Visual Abstract
Keywords: metabolic syndrome; chronic kidney disease; estimated glomerular filtration rate; heritability; twin study; abdominal obesity; cystatin C; glomerular filtration rate; risk factors; cross-sectional studies; chronic renal insufficiency; cholesterol; EGFR protein, human; receptor, epidermal growth factor; registries; humans; Renal Insufficiency, Chronic
Abstract
Background and objectives
Metabolic syndrome is a cluster of risk factors associated with CKD. By studying the genetic and environmental influences on how traits of metabolic syndrome correlate with CKD, the understanding of the etiological relationships can be improved.
Design, setting, participants, & measurements
From the population-based TwinGene project within the Swedish Twin Registry, 4721 complete twin pairs (9442 European ancestry participants) were included in this cross-sectional twin study. Metabolic syndrome-related continuous traits were measured, and the binary components as well as the status of metabolic syndrome were defined according to the National Cholesterol Education Program-Adult Treatment Panel III. The eGFR was calculated by cystatin C-based equations from the CKD epidemiology collaboration group, and CKD was defined by eGFR<60 ml/min per 1.73 m2. Genetic and environmental contributions to the correlations between traits of metabolic syndrome and CKD were estimated by using twin-based bivariate structural equation models.
Results
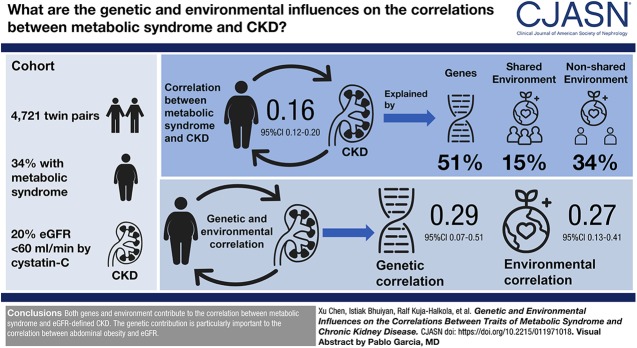
The correlation between metabolic syndrome and eGFR-defined CKD was 0.16 (95% confidence interval [95% CI], 0.12 to 0.20), out of which 51% (95% CI, 12% to 90%) was explained by genes, whereas 15% (95% CI, 0% to 42%) and 34% (95% CI, 16% to 52%) was explained by the shared and nonshared environment, respectively. The genetic and environmental correlations between metabolic syndrome and CKD were 0.29 (95% CI, 0.07 to 0.51) and 0.27 (95% CI, 0.13 to 0.41), respectively. For the correlation between abdominal obesity and eGFR, 69% (95% CI, 10% to 100%) was explained by genes and 23% (95% CI, 5% to 41%) was explained by environment. The genetic correlation between abdominal obesity and eGFR was −0.30 (95% CI, −0.54 to −0.06), whereas the environmental correlation was −0.14 (95% CI, −0.22 to −0.06).
Conclusions
Both genes and environment contribute to the correlation between metabolic syndrome and eGFR-defined CKD. The genetic contribution is particularly important to the correlation between abdominal obesity and eGFR.
Introduction
CKD is a major health problem affecting 8%–16% of the world’s population (1), and the eGFR is widely used in clinical diagnosis and treatment for CKD (2). Metabolic syndrome is a cluster of cardiovascular risk factors including abdominal obesity, hyperglycemia, dyslipidemia, and hypertension (3,4). Several cross-sectional and prospective studies have shown that metabolic syndrome and its related components are associated with both prevalent and incident CKD (5–8).
Heritability is a parameter that represents the proportion of variation in a phenotypic trait that is explained by genes (9). Heritability has traditionally been estimated from family or twin studies, although single nucleotide polymorphism (SNP)–based methods have recently been developed in parallel to large genome-wide association studies (10). Whereas a twin study can capture all genetic factors, and with adequate power to distinguish additive and nonadditive genetic effects (11); the SNP-based methods can only capture the “chip heritability,” meaning that genetic variation due to rare SNPs, copy number variations, and epigenetic factors are not accounted for (12,13). This leads to an underestimation of heritability, so called “missing heritability,” compared with the heritability from a twin study (14). Therefore, twin study still plays an important role to estimate heritability even in the genomic era, especially until whole-genome sequencing data become widely available. As an example, previous family and twin studies reported that the heritabilities of eGFR and CKD are roughly 50% (15,16), whereas the SNP-based heritability of eGFR is only 7.6% in 312,468 individuals (17).
Heritabilities of metabolic syndrome and its related traits are in the range from 24% to 60% (18,19). Since several genetic variants are shared between metabolic syndrome–related traits (such as BP, lipids, and insulin resistance), it would be of interest to disentangle the combined effect of metabolic syndrome on CKD. It also can improve the understanding of the etiological relationships by studying the genetic and environmental influences on correlations between the different traits. Therefore, in a large Swedish twin population, we aimed to systematically quantify the genetic and environmental influences on the phenotypic variation and covariation (correlation) for traits of metabolic syndrome and CKD.
Materials and Methods
Study Population, Sampling and Measurements
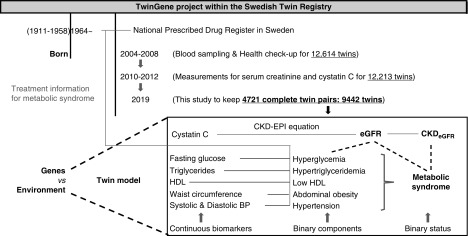
Participants in this study were from the population-based TwinGene project within the Swedish Twin Registry (20), including 12,614 Swedish twins (European ancestry) born between 1911 and 1958 (Figure 1). They had previously participated in the Screening Across the Lifespan Twin study, a computer-assisted telephone interview conducted between 1998 and 2002. The zygosity was identified by self-reported childhood resemblance and DNA markers. Both twins within a pair had to be alive and consent for future participation. All TwinGene participants have given informed consent, and this study has been approved by the regional ethical review board in Stockholm.
Figure 1.
Procedure for the study design. Participants in this study are from the population-based TwinGene project within the Swedish Twin Registry, including 12,614 Swedish twins (European ancestry) born between 1911 and 1958. Between 2004 and 2008, the participants’ height, weight, hip and waist circumference, as well as the BP were measured. Serum levels of fasting glucose and lipids were measured by routine methods on a semi-automated biochemistry analyzer. During 2010–2012, serum levels of creatinine and cystatin C were measured. Twin order was randomly assigned and the singletons were removed. Finally, 4721 complete twin pairs (1327 monozygotic, 1780 same-sex dizygotic, and 1614 opposite-sex dizygotic pairs) with both creatinine and cystatin C were used in this cross-sectional twin study. CKD-EPI, CKD Epidemiology Collaboration.
Between 2004 and 2008, the participants donated blood samples at their local health care facility after overnight fasting, and their height, weight, hip and waist circumference, as well as BP were measured. The blood samples were sent to Karolinska Institutet Biobank within 24 hours, and serum samples were aliquoted and stored in cryotubes under −80°C. Serum levels of fasting glucose and lipids were measured by routine methods on a semi-automated biochemistry analyzer (Beckman Coulter). Serum levels of creatinine were measured by enzymatic method on the Architect c8000 and Architect c16000 clinical chemistry analyzers, and serum levels of cystatin C were measured by the particle-reinforced immunoturbidimetric method on the Architect ci8200 clinical chemistry and immunoassay analyzer (Abbott Laboratories) (16).
Twin order was randomly assigned and singletons were removed, and finally 4721 complete twin pairs (1327 monozygotic, 1780 same-sex dizygotic, and 1614 opposite-sex dizygotic pairs) with both creatinine and cystatin C measurements were used in the twin study.
Definition of Metabolic Syndrome and CKD
The National Cholesterol Education Program-Adult Treatment Panel (NCEP-ATP) III guideline was used to define metabolic syndrome (4). Metabolic syndrome cases were identified if more than three of the following criteria were met: abdominal obesity (waist circumference >40 inches for men, >35 inches for women), hyperglycemia (fasting glucose ≥100 mg/dl or any antidiabetic treatment), hypertriglyceridemia (triglycerides ≥150 mg/dl or any triglyceride-lowering treatment), low HDL (HDL <40 mg/dl for men, <50 mg/dl for women, or any lipid-lowering treatments), and hypertension (systolic BP >130 mm Hg or diastolic BP >85 mm Hg, or any antihypertensive treatment). Pharmacologic treatments were identified from the National Prescribed Drug Register in Sweden, by using the Anatomic Therapeutic Chemical classification codes: (1) for hypertension (antihypertensive [C02], diuretics [C03], β-blocking agents [C07], calcium channel blockers [C08], and agents acting on the renin-angiotensin system [C09]), (2) for hyperglycemia (A10), and (3) for dyslipidemia (only fibrates were used to decrease triglycerides and increase HDL in Sweden; Anatomic Therapeutic Chemical code C10AB).
The eGFR was calculated by using the cystatin C–based equations (Supplemental Table 1) from the CKD Epidemiology Collaboration group (21); CKD cases were defined as eGFR≤60 ml/min per 1.73 m2.
Statistical Analyses
Data handling, descriptive statistics, and correlation analyses were performed in SAS v9.4 (SAS Institute Inc.). Means and SD were used to describe the characteristics of participants and the distributions of traits. Spearman correlations coefficients (rho) were estimated among the metabolic syndrome related biomarkers/components, metabolic syndrome, eGFR, and CKD; correlated pairs with |rho|≥0.15 were further investigated in the bivariate twin models (22).
Twin-Based Structural Equation Model
On the basis of the relatedness within twin pair (on average, cotwins within the same monozygotic pair share 100% of segregating genes and cotwins within the same dizygotic pair share 50%), genomic matrices were constructed in the structural equation model. The maximum likelihood methods were used to decompose the phenotypic variation (difference in population) and covariation (correlation between two traits) into a maximum of three genetic and environmental components: additive genetic effects (A; the sum of the effects of loci), dominant genetic effects (D; interactions between alleles within the same locus) or common environmental effects (C; shared environment contributes to the similarities between relatives living together), and unique environment effects (E; nonshared environment contributes to the dissimilarities within family members). Liability-threshold model was used for binary traits. The Akaike information criterion (AIC) was used to compare the fitting goodness of ACE (a model including A, C, and E components), ADE (including A, D, and E components), and AE (including A and E components) models, and the model with the lowest AIC value was defined as the best-fitted one (23). The proportions of the different A, D or C, E components contributing to the variation of each trait (a2, d2 or c2, e2), and similarly contributing to the covariation between two traits (bivariate a2, bivariate d2 or bivariate c2, bivariate e2) were estimated in the best-fitted model. Thus, univariate heritability (equal to a2, the proportion of the phenotype variance explained by A), bivariate heritability (equal to bivariate a2, the proportion of the correlation between two phenotypes explained by A), as well as genetic and environmental correlations were estimated, respectively. All twin-based structural equation model analyses were performed by OpenMx package (version 2.7.18) in R 3.4.1.
Results
Descriptive Statistics
In total, 4721 complete pairs consisting of 9442 participants were included in this study. The mean age was 65±8 years, and 44% of the participants were men (Table 1). According to the NCEP-ATP III guideline, 3180 individuals (34% of the participants) were defined as having metabolic syndrome. By using the cut-off value of eGFR<60 ml/min per 1.73 m2, 1896 individuals (20% of the participants) were defined as having CKD (Table 2).
Table 1.
Characteristics of participants from the population-based TwinGene project within the Swedish Twin Registry
| Characteristic | All | Men | Women | |||
|---|---|---|---|---|---|---|
| N | Mean±SD | N | Mean±SD | N | Mean±SD | |
| Age, yr | 9442 | 65±8 | 4183 | 65±8 | 5259 | 65±8 |
| Waist circumference, inch | 9379 | 36±5 | 4158 | 38±4 | 5221 | 34±5 |
| Glucose, mg/dl | 9438 | 100±21 | 4182 | 104±24 | 5256 | 98±19 |
| Triglycerides, mg/dl | 9440 | 119±72 | 4182 | 126±81 | 5258 | 115±63 |
| HDL, mg/dl | 9441 | 55±16 | 4183 | 48±13 | 5258 | 60±16 |
| Systolic BP, mm Hg | 8959 | 139±20 | 3955 | 139±19 | 5004 | 138±20 |
| Diastolic BP, mm Hg | 8958 | 82±11 | 3954 | 83±11 | 5004 | 81±10 |
| Creatinine, mg/dl | 9442 | 0.9±0.3 | 4183 | 1.0±0.3 | 5259 | 0.8±0.2 |
| Cystatin C, mg/L | 9442 | 1.01±0.26 | 4183 | 1.04±0.27 | 5259 | 0.99±0.24 |
| eGFR, ml/min per 1.73 m2 | 9442 | 76±19 | 4183 | 77±20 | 5259 | 76±18 |
eGFR calculated by the cystatin C–based equations from the CKD Epidemiology Collaboration group.
Table 2.
Numbers of participants with metabolic syndrome (related components) and eGFR-defined CKD
| Traits | No. (%) of All Participants | No. (%) of Men | No. (%) of Women | No. (%) with Missing Data |
|---|---|---|---|---|
| Hypertension treatment | 5979 (63) | 2697 (64) | 3282 (62) | 0 |
| Diabetes treatment | 1040 (11) | 597 (14) | 443 (8) | 0 |
| Abdominal obesity | 3397 (36) | 1170 (28) | 2227 (42) | 63 |
| Hyperglycemia | 3491(37) | 1907 (46) | 1584 (30) | 2 |
| Hypertriglyceridemia | 2240 (24) | 1115 (27) | 1125 (21) | 2 |
| Low HDL | 2703 (29) | 1392 (33) | 1311 (25) | 1 |
| Dyslipidemia | 3665 (39) | 1857(44) | 1808 (34) | 1 |
| Hypertension | 7446 (79) | 3393(81) | 4053 (77) | 167 |
| Metabolic syndrome | 3180 (34) | 1529(37) | 1651 (31) | 0 |
| CKD | 1896 (20) | 822 (20) | 1074 (20) | 0 |
In total, 9442 twins (4183 men and 5259 women) are included in the twin study. eGFR calculated by the cystatin C–based equations from the CKD Epidemiology Collaboration group; CKD cases defined by eGFR<60 ml/min per 1.73 m2. Percentages of numbers of participants who meet the criteria in each group are presented in the brackets. Metabolic syndrome is defined by the National Cholesterol Education Program-Adult Treatment Panel III guideline, cases are identified if more than three of the following criteria are met: abdominal obesity (waist circumference >40 inches for men, >35 inches for women), hyperglycemia (fasting glucose ≥100 mg/dl or any antidiabetic treatment), hypertriglyceridemia (triglycerides ≥150 mg/dl or any triglyceride-lowering treatment), low HDL (HDL <40 mg/dl for men, <50 mg/dl for women, or any lipid-lowering treatments), and hypertension (systolic BP >130 mm Hg or diastolic BP >85 mm Hg, or any antihypertensive treatment).
Relative Importance of Genes and Environment
The intrapair correlations for the metabolic syndrome– and CKD-related traits are presented by twin zygosity, and the phenotypic variation of each trait was decomposed into its genetic and environmental components within the corresponding best-fitted univariate twin model (Table 3). For all traits except for CKD and hypertension, the intrapair correlations were more than twice as large in monozygotic twins compared with dizygotic twins, indicating contributions from the dominant genetic effects (D). The ADE model was the best-fitted model for metabolic syndrome, waist circumference, fasting glucose, triglycerides, systolic BP, eGFR, abdominal obesity, and hyperglycemia; the estimates of dominant genetic variance (d2) were statistically significant (P value <0.05) for all these traits except for systolic BP. The ACE model was the best-fitted model for CKD, whereas the AE model was the best-fitted model for HDL, diastolic BP, hypertriglyceridemia, low HDL, dyslipidemia, and hypertension.
Table 3.
Univariate decomposition analyses in the twin study
| Traits | Intrapair Correlation | Best-Fitted Model | Additive Genetic Variance, a2(SEM),% | Dominant Genetic Variance, d2(SEM),% | Shared Environmental Variance, c2(SEM),% | |
|---|---|---|---|---|---|---|
| Monozygotic Twins | Dizygotic Twins | |||||
| Metabolic syndrome | 0.41 | 0.15 | ADE | 36 (11) | 26 (13) | |
| CKD | 0.49 | 0.32 | ACE | 41 (8) | 33 (6) | |
| Continuous traits | ||||||
| Waist circumference | 0.65 | 0.23 | ADE | 25 (6) | 40 (7) | |
| Fasting glucose | 0.49 | 0.20 | ADE | 29 (7) | 23 (7) | |
| Triglycerides | 0.55 | 0.24 | ADE | 41 (7) | 14 (7) | |
| HDL | 0.66 | 0.31 | AE | 65 (1) | ||
| Systolic BP | 0.42 | 0.17 | ADE | 29 (8) | 13 (7) | |
| Diastolic BP | 0.39 | 0.17 | AE | 37 (2) | ||
| eGFR | 0.58 | 0.25 | ADE | 41 (7) | 18 (7) | |
| Components of metabolic syndrome | ||||||
| Abdominal obesity | 0.49 | 0.14 | ADE | 21(11) | 49(12) | |
| Hyperglycemia | 0.45 | 0.16 | ADE | 39 (11) | 27(12) | |
| Hypertriglyceridemia | 0.40 | 0.15 | AE | 60 (3) | ||
| Low HDL | 0.44 | 0.20 | AE | 66 (3) | ||
| Dyslipidemia | 0.42 | 0.18 | AE | 60 (3) | ||
| Hypertension | 0.42 | 0.21 | AE | 68 (3) | ||
The Pearson and Spearman correlation coefficients are used for continuous and binary traits, respectively. The Akaike information criterion (AIC) is used to compare the goodness of fitting of ACE (including A, C, and E components), ADE (including A, D, and E components), and AE (including A and E components) models, and the most parsimonious one is defined by the lowest AIC value. Metabolic syndrome is defined by the National Cholesterol Education Program-Adult Treatment Panel III guideline, cases are identified if more than three of the following criteria are met: abdominal obesity (waist circumference >40 inches for men, >35 inches for women), hyperglycemia (fasting glucose ≥100 mg/dl or any antidiabetic treatment), hypertriglyceridemia (triglycerides ≥150 mg/dl or any triglyceride-lowering treatment), low HDL (HDL, <40 mg/dl for men, <50 mg/dl for women, or any lipid-lowering treatments), and hypertension (systolic BP >130 mm Hg or diastolic BP >85 mm Hg, or any antihypertensive treatment). CKD was defined by eGFR (cases with eGFR <60 ml/min per 1.73 m2); eGFR was calculated from the cystatin C–based equations from the CKD Epidemiology Collaboration group. A, additive genetic effects; D, dominant genetic effects; C, common environmental effects.
For metabolic syndrome, the narrow-sense heritability (a2, additive genetic variance) was 36% (95% confidence interval [95% CI], 14% to 58%), but significant dominant genetic variance (d2) was also identified (d2=26%; 95% CI, 1% to 52%). For eGFR-defined CKD, the estimate of a2 was 41% (95% CI, 25% to 57%), and significant contribution from the shared environmental variance (c2) was also identified (c2=33%; 95% CI, 11% to 45%).
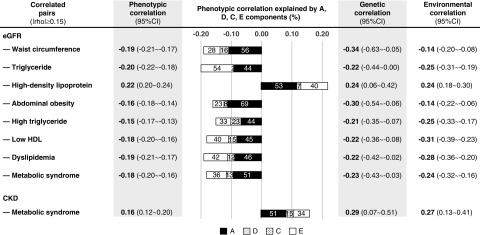
From the Spearman correlation matrix (Supplemental Figure 1), eGFR and CKD displayed moderate correlations with most, but not all of the metabolic syndrome related traits/components. Therefore, the bivariate decomposition analyses were performed for the nine correlated pairs with the absolute Spearman correlation coefficient ≥0.15 (Figure 2). In twin studies, the ADE model was the best-fitted for six out of nine correlated pairs; the ACE model was the best-fitted for the remaining three pairs (Supplemental Table 2). However, most of the estimates of bivariate d2 and bivariate c2 were nonsignificant, except for the pair of eGFR–high triglyceride (bivariate c2=23%; 95% CI, 1% to 45%). The estimates of additive genetic correlation and nonshared environmental correlation were significant for all nine correlated pairs.
Figure 2.
Bivariate decomposition analyses. Phenotypic correlations between traits of metabolic syndrome and CKD are partitioned to their genetic and environmental components. The phenotypic correlation coefficient between each pair is presented by the whole length of each bar, and the proportions of phenotypic correlation explained by different components are presented by the length of different bars. The maximum likelihood methods were used to estimate the proportion of A (additive genetic effects, indicated in black), D (dominant genetic effects, indicated in gray) or C (shared environmental effects, indicated in black dots), E (unique environmental effects, indicated in white), contributing to the correlation between two traits. Liability-threshold model was used for binary traits (CKD, metabolic syndrome, abdominal obesity, high triglyceride, low HDL, dyslipidemia). The AIC was used to compare the goodness of fitting of ACE (a model including A, C, and E components), ADE (including A, D, and E components), and AE (including A and E components) models, and the most parsimonious one was defined by the lowest AIC value. Significant estimates are in bold (P value <0.05).
For the phenotypic correlation between CKD and metabolic syndrome (Figure 2), 51% was explained by genes (bivariate a2, with 95% CI, 12% to 90%), whereas 15% was explained by common environment (bivariate c2 was nonsignificant, with 95% CI, 0% to 42%) and 34% was explained by nonshared environment (bivariate e2, with 95% CI, 16% to 52%); their genetic and environmental correlation was 0.29 (95% CI, 0.07 to 0.51) and 0.27 (95% CI, 0.13 to 0.41), respectively.
There were particularly strong genetic influences on the correlations between eGFR and two obesity traits (waist circumference and its defined abdominal obesity), with 56% and 69% of the covariation explained by genetic effects, and genetic correlations of 0.34 and −0.30, respectively. For eGFR and other five lipid-related traits (triglyceride, HDL, high triglyceride, low HDL, and dyslipidemia), the environmental correlations range from −0.25 to 0.24 and genetic correlations range from −0.22 to 0.24.
Discussion
In this twin study with 4721 complete twin pairs, we investigated the genetic and environmental influences on the correlations between metabolic syndrome, its components, and CKD. Around 51% of the phenotypic correlation between metabolic syndrome and CKD was explained by genes, 15% was explained by shared environment, and 34% was explained by nonshared environment. The genetic contributions seem to be important to the covariation between eGFR and abdominal obesity traits, whereas the opposite situation is observed for the correlation between eGFR and lipid-related traits.
Our twin models showed that both genetic and nonshared environmental factors significantly contribute to the correlation between metabolic syndrome and CKD. Previous family studies have only investigated the genetic and environmental correlations between eGFR and metabolic syndrome-related continuous traits, but not the proportions explained by genetic and environmental components. Although some of the nonshared environmental correlations were significant, they did not observe any significant genetic correlations except for with HDL (24,25). In contrast to previous family studies, our study suggests that genetic influences are particularly strong for the correlations between metabolic syndrome–related traits and CKD. One explanation is that previous studies used the creatinine-based Modification of Diet in Renal Disease equation to calculate eGFR, whereas this study used the cystatin C–based CKD Epidemiology Collaboration equation. Because cystatin C is not influenced by muscle mass and dietary protein intake, it may be a less biased marker to calculate eGFR compared with creatinine, especially in this slightly elderly population (26). In this study, moderate correlations were found between cystatin C–based eGFR and metabolic traits, whereas the correlations between creatinine-based eGFR and metabolic traits were weak and nonsignificant (Supplemental Figure 1).
Regarding the significant genetic effects to the correlation between metabolic syndrome and CKD, another explanation is probably that more twin pairs were included in our study, and in particular more twins with CKD, which render our study a larger power to detect and disentangle these effects. Our findings should also be put in the context of previous genomic studies that have indicated certain shared genetics between each single metabolic syndrome component and CKD (or eGFR), as well as having causal effects of CKD. Both obesity and type 2 diabetes have been causally associated with CKD in Mendelian randomization studies (27,28). A lower HDL has also been reported to be causal for impaired kidney function, whereas the evidence for a negative effect of triglycerides on eGFR is still lacking (29). The observed genetic contribution to the correlation in the current study is compatible with a direct effect of metabolic syndrome on CKD. Because this implication is also supported by the above-mentioned studies, alternate interpretations such as pleiotropic effects, that a single gene influences both traits but that they are otherwise unrelated of each other, are less likely. In this study, a stronger genetic contribution was observed to the correlation between eGFR and abdominal obesity compared with the other components of metabolic syndrome. Interestingly, in a previous population-based study, cystatin C was positively associated with incident abdominal obesity but not with other components of the metabolic syndrome, suggesting some shared etiology underlying the variation of these two traits (30). Whether this is specific for abdominal obesity or general obesity is not known, but we have recently reported that general obesity (defined by body mass index) is also associated with incident CKD but independent of genetic confounding (31).
AIC is commonly used to evaluate the model fitting, where lower AIC value indicates better fitting (23). In this study, the ADE model was the best-fitted model for six pairs, but none of the dominant genetic effects were significant (Figure 2), probably because the sample size of our study is still not large enough to disentangle the additive and nonadditive genetic effects, especially for the correlation between binary traits. For metabolic syndrome and CKD, the ACE model had a lower AIC than the ADE and AE models. The difference of model fitting between ACE and AE was statistically significant (P value=3.7×10−6), which indicates that some nonadditive genetic or shared environmental effects contribute to this correlation. The estimate of bivariate shared environment variance was not significant, perhaps also because the binary traits and bivariate analyses require an even larger sample size to obtain adequate power. However, twin study can estimate the genetic and environmental contribution without measuring any genes and environmental exposures. The estimates of nonshared environmental contribution in our study are quite robust, with an SEM<9% (Supplemental Table 2).
This is the first study to investigate the relative importance of genes and environment on the correlation between metabolic syndrome and CKD. The definition for metabolic syndrome is widely accepted, the data are from registers and not self-reported, and all of the measurements are done in a similar way within one laboratory. Our results indicate that the observed genetic contribution to the correlation between metabolic syndrome and eGFR-defined CKD in our study is compatible with a direct effect of metabolic syndrome on CKD. The genetic contribution is particularly important for the correlation between abdominal obesity and eGFR, which suggests a central role for abdominal obesity in the link between metabolic syndrome and CKD.
Disclosures
Dr. Bhuiyan, Dr. Chen, Dr. Kuja-Halkola, Dr. Magnusson, and Dr. Svensson have nothing to disclose.
Funding
The Swedish Twin Registry is managed by Karolinska Institutet and receives funding through the Swedish Research Council (2017-00641). This study was supported by the Swedish Society of Medicine (SLS-412071) and Stiftelsen för Njursjuka.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11971018/-/DCSupplemental.
Supplemental Table 1. Equation to calculate the eGFR (ml/min per 1.73 m2).
Supplemental Table 2. Bivariate decomposition analyses in twin study.
Supplemental Figure 1. Spearman correlation matrix.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Stevens LA, Levey AS: Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20: 2305–2313, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr., Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute : Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112: 2735–2752, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults : Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Kurella M, Lo JC, Chertow GM: Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol 16: 2134–2140, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J: The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 140: 167–174, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD: Metabolic syndrome and kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol 6: 2364–2373, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huh JH, Yadav D, Kim JS, Son JW, Choi E, Kim SH, Shin C, Sung KC, Kim JY: An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism 67: 54–61, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Visscher PM, Hill WG, Wray NR: Heritability in the genomics era--concepts and misconceptions. Nat Rev Genet 9: 255–266, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, Yang J: 10 years of GWAS discovery: Biology, function, and translation. Am J Hum Genet 101: 5–22, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Kuja-Halkola R, Rahman I, Arpegård J, Viktorin A, Karlsson R, Hägg S, Svensson P, Pedersen NL, Magnusson PK: Dominant genetic variation and missing heritability for human complex traits: Insights from twin versus genome-wide common SNP models. Am J Hum Genet 97: 708–714, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM: Finding the missing heritability of complex diseases. Nature 461: 747–753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourrat P, Lu Q, Jablonka E: Why the missing heritability might not be in the DNA. BioEssays 39: 1700067, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH: Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet 11: 446–450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raggi P, Su S, Karohl C, Veledar E, Rojas-Campos E, Vaccarino V: Heritability of renal function and inflammatory markers in adult male twins. Am J Nephrol 32: 317–323, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arpegård J, Viktorin A, Chang Z, de Faire U, Magnusson PK, Svensson P: Comparison of heritability of Cystatin C- and creatinine-based estimates of kidney function and their relation to heritability of cardiovascular disease. J Am Heart Assoc 4: e001467, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris AP, Le TH, Wu H, Akbarov A, van der Most PJ, Hemani G, Smith GD, Mahajan A, Gaulton KJ, Nadkarni GN, Valladares-Salgado A, Wacher-Rodarte N, Mychaleckyj JC, Dueker ND, Guo X, Hai Y, Haessler J, Kamatani Y, Stilp AM, Zhu G, Cook JP, Ärnlöv J, Blanton SH, de Borst MH, Bottinger EP, Buchanan TA, Cechova S, Charchar FJ, Chu PL, Damman J, Eales J, Gharavi AG, Giedraitis V, Heath AC, Ipp E, Kiryluk K, Kramer HJ, Kubo M, Larsson A, Lindgren CM, Lu Y, Madden PAF, Montgomery GW, Papanicolaou GJ, Raffel LJ, Sacco RL, Sanchez E, Stark H, Sundstrom J, Taylor KD, Xiang AH, Zivkovic A, Lind L, Ingelsson E, Martin NG, Whitfield JB, Cai J, Laurie CC, Okada Y, Matsuda K, Kooperberg C, Chen YI, Rundek T, Rich SS, Loos RJF, Parra EJ, Cruz M, Rotter JI, Snieder H, Tomaszewski M, Humphreys BD, Franceschini N: Trans-ethnic kidney function association study reveals putative causal genes and effects on kidney-specific disease aetiologies. Nat Commun 10: 29, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin HF, Boden-Albala B, Juo SH, Park N, Rundek T, Sacco RL: Heritabilities of the metabolic syndrome and its components in the Northern Manhattan Family Study. Diabetologia 48: 2006–2012, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayoumi RA, Al-Yahyaee SA, Albarwani SA, Rizvi SG, Al-Hadabi S, Al-Ubaidi FF, Al-Hinai AT, Al-Kindi MN, Adnan HT, Al-Barwany HS, Comuzzie AG, Cai G, Lopez-Alvarenga JC, Hassan MO: Heritability of determinants of the metabolic syndrome among healthy Arabs of the Oman family study. Obesity (Silver Spring) 15: 551–556, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Magnusson PK, Almqvist C, Rahman I, Ganna A, Viktorin A, Walum H, Halldner L, Lundström S, Ullén F, Långström N, Larsson H, Nyman A, Gumpert CH, Råstam M, Anckarsäter H, Cnattingius S, Johannesson M, Ingelsson E, Klareskog L, de Faire U, Pedersen NL, Lichtenstein P: The Swedish Twin Registry: Establishment of a biobank and other recent developments. Twin Res Hum Genet 16: 317–329, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Kuja-Halkola R, Chang Z, Karlsson R, Hägg S, Svensson P, Pedersen NL, Magnusson PKE: Genetic and environmental contributions to the covariation between cardiometabolic traits. J Am Heart Assoc 7: e007806, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.deLeeuw J: Introduction to akaike (1973) information theory and an extension of the maximum likelihood principle. In: Breakthroughs in Statistics, Vol. 1, edited by Kotz S, Johnson NL. , New York, Springer, 1992, pp 599–609 [Google Scholar]
- 24.MacCluer JW, Scavini M, Shah VO, Cole SA, Laston SL, Voruganti VS, Paine SS, Eaton AJ, Comuzzie AG, Tentori F, Pathak DR, Bobelu A, Bobelu J, Ghahate D, Waikaniwa M, Zager PG: Heritability of measures of kidney disease among zuni Indians: The zuni kidney project. Am J Kidney Dis 56: 289–302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song YM, Sung J, Lee K: Longitudinal relationships of metabolic syndrome and obesity with kidney function: Healthy Twin Study. Clin Exp Nephrol 19: 887–894, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Fan L, Levey AS, Gudnason V, Eiriksdottir G, Andresdottir MB, Gudmundsdottir H, Indridason OS, Palsson R, Mitchell G, Inker LA: Comparing GFR estimating equations using cystatin C and creatinine in elderly individuals. J Am Soc Nephrol 26: 1982–1989, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu M, Bi Y, Huang Y, Xie L, Hao M, Zhao Z, Xu Y, Lu J, Chen Y, Sun Y, Qi L, Wang W, Ning G: Type 2 diabetes, diabetes genetic score and risk of decreased renal function and albuminuria: A mendelian randomization study. EBioMedicine 6: 162–170, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todd JN, Dahlström EH, Salem RM, Sandholm N, Forsblom C, McKnight AJ, Maxwell AP, Brennan E, Sadlier D, Godson C, Groop PH, Hirschhorn JN, Florez JC; FinnDiane Study Group : Genetic evidence for a causal role of obesity in diabetic kidney disease. Diabetes 64: 4238–4246, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanktree MB, Thériault S, Walsh M, Paré G: HDL cholesterol, LDL cholesterol, and triglycerides as risk factors for CKD: A mendelian randomization study. Am J Kidney Dis 71: 166–172, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Magnusson M, Hedblad B, Engström G, Persson M, Nilsson P, Melander O: High levels of cystatin C predict the metabolic syndrome: The prospective Malmö Diet and Cancer Study. J Intern Med 274: 192–199, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Kuja-Halkola R, Chen X, Magnusson PKE, Svensson P, Carrero JJ: Higher body mass index is associated with incident diabetes and chronic kidney disease independent of genetic confounding. Kidney Int 95: 1225–1233, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.