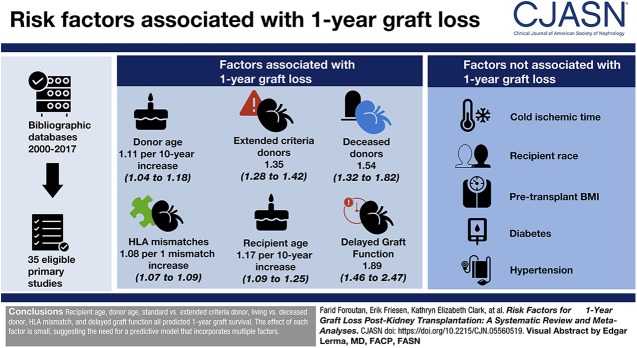
Visual Abstract
Keywords: Transplantation, chronic allograft failure, Epidemiology and outcomes, humans, delayed graft function, kidney transplantation, graft survival, risk factors, cold ischemia, body mass index, living donors, HLA antigens, transplant recipients, diabetes mellitus, hypertension, bibliographic databases, attention
Abstract
Background and objectives
With expansion of the pool of kidney grafts, through the use of higher-risk donors, and increased attention to donor management strategies, the 1-year graft survival rate is subject to change. It is, therefore, useful to elucidate 1-year graft survival rates by dissecting the characteristics of the low-risk and high-risk kidney transplant cases. The objective of our study was to evaluate factors purported to influence the risk of 1-year graft loss in kidney transplant recipients.
Design, setting, participants, & measurements
We searched bibliographic databases from 2000 to 2017 and included observational studies that measured the association between donor, recipient, the transplant operation, or early postoperative complications, and 1-year death-censored graft loss.
Results
We identified 35 eligible primary studies, with 20 risk factors amenable to meta-analysis. Six factors were associated with graft loss, with moderate to high degree of certainty: donor age (hazard ratio [HR], 1.11 per 10-year increase; 95% confidence interval [95% CI], 1.04 to 1.18), extended criteria donors (HR, 1.35; 95% CI, 1.28 to 1.42), deceased donors (HR, 1.54; 95% CI, 1.32 to 1.82), number of HLA mismatches (HR, 1.08 per one mismatch increase; 95% CI, 1.07 to 1.09), recipient age (HR, 1.17 per 10-year increase; 95% CI, 1.09 to 1.25), and delayed graft function (HR, 1.89; 95% CI, 1.46 to 2.47) as risk factors for 1-year graft loss. Pooled analyses also excluded, with a high degree of certainty, any associations of cold ischemia time, recipient race, pretransplant body mass index, diabetes, and hypertension with 1-year graft loss.
Conclusions
Recipient age, donor age, standard versus extended criteria donor, living versus deceased donor, HLA mismatch, and delayed graft function all predicted 1-year graft survival. The effect of each risk factor is small.
Introduction
In patients with ESKD receiving kidney replacement therapy transplant enormously improves quality of life and survival (1), However, because of the high demand and limited supply of available kidneys, many patients will undergo dialysis for up to 11 years or more before kidney transplant (2).
After transplantation, maximizing graft longevity becomes a focus of care. Graft loss results in return to dialysis, retransplantation, or death. Kidney transplant recipients have the highest rate of graft survival among all organs transplanted: 92% 1-year graft survival for kidneys transplanted from deceased donors (3). With expansion of the pool of kidney grafts, through the use of higher risk donors, and increased attention to donor management strategies, 1-year graft survival may change. It is therefore useful to identify low-risk and high-risk kidney transplant cases.
Prognostic studies can guide clinicians and patients in better understanding factors associated with a higher risk of graft loss in the first-year post-transplantation. Although formal risk prediction models can inform prognosis, existing models in kidney transplant perform poorly: the discriminatory performance of existing models ranges from 0.54 to 0.72, either below or marginally above the minimal threshold (0.6) for acceptable performance (4,5). The limited performance of current models may result from including risk factors useful in one cohort but not in others because of varied management protocols across centers and over time, varied or suboptimal adjustment for covariates, or risk of bias in the primary studies. A systematic review and meta-analysis of studies assessing these factors improves the precision of their associations and allow for exploration of potential sources of discrepancy between studies. Because a systematic review and meta-analysis could guide the development of a prediction model with useful discrimination and calibration (6), we undertook a review to assess the predictive power of key risk factors for kidney graft survival at 1 year post-transplant.
Materials and Methods
Data Sources and Searches
With the help of an information specialist we searched bibliographic databases in February of 2017 (Supplemental Appendix 1). Specifically, we searched MEDLINE, EMBASE, Cochrane central register for controlled trials, and Cochrane database for systematic reviews for citations between the years 2000–2017.
Study Selection and Data Extraction
Supplemental Appendix 2 provides details of the selection process and data extraction. Briefly, we selected observational studies of adult (≥18 years) kidney recipients receiving their first transplant, including studies evaluating the association between any risk factors and 1-year graft loss using multivariable analysis. We did not restrict by language or publication status. We included identified abstracts that met our inclusion criteria and provided enough information to contribute to our study. We also relied on the expertise of our clinical experts to inform us of any unpublished data not captured by our search strategy. From the final set of eligible studies, data abstractors recorded data from each study directly into a structured and pretested excel database.
Risk of Bias of Individual Studies
We assessed the risk of bias of individual studies using the Quality in Prognostic Studies instrument (7). When we judged five or more of the six Quality in Prognostic Studies domains to be at low risk of bias, we classified the overall risk of bias as low; otherwise, we considered at high risk of bias.
Data Synthesis and Statistical Analyses
We conducted meta-analysis for any risk factor evaluated in two or more studies. When a risk factor was addressed by only one study, we present the reported point estimate and 95% confidence interval [95% CI]. The included studies reported point estimates and 95% CIs as hazard ratios (HRs), odds ratios (ORs), or relative risks (RRs). Because of the low risk of graft loss within the first year after transplantation, we included ORs and RRs in the same meta-analysis without conversion (8,9). To combine studies that reported HRs with those reporting ORs or RRs, we conducted subgroup comparisons. When we observed a clinically or statistically significant difference between binary (e.g., OR or RR) and time-to-event measures (e.g., HR), we converted the OR or RR to HR using baseline risk estimates from the individual studies. When studies did not provide baseline risks, we utilized the average risk, prevalence of the risk factor, and the relative effect to estimate the baseline risk (10). The Supplemental Material includes further details of the data synthesis.
We addressed statistical heterogeneity through visual inspection of forest plots, looking for the consistency of point estimates and the extent of overlap in confidence intervals. Heterogeneity was not assessed with the I2 statistic, which is not useful in observational studies with a very large sample size (11).
This review addressed two possible subgroup analyses: risk of bias and outcome definition. The Supplemental Material presents our hypotheses for these two subgroup analyses.
When the subgroup analysis for risk of bias and outcome definition showed a significant difference across groups, we focused the analysis on studies at low risk of bias and/or those assessing death censored graft failure and applied the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) assessment only to these studies. We applied a two-sided P value of ≤0.05 to denote statistical significance. STATA’s metan function provided the platform for conducting all statistical analyses (12).
Certainty in the Body of Evidence
To assess the certainty of evidence across all studies related to a given risk factor, we used GRADE approach that rates the certainty of evidence as high, moderate, low, or very low considering issues of risk of bias, imprecision, inconsistency, indirectness, and publication bias (11). We assessed publication bias using visual inspection of funnel plots.
Results
Study Selection and Characteristics
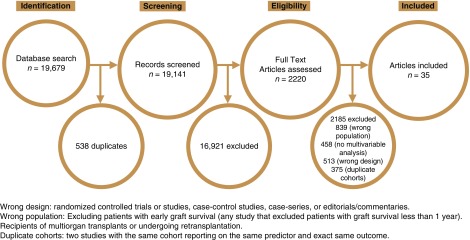
The literature search identified 19,679 unique citations, of which 2220 citations required full text review; 35 studies ultimately proved eligible (Figure 1) (13–47). Supplemental Table 1 provides a summary of study characteristics. The individual studies included patients from Canada, Denmark, Germany, Ireland, Italy, Japan, Norway, Portugal, Spain, South Korea, Taiwan, United Kingdom, and United States.
Figure 1.
Total of 35 studies included in systematic review and meta-analysis.
Risk of Bias of Individual Studies
Of the 35 eligible studies, reviewers judged 18 to have high risk of bias (Supplemental Table 2) (13,18,19,21–23,25,27,28,35–42,45), most commonly because of limitations in statistical analysis (over fitting of the regression models, building a multivariable model on the basis of level of significance in univariable analysis, and inclusion of collinear variables) and reporting (such as only reporting on the significant risk factors). Among the included studies, the authors included an average of 11 variables (SD of 6, minimum of three, and maximum of 23). Across the many risk factors included in this review, only the subgroup analyses for risk of bias in recipient diabetes and delayed graft function showed statistically significant different effect estimates in studies at high versus low risk of bias. For these, we only utilized estimates from low risk of bias studies.
Meta-Analyses of Donor Factors
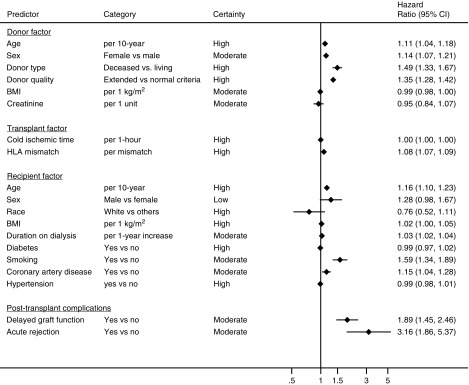
The review assessed six donor characteristics; five were independently associated with 1-year graft loss in the original studies and also proved predictive in the meta-analysis (Table 1): donor type (HR, 1.54 for deceased donors; 95% CI, 1.32 to 1.82; high certainty), donor quality (HR, 1.35 for extended criteria donors; 95% CI, 1.28 to 1.42; moderate certainty due to risk of bias), donor age (HR, 1.11 per 10-year increase; 95% CI, 1.04 to 1.18; high certainty), donor sex (HR, 1.10 for female sex; 95% CI, 1.07 to 1.21; moderate certainty due to serious inconsistency), and donor body mass index (BMI) (HR, 0.90 per 10 kg/m2 increase; 95% CI, 0.82 to 0.91; moderate certainty due to serious risk of bias). We observed that all studies defined extended criteria donors as >60 years of age or age 50–59 years with two of three associated risk factors: history of cerebrovascular accident, hypertension, or serum creatinine >1.5 mg/dl and delayed graft function as the need for dialysis within the first week post-transplant. We did not observe a statistically significant association between donor serum creatinine level and the risk of 1-year graft loss (Table 1). We did not detect publication bias for any of the donor factors.
Table 1.
Summary of findings table for all risk factors commonly identified among included studies
| Risk Factor | Study Results and Measurements | Absolute Effect Estimates | Certainty in Effect Estimates (Quality of Evidence) | Plain Text Summary | |
|---|---|---|---|---|---|
| Baseline | With Predictor | ||||
| Donor characteristics | |||||
| Age (10-yr increase) | Hazard ratio, 1.11 (95% CI, 1.04 to 1.18); on the basis of data from 178,043 patients in nine studies | 74 per 1000 | 82 per 1000 | High | Donor age increases graft failure |
| Difference: 8 more per 1000 (95% CI, 3 to 13 more) | |||||
| Sex (female versus male) | Hazard ratio, 1.10 (95% CI, 1.07 to 1.21); on the basis of data from 160,830 patients in seven studies | 70 per 1000 | 80 per 1000 | Moderate due to serious inconsistency | Female sex probably increases the risk for graft failure slightly |
| Difference: 10 more per 1000 (95% CI, 5 to 14 more) | |||||
| Type (deceased versus living) | Hazard ratio, 1.49 (95% CI, 1.33 to 1.67); on the basis of data from 149,433 patients in four studies | 60 per 1000 | 90 per 1000 | High | Deceased donors increase graft failure |
| Difference: 30 more per 1000 (95% CI, 21 to 38 more) | |||||
| Extended criteria donors (yes versus no) | Hazard ratio, 1.35 (95% CI, 1.28 to 1.42); on the basis of data from 145,879 patients in two studies | 72 per 1000 | 97 per 1000 | Moderate due to serious risk of bias | Extended criteria donor slightly increases graft failure |
| Difference: 25 more per 1000 (95% CI, 20 to 30 more) | |||||
| BMI (1 kg/m2 increase) | Hazard ratio, 0.99 (95% CI, 0.98 to 099); on the basis of data from 51,249 patients in two studies | 74 per 1000 | 73 per 1000 | Moderate due to serious risk of bias | Higher donor BMI probably decreases graft failure slightly |
| Difference: 1 more per 1000 (95% CI, 1 fewer to 1 more) | |||||
| Creatinine (1 U increase) | Hazard ratio, 0.95 (95% CI, 0.84 to 1.07); on the basis of data from 52,423 patients in two studies | 74 per 1000 | 70 per 1000 | Moderate due to serious indirectness | Donor creatinine probably has little or no difference on graft failure |
| Difference: 4 fewer per 1000 (95% CI, 11 fewer to 5 more) | |||||
| Transplant characteristics | |||||
| Cold ischemic time (1 h increase) | Hazard ratio, 1.00 (95% CI, 1.00 to 1.00); on the basis of data from 82,553 patients in five studies | 74 per 1000 | 74 per 1000 | High | Cold ischemic time has little or no difference on graft loss |
| Difference: 0 more per 1000 (95% CI, 0 fewer to 0 more) | |||||
| HLA mismatch (one mismatch increase) | Hazard ratio, 1.08 (95% CI, 1.07 to 1.09); on the basis of data from 171,446 patients in four studies | 74 per 1000 | 80 per 1000 | High | Increasing HLA mismatch slightly increases graft failure |
| Difference: 6 more per 1000 (95% CI, 5 to 6 more) | |||||
| Recipient characteristics | |||||
| Age (10-yr increase) | Hazard ratio, 1.16 (95% CI, 1.10 to 1.23); on the basis of data from 138,824 patients in 12 studies | 74 per 1000 | 85 per 1000 | High | Increasing recipient age slightly increases 1-yr graft loss |
| Difference: 11 more per 1000 (95% CI, 7 more to 16 more) | |||||
| Sex (male versus female) | Hazard ratio, 1.28 (95% CI, 0.98 to 1.67); on the basis of data from 176,972 patients in nine studies | 63 per 1000 | 81 per 1000 | Low due to serious inconsistency and serious publication bias | Recipient sex may have little or no difference on graft loss |
| Difference: 18 more per 1000 (95% CI, 2 fewer to 35 more) | |||||
| Race (white versus others) | Hazard ratio, 0.76 (95% CI, 0.52 to 1.11); on the basis of data from 169,596 patients in two studies | 87 per 1000 | 66 per 1000 | High | Recipient race has little or no difference on graft failure |
| Difference: 21 fewer per 1000 (95% CI, 51 fewer to 8 more) | |||||
| BMI (1 kg/m2 increase) | Hazard ratio, 1.02 (95% CI, 0.99 to 1.04); on the basis of data from 51,881 patients in four studies | 74 per 1000 | 75 per 1000 | High | Recipient BMI has little or no effect on graft failure |
| Difference: 1 more per 1000 (95% CI, 1 fewer to 3 more) | |||||
| Dialysis time (per 1-yr increase) | Hazard ratio, 1.03 (95% CI, 1.02 to 1.03); on the basis of data from 51,776 patients in three studies | 74 per 1000 | 76 per 1000 | Moderate due to serious risk of bias | Years on dialysis probably increases graft failure slightly |
| Difference: 2 more per 1000 (95% CI, 1 fewer to 2 more) | |||||
| Diabetes (yes versus no) | Hazard ratio, 0.99 (95% CI, 0.97 to 1.02); on the basis of data from 169,015 patients in two studies | 74 per 1000 | 73 per 1000 | High | Recipient diabetes has little or no difference on graft failure |
| Difference: 1 more per 1000 (95% CI, 2 fewer to 1 more) | |||||
| Smoking (ever versus never) | Hazard ratio, 1.59 (95% CI, 1.34 to 1.90); on the basis of data from 3156 patients in two studies | 65 per 1000 | 104 per 1000 | Moderate due to serious imprecision | Pretransplant recipient smoking probably increases graft failure slightly |
| Difference: 39 more per 1000 (95% CI, 23 to 55 more) | |||||
| Coronary artery disease (yes versus no) | Hazard ratio, 1.15 (95% CI, 1.03 to 1.27); on the basis of data from 81,194 patients in two studies | 73 per 1000 | 85 per 1000 | Moderate due to serious indirectness | Recipient coronary artery disease probably increases graft failure slightly |
| Difference: 12 more per 1000 (95% CI, 2 to 20 more) | |||||
| Hypertension (yes versus no) | Hazard ratio, 0.99 (95% CI, 0.98 to 1.01); on the basis of data from 169,314 patients in three studies | 74 per 1000 | 74 per 1000 | High | Pretransplant recipient hypertension has little or no difference on graft failure |
| Difference: 1 fewer per 1000 (95% CI, 2 fewer to 0) | |||||
| Post-transplant complications | |||||
| Delayed graft function (yes versus no) | Hazard ratio, 1.89 (95% CI, 1.46 to 2.47); on the basis of data from 2564 patients in four studies | 63 per 1000 | 119 per 1000 | Moderate due to serious inconsistency | Delayed graft function probably increases graft failure |
| Difference: 56 more per 1000 (95% CI, 31 to 84 more) | |||||
| Acute rejection (yes versus no) | Hazard ratio, 3.16 (95% CI, 1.86 to 5.38); on the basis of data from 48,768 patients in seven studies | 64 per 1000 | 203 per 1000 | Moderate due to serious inconsistency | Acute rejection probably increases graft failure |
| Difference: 139 more per 1000 (95% CI, 60 to 247 more) | |||||
95% CI, 95% confidence interval; BMI, body mass index.
Meta-Analyses of Transplant Process Factors
We assessed two risk factor variables characteristic of the transplant process (Figure 2, Table 1). The number of HLA mismatches was the only risk factor, for which we observed an association beyond chance, with 1-year death-censored graft loss (HR, 1.08 per one mismatch increase; 95% CI, 1.07 to 1.09; high certainty). We observed no significant association between cold ischemia time (HR, 1.00 per 1-hour increase; 95% CI, 0.99 to 1.00) and graft loss, despite five studies evaluating this variable, adjusted for recipient age, donor age (18,30,35,40,42), donor sex (40), donor cause of death (30), donor type (18,35,42), HLA mismatch (18,30,40,42), recipient sex (18,35,40), recipient BMI (30), recipient diabetes (18,35,42), pretransplant time on dialysis (18,30,35,42), history of cardiovascular comorbidities (18,35), delayed graft function (18,30), and early acute rejection (18,40,42). We did not detect publication bias for any of the transplant process factors.
Figure 2.
Eleven of 19 risk factors significantly associated with 1-year graft loss.
Meta-Analyses of Recipient Factors
We identified nine transplant recipient variables that had been investigated in two or more of the primary studies in this review (Figure 2, Table 1). Four of the nine were significantly associated with 1-year death-censored graft loss: recipient age (HR, 1.17 per 10-year increase; 95% CI, 1.09 to 1.25; high certainty), pretransplant smoking (HR, 1.59; 95% CI, 1.34 to 1.90; moderate certainty due to serious imprecision), pretransplant recipient coronary artery disease (HR, 1.15; 95% CI, 1.03 to 1.27; moderate certainty due to serious indirectness), and number of pretransplant years on dialysis (HR, 1.03 per 1-year increase; 95% CI, 1.02 to 1.03; moderate certainty due to serious risk of bias). We did not observe a statistically significant association for recipient sex, race, BMI, hypertension, or diabetes with 1-year graft loss (Figure 2, Table 1). We did not detect publication bias for any of the recipient factors.
Meta-Analyses of Post-Transplant Complications
The literature included within this review commonly identified delayed graft function and acute rejection as early post-transplant complications associated with death-censored graft loss (Figure 2, Table 1). For both, we observed a statistically significant association with 1-year graft loss: delayed graft function (HR, 1.89; 95% CI, 1.46 to 2.47; moderate certainty due to serious inconsistency) and acute rejection (HR, 3.16; 95% CI, 1.86 to 5.38; moderate certainty due to serious inconsistency). We did not detect publication bias for any of the post-transplant complication factors.
Risk Factors Addressed in a Single Study
This review identified an additional 72 candidate risk factors, each evaluated in only one study. We summarized the full list of risk factors in Supplemental Figure 1.
Discussion
Principal Findings
This review identified five risk factors, for which there is moderate to high certainty in the magnitude of association with 1-year graft loss: donor age, extended criteria donors, deceased donors, increasing number of HLA mismatches, and recipient age. We identified an additional five variables for which, with moderate certainty, there is an association with 1-year graft loss: donor sex, donor BMI, recipient’s number of years on dialysis, history of smoking, and coronary artery disease. With high certainty, the findings of this study exclude any association of the following variables with 1-year graft loss: increasing cold ischemia time, recipient age, recipient BMI, recipient diabetes, and recipient hypertension.
Strengths and Limitations
This study is the first large-scale, systematic review of studies that have conducted adjusted analyses addressing risk factors for 1-year graft loss after kidney transplantation. By only reviewing adjusted evidence, users of our estimates can multiply the HR of multiple risk factors to obtain their combined effect on the risk of 1-year graft loss. Using rigorous meta-analytic methods, the review provides precise measures, compared with any individual study, for the association of each risk factor and graft loss, informed by observational cohort studies. The use of GRADE methodology enabled us to not only report on the direction and magnitude of the association for each risk factor, but also to transparently report on the certainty of the evidence.
One limitation of this review is that we included studies identifying risk factors using Cox regression analysis for graft loss at all time points in follow-up. By doing so, we assumed that the authors of the primary studies had tested and ensured the proportional hazards assumption necessary for validity of any reported HR. The authors of the individual studies seldom reported on assessing the necessary assumptions of their regression models. As a result, our statistical analysis for the risk of bias assessment could not be fully informed by meeting the regression model assumptions.
We included United Network for Organ Sharing (UNOS) registry studies to represent all studies published from individual centers in the United States. By doing so, the quality of this review is dependent upon the quality of the UNOS registry data. Authors of single- or multicentered observational studies may have more direct control over their data collection and entry compared with large registries (48), and thus more likely to ensure data quality before analysis of risk factors. We utilized evidence from the UNOS registry as this is the source that is highly referred to by the transplant community.
Studies varied considerably in the covariates included in their predictive models (studies included 11±6 covariates in their regression models). Thus, results are vulnerable to the possibility that the effect of a particular risk factor might differ depending on which variables were included in a particular model.
In the context of identifying factors that increase the risk of graft loss in the year after transplant, the studies in this review have a fundamental limitation: potential candidates for transplant may be rejected because of patient factors that were not included. The reasons for not recommending transplant in such individuals may be the most powerful determinants of outcome. These may include, but not be limited to active infections and combination of older age with constellation of other comorbidities such as obesity, cardiovascular disease, malignancies, and irreversible obstructive or restrictive pulmonary disease (49).
Some may be surprised that cold ischemia time was not associated with 1-year graft loss. The simplest explanation for this finding is that, indeed, there is no association. Another plausible explanation is that present day use of storage techniques such as machine perfusion and preservation solutions minimize cold ischemia damage to the kidney (50,51). Additionally, studies treated cold ischemia time as a continuous variable and assumed a linear relationship between ischemia time and survival. It is possible that the relationship is non-linear. For instance, up to a certain duration, there may be no relation between ischemia time and outcome, but beyond that duration graft longevity diminshesS1, S3, S8 (13,15,20,52). Among the risk factors addressed by individual studies, we identified two that treated ischemia time as a binary variable. One studied used the threshold of 20 hours and observed an HR of 1.92 (95% CI, 1.26 to 2.91)S1. The other used a threshold of 24 hours and observed an HR of 1.27 (95% CI, 1.09 to 1.48)S3. Both of these studies suggest that ischemia time is associated with 1-year graft loss only after a long passage of time. Therefore, assuming a linear association might have put primary studies at high risk of missing such a nonlinear relationship.
Relation to Other Work
Kabore et al. (4) conducted a systematic review and meta-analysis to identify all risk prediction models for graft loss postkidney transplantation. Of the 34 identified models, only seven (53–59) specifically predict graft loss at 1-year post-transplantation. The median discrimination value, as measured by area under the curve statistics, is 0.63 (range, 0.54–0.72). One potential reason for poor discrimination is that these models use risk factors that we identified not to be associated with 1-year graft loss beyond chance. For example, Tang et al. (58) included recipient sex, race, height, weight, diabetes, history of hypertension, and cold ischemic time within their model to predict graft loss at 1-year post-transplantation. Our review excluded an association beyond chance for each of these risk factors and 1-year graft loss. This is one plausible explanation for Tang et al. observing an area under the curve of 0.54.
Contrary to their inclusion within the aforementioned risk prediction models, our review excluded an association beyond chance for factors including recipient sex (present in four of the seven aforementioned risk prediction models), race (five of seven models), BMI (four of seven models), diabetes (four of seven models), hypertension (four of seven models), donor creatinine (two of seven models), and cold ischemia time (five of seven models). Previous studies reported that female recipients have better long-term prognosis compared with men. Such better prognosis has been hypothesized to be due to hormonal protection (60). Such biologic explanations may require longer duration of follow-up (beyond 1 year) to express their effect. This review’s short follow-up time of 1 year may be the reason for lack of association beyond chance for recipient sex and graft loss. This review’s finding of a lack of association between race and graft loss can be explained by the diminishing racial disparity in kidney transplantation. Recent analysis of the Scientific Registry of Transplant Recipients registry in the United States suggests significant improvement in graft survival from 1990 to 2012, with the success rate improving in black recipients to a greater extent than improvement observed in white recipients (61).
Of all the factors not associated with graft loss, we were most surprised to find no evidence of an association between donor creatinine and 1-year graft loss. Both of the studies in this review that evaluated the prognostic importance of donor creatinine utilized nondeath-censored graft loss as their outcome. The inclusion of patient mortality may explain the lack of association between donor creatinine and mortality (it is possible that worse functioning kidneys would not be associated with patient mortality because of the availability of kidney replacement therapies in the event of graft failure). Another explanation may be that donors with high creatinine were not selected for transplantation, thus eliminating any association beyond chance. The association between donor creatinine and 1-year graft loss is partially captured by the significant association between extended criteria donor as a risk factor for 1-year graft loss. Additionally, among the risk factors addressed in only one study, we identified donor eGFR >60 ml/min per kilogram to be associated with a decrease in risk of graft loss at 1-year postkidney transplantation (Supplemental Figure 1).
Implication for Guidelines
From this review, however, it is evident that numerous recipient and donor characteristics increase the risk of graft loss postkidney transplantation. All such factors, although may be associated with graft loss beyond chance, may not be clinically important to diminish the magnitude of benefit attained from transplantation. This necessitates the need for risk prediction models to guide clinicians in selection of candidates whose risk for graft loss (disadvantaging the societal need for organ donors) may be higher than their risk of mortality on dialysis. Risk associations generated from this review may inspire or provide the foundational information necessary for development of a risk prediction model.
In conclusion, our systematic review and meta-analysis identified ten risk factors for which we have moderate or high certainty in their strength and magnitude of association. These factors include recipient age, donor age, extended criteria donors, deceased donors, and increasing number of HLA mismatches. With high certainty, we were able to establish that increasing cold ischemia time, recipient BMI, recipient diabetes, and recipient hypertension do not have large associations with 1-year graft survival. The optimal utilization of the factors we have identified as risk factors, in development of future risk prediction models, may improve discrimination and calibration. Such models in turn may guide the judgment clinicians need to make on the highest risk recipient and donor.
Data Sharing
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosures
Dr. Ribic reports grants and other fees from Astellas Pharma, Leo Pharma, and Pfizer outside the submitted work. All other authors have nothing to disclose.
Supplementary Material
Acknowledgments
Mr. Foroutan, Dr. Guyatt, and Dr. Meade conceived the study idea. Mr. Foroutan, Mr. Friesen, Ms. Clark, Ms. Motaghi, Dr. Zyla, Dr. Lee, Mr. Kamran, and Mr. Ali conducted the screening. Mr. Foroutan, Mr. Friesen, Ms. Clark, and Ms. Motaghi conducted the data extraction. Mr. Foroutan and Mr Friesen conducted the risk of bias assessment. Mr. Foroutan and Ms. Clark conducted the analyses and GRADE assessment. Mr Foroutan, Dr. Guyatt, Dr. Meade, Dr. Ribic, and Dr. Treleaven drafted and revised the manuscript. Ms. Orchanian-Cheff developed and conducted the systematic search of relevant databases.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05560519/-/DCSupplemental.
Supplemental Table 1. Characteristics and demographics of included studies.
Supplemental Table 2. Risk of bias of included studies.
Supplemental Figure 1. List of predictors identified by one individual study.
Supplemental Appendix 1. Systematic search strategy.
Supplemental Appendix 2. Details of the methods.
References
- 1.Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J: Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 11: 2093–2109, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2013 annual data report: Kidney. Am J Transplant 15[Suppl 2]: 1–34, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A, Wainright JL, Haynes CR, Snyder JJ, Kasiske BL, Israni AK: OPTN/SRTR 2016 annual data report: Kidney. Am J Transplant 18[Suppl 1]: 18–113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabore R, Haller MC, Harambat J, Heinze G, Leffondré K: Risk prediction models for graft failure in kidney transplantation: A systematic review. Nephrol Dial Transplant 32[Suppl 2]: ii68–ii76, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, McGinn T, Guyatt G: Discrimination and calibration of clinical prediction models: Users’ guides to the medical literature. JAMA 318: 1377–1384, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Alba AC, Walter SD, Guyatt GH, Levy WC, Fang J, Ross HJ, Lee DS: Predicting survival in patients with heart failure with an implantable cardioverter defibrillator: The heart failure meta-score. J Card Fail 24: 735.–, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C: Assessing bias in studies of prognostic factors. Ann Intern Med 158: 280–286, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Grant RL: Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 348: f7450, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR: Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8: 16, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kooter AJ, Kostense PJ, Groenewold J, Thijs A, Sattar N, Smulders YM: Integrating information from novel risk factors with calculated risks: The critical impact of risk factor prevalence. Circulation 124: 741–745, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schunemann H, Guyatt G: Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ 350: h870, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JAC: Metan: Fixed- and random-effects meta-analysis. Stata J 8: 3–28, 2008 [Google Scholar]
- 13.Asderakis A, Dyer P, Augustine T, Worthington J, Campbell B, Johnson RW: Effect of cold ischemic time and HLA matching in kidneys coming from “young” and “old” donors: Do not leave for tomorrow what you can do tonight. Transplantation 72: 674–678, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Lin HH, Huang CC, Huang JY, Yang CW, Wu MS, Fang JT, Yu CC, Chiang YJ, Chu SH: Impact of HCV infection on first cadaveric renal transplantation, a single center experience. Clin Transplant 18: 261–266, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Andresdottir MB, Haasnoot GW, Doxiadis II, Persijn GG, Claas FH: Exclusive characteristics of graft survival and risk factors in recipients with immunoglobulin A nephropathy: A retrospective analysis of registry data. Transplantation 80: 1012–1018, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Cardinal H, Hébert MJ, Rahme E, Houde I, Baran D, Masse M, Boucher A, Le Lorier J; Elderly Recipients Transplant Group : Modifiable factors predicting patient survival in elderly kidney transplant recipients. Kidney Int 68: 345–351, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Courtney AE, McNamee PT, Middleton D, Heggarty S, Patterson CC, Maxwell AP: Association of functional heme oxygenase-1 gene promoter polymorphism with renal transplantation outcomes. Am J Transplant 7: 908–913, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Krüger B, Böger CA, Schröppel B, Obed A, Hoffmann U, Murphy BT, Fischereder M, Holler E, Banas B, Krämer BK: Impact of NOD2/CARD15 haplotypes on the outcome after kidney transplantation. Transpl Int 20: 600–607, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Díaz JM, Gich I, Bonfill X, Solà R, Guirado L, Facundo C, Sainz Z, Puig T, Silva I, Ballarín J: Prevalence evolution and impact of cardiovascular risk factors on allograft and renal transplant patient survival. Transplant Proc 41: 2151–2155, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Ferrer F, Mota A, Alves R, Bastos C, Macário F, Figueiredo A, Santos L, Roseiro A, Parada B, Pratas J, Nunes P, Campos M: Renal transplantation with expanded criteria donors: The experience of one Portuguese center. Transplant Proc 41: 791–793, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Heldal K, Hartmann A, Leivestad T, Svendsen MV, Foss A, Lien B, Midtvedt K: Clinical outcomes in elderly kidney transplant recipients are related to acute rejection episodes rather than pretransplant comorbidity. Transplantation 87: 1045–1051, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Kayler LK, Garzon P, Magliocca J, Fujita S, Kim RD, Hemming AW, Howard R, Schold JD: Outcomes and utilization of kidneys from deceased donors with acute kidney injury. Am J Transplant 9: 367–373, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Lynch RJ, Ranney DN, Shijie C, Lee DS, Samala N, Englesbe MJ: Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg 250: 1014–1020, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Fuggle SV, Allen JE, Johnson RJ, Collett D, Mason PD, Dudley C, Rudge CJ, Bradley JA, Watson CJ; Kidney Advisory Group of NHS Blood and Transplant : Factors affecting graft and patient survival after live donor kidney transplantation in the UK. Transplantation 89: 694–701, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Moore J, McKnight AJ, Simmonds MJ, Courtney AE, Hanvesakul R, Brand OJ, Briggs D, Ball S, Cockwell P, Patterson CC, Maxwell AP, Gough SC, Borrows R: Association of caveolin-1 gene polymorphism with kidney transplant fibrosis and allograft failure. JAMA 303: 1282–1287, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Papalia T, Greco R, Lofaro D, Maestripieri S, Mancuso D, Bonofiglio R: Impact of body mass index on graft loss in normal and overweight patients: Retrospective analysis of 206 renal transplants. Clin Transplant 24: E241–E246, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Heldal K, Hartmann A, Leivestad T, Foss A, Midtvedt K: Risk variables associated with the outcome of kidney recipients >70 years of age in the new millennium. Nephrol Dial Transplant 26: 2706–2711, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Molnar MZ, Kovesdy CP, Bunnapradist S, Streja E, Mehrotra R, Krishnan M, Nissenson AR, Kalantar-Zadeh K: Associations of pretransplant serum albumin with post-transplant outcomes in kidney transplant recipients. Am J Transplant 11: 1006–1015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrier M, Lizé JF; Québec-Transplant Programs : Impact of expanded criteria donors on outcomes of recipients after kidney transplantation. Transplant Proc 44: 2227–2230, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Grosso G, Corona D, Mistretta A, Zerbo D, Sinagra N, Giaquinta A, Caglià P, Amodeo C, Leonardi A, Gula R, Veroux P, Veroux M: The role of obesity in kidney transplantation outcome. Transplant Proc 44: 1864–1868, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Molnar MZ, Streja E, Kovesdy CP, Shah A, Huang E, Bunnapradist S, Krishnan M, Kopple JD, Kalantar-Zadeh K: Age and the associations of living donor and expanded criteria donor kidneys with kidney transplant outcomes. Am J Kidney Dis 59: 841–848, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanmoku K, Matsuda Y, Yamamoto T, Tsujita M, Hiramitsu T, Goto N, Katayama A, Watarai Y, Kobayashi T, Uchida K: Clinical characteristics and outcomes of renal transplantation in elderly recipients. Transplant Proc 44: 281–283, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Andreoni KA, Forbes R, Andreoni RM, Phillips G, Stewart H, Ferris M: Age-related kidney transplant outcomes: Health disparities amplified in adolescence. JAMA Intern Med 173: 1524–1532, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Bay JT, Schejbel L, Madsen HO, Sørensen SS, Hansen JM, Garred P: Low C4 gene copy numbers are associated with superior graft survival in patients transplanted with a deceased donor kidney. Kidney Int 84: 562–569, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Brar A, Jindal RM, Elster EA, Tedla F, John D, Sumrani N, Salifu MO: Effect of peripheral vascular disease on kidney allograft outcomes: A study of U.S. Renal data system. Transplantation 95: 810–815, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Faravardeh A, Eickhoff M, Jackson S, Spong R, Kukla A, Issa N, Matas AJ, Ibrahim HN: Predictors of graft failure and death in elderly kidney transplant recipients. Transplantation 96: 1089–1096, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Nee R, Jindal RM, Little D, Ramsey-Goldman R, Agodoa L, Hurst FP, Abbott KC: Racial differences and income disparities are associated with poor outcomes in kidney transplant recipients with lupus nephritis. Transplantation 95: 1471–1478, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Lee SH, Oh CK, Shin GT, Kim H, Kim SJ, Kim SI: Age matching improves graft survival after living donor kidney transplantation. Transplant Proc 46: 449–453, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Anderson JE, Steiner RW, Mekeel KL, Chang DC, Hemming AW, Halldorson JB: ECD kidney transplantation outcomes are improved when matching donors to recipients using a novel creatinine clearance match ratio (CCMR). Clin Transplant 29: 738–746, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Dinis P, Nunes P, Marconi L, Furriel F, Parada B, Moreira P, Figueiredo A, Bastos C, Roseiro A, Dias V, Rolo F, Alves R, Mota A: Small kidneys for large recipients: Does size matter in renal transplantation? Transplant Proc 47: 920–925, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Ilori TO, Adedinsewo DA, Odewole O, Enofe N, Ojo AO, McClellan W, Patzer RE: Racial and ethnic disparities in graft and recipient survival in elderly kidney transplant recipients. J Am Geriatr Soc 63: 2485–2493, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koo EH, Jang HR, Lee JE, Park JB, Kim SJ, Kim DJ, Kim YG, Oh HY, Huh W: The impact of early and late acute rejection on graft survival in renal transplantation. Kidney Res Clin Pract 34: 160–164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adekoya AO, Halawa A: Kidneys from deceased elderly donors: Factors associated with adverse outcomes. Exp Clin Transplant 14: 32–37, 2016 [PubMed] [Google Scholar]
- 44.An JN, Ahn SV, Lee JP, Bae E, Kang E, Kim HL, Kim YJ, Oh YK, Kim YS, Kim YH, Lim CS: Pre-transplant cardiovascular risk factors affect kidney allograft survival: A multi-center study in Korea. PLoS One 11: e0160607, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huaman MA, Vilchez V, Mei X, Davenport D, Gedaly R: Donor positive blood culture is associated with delayed graft function in kidney transplant recipients: A propensity score analysis of the UNOS database. Clin Transplant 30: 415–420, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Redfield RR, Scalea JR, Zens TJ, Mandelbrot DA, Leverson G, Kaufman DB, Djamali A: The mode of sensitization and its influence on allograft outcomes in highly sensitized kidney transplant recipients. Nephrol Dial Transplant 31: 1746–1753, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Boffa C, van de Leemkolk F, Curnow E, Homan van der Heide J, Gilbert J, Sharples E, Ploeg RJ: Transplantation of kidneys from donors with acute kidney injury: Friend or foe? Am J Transplant 17: 411–419, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Ferreira-González I, Marsal JR, Mitjavila F, Parada A, Ribera A, Cascant P, Soriano N, Sánchez PL, Arós F, Heras M, Bueno H, Marrugat J, Cuñat J, Civeira E, Permanyer-Miralda G: Patient registries of acute coronary syndrome: Assessing or biasing the clinical real world data? Circ Cardiovasc Qual Outcomes 2: 540–547, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Chadban SJ, Knoll GA, Ahn C, Axelrod DA, Foster BJ, Kasiske BL, Kher V, Kumar D, Oberbauer R, Pascual J, Pilmore HL, Rodrigue JR, Segev DL, Sheerin NS, Tinckam KJ, Wong G: KDIGO Clinical Practice Guideline on the Evaluation and the Management of Candidates for Kidney Transplantation, 2018. Available at: https://kdigo.org/wp-content/uploads/2018/08/KDIGO-Txp-Candidate-GL-Public-Review-Draft-Oct-22.pdf. Accessed April 30, 2019 [DOI] [PubMed]
- 50.Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, van Kasterop-Kutz M, van der Heide JJ, Squifflet JP, van Heurn E, Kirste GR, Rahmel A, Leuvenink HG, Paul A, Pirenne J, Ploeg RJ: Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 360: 7–19, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Deng R, Gu G, Wang D, Tai Q, Wu L, Ju W, Zhu X, Guo Z, He X: Machine perfusion versus cold storage of kidneys derived from donation after cardiac death: A meta-analysis. PLoS One 8: e56368, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Postalcioglu M, Kaze AD, Byun BC, Siedlecki A, Tullius SG, Milford EL, Paik JM, Abdi R: Association of cold ischemia time with acute renal transplant rejection. Transplantation 102: 1188–1194, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bang K, Lee HK, Huh W, Lee YJ, Woon BS, Ro H, Hwang YH, Ha J, Park MH, Kim SJ, Park SK, Oh HY, Yang J, Ahn C: Assessment of deceased donor kidneys using a donor scoring system. Yonsei Med J 51: 870–876, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown TS, Elster EA, Stevens K, Graybill JC, Gillern S, Phinney S, Salifu MO, Jindal RM: Bayesian modeling of pretransplant variables accurately predicts kidney graft survival. Am J Nephrol 36: 561–569, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Krikov S, Khan A, Baird BC, Barenbaum LL, Leviatov A, Koford JK, Goldfarb-Rumyantzev AS: Predicting kidney transplant survival using tree-based modeling. ASAIO J 53: 592–600, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Lin RS, Horn SD, Hurdle JF, Goldfarb-Rumyantzev AS: Single and multiple time-point prediction models in kidney transplant outcomes. J Biomed Inform 41: 944–952, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Schold JD, Kaplan B, Baliga RS, Meier-Kriesche HU: The broad spectrum of quality in deceased donor kidneys. Am J Transplant 5: 757–765, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Tang H, Hurdle JF, Poynton M, Hunter C, Tu M, Baird BC, Krikov S, Goldfarb-Rumyantzev AS: Validating prediction models of kidney transplant outcome using single center data. ASAIO J 57: 206–212, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Kasiske BL, Israni AK, Snyder JJ, Skeans MA, Peng Y, Weinhandl ED: A simple tool to predict outcomes after kidney transplant. Am J Kidney Dis 56: 947–960, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Puoti F, Ricci A, Nanni-Costa A, Ricciardi W, Malorni W, Ortona E: Organ transplantation and gender differences: A paradigmatic example of intertwining between biological and sociocultural determinants. Biol Sex Differ 7: 35, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Purnell TS, Luo X, Kucirka LM, Cooper LA, Crews DC, Massie AB, Boulware LE, Segev DL: Reduced racial disparity in kidney transplant outcomes in the United States from 1990 to 2012. J Am Soc Nephrol 27: 2511–2518, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.