affecting T50 (model 4) and inflammation variables (model 5), results were similar to the main analyses (model 3; Supplemental Table 3). However, the statistically significant association of T50 with noncardiovascular mortality observed in model 3 was attenuated after adjustment for either set of variables, and was no longer significant.
Discussion
In this analysis of 3404 participants with CKD stages 2–4, higher serum calcification propensity, denoted by lower T50, was significantly associated with higher risks of atherosclerotic cardiovascular disease, all-cause mortality, and ESKD, independent of traditional cardiovascular disease risk factors. However, the associations between T50 and clinical events were attenuated and no longer statistically significant after further adjustment for eGFR and proteinuria. Although T50 and its components may be associated with the risk for adverse health outcomes among patients with CKD, modest magnitudes of association in multivariable-adjusted models do not overcome a strong correlation between T50 and eGFR. Still, T50 and its determinants, namely calcification promoters and inhibitors, may warrant further research as potential therapeutic targets.
Previous research on the associations of T50 with clinical events has mostly been conducted among patients with advanced CKD (9–12). In three separate analyses of kidney transplant recipients (n=699, n=1435, and n=685), lower T50 was associated with higher risks of all-cause and cardiovascular mortality (9,10), and cardiovascular disease events (12), independent of eGFR. T50 was similarly associated with all-cause mortality, myocardial infarction, and peripheral artery disease among 2785 patients undergoing hemodialysis from the Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE) trial (11). In our analysis including patients with CKD stages 2–4, T50 was not associated with most outcomes after adjustment for eGFR and proteinuria, except for noncardiovascular mortality, although this association is likely due to residual confounding, as it disappeared with additional adjustment for either inflammation or mineral metabolism variables. Conversely, Smith et al. (14) observed significant associations with all-cause mortality in 184 patients with CKD stages 3–4, even after adjustment for eGFR and proteinuria. There are several possible explanations for the divergent findings in our study. The CRIC study included a population that was younger and, on average, healthier with respect to baseline characteristics. Additionally, it is possible associations of T50 with outcomes differ depending on baseline kidney function, although we did not detect effect modification by eGFR. Research in broader patient populations is warranted to further investigate the role of T50, including among the general population.
In our study, T50 did not provide risk information independent of eGFR and proteinuria. This finding may be expected, given that eGFR was the strongest correlate of T50 in our primary models. We acknowledge the possibility that associations between T50 and events are confounded by kidney function, although alternate explanations are also biologically plausible. As a comprehensive, functional measure, T50 may contain unique, but ultimately overlapping, information compared with eGFR (19). Furthermore, disordered promoter-inhibitor balance may be an intermediate on the pathway from reduced kidney function to clinical events. Alternatively, it is possible that a lower T50 could precede further kidney function decline, which should be explored further in longitudinal analyses and in other patient populations. Still, the magnitudes of association were modest and advocating for the use of T50 in risk prediction may be premature. However, the components of disordered calcification promoter-inhibitor homeostasis, as integrated by the T50 test, may be actionable therapeutic targets. In two randomized, controlled trials of 34 patients with CKD stages 3–4 and 57 patients undergoing dialysis, magnesium supplementation increased T50 (20,21). Despite these favorable results, it is unknown whether improvements in T50 translate to decreased risks of cardiovascular disease, ESKD, or mortality.
Despite the attenuation of associations of T50 with clinical events after adjustment for eGFR and proteinuria, our results suggest T50 is associated with an atherosclerotic cardiovascular disease pathway. After adjustment for traditional cardiovascular disease risk factors (17), one SD lower T50 was associated with a 14% (95% CI, 5% to 25%) higher risk of atherosclerotic cardiovascular disease. Conversely, T50 was not associated with the risk of heart failure after adjustment for these risk factors. Our findings complement results from the EVOLVE trial, which showed significant associations of T50 with cardiovascular disease, particularly myocardial infarction, but not heart failure (11).
Atherosclerosis, commonly observed as calcification of the vessel intima, is a complex pathophysiologic phenomenon characterized by lipid deposition, endothelial dysfunction, macrophage accumulation, and inflammation (22), and is linked with higher risks of myocardial infarction and peripheral artery disease. Calcification of the vessel media shares some similar pathophysiologic mechanisms (23), but is a distinct phenomenon leading primarily to arterial stiffness (24) and heart failure (25). It is plausible that the calcification promoter-inhibitor balance information captured by the T50 test could reflect either type of calcification, but our results imply a stronger association with intimal, atherosclerotic calcification. Intimal calcification is a continuum characterized by initial, unstable microcalcifications, with progression to more stable, dense macrocalcifications (26). The T50 test reflects maturation of calciprotein particles, which may directly influence these processes (27–29). We previously reported that lower T50 was associated with greater coronary artery calcification severity and progression, but only among those with established calcification (30). Given that T50 was not associated with atherosclerotic cardiovascular disease independent of eGFR, it is possible T50 provides a readout of progressive, stable intimal macrocalcification, as detected by coronary computed tomography. Progressive macrocalcification is also associated with declining kidney function (31), which may explain the observed attenuation of the association between T50 and atherosclerotic cardiovascular disease after adjustment for eGFR. Although it is impossible to substantiate our speculation in this study, further mechanistic and translational research is warranted to explore the role of T50 in different types of calcification and cardiovascular disease pathways.
The association between T50 and ESKD was complex in our study, characterized by a large magnitude of association for early (<3 years) onset of ESKD and modest, or no, association for later (≥3 years) onset of ESKD. Although associations of T50 with ESKD in both time periods were attenuated after adjustment for baseline eGFR and proteinuria, lower T50 may be a marker of more severe disease already in progress. Those in the lowest quartile of T50 were at very high risk of ESKD compared with those in the highest quartile of T50, and progressed to ESKD much earlier, as illustrated by the Kaplan–Meier analysis. These findings may be expected, considering the strong association between T50 and eGFR observed in our study and previous studies (14,32). Lower values of T50 indicate impaired calcification promoter-inhibitor homeostasis and calcium-phosphate metabolism, which are associated with progression of CKD (33). Furthermore, impaired calcium-phosphate metabolism strongly predicts the risk of ESKD, as shown in a previous risk prediction analysis (34). Similarly to the analyses of other outcomes, the role of T50 in the progression of CKD warrants further exploration to determine if improvement of calcification promoter-inhibitor balance can prevent ESKD.
There are several strengths in this analysis. First, the CRIC study uses rigorous quality control and assurance across clinical sites, which minimizes bias. Additionally, clinical outcomes are adjudicated. Second, this analysis represents the largest sample size to date evaluating the associations of T50 with clinical events among patients with CKD stages 2–4. Third, we were able to include many covariates to evaluate the associations of T50, accounting for traditional cardiovascular disease risk factors and variables potentially related to T50, including mineral metabolism and inflammation biomarkers. However, there are several limitations. First, the T50 test is conducted in vitro, which results in synthetic calciprotein particles. Still, there are data to suggest synthetic particles are physiologically similar to particles in vivo, thus T50 may be a reasonable indicator of the calciprotein particle transformation process (35). Second, although several potential confounders of the associations between T50 and outcomes were included in our analyses, we were unable to evaluate other potentially important variables, including vitamin K and pH. Third, we had only one baseline T50 measurement per participant and limited follow-up data did not allow us to assess associations of longitudinal T50 trends with outcomes in this sample.
In conclusion, higher serum calcification propensity, denoted by lower T50, was associated with atherosclerotic cardiovascular disease, ESKD, and mortality, independent of traditional cardiovascular disease risk factors. However, these associations were attenuated and were no longer significant after adjustment for eGFR and proteinuria. Future studies should evaluate T50 in other patient populations and investigate therapeutic interventions to improve calcification promoter-inhibitor homeostasis to prevent associated morbidity and mortality.
Disclosures
Dr. Block reports receiving a grant, research support, and personal fees as an advisor and consultant and for travel from Keryx/Akebia; honoraria and personal fees as a Director and consultant and for travel from Ardylex, and nonfinancial support and personal fees as a consultant and for travel from Amgen, AstraZeneca, Kirin, and OPKO, outside of the submitted work. Dr. Block is also an employee of Reata Pharmaceuticals. Dr. de Boer reports receiving consultant fees, honoraria, or nonfinancial research support, including consulting fees from Boehringer Ingelheim and Ironwood and equipment and supplies for research from Abbott Laboratories and Medtronic, outside the submitted work. Dr. Dobre reports an R01 grant (SPRINT-Myocardial Fibrosis) from the National Heart, Lung, and Blood Institute (NHLBI); personal fees from Relypsa for the Resistant Hypertension Working Group; and personal fees from Tricida for the Metabolic Acidosis Working Group, outside the submitted work. Dr. Feldman reports consulting fees from Kyowa Hakko Kirin Co., Ltd and an Editor-in-Chief position with the American Journal of Kidney Diseases. Dr. Isakova reports receiving consulting fees from Bayer and Eli Lilly and other support from Shire. Dr. Mehta reports owning stock in Abbott Laboratories, AbbVie Inc., and Teva Pharmaceutical Industries. Dr. Pasch is an inventor of the T50 test and a stockholder of Calciscon AG (Nidau, Switzerland), which commercializes the test used in this study. Dr. Scialla reports receiving fees from Eli Lilly, GlaxoSmithKline, and Sanofi for the support of Clinical Event Committee activities outside of the submitted work. Dr. Smith reports receiving investigator-initiated research study grant funding from Amgen, Baxter, and Sanofi outside of the submitted work and that he is a stockholder of Calciscon AG. Dr. Bundy, Dr. Cai, Dr. Chen, Dr. Go, Dr. Hsu, Dr. Lash, Dr. Leonard, Dr. Rao, and Dr. Townsend have nothing to disclose.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Disorders (NIDDK; grant P30DK114857). Dr. Bundy is supported by the NHLBI Cardiovascular Epidemiology training grant T32HL069771. Dr. de Boer is supported by a grant from NIDDK (R01DK099199). Dr. Dobre is supported by a grant from NHLBI (R01HL141846). Dr. Isakova is supported by grants from NIDDK (R01DK102438, R01DK110087, and U01DK099930). Dr. Scialla is supported by a grant from NIDDK (R01DK111952). Funding for the Chronic Renal Insufficiency Cohort study was obtained under a cooperative agreement from NIDDK (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this study was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health/National Center for Advancing Translational Sciences (UL1TR000003), the Johns Hopkins Institute for Clinical and Translational Research (UL1TR000424), University of Maryland General Clinical Research Center (M01RR-16500), Clinical and Translational Science Collaborative of Cleveland (UL1TR000439), Michigan Institute for Clinical and Health Research (UL1TR000433), University of Illinois at Chicago Clinical and Translational Science Awards (UL1RR029879), Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases (P20 GM109036), and Kaiser Permanente National Institutes of Health/National Center for Research Resources University of California San Francisco Clinical and Translational Science Institute (UL1RR024131).
Supplementary Material
Acknowledgments
The authors thank the participants, investigators, and staff of the Chronic Renal Insufficiency Cohort (CRIC) study for their time and commitment. The CRIC study Principal Investigators are Dr. Lawrence J. Appel, Dr. Feldman, Dr. Go, Dr. Jiang He, Dr. Lash, Dr. Rao, Dr. Mahboob Rahman, and Dr. Townsend.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04710419/-/DCSupplemental.
Supplemental Table 1. Multivariable-adjusted, cross-sectional associations of baseline characteristics with T50.
Supplemental Table 2. Associations of T50 with atherosclerotic cardiovascular disease and heart failure events in CRIC study participants without self-reported history of cardiovascular disease.
Supplemental Table 3. Effect of adjustment for additional variables on associations of T50 with clinical events.
Supplemental Figure 1. Scatterplot and correlation coefficient of eGFR and T50.
References
- 1.Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, Chen J, He J: A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 88: 950–957, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 5.GBD 2017 Causes of Death Collaborators : Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1736–1788, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kestenbaum BR, Adeney KL, de Boer IH, Ix JH, Shlipak MG, Siscovick DS: Incidence and progression of coronary calcification in chronic kidney disease: The Multi-Ethnic Study of Atherosclerosis. Kidney Int 76: 991–998, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, Zhang X, Roy JA, Lustigova E, Nessel L, Ford V, Raj D, Porter AC, Soliman EZ, Wright JT Jr., Wolf M, He J; CRIC Investigators : Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol 2: 635–643, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasch A, Farese S, Gräber S, Wald J, Richtering W, Floege J, Jahnen-Dechent W: Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 23: 1744–1752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keyzer CA, de Borst MH, van den Berg E, Jahnen-Dechent W, Arampatzis S, Farese S, Bergmann IP, Floege J, Navis G, Bakker SJ, van Goor H, Eisenberger U, Pasch A: Calcification propensity and survival among renal transplant recipients. J Am Soc Nephrol 27: 239–248, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahle DO, Åsberg A, Hartmann A, Holdaas H, Bachtler M, Jenssen TG, Dionisi M, Pasch A: Serum calcification propensity is a strong and independent determinant of cardiac and all-cause mortality in kidney transplant recipients. Am J Transplant 16: 204–212, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Pasch A, Block GA, Bachtler M, Smith ER, Jahnen-Dechent W, Arampatzis S, Chertow GM, Parfrey P, Ma X, Floege J: Blood calcification propensity, cardiovascular events, and survival in patients receiving hemodialysis in the EVOLVE Trial. Clin J Am Soc Nephrol 12: 315–322, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bostom A, Pasch A, Madsen T, Roberts MB, Franceschini N, Steubl D, Garimella PS, Ix JH, Tuttle KR, Ivanova A, Shireman T, Gohh R, Merhi B, Jarolim P, Kusek JW, Pfeffer MA, Liu S, Eaton CB: Serum calcification propensity and fetuin-A: Biomarkers of cardiovascular disease in kidney transplant recipients. Am J Nephrol 48: 21–31, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith ER, Ford ML, Tomlinson LA, Bodenham E, McMahon LP, Farese S, Rajkumar C, Holt SG, Pasch A: Serum calcification propensity predicts all-cause mortality in predialysis CKD. J Am Soc Nephrol 25: 339–348, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI; CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr., Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr., Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines : 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 129[Suppl 2]: S49–S73, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Hsu JY, Roy JA, Xie D, Yang W, Shou H, Anderson AH, Landis JR, Jepson C, Wolf M, Isakova T, Rahman M, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Statistical methods for cohort studies of CKD: Survival analysis in the setting of competing risks. Clin J Am Soc Nephrol 12: 1181–1189, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bielesz B, Reiter T, Marculescu R, Gleiss A, Bojic M, Kieweg H, Cejka D: Calcification propensity of serum is independent of excretory renal function. Sci Rep 7: 17941, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bressendorff I, Hansen D, Schou M, Silver B, Pasch A, Bouchelouche P, Pedersen L, Rasmussen LM, Brandi L: Oral magnesium supplementation in chronic kidney disease stages 3 and 4: Efficacy, safety, and effect on serum calcification propensity-a prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int Rep 2: 380–389, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bressendorff I, Hansen D, Schou M, Pasch A, Brandi L: The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease: A randomized, controlled clinical trial. Clin J Am Soc Nephrol 13: 1373–1380, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demer LL, Tintut Y: Vascular calcification: Pathobiology of a multifaceted disease. Circulation 117: 2938–2948, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vervloet M, Cozzolino M: Vascular calcification in chronic kidney disease: Different bricks in the wall? Kidney Int 91: 808–817, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Townsend RR: Arterial stiffness and chronic kidney disease: Lessons from the chronic renal insufficiency cohort study. Curr Opin Nephrol Hypertens 24: 47–53, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Roy J, Pan Q, Go AS, Townsend RR; CRIC Study Investigators; CRIC Study Investigators : Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail 7: 709–716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Osborne MT, Tung B, Li M, Li Y: Imaging cardiovascular calcification. J Am Heart Assoc 7: 1–15, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith ER, Cai MM, McMahon LP, Pedagogos E, Toussaint ND, Brumby C, Holt SG: Serum fetuin-A concentration and fetuin-A-containing calciprotein particles in patients with chronic inflammatory disease and renal failure. Nephrology (Carlton) 18: 215–221, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Paloian NJ, Giachelli CM: A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol 307: F891–F900, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutikhin AG, Velikanova EA, Mukhamadiyarov RA, Glushkova TV, Borisov VV, Matveeva VG, Antonova LV, Filip’ev DE, Golovkin AS, Shishkova DK, Burago AY, Frolov AV, Dolgov VY, Efimova OS, Popova AN, Malysheva VY, Vladimirov AA, Sozinov SA, Ismagilov ZR, Russakov DM, Lomzov AA, Pyshnyi DV, Gutakovsky AK, Zhivodkov YA, Demidov EA, Peltek SE, Dolganyuk VF, Babich OO, Grigoriev EV, Brusina EB, Barbarash OL, Yuzhalin AE: Apoptosis-mediated endothelial toxicity but not direct calcification or functional changes in anti-calcification proteins defines pathogenic effects of calcium phosphate bions. Sci Rep 6: 27255, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bundy JD, Cai X, Scialla JJ, Dobre MA, Chen J, Hsu CY, Leonard MB, Go AS, Rao PS, Lash JP, Townsend RR, Feldman HI, de Boer IH, Block GA, Wolf M, Smith ER, Pasch A, Isakova T; CRIC Study Investigators : Serum calcification propensity and coronary artery calcification among patients with CKD: The CRIC (Chronic Renal Insufficiency Cohort) study. Am J Kidney Dis 73: 806–814, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kono K, Fujii H, Nakai K, Goto S, Shite J, Hirata K, Fukagawa M, Nishi S: Composition and plaque patterns of coronary culprit lesions and clinical characteristics of patients with chronic kidney disease. Kidney Int 82: 344–351, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Pruijm M, Lu Y, Megdiche F, Piskunowicz M, Milani B, Stuber M, Bachtler M, Vogt B, Burnier M, Pasch A: Serum calcification propensity is associated with renal tissue oxygenation and resistive index in patients with arterial hypertension or chronic kidney disease. J Hypertens 35: 2044–2052, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP: Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol 1: 825–831, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Smith ER, Hewitson TD, Hanssen E, Holt SG: Biochemical transformation of calciprotein particles in uraemia. Bone 110: 355–367, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.