Visual Abstract
Keywords: chronic dialysis, chronic hemodialysis, clinical epidemiology, dialysis, epidemiology and outcomes, ESRD, hemodialysis, kidney transplantation, humans, male, female, renal dialysis, incidence, prevalence, edetic acid, sex characteristics, rosa, renal replacement, diabetic nephropathies, chronic renal insufficiency, glomerulonephritis, registries, sex distribution
Abstract
Background and objectives
More men than women undergo kidney replacement therapy (KRT) despite a larger number of women being affected by CKD. The aim of this multinational European study was to explore whether there might be historic and geographic trends in sex-specific incidence and prevalence of various KRT modalities.
Design, setting, participants, & measurements
We assessed sex-specific differences in KRT incidence and prevalence using data from nine countries reporting to the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry for at least 40 years, during the period 1965–2015. Sex distribution data were compared with the European general population (Eurostat). Statistical methodology included basic descriptive statistics, incidence and prevalence calculations per million population (pmp), as well as their male-to-female ratios. Analyses were stratified by age group and diabetic status.
Results
We analyzed data from 230,378 patients receiving KRT (38% women). For all KRT modalities, the incidence and prevalence rates were consistently higher in men than women. For example, the KRT incidence increased from 8 pmp in 1965–1974 to 98 pmp in 2005–2015 in women, whereas it rose from 12 to 173 pmp in men during the same period. Male-to-female ratios, calculated for incident and prevalent KRT patients, increased with age (range 1.2–2.4), showing consistency over decades and for individual countries, despite marked changes in primary kidney disease (diabetes more prevalent than glomerulonephritis in recent decades). The proportion of kidney transplants decreased less with age in incident and prevalent men compared with women on KRT. Stratified analysis of patients who were diabetic versus nondiabetic revealed that the male-to-female ratio was markedly higher for kidney transplantation in patients with diabetes.
Conclusions
Since the beginning of KRT programs reporting to the ERA-EDTA Registry since the 1960s, fewer women than men have received KRT. The relative difference between men and women initiating and undergoing KRT has remained consistent over the last five decades and in all studied countries.
Introduction
Population-based studies have found that sex has a profound effect on the epidemiology of CKD (1). Although occasional reports from some countries indicate otherwise, most studies show that significantly more women than men are affected by CKD (1,2), both when defined by a lower GFR or by an increased urinary albumin-to-creatinine ratio (2). The higher CKD prevalence among women has been ascribed to biologic factors, a longer life expectancy, and to pitfalls in determination of kidney function (1,3,4).
However, the majority of patients that reach ESKD requiring kidney replacement therapy (KRT) are men (5), with a considerably lower incidence and prevalence of women on maintenance hemodialysis (HD) treatment (6–11). Interestingly, an early report from 1987 which postulated that sexual “inequality” in maintenance dialysis, along with racial and age inequalities, was due to “injustice,” (6) was not followed up until 2011 (9,12) and afterward (10). To the best of our knowledge, time trends in the sex distribution of dialysis initiation and maintenance have not been extracted systematically from an international database.
We hypothesized that if inequality and injustice (6) might account for unequal access to KRT, then the incidence and prevalence ratios of men and women on KRT might have undergone change over time and in geographic regions. Furthermore, although sex-specific mortality on KRT is similar, women with diabetes have a higher mortality risk than men (10,12), implying sex-specific KRT incidence and/or modality choice could differ by diabetes status. We explored these hypotheses in a large European KRT data set with historical registry data over a period of >50 years.
Materials and Methods
Study Population
The ERA-EDTA Registry collects data on patients treated with KRT for ESKD from national and regional kidney registries in Europe. In this study, we included data from those registries that provided individual patient data for at least 40 years. The study period was divided into five decades as follows: 1965–1974, 1975–1984, 1985–1994, 1995–2004, and 2005–2015. The following five registries contributed data for the entire study period: Austria, Finland, The Netherlands, Iceland, and Scotland (UK). In addition, the registries of Greece, Norway, Andalusia (Spain), and Catalonia (Spain) contributed data from 1975 onward.
Specifically, this analysis was performed on all patients in the registries listed above who initiated KRT between January 1, 1965, and December 31, 2015. Patients were followed from the onset of KRT until death, censoring (i.e., recovery of kidney function or loss to follow-up), or the end of the study period (December 31, 2015), whichever occurred first.
Data Collection and Definitions
The ERA-EDTA Registry collects the following data on an annual basis: date or month of birth, sex, primary kidney disease, date of onset of KRT, treatment modality at onset of KRT, treatment history, and date as well as cause of death.
Patients were categorized into the following age groups: 0–19, 20–44, 45–64, 65–74, and ≥75 years. Data on the youngest age group was unavailable for Scotland for the complete study period. Primary kidney disease was established by the nephrologist in the treating centers and classified according to the ERA-EDTA coding system, grouping patients into eight classes: glomerulonephritis, pyelonephritis, polycystic kidney disease, diabetes mellitus, hypertension, kidney vascular disease, miscellaneous, and unknown/missing.
General Population Data
To compare the sex distribution of patients receiving KRT with that of subjects of the same age who were not receiving such therapy, we used data from the general population in the nine included countries and regions over the corresponding time period as reference. These data were obtained from Eurostat, the statistical office of the European Union (13).
Statistical Analysis
Demographics and clinical characteristics were summarized using descriptive statistics, including percentages for categoric variables and mean values with SD for numeric data. There were only few missing data, with data completeness ranging from 99% for primary kidney disease to 100% for all other variables.
The incidence rate and the prevalence were calculated for the total KRT population and for subgroups based on sex, age, treatment modality (HD, peritoneal dialysis [PD], and kidney transplantation), and diabetes mellitus as the cause of ESKD. Whereas the incidence rate represents new cases of KRT in a year, the prevalence describes the number of patients alive and on KRT on December 31 of a given year. Both the incidence and the prevalence are expressed per million population (pmp) for men and women. The incidence was based on the first day of KRT and the incidence of kidney transplantation therefore represents pre-emptive transplants. Male-to-female ratios were calculated as the incidence or prevalence in men divided by the incidence or prevalence in women. All analyses were performed using SAS software version 9.4. We considered a P value of <0.05 (two-sided) as statistically significant.
Results
Patient Characteristics
During the study period, 230,378 patients were recorded as having initiated KRT, 89,132 of whom were women (39%). In Table 1, we present time trends in characteristics of patients initiating KRT. Overall, the incidence rate of KRT increased from 10 pmp in 1965–1974 to 135 pmp in 2005–2015. Whereas the incidence of KRT in women increased from 8 to 98 pmp over the same time periods, the percentage of women commencing KRT remained stable over time and was consistently lower than that of men at 37%–42%. The age of incident patients on KRT increased continuously across the decades of the study. Whereas the percentage of patients with glomerulonephritis and pyelonephritis as the underlying kidney disease decreased substantially, the percentage of patients with diabetes mellitus increased from 0.5% in the first decade to 24% in the last decade, corresponding to 0.05 pmp and 32 pmp, respectively. Furthermore, vascular diseases including renovascular disease and hypertension also contributed to an increasing number of patients receiving KRT over time. The primary treatment modality choice remained relatively stable, with HD being the most commonly used modality throughout the study period in all age groups by far, with percentages varying between 80% and 88%. We observed a peak in the percentage of PD around 1990 and a small increase in pre-emptive kidney transplantation over time.
Table 1.
Characteristics of patients commencing kidney replacement therapy
| Characteristics | 1965–1974 | 1975–1984 | 1985–1994 | 1995–2004 | 2005–2015 |
|---|---|---|---|---|---|
| Number of patients (pmp) | 3024 (10.0) | 13,627 (34.1) | 42,195 (69.9) | 71,571 (113.3) | 99,961 (134.5) |
| Age in years (mean [SD]) | 38 (13) | 47 (16) | 55 (17) | 61 (16) | 65 (16) |
| Age in years (median [IQR]) | 39 (28–48) | 49 (36–59) | 58 (44–67) | 65 (52–73) | 68 (56–77) |
| Sex: women (pmp [%]) | 8 (40) | 28 (42) | 57 (41) | 88 (39) | 97 (37) |
| Sex: men (pmp [%]) | 12 (60) | 40 (58) | 84 (59) | 139 (61) | 173 (63) |
| Primary kidney disease (pmp [%]) | |||||
| Glomerulonephritis | 5.1 (51) | 9.6 (28) | 14 (20) | 15.4 (13) | 13.9 (10) |
| Pyelonephritis | 1.6 (16) | 5.6 (17) | 8.5 (12) | 8.9 (8) | 7.3 (6) |
| Polycystic kidney disease | 0.31 (3) | 2.4 (7) | 5 (7) | 7 (6) | 7.5 (6) |
| Hypertension | 0.21 (2) | 1.5 (4) | 3.9 (7) | 11 (10) | 15 (11) |
| Diabetes mellitus | 0.05 (0.5) | 2.9 (9) | 10.7 (15) | 25.1 (22) | 32.3 (24) |
| Kidney vascular disease | 0.17 (2) | 0.6 (2) | 2.8 (4) | 6.5 (6) | 8.1 (6) |
| Miscellaneous | 2.25 (22) | 6.2 (18) | 11.2 (16) | 17.1 (15) | 20.4 (15) |
| Unknown/missing | 0.34 (3) | 5.3 (15) | 13.2 (18) | 22.4 (19) | 30 (21) |
| First KRT modality (pmp [%]) | |||||
| HD | 8.9 (88) | 29.1 (85) | 55.9 (80) | 93.9 (83) | 111.5 (83) |
| PD | 0.95 (10) | 4.3 (13) | 12.9 (18) | 17.3 (15) | 17.1 (13) |
| Kidney transplantation | 0.23 (2) | 0.79 (2) | 1.4 (2) | 2.3 (2) | 5.9 (4) |
| Country (pmp) | |||||
| Austria | 8.4 | 45.3 | 90.2 | 131.1 | 146.2 |
| Women | 6.0 | 31.8 | 75.7 | 102.2 | 102.8 |
| Men | 11.1 | 60.4 | 105.8 | 162.0 | 191.8 |
| Finland | 11.1 | 33.9 | 55.2 | 87.9 | 89.4 |
| Women | 9.3 | 28.1 | 45.8 | 65.7 | 58.6 |
| Men | 12.8 | 40.1 | 65.2 | 111.1 | 121.4 |
| Greece | 28.4 | 69.2 | 142.9 | 205.0 | |
| Women | 22.9 | 54.3 | 110.0 | 148.3 | |
| Men | 34.1 | 84.4 | 176.3 | 263.5 | |
| Iceland | 11.8 | 20.3 | 43.2 | 54.1 | 78.0 |
| Women | 11.2 | 22.3 | 34.2 | 42.3 | 60.1 |
| Men | 12.3 | 18.4 | 52.1 | 68.6 | 95.6 |
| Norway | 44.4 | 59.5 | 88.2 | 104.7 | |
| Women | 32.2 | 42.0 | 58.2 | 68.0 | |
| Men | 56.8 | 77.4 | 118.7 | 141.5 | |
| Andalusia | 63.7 | 65.3 | 110.1 | 122.2 | |
| Women | 55.1 | 55.0 | 89. 7 | 91.5 | |
| Men | 72.7 | 75.8 | 130.9 | 153.6 | |
| Catalonia | 69.0 | 91.3 | 141.6 | 146.8 | |
| Women | 60.5 | 69.9 | 105.2 | 102.3 | |
| Men | 77.9 | 113.7 | 179.3 | 192.3 | |
| The Netherlands | 11.9 | 33.5 | 66.1 | 94.6 | 116.0 |
| Women | 9.6 | 28.9 | 56.0 | 75.2 | 87.8 |
| Men | 14.1 | 38.1 | 76.4 | 114.2 | 144.7 |
| UK (Scotland) | 7.0 | 22.4 | 39.5 | 101.5 | 104.5 |
| Women | 5.1 | 17.3 | 31.1 | 84.6 | 83.7 |
| Men | 8.9 | 28.0 | 48.4 | 119.4 | 126.7 |
pmp, per million population; IQR, interquartile range; KRT, kidney replacement therapy; HD, hemodialysis; PD, peritoneal dialysis.
Certain patient characteristics differed between the sexes (Table 2). Women tended to be slightly older than men at KRT initiation and were less likely to have glomerular disease. In the last three decades, the relative contribution of diabetes mellitus as underlying kidney disease was slightly higher among women than among men, although this difference did not reach statistical significance.
Table 2.
Sex-specific characteristics of patients commencing kidney replacement therapy in historic cohorts
| Characteristics | 1965–1974 | 1975–1984 | 1985–1994 | 1995–2004 | 2005–2015 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | P value | Men | Women | P value | Men | Women | P value | Men | Women | P value | Men | Women | P value | |
| Age at onset (mean [SD]) | 39 (13) | 38 (13) | 0.12 | 47 (16) | 48 (16) | 0.0004 | 54 (17) | 56 (17) | <0.001 | 61 (16) | 62 (16) | <0.001 | 64 (16) | 65 (16) | <0.001 |
| Primary kidney disease (%) | |||||||||||||||
| Glomerulonephritis | 57 | 41 | <0.001 | 34 | 21 | <0.001 | 23 | 15 | <0.001 | 15 | 11 | <0.001 | 11 | 9 | <0.001 |
| Pyelonephritis | 10 | 25 | 12 | 23 | 10 | 15 | 7 | 9 | 5 | 6 | |||||
| Polycystic kidney disease | 3 | 4 | 6 | 9 | 6 | 8 | 6 | 7 | 5 | 7 | |||||
| Hypertension | 3 | 1 | 5 | 3 | 8 | 5 | 11 | 8 | 12 | 10 | |||||
| Diabetes mellitus | 0.6 | 0.4 | 9 | 8 | 15 | 16 | 21 | 23 | 24 | 24 | |||||
| Kidney vascular disease | 2 | 2 | 2 | 1 | 5 | 3 | 7 | 5 | 7 | 5 | |||||
| Miscellaneous | 22 | 24 | 16 | 21 | 14 | 19 | 14 | 17 | 14 | 17 | |||||
| Unknown | 3 | 3 | 15 | 14 | 18 | 18 | 19 | 19 | 20 | 21 | |||||
| First KRT modality (%) | |||||||||||||||
| HD | 89 | 87 | 0.41 | 86 | 85 | 0.21 | 80 | 79 | 0.02 | 83 | 83 | 0.46 | 83 | 83 | 0.006 |
| PD | 9 | 10 | 12 | 13 | 18 | 9 | 15 | 15 | 13 | 13 | |||||
| Kidney transplantation | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 5 | |||||
| Country (%) | |||||||||||||||
| Austria | 21 | 20 | 0.51 | 22 | 22 | 0.004 | 16 | 17 | <0.001 | 15 | 15 | <0.001 | 14 | 13 | <0.001 |
| Finland | 16 | 17 | 12 | 12 | 6 | 7 | 7 | 6 | 6 | 5 | |||||
| Greece | 11 | 10 | 17 | 16 | 22 | 21 | 25 | 25 | |||||||
| Iceland | 0.5 | 0.7 | 0.3 | 0.4 | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | |||||
| Norway | 6 | 7 | 7 | 5 | 6 | 5 | 6 | 5 | |||||||
| Andalusia | 3 | 3 | 10 | 11 | 11 | 12 | 11 | 11 | |||||||
| Catalonia | 3 | 3 | 14 | 12 | 13 | 12 | 12 | 11 | |||||||
| The Netherlands | 50 | 51 | 34 | 36 | 23 | 24 | 21 | 21 | 21 | 22 | |||||
| UK (Scotland) | 12 | 11 | 9 | 8 | 7 | 7 | 7 | 8 | 6 | 7 | |||||
Totals may be not add up to 100% due to rounding. KRT, kidney replacement therapy; HD, hemodialysis; PD, peritoneal dialysis.
Time Trends in the Incidence and Prevalence of KRT
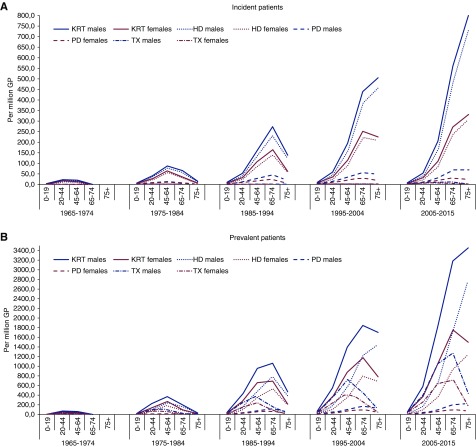
Incidence rates for the different KRT modalities are presented in Figure 1A. In all age groups and decades, more men than women started KRT. Moreover, both the HD and PD incidence increased more in men compared with women over time. Age-specific time trends in kidney transplantation as the first KRT modality showed the number of pre-emptive transplantations was markedly lower in the age group ≥75 years, when compared with younger patients. With higher age, the incidence of pre-emptive kidney transplantation in men relative to women increased (in 2005–2015: 45–64 years, 13 versus 8 pmp [male-to-female ratio 1.5]; 65–74 years, 12 versus 6 pmp [2.3]; ≥75 years, 1 versus 0.3 pmp [4.7]). However, the numbers of pre-emptive kidney transplantations were very small, especially in the earlier decades.
Figure 1.
Sex-specific time trends in kidney replacement therapy incidence and prevalence, per million population, by age group. (A) Incidence per million population for men (blue lines) and women (pink lines). (B) Prevalence per million population for men (blue lines) and women (pink lines). GP, general population; HD, hemodialysis; KRT, kidney replacement therapy; PD, peritoneal dialysis; TX, kidney transplantation.
The patterns of sex-specific prevalence largely corresponded to those of the incidence (Figure 1B). Again, the proportion of kidney transplants decreased less with age in prevalent men compared with women on KRT (in 2005–2015: 45–64 years, 1077 versus 641 cases pmp [male-to-female ratio 1.7]; 65–74 years, 1263 versus 712 pmp [1.8]; ≥75 years, 445 versus 193 pmp [2.3]).
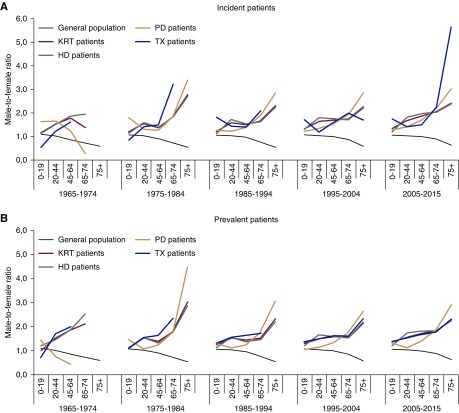
The male-to-female ratio in the general population decreased steadily with increasing age from 1.1 in the 0–19 years age group to 0.6 in the ≥75 year age group in all decades (Figure 2A). By contrast, the male-to-female ratio increased almost linearly in the overall incident KRT cohort, e.g., from 1.2 in patients aged 0–19 years to 2.4 in patients aged ≥75 years in 2005–2015. Compared with HD, where an increasing male-to-female ratio was apparent from age 20 years onward, the PD male-to-female ratios remained closer to 1.0 in the younger age groups. With regard to kidney transplantation, again, a greater increase in the male-to-female ratio by age was found for incident patients on KRT (an increase from 1.7 to 5.6 in the years 2005–2015), whereas the observation was less pronounced in prevalent patients (an increase from 1.4 to 2.3 in 2005–2015; Figure 2B). Calculation of the crude percentages of women among incident and prevalent patients receiving KRT showed comparable results (Supplemental Figure 1). The analysis of registry-specific time trends in the sex-dependent KRT incidence rates and the male-to-female ratios, by age group, yielded very similar results across the participating countries (Supplemental Figures 2 and 3). The relative decrease in KRT incidence in the oldest age groups was analyzed further by splitting the age categories ≥70 years into 5-year intervals, but the results were not meaningfully different.
Figure 2.
Time trends in male-to-female ratios for kidney replacement therapy incidence and prevalence, by age group. (A) Male-to-female ratio for the incidence of kidney replacement therapy compared with the general population (male-to-female ratios in patients initiating various modalities of KRT and in the general population). (B) Male-to-female ratio for the prevalence of kidney replacement therapy compared with the general population (male-to-female ratios in patients who are on various forms modalities of KRT and in the general population). HD, hemodialysis; KRT, kidney replacement therapy; PD, peritoneal dialysis; TX, kidney transplantation.
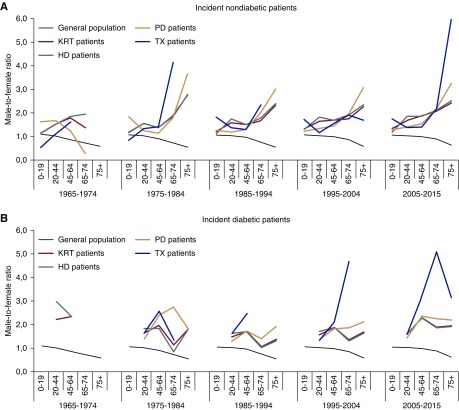
Time Trends in KRT of Patients with or without Diabetes
We next carried out a stratified analysis of patients who were diabetic versus nondiabetic (Figure 3). Notably, patients with diabetes constituted a minority of all patients receiving KRT from 1964 through 1984. The stratified data were therefore only interpreted for the last three decades (1985–1994, 1995–2004, and 2005–2015). We found the age-dependent male-to-female ratios among the incident nondiabetic KRT population were the same as for the overall KRT population (Figure 3A). Higher male-to-female ratios were present from the age of 20 years onward in patients with diabetes compared with patients without diabetes (Figure 3B). The male-to-female ratio for patients receiving a pre-emptive kidney transplantation increased markedly at ≥75 years in the nondiabetic group. This phenomenon occurred earlier (beginning at ≥45 years) in patients with diabetes. Patients with diabetes aged ≥75 years hardly received any transplants (kidney transplantation prevalence of patients with diabetes aged ≥75 years was 30 in men versus 9 pmp in women; male-to-female ratio 3.6).
Figure 3.
Time trends in male-to-female ratios for kidney replacement therapy incidence, by diabetic status and age group. (A) Male-to-female ratio in patients without diabetes compared with the general population. (B) Male-to-female ratio in patients with diabetes compared with the general population. HD, hemodialysis; KRT, kidney replacement therapy; PD, peritoneal dialysis; TX, kidney transplantation.
Discussion
In this study, we found more men than women started all types of KRT across all age strata in several European countries. We also observed that the male-to-female ratio increased with age for all KRT modalities and remained relatively stable over the last 50 years. Furthermore, diabetes mellitus as an underlying kidney disease was associated with a lower rate of pre-emptive kidney transplantation in women than in men, especially among elderly patients.
The findings that, in all European countries reporting to the ERA-EDTA Registry, a higher number and a higher percentage of men compared with women initiated any type of KRT during the last five decades, and that the proportion of women on KRT remained constant throughout the study period, are consistent with our previous report from a national HD registry (14). The reasons for these observations are currently unclear. Several explanations for the discrepancy between the sex distribution in CKD versus KRT have been proposed (1), including unequal access to KRT, competing risks of dying before KRT initiation (15), and faster progression of CKD in men than women (16,17). Because health inequalities, perhaps due to gender disparity, are potentially modifiable, it would be important to prove such a phenomenon as the underlying cause for the different sex distribution of patients with CKD versus KRT. If inequalities in access to care have prevented women from reaching KRT, these disparities would then have remained remarkably stable in Europe over the last 50 years because otherwise they would not be the most important factor explaining the observed sex differences. However, this notion is purely speculative because this study does not provide information on healthcare access.
The literature on access to healthcare in men and women with CKD is conflicting. For instance, both awareness of CKD among patients and the intensity of predialysis nephrology care were found to be lower among women (18,19). By contrast, the US Renal Data System (USRDS) reported a higher percentage of women with CKD visiting a nephrologist in 2015 (20). To date, longitudinal analyses specifically exploring this issue are lacking. Our data showed consistently rising incidence and prevalence of KRT in men over the last five decades in Europe. These findings were consistent across countries with variable healthcare policies and accessibility to kidney transplantation, such as Greece, Spain, or The Netherlands. If future studies succeed in placing the KRT findings in context of definitive healthcare access data, reasonable conclusions (or the lack thereof) might perhaps be reached.
The present findings might be explainable by those previous studies suggesting a faster progression of CKD in men (1,16,17), which could be a logical rationale for the sex-specific difference in the KRT incidence. However, two important meta-analyses analyzing this issue reached opposite conclusions (16,21), perhaps because previous studies included population-based data and referred patients indistinctively, and used both eGFR decline and KRT initiation as study outcomes to define “progression” to ESKD. Indeed, the most recent study observed a lower risk of eGFR decline in women (22). A possible slower progression to KRT among women with CKD has been ascribed to several factors, including biologic processes such as hormonal protection before menopause and differences in oxidative stress (23–25), lifestyle factors, and better adherence to medical therapy (26). However, women are also more likely to choose conservative kidney care (27).
KRT incidence rates increased with age during the last two decades and, interestingly, the same applied to the male-to-female ratios of KRT (both dialysis and kidney transplantation) also throughout all five decades. This increase did not come to a halt in the ≥75 years KRT cohort, despite the declining male-to-female ratio in the general population. The greater comorbidity burden among males may play an important role (28). Further investigations in the elderly population with advanced CKD (stage 4 or 5) will be useful in identifying potentially protective biologic factors and perhaps also behavioral factors in women underlying this phenomenon. Such investigations in CKD would be timely, because the interplay between sex-specific biology and perception is in the interest of patients and has been addressed more rigorously for other chronic conditions, such as cardiovascular disease (29–31).
Our study is the first multinational data set describing historic, sex-dependent time trends in the incidence and prevalence of PD. The prevalence of PD has risen over the last 30 years, and this has been more pronounced in men. The overall PD incidence and prevalence in this era are comparable to previously reported data (32). However, it should be noted that, unlike for HD, the incidence of PD appeared to stall in the oldest age group. A possible explanation for this finding could be that several modifiable and nonmodifiable factors—e.g., dependence on a spouse or caregiver, lower functional status, or lower cognitive function—gain more importance in the dialysis modality decision at higher ages, leading to a perceived inability of the patient to carry out PD (33). In addition, women more often live alone as their life expectancy is longer than the life expectancy of men. Our study also found the male-to-female ratio remained relatively low in young patients with PD (both incident and prevalent). This finding suggests younger women are more likely to choose PD as their primary KRT modality, perhaps due to better pre-KRT health status, better self-management, and an expected greater quality of life, despite a higher risk of infection (33–35).
This study also highlights important sex-specific differences regarding the access to kidney transplantation. First, pre-emptive transplantation (in absolute numbers) was less likely to occur in elderly women compared with elderly men. This finding can only partially be explained by the higher number of elderly men requiring KRT. Another explanation could be faster progression of CKD among men, although slower progression might actually favor pre-emptive kidney transplantation among women. Perhaps more importantly, this finding also needs to be viewed in the context of women being more likely to donate a kidney to their spouse. This hypothesis is supported by a single center study from Canada, where more than a third of the wives who were acceptable donors went on to donate a kidney to their spouse, compared with 6.5% of husbands (36).
Second, the probability of receiving a kidney transplantation (both in a pre-emptive setting and after a period on dialysis) was distinctly diminished in female patients with diabetes. Villar et al. (5) reported a female overrepresentation among patients with diabetes receiving HD. Whereas previous studies identified worse long-term outcomes for women with diabetes on KRT compared with men (10,12), it is important that future work explores why women with diabetes are less likely to undergo kidney transplantation.
Third, the finding that the male-to-female ratio in the proportion of incident kidney transplants was >1 throughout all decades and age groups (with the exception of the youngest age group [0–19 years] in the first two decades analyzed) is consistent with many previous reports from the United States (37–44), one study from Canada (45), and another study from France (46). An important analysis of USRDS data dissected the transplant process from waitlisting forward (in time) (47). The authors arrived at the conclusion that relatively fewer women than men were waitlisted for kidney transplantation, but transplant rates thereafter were similar between women and men after adjusting for preformed lymphocytotoxic antibody levels, which are usually higher in women (reviewed by Carrero et al. [1]). Further analysis should be made to determine whether sex-specific inequalities in waitlisting account for the unequal transplant rates observed in this European study.
Strengths of this study are the large sample size, the long follow-up period, and the multinational data acquisition. Furthermore, data on all KRT modalities were available, allowing us to report on sex-specific differences in access to kidney transplantation. Nevertheless, several limitations inherent to the study design require mentioning. The lack of data on vascular access and laboratory parameters precludes conclusions regarding certain modifiable factors among incident and prevalent patients on dialysis, as well as GFR at KRT initiation and adequacy of pre-KRT care. Moreover, only patients who actually received KRT were included; thus, due to the resulting survival bias, potential presumptions about patient care in the predialysis setting are hampered even further. However, the study was not designed to explore such factors. Information on lifestyle, symptoms in the period preceding KRT, indications for starting KRT, and quality of predialysis care may be indispensable when determining whether sex-specific healthcare inequalities do exist.
In conclusion, our study identified notable age-dependent differences between men and women initiating KRT in Europe during the last 50 years. Overall, men were more likely to receive KRT. Collectively, these data contrast with the well documented higher prevalence rates of earlier stages of CKD in women (1,2), which is also not fully explainable by underestimation of the GFR in women (1,3,4). The male-to-female ratios of KRT incidence remained remarkably constant over time. Future epidemiologic studies should address sex-specific KRT initiation by primary kidney disease, and evaluate potential sex differences in the competing risks of mortality versus KRT initiation in the general population (15). More detailed investigations could also explore the perception of women and men, as well as their caretakers, at the transition from pre-ESKD to KRT. Shedding more light on the fate of women and men after reaching ESKD would be indispensable as patient and physician educational programs might have to raise awareness about potential healthcare discrepancies to improve patient outcomes.
Disclosures
Dr. Cases reports receiving grants from Bristol-Myers Squibb and Vifor Fresenius; personal fees from positions on advisory boards with Astellas, AstraZeneca, and Daichii Sankyo; and personal fees from a position on an advisory board and speaker fees with Vifor Fresenius, outside of the submitted work. Dr. Finne reports lecture fees from Baxter outside of the submitted work. Dr. Hecking reports receiving grants from Astellas Pharma, Boehringer Ingelheim, FWF Austrian Science Fund, and Siemens Healthcare and personal fees from Siemens Healthcare, outside of the submitted work. Dr. Jager reports speaker fees from Vifor Fresenius outside of the submitted work. The other authors have nothing to disclose.
Funding
Dr. Antlanger and Dr. Hecking were supported by a grant from the Austrian Science Fund (KL754-B). Dr. Noordzij, Dr. van de Luijtgaarden, Dr. Jager and the ERA-EDTA Registry were supported by grants from the ERA-EDTA during the conduct of the study.
Supplementary Material
Acknowledgments
We would like to thank the patients and staff of the dialysis and transplant units for contributing the data via their national and regional kidney registries. Furthermore, we gratefully acknowledge the following registries and persons for their contribution to the data: Austrian Dialysis and Transplant Registry (OEDTR), Finnish Registry for Kidney Diseases (A. Pylsy and P. H. Groop), Hellenic Renal Registry (N. Afentakis), Icelandic ESKD Registry, Norwegian Renal Registry (T. Leivestad, A. Åsberg), Dutch Renal Registry (RENINE; L. Heuveling, S. Vogelaar), Scottish Renal Registry (all of the Scottish renal units), and the regional registries of Andalusia (SICATA; P. Castro de la Nuez on behalf of all users of SICATA) and Catalonia (RMRC; E. Arcos, J. Comas, and J. Tort). We also thank the other ERA-EDTA registry committee members not mentioned above for their advice in the analysis and the drafting of this article: C. Zoccali, P. Ambühl, M. Arici, J. De Meester, M. Evans, J. Harambat, L. Mercadal, M. Nordio, S.S. Sørensen, and E. Vidal, as well as A. Kramer and V.S. Stel in the Academic Medical Center Registry office for data collection and management.
This article was written by Dr. Antlanger, Dr. Aresté-Fosalba, Dr. Carrero, Dr. Cases, Dr. Finne, Dr. Hecking, Dr. Hemmelder, Dr. Jager, Dr. Kramar, Dr. Massy, Dr. Noordzij, Dr. Palsson, Dr. Varberg Reisaeter, Dr. Traynor, and Dr. van de Luijtgaarden, on behalf of the ERA-EDTA Registry, an official body of the ERA-EDTA.
The results presented in this article have not been published previously in whole or part.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Sex and the Incidence and Prevalence of Kidney Disease,” on pages XXX–XXX.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04400419/-/DCSupplemental.
Supplemental Figure 1. Time trends in the percentage of women among incident and prevalent kidney replacement therapy populations, compared with the general population, by age group.
Supplemental Figure 2. Sex-specific time trends in kidney replacement therapy incidence, by country and age group.
Supplemental Figure 3. Time trends in male-to-female ratios for kidney replacement therapy incidence, by country and age group.
References
- 1.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ: Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 14: 151–164, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 165: 473–481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glassock R, Delanaye P, El Nahas M: An age-calibrated classification of chronic kidney disease. JAMA 314: 559–560, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M: Age- and gender-specific reference values of estimated GFR in Caucasians: The Nijmegen Biomedical Study. Kidney Int 72: 632–637, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Villar E, Remontet L, Labeeuw M, Ecochard R: Effect of age, gender, and diabetes on excess death in end-stage renal failure. J Am Soc Nephrol 18: 2125–2134, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Kjellstrand CM, Logan GM: Racial, sexual and age inequalities in chronic dialysis. Nephron 45: 257–263, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Brynger H, Brunner FP, Chantler C, Donckerwolcke RA, Jacobs C, Kramer P, Selwood NH, Wing AJ: Combined report on regular dialysis and transplantation in Europe. X, 1979. Proc Eur Dial Transplant Assoc 17: 2–86, 1980 [PubMed] [Google Scholar]
- 8.Eggers PW, Connerton R, McMullan M: The Medicare experience with end-stage renal disease: Trends in incidence, prevalence, and survival. Health Care Financ Rev 5: 69–88, 1984 [PMC free article] [PubMed] [Google Scholar]
- 9.Carrero JJ, de Jager DJ, Verduijn M, Ravani P, De Meester J, Heaf JG, Finne P, Hoitsma AJ, Pascual J, Jarraya F, Reisaeter AV, Collart F, Dekker FW, Jager KJ: Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol 6: 1722–1730, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Hecking M, Bieber BA, Ethier J, Kautzky-Willer A, Sunder-Plassmann G, Säemann MD, Ramirez SP, Gillespie BW, Pisoni RL, Robinson BM, Port FK: Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: The Dialysis Outcomes and Practice Patterns Study (DOPPS). PLoS Med 11: e1001750, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer A, Pippias M, Noordzij M, Stel VS, Afentakis N, Ambühl PM, Andrusev AM, Fuster EA, Arribas Monzón FE, Åsberg A, Barbullushi M, Bonthuis M, Caskey FJ, Castro de la Nuez P, Cernevskis H, des Grottes JM, Garneata L, Golan E, Hemmelder MH, Ioannou K, Jarraya F, Kolesnyk M, Komissarov K, Lassalle M, Macario F, Mahillo-Duran B, Martín de Francisco AL, Palsson R, Pechter Ü, Resic H, Rutkowski B, Santiuste de Pablos C, Seyahi N, Simic Ogrizovic S, Slon Roblero MF, Spustova V, Stojceva-Taneva O, Traynor J, Massy ZA, Jager KJ: The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) registry annual report 2015: A summary. Clin Kidney J 11: 108–122, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrero JJ, de Mutsert R, Axelsson J, Dekkers OM, Jager KJ, Boeschoten EW, Krediet RT, Dekker FW; NECOSAD Study Group : Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant 26: 270–276, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Eurostat , 2018. Available at: http://ec.europa.eu/eurostat/data/database. Accessed July 11, 2018
- 14.Kainz A, Berner C, Ristl R, Simon A, Stamm T, Zitt E, Kramar R, Antlanger M, Kautzky-Willer A, Schmaldienst S, Schernhammer E, Port FK, Carrero JJ, Jager KJ, Hecking M: Sex-specific analysis of haemodialysis prevalence, practices and mortality over time: The Austrian Dialysis Registry from 1965 to 2014. Nephrol Dial Transplant 34: 1026–1035, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR; Chronic Kidney Disease Prognosis Consortium : Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: A meta-analysis. BMJ 346: f324, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neugarten J, Acharya A, Silbiger SR: Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol 11: 319–329, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Silbiger SR, Neugarten J: The role of gender in the progression of renal disease. Adv Ren Replace Ther 10: 3–14, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Fischer MJ, Stroupe KT, Kaufman JS, O'Hare AM, Browning MM, Sohn MW, Huo Z, Hynes DM: Predialysis nephrology care and dialysis-related health outcomes among older adults initiating dialysis. BMC Nephrol 17: 103, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Renal Data System : USRDS 2017 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2017 [Google Scholar]
- 21.Jafar TH, Schmid CH, Stark PC, Toto R, Remuzzi G, Ruggenenti P, Marcantoni C, Becker G, Shahinfar S, De Jong PE, De Zeeuw D, Kamper AL, Strangaard S, Levey AS: The rate of progression of renal disease may not be slower in women compared with men: A patient-level meta-analysis. Nephrol Dial Transplant 18: 2047–2053, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M, Manoharan A, Steigerwalt S, Wright J, Rahman M, Rosas SE, Saunders M, Sharma K, Daviglus ML, Lash JP; CRIC Investigators : Sex-Related disparities in CKD progression. J Am Soc Nephrol 30: 137–146, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maric C, Sandberg K, Hinojosa-Laborde C: Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol 15: 1546–1556, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Reckelhoff JF, Zhang H, Srivastava K: Gender differences in development of hypertension in spontaneously hypertensive rats: Role of the renin-angiotensin system. Hypertension 35: 480–483, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Valdivielso JM, Jacobs-Cachá C, Soler MJ: Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens 28: 1–9, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Crews DC, Kuczmarski MF, Miller ER 3rd, Zonderman AB, Evans MK, Powe NR: Dietary habits, poverty, and chronic kidney disease in an urban population. J Ren Nutr 25: 103–110, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton RL, Turner RM, Howard K, Snelling P, Webster AC: Patients who plan for conservative care rather than dialysis: A national observational study in Australia. Am J Kidney Dis 59: 419–427, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Ceretta ML, Noordzij M, Luxardo R, De Meester J, Abad Diez JM, Finne P, Heaf JG, Couchoud C, Kramar R, Collart F, Cases A, Palsson R, Reisæter AV, Rydell H, Massy ZA, Jager KJ, Kramer A: Changes in co-morbidity pattern in patients starting renal replacement therapy in Europe-data from the ERA-EDTA Registry. Nephrol Dial Transplant 33: 1794–1804, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE: Cardiovascular disease in women: Clinical perspectives. Circ Res 118: 1273–1293, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merz CN: The Yentl syndrome is alive and well. Eur Heart J 32: 1313–1315, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Lindquist R, Boucher JL, Grey EZ, Cairns B, Bobra S, Windenburg D, Konety S, Graham K, Luepker R, Hayes SN: Eliminating untimely deaths of women from heart disease: Highlights from the Minnesota Women’s Heart Summit. Am Heart J 163: 39–48.e1, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Jain AK, Blake P, Cordy P, Garg AX: Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 23: 533–544, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chanouzas D, Ng KP, Fallouh B, Baharani J: What influences patient choice of treatment modality at the pre-dialysis stage? Nephrol Dial Transplant 27: 1542–1547, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Ros S, Remón C, Qureshi AR, Quiros P, Lindholm B, Carrero JJ: Increased risk of fatal infections in women starting peritoneal dialysis. Perit Dial Int 33: 487–494, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakewell AB, Higgins RM, Edmunds ME: Quality of life in peritoneal dialysis patients: Decline over time and association with clinical outcomes. Kidney Int 61: 239–248, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman D, Donnelly S, Miller J, Stewart D, Albert SE: Gender disparity in living renal transplant donation. Am J Kidney Dis 36: 534–540, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Eggers PW: Effect of transplantation on the Medicare end-stage renal disease program. N Engl J Med 318: 223–229, 1988 [DOI] [PubMed] [Google Scholar]
- 38.Held PJ, Pauly MV, Bovbjerg RR, Newmann J, Salvatierra O Jr.: Access to kidney transplantation. Has the United States eliminated income and racial differences? Arch Intern Med 148: 2594–2600, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Kjellstrand CM: Age, sex, and race inequality in renal transplantation. Arch Intern Med 148: 1305–1309, 1988 [PubMed] [Google Scholar]
- 40.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Gaylin DS, Held PJ, Port FK, Hunsicker LG, Wolfe RA, Kahan BD, Jones CA, Agodoa LY: The impact of comorbid and sociodemographic factors on access to renal transplantation. JAMA 269: 603–608, 1993 [PubMed] [Google Scholar]
- 42.Bloembergen WE, Mauger EA, Wolfe RA, Port FK: Association of gender and access to cadaveric renal transplantation. Am J Kidney Dis 30: 733–738, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Soucie JM, Neylan JF, McClellan W: Race and sex differences in the identification of candidates for renal transplantation. Am J Kidney Dis 19: 414–419, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Segev DL, Kucirka LM, Oberai PC, Parekh RS, Boulware LE, Powe NR, Montgomery RA: Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol 20: 621–628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaubel DE, Stewart DE, Morrison HI, Zimmerman DL, Cameron JI, Jeffery JJ, Fenton SS: Sex inequality in kidney transplantation rates. Arch Intern Med 160: 2349–2354, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Bayat S, Macher MA, Couchoud C, Bayer F, Lassalle M, Villar E, Caillé Y, Mercier S, Joyeux V, Noel C, Kessler M, Jacquelinet C; REIN Registry : Individual and regional factors of access to the renal transplant waiting list in France in a cohort of dialyzed patients. Am J Transplant 15: 1050–1060, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Wolfe RA, Ashby VB, Milford EL, Bloembergen WE, Agodoa LY, Held PJ, Port FK: Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis 36: 1025–1033, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.