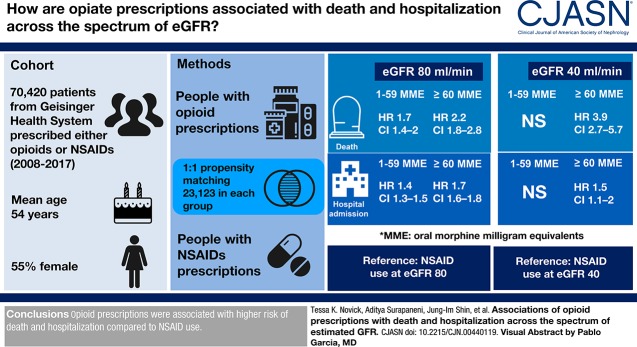
Visual Abstract
Keywords: chronic kidney disease, adult, humans, female, opioid analgesics, non-steroidal anti-inflammatory agents, risk, Pennsylvania, MME, goals, pain, cohort studies, morphine, prescriptions, hospitalization, primary health care
Abstract
Background and objectives
Most opioids undergo kidney excretion. The goal of this study was to evaluate opioid-associated risks of death and hospitalization across the range of eGFR.
Design, setting, participants, & measurements
The study population included adult primary care patients in Geisinger Health (Danville, PA) between 2008 and 2017. People receiving their first opioid prescription were propensity matched to people receiving NSAIDS (and, in sensitivity analysis, gabapentinoids) and the risk of death and hospitalization were compared, classifying opioid medication exposure as time-varying daily oral morphine milligram equivalents (MMEs) across time-varying eGFR.
Results
The propensity-matched cohort included 46,246 patients prescribed either opioids or NSAIDs between 2008 and 2017 (mean [SD] age, 54 [16] years; 56% female; 3% of black race). Prescriptions for 1–59 and ≥60 MMEs were associated with higher risk of death (HR, 1.70; 95% CI, 1.41 to 2.05 for 1–59 MMEs; HR, 2.25; 95% CI, 1.82 to 2.79 for ≥60 MMEs) and hospitalization (HR, 1.38; 95% CI, 1.30 to 1.46 for 1–59 MMEs; HR, 1.68; 95% CI, 1.56 to 1.81 for ≥60 MMEs) compared with NSAID prescriptions, when evaluated at eGFR 80 ml/min per 1.73 m2. The relative risk of death associated with ≥60 MMEs was higher at lower GFR (e.g., eGFR, 40 ml/min per 1.73 m2; HR, 3.94; 95% CI, 2.70 to 5.75; P for interaction, 0.01). When gabapentinoids were used as the comparison medication, only ≥60 MMEs were significantly associated with higher risk of death (HR, 2.72; 95% CI, 1.71 to 4.34), although both 1–59 and ≥60 MMEs were associated with risk of hospitalization (HR, 1.22; 95% CI, 1.04 to 1.43 for 1–59 MMEs; HR, 1.54; 95% CI, 1.28 to 1.86 for ≥60 MMEs).
Conclusions
The receipt of prescription opioids was associated with a higher risk of death and hospitalization compared with other pain medications, particularly with higher doses and at lower eGFR.
Introduction
Use of opioid analgesics in the United States has reached historic levels, with a fourfold increase in opioid prescribing between 1999 and 2010 (1–3). More than 2.1 million Americans are estimated to have an opioid use disorder (4), and 12% of adults with prescription opioids report misuse, defined as use without a prescription or a pattern of use other than what was directed by a physician (2). There has also been a corresponding surge in overdose deaths. In 2017, more than 49,000 Americans died from opioid overdose, the highest year on record, and over 19,000 of these deaths involved prescription opioids (5). In the general population, prescription opioid use has been associated with increased risk of death and hospitalizations (6,7).
An estimated 58% of individuals with CKD experience chronic pain, a prevalence two to three times that of the general population (8–13). Neuropathy, peripheral vascular disease, and pain syndromes unique to kidney disease such as osteodystrophy and calciphylaxis are common (9,12). However, kidney disease limits therapeutic options for pain control, in large part due to the relative contraindication to nonsteroidal anti-inflammatory drugs (NSAIDs) (14). Opioids are an alternative, and over 40% of individuals with advanced CKD have received opioid prescriptions (13). The vast majority of opioids undergo some degree of kidney excretion (15). Concentrations of both parent opioids and their metabolites can be altered in CKD, which can necessitate dose adjustment, change drug efficacy, and possibly increase risk for toxicity (15–17). Among people on dialysis, past studies have shown higher risk of death, dialysis discontinuation, hospitalizations, altered mental status, falls, and fractures with opioid prescriptions (18,19). However, little is known about the risks associated with opioid use in patients with CKD not on dialysis (8,20).
The objective of this study was to quantify the association between prescription opioids and death and hospitalization across patients with varying levels of eGFR in a large, integrated healthcare delivery system. We hypothesized that higher prescribed oral morphine milligram equivalents (MMEs) would be associated with increased risk of adverse outcomes.
Materials and Methods
Study Population
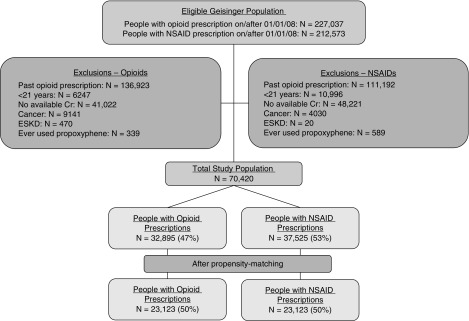
We included patients receiving primary care from Geisinger Health. Geisinger serves 44 counties in central and northeastern Pennsylvania (21). All inpatient and outpatient visits are compiled in an electronic record that includes laboratory data, prescription records, billing codes, and vital signs. The out-migration rate is approximately 1% per year (22). We included individuals who had new opioid or NSAID prescriptions on or after January 1, 2008. We excluded persons with opioid prescriptions before January 1, 2008, aged <21 years, no outpatient serum creatinine measurements within 1 year before opioid prescription, history of malignancy, ESKD, initial prescription for an opioid used to treat addiction (buprenorphine, methadone, levacetylmethadol, lofexidine, levomethadone, and diamorphine), and any prescription for propoxyphene, resulting in a study population of 70,420 individuals (Figure 1). Propoxyphene was excluded due to safety concerns and subsequent removal from the US market in 2010 (23).
Figure 1.
Study population. Cr, creatinine; NSAID, nonsteroidal anti-inflammatory drug.
This study was reviewed and approved by the Johns Hopkins University Bloomberg School of Public Health and Geisinger Medical Center Institutional Review Board.
Measurement of Opioid Prescription
Opioid prescription was assessed from the electronic medical record. For time-varying analyses, periods of medication exposure began the day of prescription, through the medication discontinuation date, plus a lag period of 15 days. For each prescription, we converted the daily dose into standardized MMEs to account for differences in potency among opioid agents (24). Every time an opioid prescription was added, discontinued, or changed, the MME exposure was recalculated. If more than one opioid was prescribed, the MMEs for each opioid were added together. Opioid exposure was then categorized as 1–59 or ≥60 MMEs to be consistent with past studies among persons with kidney disease (19). Opioid agents included: morphine, hydromorphone, codeine, dihydrocodeine, oxymorphone, hydrocodone, nalbuphine, meperidine, oxycodone, fentanyl, tramadol, tapentadol, and pentazocine. Prescriptions for opioid/NSAID combination agents were not included. Prescriptions for buprenorphine and methadone were included only during follow-up.
Measurement of Comparator Prescription
Prescriptions and reported use of NSAIDs and gabapentinoids (gabapentin, pregabalin) were abstracted from the medical record. Periods of medication exposure were defined in a similar manner to those of opioids with a lag period of 15 days.
Measurement of eGFR
Baseline serum creatinine was determined using the most proximal antecedent outpatient value to prescription start date and converted to eGFR using the CKD Epidemiology Collaboration equation (25). All creatinine values were measured using isotope-dilution mass spectrometry traceable assays according to manufacturer specifications (21). In the time-varying analyses, eGFR was updated every time an outpatient serum creatinine was measured.
Measurement of Other Covariates
All demographic and clinical covariates were ascertained using electronic medical record data. Demographic variables included age, sex, and race. Clinical variables—including body mass index (kg/m2), systolic BP, diastolic BP, HDL level (mmol/L), total cholesterol level (mmol/L), and random glucose (mmol/L)—were based on the most proximal measurements that occurred on or before the start date and analyzed as continuous variables. Current and former use of cigarettes and current alcohol consumption were defined by self-report (yes/no) using the same time frame. Use of medications for hypertension, diabetes, and dyslipidemia were defined as receiving a prescription in the year before start date, and were analyzed as dichotomous variables (yes/no). Medical comorbidities, including coronary artery disease, congestive heart failure, peripheral arterial disease, major depressive disorder, diabetes, hypertension, osteoporosis, myalgias, and history of amputations were defined using documentation of International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes (Supplemental Table 1). Insurance status was defined using five categories of the most commonly used insurance plans (Blue Cross/Blue Shield, “commercial,” Geisinger Health Plan, Medicare, and “other”).
Outcomes
Outcomes included death and hospitalization, which were ascertained from the electronic medical record. Hospitalization was defined as the first inpatient stay lasting >1 day (from admission date to discharge date). Hospitalizations for <1 day were excluded because they likely reflect emergency department visits, observation visits, or elective procedures. There was no maximum length of stay imposed.
Development of Propensity Scores and Matching Protocol
We used 1:1 propensity matching to construct a cohort of similar groups of individuals who received new opioid prescriptions (without active NSAIDs) and had prescriptions for NSAIDs (without history of opioid prescriptions). We calculated propensity scores for the receipt of an opioid prescription (yes/no) using logistic regression and the following variables: initial prescription year; age; sex; black race; initial eGFR; body mass index; systolic BP; total cholesterol; random glucose; smoking status; alcohol use; cardiovascular disease; congestive heart failure; peripheral artery disease; major depressive disorder; diabetes; hypertension; osteoporosis; myalgias; insurance status; prescription for gabapentinoids; and medications for hypertension, dyslipidemia, and diabetes. Total cholesterol was included in the propensity score as it is a risk factor for cardiovascular disease and, in turn, hospitalization and death. Matching was performed without replacement using caliper sizes of 0.001. Propensity scores were calculated once at the time of medication initiation.
Statistical Analyses
We compared baseline characteristics across patients using standardized mean differences. We used Cox proportional hazards regression to estimate the association between opioid MMEs and outcomes across eGFR, which was modeled with linear splines using knots at 45, 60, and 90 ml/min per 1.73 m2. We included an interaction term between category of MME and each spline piece of eGFR to evaluate for differences in hazard ratios (HRs) at different levels of eGFR, reporting interactions at a specific point. Patients were followed until the event of interest, or administratively censored on February 1, 2017. Individuals were additionally censored if they developed cancer, ESKD, or switched medication groups.
We conducted several sensitivity analyses. We compared individuals with opioid prescriptions (without a history of gabapentinoid prescriptions) to people with gabapentinoid prescriptions (without a history of opioid prescriptions) as an alternative active comparator. In the gabapentinoid analysis, individuals with opioid prescriptions were matched 2:1 to individuals with gabapentinoid prescriptions, using caliper sizes of 0.0001, and the propensity score included the same variables as in the primary analysis (except prescriptions for gabapentinoids). We performed an analysis on the propensity-matched cohort defining medication exposure based only on initial prescription and calculating eGFR once using the outpatient serum creatinine measurement that was most proximal to the initial prescription. We also analyzed the full study population without propensity matching and adjusted for covariables included in the propensity score. We performed a competing risk analysis for hospitalization, accounting for the competing event of death using the method of Fine and Gray (26). Finally, we repeated the primary analysis for risk of hospitalization, censoring on last clinic visit to evaluate for potential loss to follow-up.
All analyses were performed using Stata version 14.2 (StataCorp, College Station, TX). A P value <0.05 was considered statistically significant.
Results
Study Population and Opioid Characteristics in the Propensity-Matched Cohort
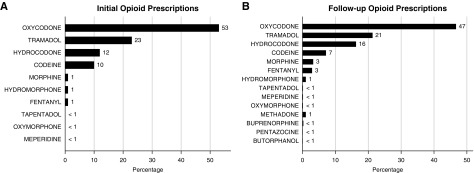
There were 32,895 patients who received initial opioid prescriptions and 37,525 who received NSAID prescriptions during the study period. Close matches were successfully identified for 23,123 individuals with opioid prescriptions (Table 1). In the propensity-matched cohort of 46,246 individuals, the mean (SD) age was 54 (16), 25,852 (56%) were women, and 1382 (3%) were of black race. There were no meaningful differences in baseline characteristics of patients with opioid and NSAID prescriptions after propensity matching; however, the matched patients receiving opiate prescriptions were healthier than the full population of patients receiving opiate prescriptions (Supplemental Figures 1 and 2). The median (interquartile range) number of opioid prescriptions during follow-up was 2 (1–4) per person, and the median (interquartile range) number of serum creatinine measurements was 5 (2–10) per person. There were 25,172 initial opioid prescriptions for 1–59 MMEs, and 7723 initial prescriptions for ≥60 MMEs. The most common initially prescribed opioids were oxycodone, tramadol, hydrocodone, and codeine (Figure 2A). There were increases in the proportion of fentanyl, hydromorphone, and morphine in the subsequent 167,509 prescriptions (Figure 2B).
Table 1.
Characteristics of Geisinger Health System patients at the time of prescription for an opioid or nonsteroidal anti-inflammatory drug, before and after propensity matching
| Covariables | Before Propensity Matching | After Propensity Matching | ||||
|---|---|---|---|---|---|---|
| People Prescribed Opioids (n=32,895) | People Prescribed NSAIDs (n=37,525) | Standardized Mean Difference | People Prescribed Opioids (n=23,123) | People Prescribed NSAIDs (n=23,123) | Standardized Mean Difference | |
| Age, mean (SD) | 57 (17) | 52 (16) | 35 | 54 (17) | 54 (16) | −1 |
| Female (N [%]) | 17,665 (54%) | 21,375 (57%) | −7 | 12,920 (56%) | 12,932 (56%) | 0 |
| Black race (N [%]) | 843 (3%) | 1317 (4%) | −6 | 674 (3%) | 708 (3%) | −1 |
| Baseline eGFR (mean in ml/min per 1.73 m2 [SD]) | 84 (25) | 92 (21) | −33 | 89 (23) | 89 (21) | 1 |
| Body mass index (mean in kg/m2 [SD]) | 30.8 (7.7) | 31.2 (7.4) | −6 | 31.0 (7.6) | 31.0 (7.1) | 0 |
| Systolic BP (mean in mm Hg [SD]) | 128 (18) | 125 (16) | 15 | 127 (17) | 127 (16) | 1 |
| Diastolic BP (mean in mm Hg [SD]) | 74 (11) | 75 (10) | −9 | 75 (10) | 75 (10) | −5 |
| HDL (mean in mg/dl [SD]) | 50 (16) | 51 (16) | −7 | 51 (12) | 51 (13) | −3 |
| Total cholesterol (mean in mg/dl [SD]) | 183 (42) | 190 (40) | −18 | 187 (31) | 186 (32) | 0 |
| Random glucose (mean in mg/dl [SD]) | 108 (35) | 102 (28) | 18 | 105 (31) | 105 (31) | −1 |
| Alcohol consumption (N [%]) | 13,147 (45%) | 17,145 (51%) | −12 | 11,193 (48%) | 11,242 (49%) | 0 |
| Current cigarette use (N [%]) | 6721 (20%) | 7841 (21%) | −1 | 5097 (22%) | 5076 (22%) | 0 |
| Former cigarette use (N [%]) | 10,340 (31%) | 9955 (27%) | 11 | 6845 (30%) | 6811 (29%) | 0 |
| Antihypertensive prescription (N [%]) | 18,509 (56%) | 16,208 (43%) | 26 | 11,299 (49%) | 11,399 (49%) | −1 |
| Antidyslipidemic prescription (N [%]) | 13,227 (40%) | 11,982 (32%) | 17 | 8035 (35%) | 8212 (36%) | −2 |
| Antidiabetic prescription (N [%]) | 6730 (20%) | 5121 (14%) | 18 | 3741 (16%) | 3817 (17%) | −1 |
| Coronary artery disease (N [%]) | 5560 (17%) | 2251 (6%) | 35 | 1921 (8%) | 2027 (9%) | −2 |
| Congestive heart failure (N [%]) | 1643 (5%) | 377 (1%) | 24 | 295 (1%) | 349 (2%) | −2 |
| Cardiovascular disease (N [%]) | 2544 (8%) | 1098 (3%) | 22 | 2907 (13%) | 3092 (13%) | −2 |
| Peripheral artery disease (N [%]) | 1154 (4%) | 428 (1%) | 16 | 361 (2%) | 388 (2%) | −1 |
| Major depressive disorder (N [%]) | 5153 (16%) | 5816 (15%) | 0 | 3775 (16%) | 3768 (16%) | 0 |
| Diabetes (N [%]) | 3676 (11%) | 2441 (7%) | 17 | 1899 (8%) | 1945 (8%) | −1 |
| Hypertension (N [%]) | 9205 (28%) | 7484 (20%) | 19 | 5543 (24%) | 5570 (24%) | 0 |
| Osteoporosis (N [%]) | 2319 (7%) | 1925 (5%) | 8 | 1419 (6%) | 1438 (6%) | 0 |
| Myalgias (N [%]) | 1792 (5%) | 2317 (6%) | −3 | 1358 (6%) | 1369 (6%) | 0 |
| Amputations (N [%]) | 90 (0%) | 7 (0%) | 7 | 35 (0%) | 5 (0%) | 4 |
Percentages reflect column-wide percentages. NSAIDs, nonsteroidal anti-inflammatory drugs.
Figure 2.
Opioid prescriptions at initial prescription and throughout follow-up. (A) Distribution of initial opioid prescriptions. (B) Distribution of opioids prescribed throughout follow up.
Risk of Adverse Outcomes in the Propensity-Matched Cohort
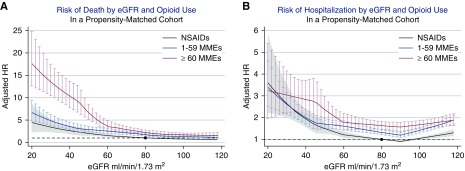
In the propensity-matched cohort, there were 2457 deaths and 9147 first hospitalizations, corresponding to 12 deaths and 54 incident hospitalizations per 1000 person-years (Table 2). Exposure to higher MMEs was associated with higher risk of both death and hospitalization (Figure 3). Compared with individuals with NSAID prescriptions and at an eGFR of 80 ml/min per 1.73 m2, the HR for death was 1.70 (95%, CI 1.41 to 2.05) for 1–59 MME, and 2.25 (95% CI, 1.82 to 2.79) for ≥60 MMEs. Higher opioid doses were associated with higher relative risk of death at lower levels of eGFR. For example, compared with individuals with NSAID prescriptions and at an eGFR of 40 ml/min per 1.73 m2, the HR for death for individuals with ≥60 MMEs was 3.94 (95% CI, 2.70 to 5.75; P for point-wise interaction of 0.01, comparing eGFR 40 with 80 ml/min per 1.73 m2).
Table 2.
Death and hospitalization events by category of time-varying eGFR and medication status
| Time-varying eGFR | eGFR ≥90 ml/min per 1.73 m2 | eGFR ≥60 to <90 ml/min per 1.73 m2 | eGFR ≥30 to <60 ml/min per 1.73 m2 | eGFR <30 ml/min per 1.73 m2 |
|---|---|---|---|---|
| Death | ||||
| NSAID use: events | 93 | 127 | 71 | 11 |
| NSAID use: incident rates | 4.6 (3.8–5.7) | 9.2 (7.7–10.9) | 27.7 (22.0–35.0) | 93.8 (52.0–169.4) |
| Opiate use (<60 MMEs): events | 175 | 303 | 195 | 45 |
| Opiate use (<60 MMEs): incident rates | 8.2 (7.1–9.5) | 19.2 (17.2–21.5) | 46.7 (40.6–53.7) | 156.3 (116.7–209.3) |
| Opiate use (≥60 MMEs): events | 82 | 149 | 102 | 39 |
| Opiate use (≥60 MMEs): incident rates | 9.8 (7.9–12.2) | 26.1 (22.2–30.6) | 106.7 (87.9–129.6) | 388.4 (283.8–531.7) |
| Incident hospitalization | ||||
| NSAID use: events | 745 | 628 | 184 | 18 |
| NSAID use: incident rates | 48.4 (45.0–52.0) | 57.3 (53.0–62.0) | 100.7 (87.1–116.3) | 400.4 (252.3–635.5) |
| Opiate use (<60 MMEs): events | 1146 | 851 | 330 | 38 |
| Opiate use (<60 MMEs): incident rates | 65.8 (62.1–69.7) | 71.0 (66.4–76.0) | 115.3 (103.5–128.4) | 266.8 (194.1–366.7) |
| Opiate use (≥60 MMEs): events | 451 | 335 | 93 | 15 |
| Opiate use (≥60 MMEs): incident rates | 73.4 (66.9–80.5) | 87.2 (78.4–97.1) | 158.7 (129.5–194.5) | 390.1 (235.2–647.1) |
Ranges in parentheses refer to 95% confidence intervals. There were an additional 1065 deaths and 4313 hospitalizations that occurred during follow-up when participants were prescribed neither opiates nor NSAIDs (9.8 deaths per 1000 person-years and 44.4 hospitalizations per 1000 person-years). NSAID, nonsteroidal anti-inflammatory drug; MMEs, daily oral morphine milligram equivalents.
Figure 3.
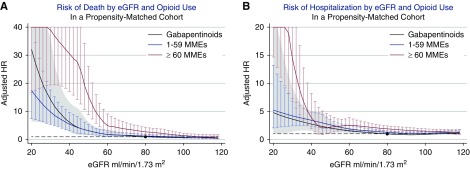
Risk of death and hospitalization: opioids versus nonsteroidal anti-inflammatory drugs. (A) Adjusted hazard ratios (HRs) for death and (B) hospitalization in those with ≥60 oral morphine milligram equivalents (MMEs) (red line), 1–59 MMEs (blue line), and NSAID prescriptions (black line), compared with a reference group of individuals with an NSAID prescription and eGFR of 80 ml/min per 1.73 m2. NSAID, nonsteroidal anti-inflammatory drug.
For the outcome of first hospitalization, compared with individuals with NSAID prescriptions and eGFR of 80 ml/min per 1.73 m2, patients receiving prescriptions for 1–59 MME had 1.38-times higher risk (95% CI, 1.30 to 1.46), and patients receiving prescriptions for ≥60 MMEs had 1.68-times higher risk (95% CI, 1.56 to 1.81). The risk of hospitalization associated with opiate prescription did not differ by level of eGFR. For example, compared with individuals with NSAID prescriptions and at an eGFR of 40 ml/min per 1.73 m2, the HR for hospitalization for individuals with ≥60 MMEs was 1.46 (95% CI, 1.05 to 2.02; P for interaction, 0.53).
Sensitivity Analyses
When compared with gabapentinoid prescriptions, risk of death was higher for ≥60 MMEs (HR, 2.72; 95% CI, 1.71 to 4.34 at eGFR 80 ml/min per 1.73 m2), but there was no significant difference in risk of death with lower opioid doses (HR, 1.25; 95% CI, 0.81 to 1.94 for 1–59 MME). Risks of hospitalization were higher for all opioid doses (HR, 1.22; 95% CI, 1.04 to 1.43 for 1–59 MME; HR, 1.54; 95% CI, 1.28 to 1.86 for ≥60 MMEs) (Figure 4, Supplemental Figures 3 and 4, and Supplemental Table 2). Results from the analyses using only baseline prescription status and eGFR were consistent with the time-varying analyses, although there was no difference in the association between opioid prescription and adverse outcomes by eGFR (Supplemental Figure 5). Results from fully adjusted models were consistent with the primary analysis (Supplemental Figure 6). Results of the competing risk analysis for hospitalization showing higher risk of hospitalization with higher prescribed dose were weak or null at lower eGFR when accounting for competing risk of death (Supplemental Figure 7). Results from the analysis censoring on last clinic date were similar to the primary analysis for risk of hospitalization (Supplemental Figure 8).
Figure 4.
Risk of death and hospitalization: opioids versus gabapentinoids. (A) Adjusted hazard ratios (HRs) for death and (B) hospitalization in those with ≥60 oral morphine milligram equivalents (MMEs) (red line), 1–59 MMEs (blue line), and gabapentinoid prescriptions (black line), compared with a reference group of individuals with a gabapentinoid prescription and eGFR of 80 ml/min per 1.73m2.
Discussion
In this community-based cohort of patients receiving care at a tertiary health system, we demonstrate higher risk of death and hospitalization with opioid prescriptions compared with other medications used to treat pain, with the highest risks in people with lower eGFR. Risks increased with higher dose of opioid. Coupled with previous studies demonstrating opioid use is highest in patients with lower eGFR (13), our results suggest patients with reduced eGFR constitute a particularly high-risk population that merits targeting for education and harm-mitigation efforts.
Literature on risks associated with opioid use in kidney disease has largely focused on the dialysis population, and little is known about predialysis CKD. In observational studies on patients on dialysis, opioid use has been associated with increased risk for altered mental status (19), fractures (19,27), poor sleep (28), death (18), hospitalization (18), and dialysis discontinuation (18). In a prospective cohort of 140,899 Medicare-covered patients receiving hemodialysis in 2011, the risk of emergency room visit or hospitalization for fracture increased by 4% for every 60-mg increase in oral morphine equivalents (19). In kidney transplant recipients, opioid prescriptions were associated with increased mortality and allograft loss (29). Our study expands the literature as one of the first to evaluate the risk of death and hospitalization across levels of eGFR, with specific attention to patients with predialysis reduced eGFR.
Opioids may lead to higher risk of death and hospitalization through increased risk for dependence, substance use disorders, over-sedation, and respiratory depression (30–36). Mental illness may also play a role, as it is often associated with opioid misuse (37–39). People with kidney disease may face even higher risks given that kidney excretion of opioids is impaired with possible accumulation and toxicity. (14,17,20,30,40,41) Higher endogenous exposure may heighten associated risks such as hospitalizations and risk of death (41). Of note, in this study, our comparison medications may also not be benign: NSAIDs are often discouraged in CKD, and gabapentin itself undergoes kidney excretion.
There is minimal guidance on pain treatment in the setting of CKD. For the general population, the Center for Disease Control (CDC) and the American Society of Interventional Pain Physicians (ASIPP) recommend initial treatment with nonopioid pharmacologic therapy (such as NSAIDs, acetaminophen, anticonvulsants, and serotonin-NE reuptake inhibitors), and nonpharmacologic therapy (such as exercise therapy, acupuncture, massage therapy, and cognitive behavioral therapy) (42–44). Both the CDC and ASIPP advocate for the use of the World Health Organization’s three-step analgesic ladder, which categorizes pain as mild, moderate, or severe as a way to guide initiation and escalation of treatment (45). If opioids are initiated for chronic pain, immediate-release formulations are preferred over long-acting ones, and doses should not exceed a ceiling of 90 MMEs per day (42–44).
Recommendations for patients with CKD are similar to those in the general population, with the added guidance for frequent monitoring of kidney function and reduction in opioid dose as eGFR declines (42–44). However, optimal dosing of opioids in the setting of CKD is unknown. Due to uncertain pharmacokinetics in patients with CKD, some experts recommend the preferential use of short-acting opioids in CKD—that is, tramadol, oxycodone, and hydromorphone for moderate pain, and fentanyl and methadone for severe pain—with avoidance of morphine, codeine, hydrocodone, tapentadol, and meperidine (14,17,20,30,40). However, there are limited data on tramadol and oxycodone in the setting of reduced kidney function (19). The active metabolites of morphine and codeine are cleared by kidney and have been shown to cause respiratory and central nervous system depression, hypotension, myoclonus, and seizures, as well as lethal intoxication in CKD (30–36). Hydrocodone and tapentadol are not recommended in CKD due to high reliance on kidney excretion (85%–99%) (46,47).
Strengths and Limitations
Strengths of our study include a large data set that maximized power and use of an active comparator with propensity matching to ensure comparison groups were as similar as possible other than their opioid prescription status. We conducted sensitivity analyses using gabapentinoid prescriptions as an alternative active comparator, an analysis using only baseline prescription status and covariates, and one for hospitalization using competing risk methodologies.
This study had several limitations, some of which are inherent to electronic medical record data. We used ICD codes to measure covariables, leaving the potential for misclassification. Our exposure was defined using prescription records, and we potentially missed prescriptions from providers outside Geisinger and over-the-counter NSAIDs. However, Geisinger patients tend to receive most inpatient and outpatient care within the same system, maximizing completeness of exposure ascertainment and follow-up. Opioid exposure was determined by opioid prescription and not pharmacy fill data. Propensity matching served to minimize differences between groups but may limit generalizability to the broader Geisinger Health population receiving opioid prescriptions. Individuals were matched on the presence of opioid prescription, not on MME, to increase the number of individuals available to match. We cannot fully account for reasons a medication was selected for treatment (e.g., type of pain, frailty status, risk of gastrointestinal bleeding). We did not update information on covariates over time, only prescription status and eGFR. We did not have information on cause of death. As with all observational studies, residual confounding is possible, and we cannot ascertain causality.
In conclusion, our study showed that opioid prescriptions were associated with higher risk of death and hospitalization compared with NSAID use, particularly in people with reduced eGFR. The manifest clinical question is how to better treat pain in a population with limited therapeutic options. More research is needed on optimal opioid dosing in patients with reduced kidney function, and on dosing and risks associated with nonopioid therapies. For patients with reduced eGFR and opioid prescriptions, counseling about the possible risks associated with opioids, promotion of nonpharmacologic therapies, and regular consideration of whether the benefit of pain control outweighs such risks is crucial.
Disclosures
Dr. Alexander reports that he was past chair of the Food and Drug Administration’s Peripheral and Central Nervous System Advisory Committee; has served as a paid advisor to IQVIA; is a cofounding principal and equity holder in Monument Analytics, a healthcare consultancy whose clients include the life sciences industry, as well as plaintiffs in opioid litigation; and is a member of OptumRx’s National Pharmacy & Therapeutics Committee. These associations have been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. Dr. Grams reports travel expenses paid by Dialysis Clinic Inc. and Kidney Disease Improving Global Outcomes. Dr. Inker reports receiving grants from the National Institutes of Health (NIH), the National Kidney Foundation, Omeros, and Reata Pharmaceuticals for research and contracts with the Paul Teschan Research Fund and Tufts Medical Center, outside of the submitted work. Dr. Inker also reports participation in consulting agreements with Omeros and Tricida and a position on the medical advisory board for Alport Syndrome Foundation. Dr. Inker further reports a patent issued for precise estimation of GFR from multiple biomarkers (PCT/US2015/044567). Dr. Chang, Dr. Novick, Dr. Shin, Dr. Surapaneni, and Dr. Wright have nothing to disclose.
Funding
Research reported in this publication was supported by grants from NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to Dr. Coresh and Dr. Inker (R01 DK100446) and Dr. Grams and Dr. Inker (R01 DK115534). Dr. Novick was supported by NIH/NIDDK under award number T32DK7732-24.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Safe and Effective Management of Pain in People with CKD,” on pages 1551–1553.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00440119/-/DCSupplemental.
Supplemental Table 1. ICD codes.
Supplemental Table 2. Population characteristics for opioids versus gabapentinoids analysis.
Supplemental Figure 1. Propensity-matching for opioid versus NSAIDs analysis.
Supplemental Figure 2. Distribution of propensity scores for opioid prescription.
Supplemental Figure 3. Sensitivity analysis study population: opioids versus gabapentinoids.
Supplemental Figure 4. Propensity-matching for opioid versus gabapentinoid analysis.
Supplemental Figure 5. Sensitivity analysis: opioids versus NSAIDs using initial prescription and eGFR.
Supplemental Figure 6. Sensitivity analysis: fully adjusted opioids versus NSAIDs.
Supplemental Figure 7. Sensitivity analysis: competing risk analysis for hospitalizations.
Supplemental Figure 8. Sensitivity analysis: risk of hospitalization censoring on last clinic visit.
References
- 1.Daubresse M, Chang HY, Yu Y, Viswanathan S, Shah ND, Stafford RS, Kruszewski SP, Alexander GC: Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care 51: 870–878, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM: Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 National survey on drug use and health. Ann Intern Med 167: 293–301, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Alexander GCFS, Frattaroli S, Gielen AC, editors: The Opioid Epidemic: From Evidence to Impact, Baltimore, MD, Johns Hopkins Bloomberg School of Public Health, 2017 [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration: Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17-504, NSDUH Series H-52), Rockville, MD, Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, 2017 [Google Scholar]
- 5.National Institute on Drug Abuse: Overdose Death Rates. 2018. Available at: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed November 26, 2018
- 6.Rudd RA, Aleshire N, Zibbell JE, Gladden RM: Increases in drug and opioid overdose deaths--United States, 2000-2014. MMWR Morb Mortal Wkly Rep 64: 1378–1382, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Rudd RA, Seth P, David F, Scholl L: Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep 65: 1445–1452, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Davison SN, Koncicki H, Brennan F: Pain in chronic kidney disease: A scoping review. Semin Dial 27: 188–204, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Davison SN: Pain in hemodialysis patients: Prevalence, cause, severity, and management. Am J Kidney Dis 42: 1239–1247, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Davison SN, Mayo PR: Pain management in chronic kidney disease: The pharmacokinetics and pharmacodynamics of hydromorphone and hydromorphone-3-glucuronide in hemodialysis patients. J Opioid Manag 4: 335–336, 339–344, 2008. [PubMed]
- 11. Davison SN: Pain, analgesics, and safety in patients with CKD. Clin J Am Soc Nephrol 10: 350–352, 2015. [DOI] [PMC free article] [PubMed]
- 12.Wu J, Ginsberg JS, Zhan M, Diamantidis CJ, Chen J, Woods C, Fink JC: Chronic pain and analgesic use in CKD: Implications for patient safety. Clin J Am Soc Nephrol 10: 435–442, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novick TK, Surapaneni A, Shin JI, Ballew SH, Alexander GC, Inker LA, Chang AR, Grams ME: Prevalence of opioid, gabapentinoid, and NSAID use in patients with CKD. Clin J Am Soc Nephrol 13: 1886–1888, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whelton A: Nephrotoxicity of nonsteroidal anti-inflammatory drugs: Physiologic foundations and clinical implications. Am J Med 106[5B]: 13S–24S, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Smith HS: The metabolism of opioid agents and the clinical impact of their active metabolites. Clin J Pain 27: 824–838, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Portenoy RK, Foley KM, Stulman J, Khan E, Adelhardt J, Layman M, Cerbone DF, Inturrisi CE: Plasma morphine and morphine-6-glucuronide during chronic morphine therapy for cancer pain: Plasma profiles, steady-state concentrations and the consequences of renal failure. Pain 47: 13–19, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Koncicki HM, Unruh M, Schell JO: Pain management in CKD: A guide for nephrology providers. Am J Kidney Dis 69: 451–460, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Kimmel PL, Fwu CW, Abbott KC, Eggers AW, Kline PP, Eggers PW: Opioid prescription, morbidity, and mortality in United States dialysis patients. J Am Soc Nephrol 28: 3658–3670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL: Opioid analgesics and adverse outcomes among hemodialysis patients. Clin J Am Soc Nephrol 13: 746–753, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koncicki HM, Brennan F, Vinen K, Davison SN: An approach to pain management in end stage renal disease: Considerations for general management and intradialytic symptoms. Semin Dial 28: 384–391, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, Lewis M, Kramer H, Hartle JE, Carey D, Appel LJ, Grams ME: Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int 90: 164–171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy J, Shah NR, Wood GC, Townsend R, Hennessy S: Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for hypertension on clinical end points: A cohort study. J Clin Hypertens (Greenwich) 14: 407–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration: FDA Drug Safety Communication: FDA Recommends Against the Continued Use of Propoxyphene, Silver Spring, MD, US Food and Drug Administration, 2010 [Google Scholar]
- 24.Center for Medicare and Medicaid Services: Opioid Morphine Eq Conversion Factors. Available at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed March 5, 2019
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC, Fine JP: Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 36: 4391–4400, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL: Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, Kurokawa K, Rayner HC, Furniss AL, Port FK, Saran R: Sleep quality predicts quality of life and mortality risk in haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 23: 998–1004, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Abbott KC, Fwu CW, Eggers PW, Eggers AW, Kline PP, Kimmel PL: Opioid prescription, morbidity, and mortality in US transplant recipients. Transplantation 102: 994–1004, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murtagh FE, Chai MO, Donohoe P, Edmonds PM, Higginson IJ: The use of opioid analgesia in end-stage renal disease patients managed without dialysis: Recommendations for practice. J Pain Palliat Care Pharmacother 21: 5–16, 2007 [PubMed] [Google Scholar]
- 31.Bodd E, Jacobsen D, Lund E, Ripel A, Mørland J, Wiik-Larsen E: Morphine-6-glucuronide might mediate the prolonged opioid effect of morphine in acute renal failure. Hum Exp Toxicol 9: 317–321, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Angst MS, Bührer M, Lötsch J: Insidious intoxication after morphine treatment in renal failure: Delayed onset of morphine-6-glucuronide action. Anesthesiology 92: 1473–1476, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Lagas JS, Wagenaar JF, Huitema AD, Hillebrand MJ, Koks CH, Gerdes VE, Brandjes DP, Beijnen JH: Lethal morphine intoxication in a patient with a sickle cell crisis and renal impairment: Case report and a review of the literature. Hum Exp Toxicol 30: 1399–1403, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Guay DR, Awni WM, Findlay JW, Halstenson CE, Abraham PA, Opsahl JA, Jones EC, Matzke GR: Pharmacokinetics and pharmacodynamics of codeine in end-stage renal disease. Clin Pharmacol Ther 43: 63–71, 1988 [DOI] [PubMed] [Google Scholar]
- 35.Szeto HH, Inturrisi CE, Houde R, Saal S, Cheigh J, Reidenberg MM: Accumulation of normeperidine, an active metabolite of meperidine, in patients with renal failure of cancer. Ann Intern Med 86: 738–741, 1977 [DOI] [PubMed] [Google Scholar]
- 36.Hochman MS: Meperidine-associated myoclonus and seizures in long-term hemodialysis patients. Ann Neurol 14: 593, 1983 [DOI] [PubMed] [Google Scholar]
- 37.Han B, Compton WM, Blanco C, Jones CM: Prescription opioid use, misuse, and use disorders in U.S. Adults. Ann Intern Med 168: 383–384, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Mojtabai R, Amin-Esmaeili M, Nejat E, Olfson M: Misuse of prescribed opioids in the United States. Pharmacoepidemiol Drug Saf 28: 345–353, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Han B, Compton WM, Blanco C, Jones CM: Correlates of prescription opioid use, misuse, use disorders, and motivations for misuse among US adults. J Clin Psychiatry 79: 17m11973, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Henrich WL, Agodoa LE, Barrett B, Bennett WM, Blantz RC, Buckalew VM Jr, D’Agati VD, DeBroe ME, Duggin GG, Eknoyan G: Analgesics and the kidney: Summary and recommendations to the Scientific Advisory Board of the National Kidney Foundation from an Ad Hoc Committee of the National Kidney Foundation. Am J Kidney Dis 27: 162–165, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Davison SN: Clinical pharmacology considerations in pain management in patients with advanced kidney failure. Clin J Am Soc Nephrol 14: 917–931, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dowell D, Haegerich TM, Chou R: CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA 315: 1624–1645, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manchikanti L, Abdi S, Atluri S, Balog CC, Benyamin RM, Boswell MV, Brown KR, Bruel BM, Bryce DA, Burks PA, Burton AW, Calodney AK, Caraway DL, Cash KA, Christo PJ, Damron KS, Datta S, Deer TR, Diwan S, Eriator I, Falco FJ, Fellows B, Geffert S, Gharibo CG, Glaser SE, Grider JS, Hameed H, Hameed M, Hansen H, Harned ME, Hayek SM, Helm S 2nd, Hirsch JA, Janata JW, Kaye AD, Kaye AM, Kloth DS, Koyyalagunta D, Lee M, Malla Y, Manchikanti KN, McManus CD, Pampati V, Parr AT, Pasupuleti R, Patel VB, Sehgal N, Silverman SM, Singh V, Smith HS, Snook LT, Solanki DR, Tracy DH, Vallejo R, Wargo BW; American Society of Interventional Pain Physicians: American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part I--evidence assessment. Pain Physician 15[Suppl]: S1–S65, 2012 [PubMed] [Google Scholar]
- 44.Manchikanti L, Kaye AM, Knezevic NN, McAnally H, Slavin K, Trescot AM, Blank S, Pampati V, Abdi S, Grider JS, Kaye AD, Manchikanti KN, Cordner H, Gharibo CG, Harned ME, Albers SL, Atluri S, Aydin SM, Bakshi S, Barkin RL, Benyamin RM, Boswell MV, Buenaventura RM, Calodney AK, Cedeno DL, Datta S, Deer TR, Fellows B, Galan V, Grami V, Hansen H, Helm Ii S, Justiz R, Koyyalagunta D, Malla Y, Navani A, Nouri KH, Pasupuleti R, Sehgal N, Silverman SM, Simopoulos TT, Singh V, Solanki DR, Staats PS, Vallejo R, Wargo BW, Watanabe A, Hirsch JA: Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician 20[2S]: S3–S92, 2017 [PubMed] [Google Scholar]
- 45.Barakzoy AS, Moss AH: Efficacy of the world health organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol 17: 3198–3203, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Hartrick CT: Tapentadol immediate-release for acute pain. Expert Rev Neurother 10: 861–869, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Terlinden R, Ossig J, Fliegert F, Lange C, Göhler K: Absorption, metabolism, and excretion of 14C-labeled tapentadol HCl in healthy male subjects. Eur J Drug Metab Pharmacokinet 32: 163–169, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.