CKD is a common complication in people with diabetes (1) and contributes to approximately half of all incident cases of ESKD in the developed world (2). CKD occurs in approximately 40% of people with type 2 diabetes (3); given the health-related effects of CKD, there is great need for interventions to prevent and/or slow its progression.
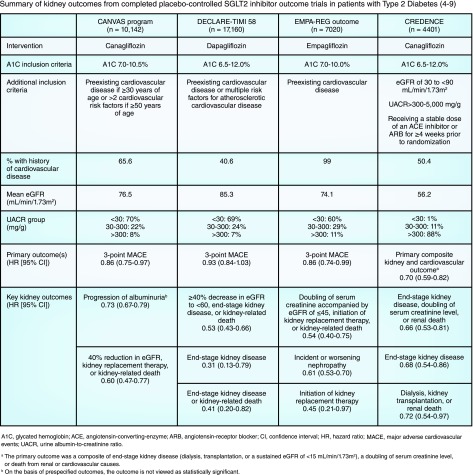
Over the past decade, multiple cardiovascular outcome trials with antihyperglycemic agents have completed. As a result, the sodium-glucose cotransporter 2 (SGLT2) inhibitor class has received much attention due to their reported cardiovascular and kidney benefits. The BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients trial was the first outcome trial to report improvements in cardiovascular and kidney outcomes, using the SGLT2 inhibitor empagliflozin; subsequently, the Canagliflozin Cardiovascular Assessment Study (CANVAS) program and the Dapagliflozin Effect on Cardiovascular Events-Thrombosis in Myocardial Infarction 58 trial also demonstrated significant reductions in cardiovascular and kidney outcomes for canagliflozin and dapagliflozin, respectively (Figure 1) (4–8). Although these trials provide exciting results related to kidney outcomes, they enrolled a relatively small fraction of patients with CKD at baseline and were not primarily designed to assess kidney end points, but rather included secondary kidney outcome measures (4–8). On the basis of these secondary kidney outcomes, however, the American Diabetes Association recommended in their 2019 Standards of Medical Care that an SGLT2 inhibitor (or GLP-1 receptor agonist) shown to reduce the risk of CKD progression, cardiovascular events, or both, be considered in patients with type 2 diabetes and CKD not meeting individualized glycemic goals with metformin and lifestyle interventions (1).
Figure 1.
Summary of kidney outcomes from completed placebo-controlled SGLT2 inhibitor outcome trials in patients with type 2 diabetes (4-9).
In contrast to the previously completed cardiovascular outcome trials, the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial was designed to assess the effects of the SGLT2 inhibitor canagliflozin primarily on kidney outcomes in participants with type 2 diabetes and albuminuric CKD (Figure 1) (9). CREDENCE was a double-blind, randomized trial of canagliflozin versus placebo in people with type 2 diabetes with a baseline eGFR of 30–<90 ml/min per 1.73 m2 and a urine albumin-to-creatinine ratio of >300–5000 mg/g. All participants were also receiving a stable dose of either an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB). The primary outcome for the trial was a composite of ESKD (dialysis, kidney transplantation, or a sustained eGFR <15 ml/min per 1.73 m2), doubling of serum creatinine, or death from kidney or cardiovascular causes. A total of 4401 participants were enrolled when the study was stopped early because of a signal for efficacy during a planned interim analysis, with a median participant follow-up of 2.62 years. Approximately 50% of participants had a history of cardiovascular disease. The risk of the primary outcome was reduced by 30% with canagliflozin treatment (hazard ratio, 0.70; 95% confidence interval, 0.59 to 0.82; P<0.001). Findings for the kidney-specific composite outcome (ESKD, doubling of creatinine, or kidney-related death) was additionally positive (hazard ratio, 0.66; 95% confidence interval 0.53 to 0.81; P<0.001). Canagliflozin treatment was also associated with a lower risk for several cardiovascular-related outcomes.
So how does CREDENCE inform current use of SGLT2 inhibitors in CKD? We believe there are several important lessons to be taken from the CREDENCE trial:
Canagliflozin may be considered a reasonable option for use in patients with type 2 diabetes and albuminuric CKD who are already receiving renin-angiotensin system blockade
CREDENCE demonstrated clear benefit of canagliflozin treatment on important cardiovascular and kidney outcomes in a population with type 2 diabetes and albuminuric CKD. Importantly, these improvements are observed on the background of ACE inhibitor/ARB therapy.
However, people with type 2 diabetes can often present with low eGFR in the absence of albuminuria and although similar benefits may be postulated in this population, more research is needed (2). It is also currently unknown if the kidney benefits observed in CREDENCE would be seen in patients with eGFR<30 ml/min per 1.73 m2 and/or urine albumin-to-creatinine ratio values <300 mg/g. Although CREDENCE excluded these participants, the study results demonstrated that canagliflozin prevented sustained eGFR <15 ml/min per 1.73 m2. Findings from ongoing outcome studies with other SGLT2 inhibitors in patients with type 2 diabetes and CKD will be helpful to substantiate a class effect. Indeed, although not statistically significant in the subgroup of CREDENCE participants with eGFR>60 ml/min per 1.73 m2, SGLT2 inhibitors may have a role in kidney protection at higher eGFR as supported by secondary outcomes from completed cardiovascular outcome trials (4–8). Notably, the Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with Chronic Kidney Disease and The Study of Heart and Kidney Protection with Empagliflozin studies are testing SGLT2 inhibitor use in populations inclusive of patients with nondiabetic kidney disease (10).
CREDENCE informs our understanding regarding the safety of SGLT2 inhibitor use in patients with CKD
CREDENCE provides additional information on the safety profile for canagliflozin (9). Although findings from the CANVAS program raised concern about risk of amputation and fracture with canagliflozin treatment (7), neither were found to be increased relative to placebo in CREDENCE, which enrolled a population at higher expected risk for both events; of note, median follow-up time was similar in the two studies. It remains unclear whether these different event rates may be because of differences in populations studied or protocols used to capture these adverse events within the trials, respectively. If amputation is of clinical concern, it seems appropriate to avoid use in patients with severe peripheral vascular disease or a history of amputation.
Current SGLT2 inhibitor labeling recommends dose reductions on the basis of eGFR because of diminished glucose-lowering effect with reduced kidney function, with use not recommended for empagliflozin, canagliflozin, and dapagliflozin in patients with an eGFR<45 ml/min per 1.73 m2. Findings from CREDENCE demonstrate that canagliflozin use in patients with an eGFR below this threshold is safe and provides kidney benefit. Indeed, participants in CREDENCE continued canagliflozin treatment per protocol until initiation of dialysis or kidney transplantation. Although the initial labeling for canagliflozin included a warning for hyperkalemia, this was not noted in CREDENCE or other SGLT2 inhibitor cardiovascular outcome trials. This finding suggests that SGLT2 inhibitors may offer an alternative for patients unable to tolerate ACE/ARB therapy because of hyperkalemia, although further evidence is needed to demonstrate improvement in kidney outcomes in the absence of background ACE/ARB use.
Although the absolute number of diabetic ketoacidosis (DKA) events were small in both groups, there was a near 10-fold increased relative risk of DKA with canagliflozin treatment. Interestingly, the DKA event rate was two-fold higher in CANVAS, but did not reach statistical significance in the canagliflozin-treated group. Nonetheless, this finding of higher risk for DKA is generally consistent with other clinical trials with SGLT2 inhibitors and reinforces the need for education about recognition of DKA signs and symptoms, sick day care, and the importance of seeking prompt medical attention.
Conclusions
In conclusion, CREDENCE provides long-awaited evidence in support of SGLT2 inhibition to delay the progression of CKD in patients with type 2 diabetes and kidney disease. A better understanding of the mechanisms underlying the cardiovascular and kidney protective benefits of SGLT2 inhibitors is still needed (10). Findings from ongoing SGLT2 inhibitor trials are likewise eagerly awaited to further our knowledge on how best to integrate these therapies into the care of patients with CKD to improve outcomes. Additional data are also needed for populations underrepresented in studies to date including racial/ethnic minority populations and younger patients where the lifetime risk of CKD is significant. Longer-duration studies in patients with CKD will ultimately inform the long-term safety of SGLT2 inhibitor use.
Disclosures
Dr. Kalyani and Dr. Neumiller have nothing to disclose.
Acknowledgments
The content of this article does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed therein lies entirely with the authors.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.American Diabetes Association: 11. Microvascular complications and foot care: Standards of medical care in diabetes-2019. Diabetes Care 42[Suppl 1]: S124–S138, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME: Diabetic kidney disease: A report from an ADA Consensus Conference. Am J Kidney Dis 64: 510–533, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Alicic RZ, Rooney MT, Tuttle KR: Diabetic kidney disease: Challenges, progress and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators: Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators: Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group: Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators: Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJL, Zelniker TA, Dwyer JP, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Kato ET, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Raz I: Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: An analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 7: 606–617, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Alicic RZ, Neumiller JJ, Johnson EJ, Dieter B, Tuttle KR: Sodium-glucose cotransporter 2 inhibition and diabetic kidney disease. Diabetes 68: 248–257, 2019 [DOI] [PubMed] [Google Scholar]