Visual Abstract
Keywords: endothelium, phosphate, microvascular dysfunction, capillaroscopy, laser-Doppler flowmetry, retinal microvessels, humans, microscopic angioscopy, hyperemia, cross-sectional studies, reference values, linear models, dilatation, hot temperature, venules, arterioles, capillaries, retinal vessels, phosphates, cohort studies, male, female
Abstract
Background and objectives
Higher serum phosphate is associated with cardiovascular events and all-cause mortality. Explanations of this association have focused on large vessel calcification and stiffness. Studies suggest that a higher serum phosphate induces microvascular dysfunction, but relationships in humans with direct measures of microvascular function are lacking.
Design, setting, participants, & measurements
We performed a cross-sectional analysis of 3189 community-living participants that underwent skin capillaroscopy, laser-Doppler flowmetry, and flicker light–induced retinal vessel responses. We used linear regression to assess the association between serum phosphate and each microvascular outcome. The primary outcome was skin capillary recruitment during postocclusive peak reactive hyperemia by capillaroscopy. Secondary outcomes included capillary recruitment during venous congestion, heat-induced skin hyperemic response, flicker light–induced retinal arteriolar, and venular dilation.
Results
The mean age of the cohort was 59±8 years, 48% were women, 7% had an eGFR <60 ml/min per 1.73 m2, and the mean serum phosphate concentration was 3.2±0.5 mg/dl. A 1 mg/dl higher serum phosphate was independently associated with a 5.0% lower postocclusive capillary recruitment (95% CI, −10.0% to −0.1%). Results were similar for capillary recruitment with venous congestion (−4.5%; 95% CI, −9.8% to 0.7%). A 1 mg/dl higher serum phosphate was also independently associated with a 0.23% lower retinal venular dilation in response to flicker light (95% CI, −0.44% to −0.02%). A higher serum phosphate was not associated with change in flicker light–induced retinal arteriolar dilation or heat-induced skin hyperemic response, however a higher serum phosphate was associated with a lower heat-induced skin hyperemic response among men (−149% [95% CI, −260 to −38] per 1 mg/dl higher serum phosphate) but not women (P interaction, 0.01).
Conclusions
Higher serum phosphate concentrations, even within the normal range, are associated with microvascular dysfunction in community-living individuals.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2019_09_20_CJN02610319.mp3
Introduction
Higher serum phosphate concentrations are independently associated with cardiovascular events and all-cause mortality. These associations are evident in the general population, in persons with CKD, in persons with overt hyperphosphatemia, and in persons with slightly higher serum phosphate concentrations that remain within the normal laboratory reference range (1–5). Evaluation of potential mechanisms linking hyperphosphatemia with cardiovascular events and mortality has largely focused on large-vessel alterations including arterial calcification and stiffness (6,7). However, recent studies have suggested that higher phosphate exposure may also contribute to microvascular dysfunction (8,9). In vitro studies have demonstrated that phosphate inhibits nitric oxide production by reducing nitric oxide synthase expression (9). The few available clinical studies are also supportive, but have largely relied on flow-mediated dilation (FMD) as the marker of endothelial dysfunction (10,11). FMD uses flow through large vessels (typically the brachial or femoral artery) to infer information about bioavailable nitric oxide downstream in the microvasculature, an important determinant of endothelial function (12). To our knowledge, no studies have yet evaluated the relationship between phosphate and microvascular function by direct imaging of the microvasculature or assessment of dynamic changes in dilation of microvasculature in response to stimuli.
Skin capillaroscopy, skin laser-Doppler flowmetry, and flicker light–induced retinal vessel dilation are reproducible noninvasive markers of microvascular function that more directly evaluate the arterioles, venules, and capillaries. Direct skin capillaroscopy is a technique in which an intravital microscope is used in the direct assessment of the dermal capillaries, both at rest and in response to arterial occlusion or venous congestion (13). Laser-Doppler flowmetry measures skin arteriolar and venular flow and changes in response to local heating of the skin, a process partially mediated by nitric oxide (14). Abnormalities in skin capillaroscopy, laser-Doppler flowmetry, and retinal diameter have all been associated with increased cardiovascular risk in select populations (15–18). Retinal arterioles and venules dilate in response to flicker flight (with the magnitude of dilation thought to be mediated by nitric oxide), which can thus serve as an additional measure of microvascular function (19). These microvascular function measurement techniques have been demonstrated to be altered in persons with hypertension, albuminuria, and (pre)diabetes (13,20,21).
In a population-based cohort, we evaluated the cross-sectional association between serum phosphate concentration and microvascular function using skin capillaroscopy, laser-Doppler flowmetry, and flicker light–induced retinal vessel dilation. We hypothesized that higher serum phosphate concentrations, even within the normal range, would be associated with diminished microvascular function.
Materials and Methods
Study Population
The Maastricht study is an observational, prospective, population-based, cohort study. The rationale and methodology have been described previously (22). In brief, the study focuses on the etiology, pathophysiology, complications, and comorbidities of type 2 diabetes mellitus and is characterized by an extensive phenotyping approach. Eligible participants were aged 40–75 years and living in the southern part of The Netherlands. Participants were recruited through mass media campaigns, from the municipal registries, and the regional diabetes patient registry via mailings. Recruitment was stratified according to known type 2 diabetes mellitus status, with an oversampling of individuals with type 2 diabetes mellitus for efficiency. We report a cross-sectional analysis of 3451 participants that underwent phosphate measurement as well as skin capillaroscopy, laser-Doppler flowmetry, or flicker light–induced retinal vessel dilation between November 2010 and September 2013 (20). Any individual that provided a blood sample and underwent at least one of these measurements was included in this analysis. From the initial 3451 participants, 862 underwent capillaroscopy, 1647 underwent laser-Doppler flowmetry, and 2261 underwent retinal vessel analysis. The baseline visit and collection of venous blood samples used for phosphate measurements occurred within 3 months of the microvascular function measurements. The study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sport of The Netherlands (permit 131088-105234-PG). All participants gave written informed consent. There were 659, 1306, and 1834 participants included in the final capillaroscopy, laser-Doppler flowmetry, and retinal vessel analyses, respectively (Figure 1). The main reasons for the differences in participant numbers within each measurement technique were logistic issues and image quality (23).
Figure 1.
Maastricht population for each microvascular end point. BMI, body mass index; LDF, laser-Doppler flowmetry.
Serum Phosphate Measurement
Participants had fasted overnight at the time of blood sampling. Samples were stored at −80°C from collection until testing in 2017–2018. Serum phosphate was measured spectrophotometrically by using an automated analyzer (Cobas 8000, C702 module; Roche). Analytical variation of phosphate in serum, estimated from internal quality controls, was 4%. The detectable range was 0.31–20 mg/dl. Among adults, the normal range for this device is 2.5–4.5 mg/dl.
Microvascular Measurements
For a full description of microvascular measurements, see Supplemental Methods. All participants were asked to refrain from ingesting caffeine or smoking for 3 hours before measurement. A light meal (breakfast or lunch), low in fat content, was allowed at least 90 minutes before the start of the measurements.
Skin Capillaroscopy
Details of the skin capillaroscopy measurements have been described previously (20). Briefly, measurements were performed in a quiet, temperature-controlled room (24°C) with participants in the supine position. Capillaries were visualized in the dorsal skin of the third and fourth finger of the right hand using a digital microscope (Capiscope; KK Technology, Honiton, UK). Capillaries were visualized 4.5 mm proximal to the terminal row of capillaries at the nail fold. All capillary density measurements were made on two fingers, and the average was used for each individual in analysis. Capillary density was first measured at baseline. Next, a finger cuff was inflated to 260 mm Hg to occlude arterial flow for 4 minutes and then released. Capillary density was then measured for 15 seconds. Venous congestion was then applied by inflating the cuff to 60 mm Hg for 2 minutes and all capillaries were counted for 15 seconds in the second minute of occlusion. Operators performing the capillaroscopy were blinded to the measurements and analysis. Considering the known associations between albuminuria (an indicator of microvascular dysfunction) and changes in skin capillaroscopy (20), and to minimize multiple comparisons, we chose to evaluate the percentage change in response to arterial occlusion as our primary outcome a priori. Percentage change was calculated as (peak value-baseline value)/(baseline value) ×100. We also evaluated the percentage change from baseline to venous congestion in secondary analyses.
Laser-Doppler Flowmetry
Details of the laser-Doppler flowmetry measurements have been described previously (21). Briefly, skin blood flow was measured using a laser-Doppler flowmetry system (Periflux 5000; Perimed, Järfalla, Sweden) equipped with a thermostatic probe (PF 457; Perimed). The thermostatic probe was placed at the dorsal side of the wrist of the left arm, with care to avoid any visible large blood vessels. Blood flow was measured at baseline for 2 minutes and then skin was heated to 44°C for 23 minutes. The heat-induced skin hyperemic response was expressed as the percentage increase in average perfusion units during the 23-minute heating phase over the average baseline perfusion units. The percentage change was calculated as (peak value−baseline value)/baseline value×100.
Retinal Vessel Dilation
Details of the retinal vessel dilation response to flicker-light measurements have been described previously (21). Briefly, retinal arteriolar and venular dilation response to flicker light was measured in a dimly lit room using a Dynamic Vessel Analyzer (IMEDOS, Jena, Germany). First, pupils were dilated with 0.5% tropicamide and 2.5% phenylephrine at least 15 minutes before the start of the examination. Participants with an intraocular pressure of >30 mm Hg were excluded. Participants were asked to focus on a needle inside a retinal camera (FF450; Carl Zeiss GmbH, Jena, Germany) and the fundus of the eye was examined under green light. Straight vessel segments of 1.5 mm were examined and the diameter was measured over 50 seconds using the Dynamic Vessel Analyzer. Baseline diameter was calculated as the average diameter between seconds 20 and 50 of the baseline period, and was expressed in measurement units in which 1 U was equal to 1 µm of the Gullstrand eye. Next, flicker-light stimulation was performed for 40 seconds (12.5 Hz, bright-to-dark contrast ratio of 25:1), followed by a 60 second recovery period, and then vessel diameter was remeasured. The percentage dilation above baseline was based on the average dilation as compared with the baseline diameter. Percentage change was calculated as (peak value−baseline value)/baseline value×100.
Covariate Measurements
To assess glucose metabolism status, participants who were not using insulin and had fasting glucose of <200 mg/dl underwent a standardized 2-hour, 75-g oral glucose tolerance test after an overnight fast. Glucose metabolism status was defined according to the World Health Organization 2006 criteria as normal glucose metabolism, impaired fasting glucose, impaired glucose tolerance (combined as prediabetes), and type 2 diabetes mellitus (20).
History of cardiovascular disease, duration of diabetes, menopausal status, physical activity (h/wk), and smoking status (never, former, current) were assessed by questionnaire (22). Use of lipid-modifying, antihypertensive, and glucose-lowering medication as well as postmenopausal hormone replacement therapy was assessed during a medication interview where generic name, dose, and frequency were registered (22). We measured weight, height, body mass index, waist circumference, office and ambulatory 24-hour BP, plasma glucose levels, serum creatinine, spot urine albumin/creatinine ratios (twice), glycated hemoglobin A1c, and plasma lipid profile as described elsewhere (22). eGFR was calculated using the four-variable Modification of Diet in Renal Disease equation (24). The presence of retinopathy was assessed in both eyes by using fundus photographs taken with an Auto Fundus Camera (model AFC-230; Nidek, Gamagori, Japan) (21).
Statistical Methods
Participant characteristics were compared across serum phosphate quartiles. Multiple linear regression was used to assess the association of serum phosphate with each measure of microvascular function. In companion analyses, we evaluated quartiles of phosphate, setting the lowest as the reference category, to assess the functional form of the relationships. We developed a sequence of models. Model 1 adjusted for age and sex. Model 2 additionally adjusted for body mass index, smoking status, 24-hour ambulatory systolic BP, use of antihypertensives, use of lipid-modifying agents, glucose metabolism status, eGFR, and serum calcium. We assessed for sex, diabetes, and CKD interactions by including multiplicative interaction terms in model 2. Lastly, we performed post-hoc sensitivity analyses additionally adjusting for office BP instead of ambulatory BP, antihypertensive class, as well as household income and size. Analyses were conducted in Stata SE version 14.1 (College Station, TX). P values <0.05 were considered statistically significant for all analyses including interaction terms.
Results
The mean age of the 3189 participants was 59±8 years, 48% were women and 7% had an eGFR of <60 ml/min per 1.73 m2. The mean serum phosphate concentration was 3.2±0.5 mg/dl, and 257 (8%) participants had serum phosphate concentrations ≥4.0 mg/dl. Baseline characteristics across quartiles of serum phosphate are shown in Table 1. Baseline characteristics for the subcohorts that underwent each microvascular measurement are reported in Supplemental Table 1. Compared with persons in the lowest quartile, those with higher serum phosphate were more often women, current smokers, had lower BP, were less likely to be taking antihypertensive medications, were more likely to have retinopathy, and had slightly higher serum calcium concentrations.
Table 1.
Baseline characteristics of participants by serum phosphate quartiles
| Characteristics | Phosphate Quartiles (Range) | |||
|---|---|---|---|---|
| Q1 (1.6–2.8 mg/dl) | Q2 (2.9–3.2 mg/dl) | Q3 (3.2–3.6 mg/dl) | Q4 (3.6–5.2 mg/dl) | |
| n=809 | n=855 | n=781 | n=744 | |
| Age (yr [±SD]) | 60 (9) | 60 (8) | 60 (8) | 59 (8) |
| Male (n [%]) | 644 (80) | 549 (64) | 307(39) | 159 (21) |
| BMI (kg/m2 [±SD]) | 28 (4.2) | 27 (4.3) | 27 (4.5) | 26 (5.1) |
| Smoking (n [%]) | ||||
| Never | 283 (35) | 297 (36) | 260 (34) | 226 (31) |
| Former | 433 (54) | 429 (51) | 394 (51) | 375 (52) |
| Current | 82 (10) | 110 (13) | 118 (15) | 123 (17) |
| Diabetes status (n [%])a | ||||
| Normal | 424 (52) | 462 (54) | 453 (58) | 438 (59) |
| Prediabetes | 152 (19) | 119 (14) | 104 (13) | 92 (12) |
| Type 2 diabetes | 233 (29) | 274 (32) | 224 (29) | 214 (29) |
| HbA1C (% [±SD]) | 5.9 (0.9) | 5.9 (0.9) | 5.9 (0.9) | 6.0 (1.0) |
| Retinopathy (n [%]) | 10 (1) | 12 (2) | 12 (2) | 16 (2) |
| Cardiovascular disease (n [%]) | 117 (15) | 145 (17) | 131 (17) | 118 (17) |
| BP (mm Hg [±SD]) | ||||
| Office systolic BP | 139 (17) | 136 (18) | 134 (18) | 130 (18) |
| Office diastolic BP | 79 (10) | 76 (10) | 75 (10) | 74 (10) |
| 24-h systolic BP | 122 (11) | 120 (12) | 118 (12) | 116 (11) |
| 24-h diastolic BP | 76 (7) | 74 (7) | 73 (7) | 72 (7) |
| BP medication (n [%]) | 331 (41) | 380 (45) | 304 (39) | 270 (36) |
| Hyperlipidemia medication (n [%]) | 303 (37) | 337 (40) | 273 (35) | 257 (35) |
| eGFR (ml/min per 1.73 m2 [±SD]) | 81 (18) | 82 (17) | 81 (16) | 80 (17) |
| CKD stage (n [%]) | ||||
| None (eGFR >60 ml/min per 1.73 m2) | 760 (94) | 782 (91) | 722 (92) | 695 (93) |
| CKD stage 3a | 45 (6) | 63 (7) | 54 (7) | 39 (5) |
| CKD stage 3b | 4 (0.5) | 10 (1) | 5 (1) | 8 (1) |
| CKD stage 4 | 0 (0) | 0 (0) | 0 (0) | 2 (0.3) |
| Urine albumin/creatinine ratio (mg/g [±IQR]) | 4 (2–8) | 5 (3–9) | 5 (3–10) | 5 (2–8) |
| Calcium (mg/dl [±SD]) | 9.3 (0.4) | 9.3 (0.3) | 9.4 (0.3) | 9.4 (0.3) |
The upper range of Q2 was 3.19, the lower range of Q3 was 3.22, the upper range of Q3 was 3.56, and the lower range of Q4 was 3.60.
BMI, body mass index; HbA1C, hemoglobin A1c; IQR, interquartile range.
Glucose metabolism status was assessed by an oral glucose tolerance test and defined according to the World Health Organization 2006 criteria as normal glucose metabolism, impaired fasting glucose, impaired glucose tolerance (combined as prediabetes), and type 2 diabetes mellitus.
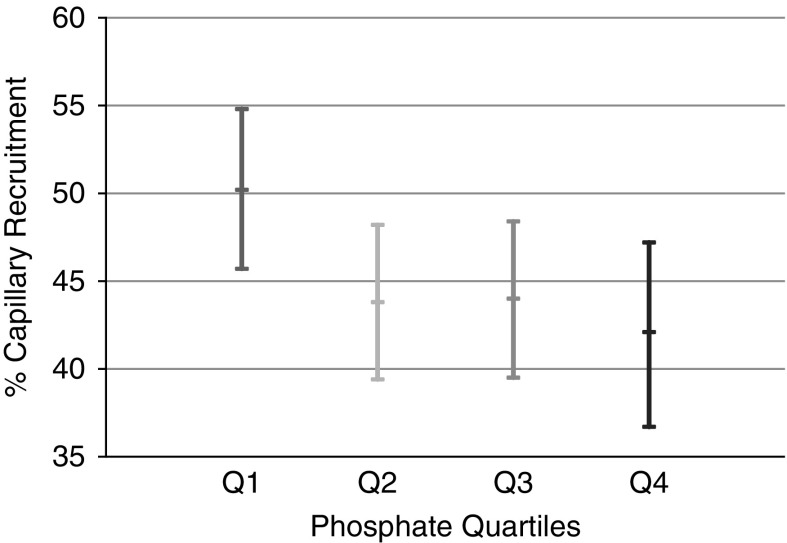
Among the 659 individuals with available serum phosphate and capillaroscopy measurements, the mean postocclusive capillary recruitment was 45%±29%. In model 1, higher serum phosphate was associated with statistically significantly less capillary recruitment during postocclusive peak reactive hyperemia (−5.2% per 1 mg/dl higher phosphate). In the fully adjusted model, this association remained essentially unchanged (Table 2). When phosphate was evaluated by quartiles, the relationship with postocclusive reactive hyperemia was fairly linear and, compared with persons in the lowest quartile, persons in the highest phosphate quartile recruited 8.4% less capillaries in response to arterial occlusion (Figure 2). This association was similar in either sex, in individuals with and without diabetes, and with and without CKD (Supplemental Table 2).
Table 2.
Association of phosphate with microvascular measurements
| Measurement Used (% [95% CI]) | Measurement Results per Phosphate Range Quartile | Per 1 mg/dl Higher Serum Phosphate | Pa | |||
|---|---|---|---|---|---|---|
| Q1 (1.6–2.8 mg/dl) | Q2 (2.9–3.2 mg/dl) | Q3 (3.2–3.6 mg/dl) | Q4 (3.6–5.2 mg/dl) | |||
| Capillary recruitment during postocclusive reactive hyperemia (n=659) | ||||||
| Model 1 | Reference | −7.1 (−13.3 to −0.8) | −7.1 (−13.6 to −0.6) | −8.7 (−15.9 to −1.6) | −5.2 (−10.1 to −0.4) | 0.04 |
| Model 2 | Reference | −6.6 (−12.8 to −0.3) | −6.3 (−12.8 to 0.3) | −8.4 (−15.6 to −1.1) | −5.0(−10.0 to −0.1) | 0.04 |
| Capillary recruitment during venous congestion (n=659) | ||||||
| Model 1 | Reference | −5.6 (−12.3 to 1.0) | −6.0 (−12.9 to 0.90) | −8.1 (−15.7 to −0.5) | −4.7 (−9.8 to 0.5) | 0.08 |
| Model 2 | Reference | −5.2 (−11.7 to 1.6) | −5.0 (−11.9 to 2.0) | −7.9 (−15.6 to −0.1) | −4.5 (−9.8 to 0.7) | 0.09 |
| Heat-induced skin hyperemic response (n=1306) | ||||||
| Model 1 | Reference | −132 (−242 to −22) | −112 (−230 to 6) | −120 (−249 to 8) | −53 (−140 to 35) | 0.24 |
| Model 2 | Reference | −117 (−226 to −7) | −92 (−211 to 27) | −81 (−211 to 415) | −25 (−113 to 63) | 0.57 |
| Retinal arteriolar dilation (n=1834) | ||||||
| Model 1 | Reference | −0.18 (−0.53 to 0.18) | −0.12 (−0.50 to 0.25) | −0.21 (−0.61 to 0.20) | −0.19 (−0.47 to 0.07) | 0.15 |
| Model 2 | Reference | −0.11 (−0.46 to 0.24) | −0.05 (−0.43 to 0.33) | −0.09 (−0.50 to 0.32) | −0.12 (−0.30 to 0.15) | 0.39 |
| Retinal venular dilation (n=1834) | ||||||
| Model 1 | Reference | −0.02 (−0.29 to 0.26) | −0.25 (−0.54 to 0.05) | −0.41 (−0.73 to −0.10) | −0.26 (−0.47 to −0.05) | 0.01 |
| Model 2 | Reference | 0.03 (−0.24 to 0.31) | −0.20 (−0.49 to 0.10) | −0.35 (−0.66 to −0.03) | −0.23 (−0.44 to −0.02) | 0.03 |
The upper range of Q2 was 3.19, the lower range of Q3 was 3.22, the upper range of Q3 was 3.56, and the lower range of Q4 was 3.60.
Model 1 adjusted for age and sex; model 2 additionally adjusted for body mass index, smoking status, 24-h ambulatory systolic BP, use of antihypertensives, use of lipid-modifying agents, glucose metabolism status, eGFR, and serum calcium.
P values reported for the continuous analysis.
Figure 2.
Percentage capillary recruitment during postocclusive reactive hyperemia is lower at higher phosphate quartiles. Phosphate quartile ranges were (Q1) 1.6–2.8 mg/dl, (Q2) 2.9–3.2 mg/dl, (Q3) 3.2–3.6 mg/dl, and (Q4) 3.6–5.2 mg/dl. Values presented are adjusted for age, sex, smoking status, 24-hour ambulatory systolic BP, use of antihypertensives, use of lipid modifying agents, diabetes status, eGFR, and serum calcium.
Next, we evaluated skin capillary recruitment during venous congestion. Among the 659 individuals, the mean percentage recruitment during venous congestion was 46%±31%. Similar to the postocclusive response, in model 1, serum phosphate was inversely associated with capillary recruitment during venous congestion (−4.7% per 1 mg/dl higher in phosphate) but this association did not reach statistical significance (Table 2). In the fully adjusted model the association with capillary recruitment during venous congestion remained similar. There was no significant interaction of sex, diabetes, or CKD status and phosphate for the capillary recruitment endpoint (Supplemental Table 2).
We next evaluated laser-Doppler flow in forearm skin. The mean heat-induced skin hyperemia was 1120%±762% among the 1306 individuals with laser-Doppler flowmetry and serum phosphate data. Serum phosphate was not associated with heat-induced skin hyperemic response across the series of models. (Table 2). However, we observed that the association differed by sex (P interaction, 0.01). In analyses stratified by sex, we found no association between serum phosphate and heat-induced skin hyperemia among women. However, serum phosphate was strongly associated with heat-induced skin hyperemia among men, such that a 1 mg/dl higher phosphate was associated with a 148% lower heat-induced skin hyperemic response (Table 3). We found no evidence of interaction with diabetes or CKD status.
Table 3.
Association of serum phosphate with heat-induced skin hyperemic response, stratified by sex
| Sex | Change per 1 mg/dl Higher Phosphate (% [95% CI])a | P Value | P Interaction |
|---|---|---|---|
| Men | −149 (−260 to −38) | 0.01 | 0.01 |
| Women | 88 (−55 to 230) | 0.23 |
Adjusted for age, sex, body mass index, smoking status, 24-h ambulatory systolic BP, use of antihypertensives, use of lipid-modifying agents, diabetes status, eGFR, and serum calcium.
The mean retinal arteriolar and venular dilation were 3.0%±2.8% and 3.9%±2.2%, respectively, among the 1834 individuals with both retinal imaging and serum phosphate data. Serum phosphate was not associated with flicker light–induced retinal arteriolar dilation in either the demographic or fully adjusted model (P=0.15 and 0.39 for models 1 and 2, respectively) (Table 2). Serum phosphate was, however, inversely associated with flicker light–induced venular dilation in both model 1 and the fully adjusted model, such that a 1 mg/dl higher serum phosphate was associated with a 0.23% lower venular diameter in the fully adjusted model. When evaluating phosphate quartiles, the relationship with flicker light–induced retinal venule dilation appeared fairly linear across increasing quartiles. There were no statistically significant interactions between sex (Supplemental Table 2), diabetes, or CKD status and either retinal imaging outcome.
Considering ambulatory BP data were missing in 380 participants, we performed sensitivity analyses using office BP. Associations using these measurements were essentially unaltered. Similarly, further adjustment for antihypertensive class, household income, and household size did not significantly alter the association of serum phosphate with any of the measured outcomes.
Discussion
In a large, well characterized cohort of community-living men and women, we demonstrate that a higher serum phosphate concentration, even within the normal reference range, is associated with lower capillary recruitment. We also found higher serum phosphate concentrations were associated with lower flicker light–induced retinal venular dilation. Lastly, we also found serum phosphate to be associated with a lower heat-induced skin hyperemic response among men. Overall, these findings demonstrate that higher serum phosphate concentrations are independently associated with worse microvascular function in community-living individuals.
Putative relationships of phosphate with microvascular disease are only just emerging. Multiple in vitro studies have found that endothelial cells exposed to high phosphate concentrations have reduced amounts of endothelial nitric oxide synthase leading to a reduced vasodilation (8,9). These studies suggest phosphate may be directly causative of impaired microvascular function. In a rodent model of CKD and endothelial dysfunction, a low phosphate diet improved vasodilation of the thoracic aorta through induction of increased activation of endothelial nitric oxide synthase (25). Other studies have reported a link between higher serum phosphate and downregulation of Annexin II, a key protein in several biologic processes including endothelial cell adhesion and angiogenesis (26). Endothelial cell apoptosis has also been observed in the setting of hyperphosphatemia (27). Alternatively, α-Klotho deficiency or other emerging pathways may be contributing to both a higher serum phosphate and impaired microvascular function (28). Thus higher phosphate may be associated with impaired microvascular function via multiple mechanisms.
Human studies evaluating the relationship between phosphate and microvascular function have had small sample sizes and have used FMD, which reflects nitric oxide production but uses changes in large vessels to infer potential changes in downstream microvasculature (10,11). In a study among 100 persons with severe CKD, individuals were randomized to 8 weeks of an intestinal phosphate binder, which limits dietary phosphate absorption. These patients had significantly elevated serum phosphate levels at baseline. Those randomized to the binder had significant improvement in brachial artery FMD compared with their baseline FMD measurement (11). Similarly, Shuto et al. fed 11 healthy volunteers meals containing 400 and 1200 mg of phosphate, using a crossover design, and performed brachial artery FMD measurements 2 hours after each meal. The high-phosphate meal induced significantly higher serum phosphate concentrations, although the average peak phosphate was still around the high end of the normal range (4.6 mg/dl). Compared with the low-phosphate meal, the high-phosphate meal induced significantly less arterial dilation by FMD (10). Thus, these findings suggested that not only does phosphate impair endothelial function, but it can do so acutely, even in healthy persons without CKD. Similarly, in another small, controlled trial of healthy subjects randomized to 11 weeks of a high-phosphate diet versus a low-phosphate diet combined with a phosphate binder, those in the high-phosphate group experienced a 4 mm Hg increase in systolic BP compared with those in the low-phosphate group, possibly due to the effects of phosphate on the microvasculature (29). The persons randomized to the high-phosphate diet still had an average phosphate in the normal range (around 4 mg/dl). In larger community-living populations, Mehta et al. reported an association between higher serum phosphate and prevalence of retinopathy, even among persons with a serum phosphate in the normal range in the Multi-Ethnic Study of Atherosclerosis, a finding confirmed in our study (30). In that study, serum phosphate was not associated with central retinal arteriolar or venular diameter; however, these measurements were made without flicker-light stimulation, thus this would only reflect the baseline diameter. Higher serum phosphate has also been associated with cerebral microvascular disease and there have been conflicting studies regarding an association between serum phosphate and albuminuria, a marker of microvascular disease in the kidney (31–33). All of these studies accounted for kidney function, sex, and other key confounders. Our study is consistent with these prior works and we extend these findings by evaluating three distinct techniques that more directly assess microvascular function concurrently, and demonstrate by multiple direct metrics of the microvasculature that higher serum phosphate is associated with impaired microvascular function among healthy individuals.
Older women have higher serum phosphate levels than older men (7,34–36). In rodent models, estrogen induces phosphaturia with a resultant decline in serum phosphate (37). As women transition through menopause and estrogen levels decrease, serum phosphate levels rise, likely due to the loss of estrogen-induced phosphaturia (38). Panwar et al. (39) recently reported that sex modified the relationship between fibroblast growth factor 23 (a hormone regulating serum phosphate) and coronary heart disease. Considering the known differences in phosphate handling between older men and women, we evaluated whether the effects of phosphate on the microvasculature were modified by sex. We found a significant interaction with the heat-induced skin hyperemic response: higher phosphate was strongly negatively associated with the heat-induced skin hyperemic response among men but not women. Whether this finding is reflective of differences in estrogen levels and associated phosphate homeostatic control, whether sex differences exist in younger persons, or whether this finding was due to chance, requires further investigation. The very low P value observed for the interaction argues against chance as the likely explanation.
The Maastricht study is unique in that there were several distinct microvascular imaging techniques performed on >3000 participants with a range of comorbidities. The concurrent availability of demographics, cardiovascular risk factor status, and kidney function are additional key strengths. This study also has important limitations. First, as the design is cross-sectional, the temporal directions of associations are uncertain and there may be issues of residual confounding for which we could not account. Second, this was a community-living population, which increases generalizability but results in a fairly healthy study population. It is unclear if these findings can be extended to patients with more advanced CKD or overt hyperphosphatemia. Third, although we found a strong association of serum phosphate with capillary recruitment postarterial occlusion (our prespecified primary outcome), the association between serum phosphate and microvascular function did not reach statistical significance for all end points, despite the direction of point estimates being consistent across all end points irrespective of significant P values. Similarly, the magnitude of the association for the retinal vein dilation outcome appears small (0.23% per 1 mg/dl higher serum phosphate,). However, for point of reference, the difference in retinal vessel dilation between persons with normal glucose metabolism and persons with prediabetes was shown to be 0.2%, growing to 0.6% when comparing persons with normal glucose metabolism to those with type 2 diabetes (17). Lastly, there was a significant amount of missing covariate data. However, this was almost entirely due to lack of ambulatory BP measurements. When analyzing the more complete cohort using office BP instead of ambulatory BP, our results were essentially unchanged.
In conclusion, beyond relationships with large-vessel arterial disease, higher serum phosphate is independently associated with impaired microvascular function in community-living individuals predominantly with normal kidney function and with serum phosphate concentrations within the normal laboratory reference range. These findings suggest pathways linking serum phosphate concentrations with cardiovascular disease and mortality may be more complex than phosphate simply promoting arterial calcification and stiffness. The findings also suggest there may be novel avenues to target the effects of phosphate on the vasculature beyond effects in large arteries. Further investigation is needed to evaluate if these associations may be causative. If confirmed, these findings would have important clinical implications regarding phosphate intake in the general population and for the development of novel strategies to improve vascular health by targeting microvascular function.
Disclosures
Dr. Ix reports receiving an Investigator Initiated Research Grant from Baxter International outside of the submitted work. Dr. Webers reports receiving grants from Alcon/Novartis and Santen and personal fees from Alcon/Novartis, Santen, and Thea Pharma, outside of the submitted work. Dr. Berendschot, Dr. Dagnelie, Dr. Ginsberg, Dr. Houben, Dr. Kooman, Dr. Malhotra, and Dr. Stehouwer have nothing to disclose.
Funding
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases awarded to Dr. Ginsberg (F32 DK116476 and K23 DK118197) and Dr. Ix (K24 DK110427). Dr. Ginsberg was also supported by the National Institutes of Health Loan Repayment Program. Dr. Ix was supported by an Established Investigator Award from the American Heart Association (14EIA18560026). The Maastricht study was supported by the Cardiovascular Center (CVC, Maastricht, The Netherlands); Care and Public Health Research Institute (CAPHRI) (Maastricht, The Netherlands); CARIM School for Cardiovascular Diseases (Maastricht, The Netherlands); the Dutch Ministry of Economic Affairs; the European Regional Development Fund (31O.041) via OPZuid, the province of Limburg; Health Foundation Limburg (Maastricht, The Netherlands); NUTRIM School for Nutrition and Translational Research in Metabolism (Maastricht, The Netherlands); the Pearl String Initiative Diabetes (Amsterdam, The Netherlands); Stichting Annadal (Maastricht, The Netherlands); Stichting De Weijerhorst (Maastricht, The Netherlands); and by unrestricted grants from Janssen-Cilag (Tilburg, The Netherlands), Novo Nordisk Farma (Alphen aan den Rijn, The Netherlands), and Sanofi-Aventis Netherlands (Gouda, The Netherlands).
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02610319/-/DCSupplemental.
Supplemental Table 1. Baseline characteristics of participants by subcohort.
Supplemental Table 2. Association of phosphate with microvascular function stratified by sex.
References
- 1.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 2.O’Seaghdha CM, Hwang SJ, Muntner P, Melamed ML, Fox CS: Serum phosphorus predicts incident chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant 26: 2885–2890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators : Relation between serum phosphate level and cardiovascular event rate in people with coronary disease [published correction appears in Circulation 116: e556, 2007]. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA: Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20: 397–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR: Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol 4: 609–615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens KK, Denby L, Patel RK, Mark PB, Kettlewell S, Smith GL, Clancy MJ, Delles C, Jardine AG: Deleterious effects of phosphate on vascular and endothelial function via disruption to the nitric oxide pathway. Nephrol Dial Transplant 32: 1617–1627, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng A, Wu T, Zeng C, Rakheja D, Zhu J, Ye T, Hutcheson J, Vaziri ND, Liu Z, Mohan C, Zhou XJ: Adverse effects of simulated hyper- and hypo-phosphatemia on endothelial cell function and viability. PLoS One 6: e23268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, Nakaya Y, Takeda E: Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 20: 1504–1512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Mallamaci F, Zoccali C: Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: A randomized clinical trial. Am J Kidney Dis 59: 177–185, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH: Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension 63: 376–382, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Triantafyllou A, Anyfanti P, Pyrpasopoulou A, Triantafyllou G, Aslanidis S, Douma S: Capillary rarefaction as an index for the microvascular assessment of hypertensive patients. Curr Hypertens Rep 17: 33, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Roustit M, Cracowski JL: Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci 34: 373–384, 2013 [DOI] [PubMed] [Google Scholar]
- 15.IJzerman RG, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serné EH, Stehouwer CD: Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest 33: 536–542, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Kruger A, Stewart J, Sahityani R, O’Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS: Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: Correlation with cardiovascular risk. Kidney Int 70: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD: Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The atherosclerosis risk in communities study. JAMA 287: 1153–1159, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Wang JJ, Liew G, Wong TY, Smith W, Klein R, Leeder SR, Mitchell P: Retinal vascular calibre and the risk of coronary heart disease-related death. Heart 92: 1583–1587, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo M, Wang L, Bill A: The role of nitric oxide in hyperaemic response to flicker in the retina and optic nerve in cats. Acta Ophthalmol Scand 75: 232–235, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Martens RJ, Henry RM, Houben AJ, van der Kallen CJ, Kroon AA, Schalkwijk CG, Schram MT, Sep SJ, Schaper NC, Dagnelie PC, Muris DM, Gronenschild EH, van der Sande FM, Leunissen KM, Kooman JP, Stehouwer CD: Capillary rarefaction associates with albuminuria: The Maastricht study. J Am Soc Nephrol 27: 3748–3757, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sörensen BM, Houben AJ, Berendschot TT, Schouten JS, Kroon AA, van der Kallen CJ, Henry RM, Koster A, Sep SJ, Dagnelie PC, Schaper NC, Schram MT, Stehouwer CD: Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: The Maastricht study. Circulation 134: 1339–1352, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Schram MT, Sep SJ, van der Kallen CJ, Dagnelie PC, Koster A, Schaper N, Henry RM, Stehouwer CD: The Maastricht study: An extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol 29: 439–451, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Martens RJH, Houben AJHM, Kooman JP, Berendschot TTJM, Dagnelie PC, van der Kallen CJH, Kroon AA, Leunissen KML, van der Sande FM, Schaper NC, Schouten JSAG, Schram MT, Sep SJS, Sörensen BM, Henry RMA, Stehouwer CDA: Microvascular endothelial dysfunction is associated with albuminuria: The Maastricht study. J Hypertens 36: 1178–1187, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Van TV, Watari E, Taketani Y, Kitamura T, Shiota A, Tanaka T, Tanimura A, Harada N, Nakaya Y, Yamamoto H, Miyamoto K, Takeda E: Dietary phosphate restriction ameliorates endothelial dysfunction in adenine-induced kidney disease rats. J Clin Biochem Nutr 51: 27–32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Marco GS, König M, Stock C, Wiesinger A, Hillebrand U, Reiermann S, Reuter S, Amler S, Köhler G, Buck F, Fobker M, Kümpers P, Oberleithner H, Hausberg M, Lang D, Pavenstädt H, Brand M: High phosphate directly affects endothelial function by downregulating annexin II. Kidney Int 83: 213–222, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D, Pavenstädt H: Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol 294: F1381–F1387, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Mazzotta C, Manetti M, Rosa I, Romano E, Blagojevic J, Bellando-Randone S, Bruni C, Lepri G, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M: Proangiogenic effects of soluble α-Klotho on systemic sclerosis dermal microvascular endothelial cells. Arthritis Res Ther 19: 27, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammad J, Scanni R, Bestmann L, Hulter HN, Krapf R: A controlled increase in dietary phosphate elevates BP in healthy human subjects. J Am Soc Nephrol 29: 2089–2098, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta R, Hodakowski A, Cai X, Lee KE, Kestenbaum BR, de Boer IH, Fawzi A, Wong TY, Ix J, Klein B, Klein R, Isakova T: Serum phosphate and retinal microvascular changes: The multi-ethnic study of atherosclerosis and the beaver dam eye study. Ophthalmic Epidemiol 24: 371–380, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung CP, Peng LN, Chou KH, Liu LK, Lee WJ, Lin CP, Chen LK, Wang PN: High circulatory phosphate level is associated with cerebral small-vessel diseases. Transl Stroke Res 10: 265–272, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Oh SW, Heo NJ, Chin HJ, Na KY, Kim S, Chae DW: Serum phosphorus as a predictor of low-grade albuminuria in a general population without evidence of chronic kidney disease. Nephrol Dial Transplant 27: 2799–2806, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Ellam T, Fotheringham J, Wilkie ME, Francis SE, Chico TJ: Bone mineral metabolism parameters and urinary albumin excretion in a representative US population sample [published correction appears in PLoS One 9: e97392, 2014]. PLoS One 9: e88388, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foley RN, Collins AJ, Ishani A, Kalra PA: Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 156: 556–563, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr., Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Meng J, Ohlsson C, Laughlin GA, Chonchol M, Wassel CL, Ljunggren O, Karlsson MK, Mellstrom D, Orwoll ES, Barrett-Connor E, Ix JH; Osteoporotic Fractures in Men (MrOs) Study Group : Associations of estradiol and testosterone with serum phosphorus in older men: The Osteoporotic Fractures in Men study. Kidney Int 78: 415–422, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faroqui S, Levi M, Soleimani M, Amlal H: Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int 73: 1141–1150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ix JH, Chonchol M, Laughlin GA, Shlipak MG, Whooley MA: Relation of sex and estrogen therapy to serum fibroblast growth factor 23, serum phosphorus, and urine phosphorus: The Heart and Soul study. Am J Kidney Dis 58: 737–745, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panwar B, Judd SE, Wadley VG, Jenny NS, Howard VJ, Safford MM, Gutiérrez OM: Association of fibroblast growth factor 23 with risk of incident coronary heart disease in community-living adults. JAMA Cardiol 3: 318–325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.