Abstract
Objectives:
Screening for hepatitis C virus (HCV) infection in jail provides an opportunity to educate and offer care to a high-risk population. We aimed to (1) estimate the prevalence of HCV infection in jail; (2) describe the demographic characteristics, risk factors, and pre-incarceration health insurance status associated with HCV infection; and (3) examine the implementation of HCV screening in jail.
Methods:
We conducted a retrospective analysis of an opt-out HCV screening program with HCV RNA confirmation and patient education at the Dallas County Jail from April 1 through November 2, 2017. We extracted data on test results, demographic characteristics, and release destination from electronic medical records. A nurse navigator recorded data on patient self-reported risk factors and pre-incarceration health insurance status.
Results:
Of 4089 incarcerated persons screened, 708 (17.3%) had a positive HCV antibody result. Of these, 641 (90.5%) had an HCV RNA test ordered; 576 (89.9%) had RNA tests completed, of whom 413 (71.7%) had a positive HCV RNA result. Of these 413, 352 (85.2%) received patient education. Half of HCV RNA-positive incarcerated persons (n = 207, 50.1%) were born outside the birth cohort (1945-1965). Among those with HCV infection, commonly reported risk factors were injection drug use (168 of 352; 47.8%) and tattoos (82 of 352; 23.4%). Most incarcerated persons with HCV infection (284 of 350; 81.1%) did not have health insurance. HCV antibody prevalence was higher among incarcerated persons released to prison (232 of 961; 24.1%) than to outside agencies (38 of 403; 9.4%) or the community (178 of 1026; 17.4%).
Conclusions:
Screening for HCV with RNA confirmation in jail provides an opportunity for disease education, transmission prevention, and navigation to HCV treatment. Future efforts should examine post-incarceration linkage to care.
Keywords: hepatitis C, incarceration, prevalence, risk factors, health insurance
Hepatitis C virus (HCV) is a common blood-borne disease estimated to affect more than 4.1 million persons in the United States1; the prevalence of HCV infection is 1.8% in Texas.2 The rate of HCV infection increased in recent years to nearly 34 000 new cases in 2015 as a result of the opioid epidemic, because HCV is highly transmittable through injection drug use (IDU).3,4 Complications from HCV infection include liver cirrhosis, hepatocellular carcinoma, and the need for liver transplant.5 In 2013, HCV infection accounted for nearly 20 000 deaths in the United States, more than all other reportable infectious diseases combined.4 The introduction of direct-acting antiviral medications, which have high cure rates and few side effects, has led to increased efforts to diagnose, treat, and cure HCV infection to lower the disease burden, prevent transmission, and decrease mortality.6,7
Persons in jails and prisons are disproportionately affected by HCV infection; prevalence is more than 10 times higher among persons who are incarcerated (range, 9.6%-41.1%)8 than among persons in the community (range, 0.5%-2.4%).9 In addition, according to a survey of US state correctional departments in 2006, an estimated 30% of persons with HCV infection in the United States have spent time in a correctional facility.8 With the high prevalence of HCV infection in correctional settings and limited access to health care before incarceration, testing in jails and prisons provides a key opportunity to identify persons with HCV infection and provide education on disease prevention and treatment.
The HCV care cascade describes the progression from HCV screening through completion of treatment and includes (1) HCV antibody detection, (2) chronic infection confirmation with HCV RNA testing, (3) referral to specialty care, (4) specialist evaluation, (5) treatment initiation, (6) treatment completion, and (7) sustained virologic response.10 HCV antibody screening is recommended by the World Health Organization for all incarcerated persons and by the US Federal Bureau of Prisons for all persons incarcerated in state and federal prisons. However, as of 2015, only 17 states offered routine opt-out HCV testing in prisons, and data documenting rates of testing in jails were limited.11-13 This low rate of HCV screening in prisons is due in part to such barriers as challenges implementing testing, the cost of antibody and RNA tests, and the high cost of treatment.14 An additional barrier in jails is the short length of stay for most incarcerated persons. The average length of incarceration in jail in the United States is 25 days, which necessitates efficient processes to screen and educate incarcerated persons on their status before release.15 Few studies have examined the implementation of HCV screening and testing in jail, and minimal data exist on release destination and health insurance status of HCV-positive (by antibody or by RNA testing) persons after incarceration.
Opt-out HCV antibody screening was implemented at the Dallas County Jail beginning in June 2015. In April 2017, the jail added HCV RNA testing and a dedicated nurse navigator position.16 The objectives of our study were to (1) estimate the prevalence of HCV infection (by antibody and RNA testing) at the Dallas County Jail; (2) describe the demographic characteristics, risk factors for HCV infection, and pre-incarceration health insurance status in this population; and (3) examine the implementation of HCV screening and testing in the jail.
Methods
We conducted a retrospective analysis of an opt-out HCV screening program in the Dallas County Jail in Dallas, Texas, from April 1 through November 2, 2017. The Dallas County Jail is one of the largest jails in the United States, serves as both a city and county jail, and typically houses about 5200 persons at a time, with about 275 persons entering and being released daily. Most persons in the Dallas County Jail are male (78%), non-Hispanic black (49%), and the mean age is 35 (C. Berry, personal communication, December 4, 2018).
The Dallas County Jail began offering routine opt-out HCV antibody screening and confirmatory HCV RNA testing at the time of blood draw for incarcerated persons starting April 1, 2017. Approximately 10% of the jail population undergoes blood draws ordered for various reasons. A phlebotomist manually reviews each person’s medical record for all previous laboratory tests at the jail before screening to avoid duplication. For the first 3 months of the study, if the HCV antibody result was positive, the nurse navigator manually ordered an HCV RNA test, triggering an additional blood draw. In July 2017, this process was altered to simultaneous collection of HCV antibody and HCV RNA as a single blood draw, with the HCV RNA sample stored and automatically run by the laboratory if the HCV antibody test was positive. A full-time dedicated nurse navigator notified patients who had positive HCV RNA test results and provided disease education, prevention counseling, and information about linkage to HCV care. The nurse navigator provided a hotline number for patients to call after release to reach the nurse navigator. The nurse navigator followed up with incarcerated persons after their release into the community by telephone, assisted in setting up medical financial assistance, and assisted in scheduling laboratory and radiology tests with providers before appointments at a community liver clinic. The nurse navigator sent a certified letter to incarcerated persons who had a positive HCV RNA result who were released before receiving education to notify them of an abnormal laboratory result. In several instances, these persons could not be reached. Because this project was focused on quality improvement of a guideline-recommended practice (ie, HCV testing in jail), the University of Texas Southwestern Internal Review Board considered it exempt from review board review.
The laboratory performed HCV antibody testing using an HCV antibody enzyme immunoassay (Roche, Basel, Switzerland) and HCV RNA testing using a Cobas AmpliPrep/Cobas TaqMan HCV Test version 2.0 (Roche, Basel, Switzerland). Testing ran from April 1 through November 2, 2017. An HCV antibody test result of >0.9 signal-to-cutoff ratio is positive, and an HCV RNA test result with any detectable RNA (>15 IU/mL) is positive. We calculated estimated HCV antibody prevalence by dividing the number of positive HCV antibody tests by the total number of antibody tests performed. We calculated HCV RNA positivity by dividing the number of positive RNA tests by the total number of persons who received screening. Sixty-seven of 708 HCV antibody-positive persons were released before they received a confirmatory HCV RNA test, so an overall HCV RNA positivity rate was extrapolated by multiplying the number of positive HCV antibody tests by the proportion of HCV RNA tests that were positive. We also calculated the percentage of HCV antibody-positive persons who received an HCV RNA test by dividing the number of HCV RNA tests by the number of HCV antibody-positive persons before and after switching to a single blood draw.
We extracted data on the following demographic characteristics from electronic medical records and the Adult Information System used by the Dallas County Sheriff Department: age (born between 1945 and 1965 [the birth cohort], born before 1945, or born after 1965); sex (male or female); race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, or other); HCV antibody and RNA test results; and release location (community, Texas Department of Criminal Justice, or agency). We considered persons born from 1945 through 1965 (baby boomers) to be in the birth cohort. For HCV RNA-positive persons, a nurse navigator recorded self-reported HCV risk factors and pre-incarceration health insurance status. We categorized self-reported risk factors into the following mutually exclusive groups: IDU, tattoo, both IDU and tattoo, blood exposure (includes blood transfusion), sexual contact, other, or unknown. We categorized health insurance as uninsured, Medicaid, Medicare or dual insurance (ie, Medicaid and Medicare), and US Department of Veterans Affairs.
We categorized release location as (1) release to community (including bail or probation), (2) release to the Texas Department of Criminal Justice (prison system), or (3) release to an agency (eg, special program, psychiatric facility). We also calculated the estimated prevalence of HCV antibody for each release location by dividing the number of HCV antibody-positive persons released to a location by the total number of HCV antibody-tested persons released to that location during the study period. We compared HCV antibody-positive prevalence by release to prison or elsewhere using the Pearson χ2 test of significance, with P < .05 considered significant. We conducted all data analyses by using Stata version 14.17
Results
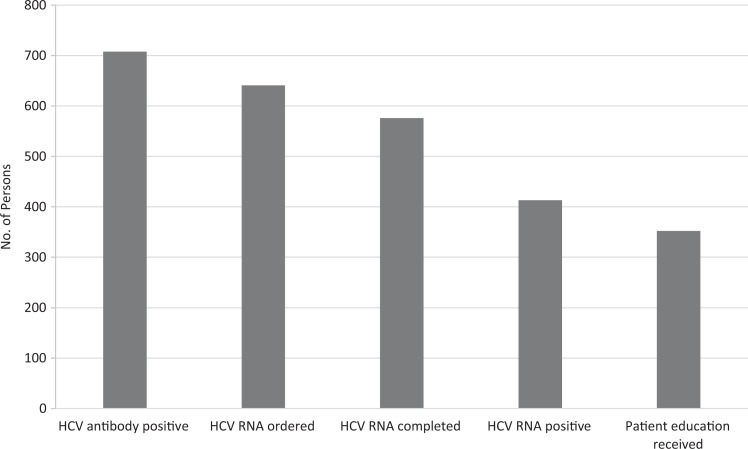
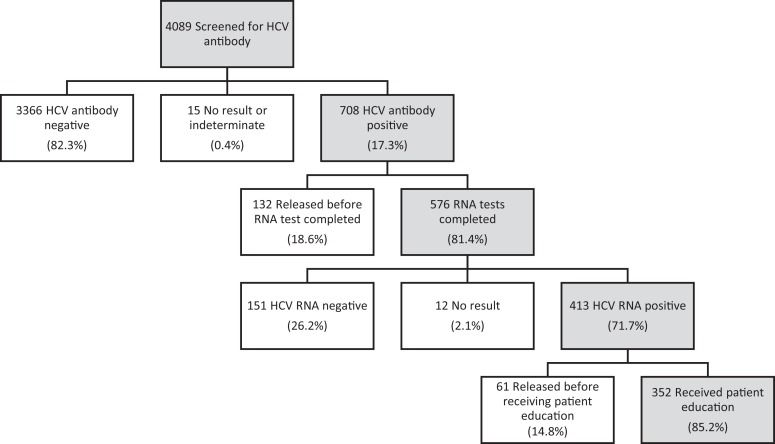
Of 4089 incarcerated persons screened with an HCV antibody test, 708 (17.3%) had a positive HCV antibody result. Of the 708 positive results, 641 (90.5%) had a confirmatory HCV RNA test ordered, of which 576 (89.9%) were completed. Four hundred thirteen of 576 (71.7%) incarcerated persons had a positive HCV RNA result, of which 352 (85.2%) received patient education (Figure 1). HCV RNA positivity was confirmed in 413 of 4089 (10.1%) persons screened for HCV antibody (Figure 2). If HCV RNA test results included all persons with a positive HCV antibody result, before confirmatory RNA testing (n = 708), the estimated HCV RNA positivity was 12.4% (708/4089 × 71.7%). After switching to a single blood draw, the proportion of HCV antibody-positive persons who underwent HCV RNA testing increased: 66.5% (276 of 415) of HCV antibody-positive persons received HCV RNA testing from April 1 through July 9, 2017, whereas 94.6% (283 of 299) of HCV antibody-positive persons received HCV RNA testing from July 10 through November 2, 2017.
Figure 1.
Hepatitis C virus (HCV) screening, testing, and notification among 4089 incarcerated persons at the Dallas County Jail, April 1 through November 2, 2017.
Figure 2.
Hepatitis C virus (HCV) antibody testing, HCV RNA quantitative testing, and patient education among incarcerated persons screened for HCV antibody at the Dallas County Jail, April 1 through November 2, 2017.
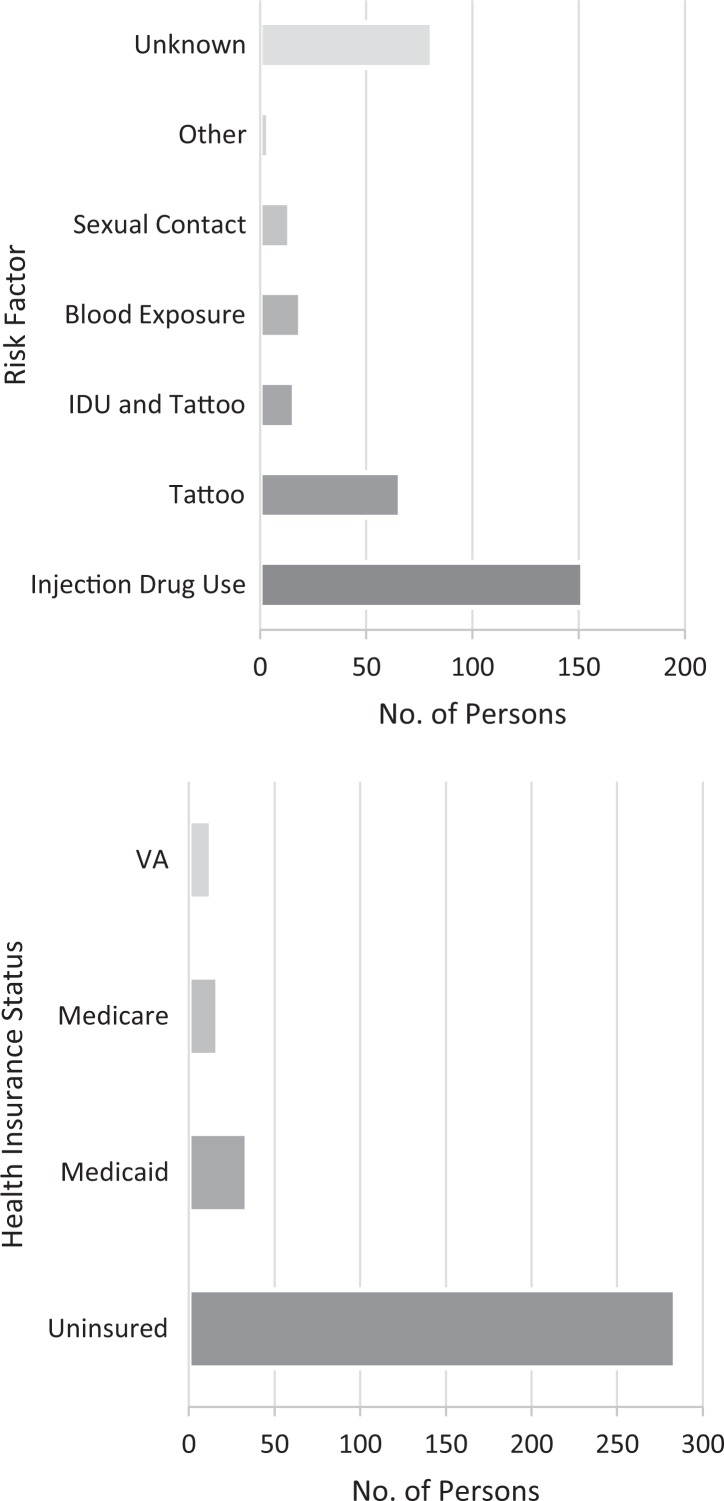
Of 413 persons who tested HCV RNA positive, the median age was 49.1, 207 (50.1%) were born in the birth cohort, 332 (80.6%) were male, and 191 (46.3%) were non-Hispanic black, followed by 161 (39.0%) non-Hispanic white, 60 (14.5%) Hispanic, and 1 (0.2%) unknown (Table 1). Self-reported risk factors among 352 HCV RNA-positive persons were IDU (43.2%), tattoos (18.8%), both IDU and tattoos (4.6%), blood exposure (5.4%), sexual contact (4.0%), unknown (23.0%), and other (1.1%). Reported health insurance types before incarceration were uninsured (81.8%), Medicaid (9.8%), Medicare or dual insurance (ie, Medicare and Medicaid; 4.9%), and US Department of Veterans Affairs (3.7%) (Figure 3).
Table 1.
Age, sex, and race/ethnicity of incarcerated persons screened through opt-out hepatitis C virus (HCV) testing, by screening outcome, at the Dallas County Jail, April 1 through November 2, 2017
| Characteristic | Total | HCV Antibody Positive | HCV Antibody Negative | HCV Antibody Positive, RNA Positive | HCV Antibody Positive, RNA Negative |
|---|---|---|---|---|---|
| Total | 4089 (100.0) | 708 (17.3) | 3366 (82.3) | 413 (58.3) | 151 (21.3) |
| Age, mean (SD), y | 38.8 (12.2) | 47.7 (12.2) | 36.9 (11.3) | 49.1 (11.7) | 45.4 (12.6) |
| Birth cohort | |||||
| Born 1945-1965 | 735 (18.0) | 311 (43.9) | 420 (12.5) | 206 (49.9) | 51 (33.8) |
| Born before 1945 or after 1965 | 3354 (82.0) | 397 (56.1) | 2946 (87.5) | 207 (50.1) | 100 (66.2) |
| Sexa | |||||
| Male | 3166 (77.5) | 541 (76.5) | 2615 (77.8) | 332 (80.6) | 106 (70.2) |
| Female | 917 (22.5) | 166 (23.5) | 746 (22.2) | 80 (19.4) | 45 (29.8) |
| Race/ethnicity | |||||
| Non-Hispanic white | 1178 (28.8) | 308 (43.5) | 869 (25.8) | 161 (39.0) | 86 (57.0) |
| Non-Hispanic black | 2266 (55.4) | 296 (41.8) | 1957 (58.1) | 191 (46.2) | 49 (32.5) |
| Hispanic | 619 (15.1) | 102 (14.4) | 516 (15.3) | 60 (14.5) | 16 (10.6) |
| Unknown | 26 (0.6) | 2 (0.3) | 24 (0.7) | 1 (0.2) | 0 |
Abbreviation: SD, standard deviation. a Missing data for sex resulted in different denominators for the total (n = 4083), number HCV antibody positive (n = 707), number HCV antibody negative (n = 3361), and number HCV antibody positive and RNA positive (n = 412).
Figure 3.
Self-reported risk factors and health insurance before incarceration among incarcerated persons testing RNA positive for hepatitis C virus infection at the Dallas County Jail, April 1 through November 2, 2017. Abbreviations: IDU, injection drug use; VA, US Department of Veterans Affairs.
In addition, the prevalence of HCV antibody positivity among persons released to the Texas Department of Criminal Justice (232 of 961; 24.1%) was significantly higher than among persons released to the community (178 of 1026; 17.4%) or an agency (38 of 403; 9.4%) (P < .001) (Table 2). Of persons who tested HCV RNA positive and were released during the study period, 151 were released to the Texas Department of Criminal Justice, 99 were released to the community, and 18 were released to an agency.
Table 2.
Prevalence of hepatitis C virus (HCV) antibody among incarcerated persons, by release destination from the Dallas County Jail, April 1, 2017, through February 2, 2018
| Release Destination | No. of Persons Screened for HCV Antibody | HCV Antibody Positive, No. (%) |
|---|---|---|
| Total screeneda | 4089 | 708 (17.3) |
| Communityb | 1026 | 178 (17.4) |
| Texas Department of Criminal Justice | 961 | 232 (24.1) |
| Agencyc | 403 | 38 (9.4) |
a Persons screened from April 1 through November 2, 2017, regardless of release status.
b Released to community, including probation, during the study period.
c Released to special program or psychiatric health facility during the study period.
Discussion
We found that 17.3% of the Dallas County Jail population was HCV antibody positive, and the estimated percentage of the jail population with detectable HCV RNA was 12.4%. The prevalence of HCV infection is 1.8% in Texas.2 Our findings are consistent with estimates that HCV prevalence among persons in prisons and jails is up to 10 times higher than in the community.18,19 We found that 71.7% of incarcerated persons who had a positive HCV antibody test had detectable HCV RNA, which is slightly lower than estimates indicating that 74%-86% of persons with HCV infection develop chronic infection.20 Few studies report the percentage of HCV antibody–positive persons in jail and prison who are HCV RNA positive, although estimates range from 65% to 90%.10,21,22 Understanding what proportion of the US population has a detectable HCV RNA level is important in determining the overall disease burden and for budgeting resources for continuity of HCV care.
In our study, half of incarcerated persons who were confirmed HCV RNA positive were born outside of the birth cohort, which is consistent with findings of HCV antibody positivity in this population16 and highlights the importance of expanding screening practices to include younger generations. Recent literature shows that HCV incidence rates among young adults born after 1965 are as high as or higher than HCV incidence rates among baby boomers, and most persons who inject drugs who become infected with HCV are infected as young adults, making this a key population in which to expand screening to identify new cases.23,24
In our study, common self-reported risk factors for HCV infection were IDU, tattoos, or both. IDU is the highest risk factor worldwide for developing HCV infection, and substance use disorders are common among persons in jails and prisons, with a prevalence of 30% to 51% worldwide.25,26 The commonly reported risk factor of IDU combined with the increased spread of HCV infection associated with the opioid epidemic indicates the need for a multidisciplinary approach to address common comorbidities that prevent treatment of HCV infection, such as substance use disorders.27 In addition, a recent meta-analysis confirmed a strong association between tattooing and HCV infection and highlighted reusing tattooing needles as a common practice by incarcerated persons,28 reinforcing the need for patient education and prevention counseling.
In our study, most incarcerated persons who were confirmed HCV RNA positive did not have health insurance, posing challenges for HCV continuity of care after release to the community. Lack of health insurance is a main barrier to obtaining HCV treatment, and the cost effectiveness of screening programs hinges on achieving sustained virologic response.29,30 Texas leads the United States in the number and percentage of persons who lack health insurance,31 and even persons with Medicaid coverage must progress to significant liver fibrosis, a preventable complication of HCV infection, and take a drug screening test to qualify for treatment.32
We found that the estimated prevalence of HCV antibody was higher among persons who were released to prison than among persons who were released to the community or an agency. This finding supports findings that the prevalence of HCV antibody is higher among persons in prisons than among persons in jails.33 Although data on criminal charges were not available in our data set, we suggest that this difference between persons who are sentenced to prison and persons who are released to the community may be related to a longer duration of substance use and recurring or more severe drug-related crimes among those sentenced to prisons, resulting in an increased risk for both HCV infection and a prison sentence. Higher HCV prevalence among persons entering prison, compared with persons entering jails, has further implications for ensuring that persons are not lost to follow-up because they transfer to prisons. Jails need additional infrastructure to notify prisons of persons’ HCV status and to prevent unnecessary testing duplication. In addition, teaching persons to self-advocate can facilitate initiation of future medical care. Long-term incarceration may be an opportunity to provide HCV treatment. Although the high costs of treatment are prohibitive to facilities that would pay to treat incarcerated persons (eg, jails, prisons), costs are declining and policy on treatment eligibility is changing, with more inclusive criteria resulting in increased use of HCV treatment.34
To reduce the burden of HCV infection and transmission, persons with HCV infection need to be linked to care, receive treatment, and achieve sustained virologic response. In our study, the nurse navigator played a key role in reaching out to patients after they were released to the community and helping them connect to care. Post-incarceration linkage to care is challenging because of issues such as unstable housing, lack of transportation, relapse to substance use, and paperwork needed to establish medical care and will require identifying and addressing barriers to care, in addition to providing strong patient support.2 Future research will examine post-incarceration linkage-to-care processes and data from linkage-to-care and clinical outcomes.
Limitations
This study had several limitations. First, screening was limited to a single site; thus, the results may not be generalizable to other populations. Second, screening was offered to all persons already undergoing a blood draw for chronic medical conditions (only 10% of the total jail population) rather than a random sample; as such, our results may not reflect the true prevalence of HCV infection in this population. However, the demographic characteristics of persons tested were representative of the total jail population, and our results likely approximated the overall prevalence of HCV infection at this jail. Third, because risk factors were self-reported, it is possible that IDU was underreported because of its illicit nature and fear of self-incrimination. Fourth, “tattoo” as a risk factor did not specify whether tattoos were acquired in jail or prison or at a tattoo parlor, which limited our ability to further specify the origin of, or suggest prevention interventions for, tattoo-associated HCV infections. Fifth, because of the short duration of incarceration and unknown release dates for our population, our facility does not provide HCV treatment. However, most HCV RNA-positive persons received disease education and transmission prevention, and our nurse navigator continues to work with HCV-infected persons post-release to connect them to care.
Conclusions
Several findings from this study are notable. First, we found that HCV screening with RNA confirmation and patient education is feasible in a jail setting. Second, because of the high prevalence of HCV infection among incarcerated persons, they are a key population in which to conduct screening and link patients to care after release to the community. Third, half of the HCV RNA-positive incarcerated persons in our study were not in the birth cohort, which warrants expansion of screening practices to persons born after 1965. Fourth, common risk factors of IDU and tattoos may require using a multidisciplinary approach to post-incarceration care and patient counseling on HCV transmission while incarcerated. Fifth, most patients with confirmed HCV infection did not have health insurance, posing challenges for HCV continuity of care after release. The high prevalence of HCV antibody-positive persons in prison suggests the need for improved jail infrastructure for notifying prisons of HCV testing results. Future efforts should examine post-incarceration linkage to treatment.
Acknowledgments
The authors thank the Parkland Health and Hospital System team members Patrick M. Jones, Tina M. Hill, Samsher Rawal, Leah Esseltine, Jeanette Hill, Ulysses Prioleau, Ken Dobbs, and Kyung Tae Kim for their contributions to this project.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: A.E.N. is supported by funding from the National Institute of Allergy and Infectious Diseases (K23 AI 112477). This study was funded in part through the Gilead FOCUS program, a branch of the Gilead Sciences Government Affairs division, which partners with health care organizations in implementing hepatitis C virus screening and best practice models. FOCUS supports activities up to first medical appointment and, thus, was not involved in FOCUS partners’ decisions for subsequent patient care, the design of this study, or preparation of this article.
ORCID iD: Caroline M. Abe, BS  https://orcid.org/0000-0002-4128-5538
https://orcid.org/0000-0002-4128-5538
References
- 1. Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013-2016. Hepatology. 2019;69(3):1020–1031. doi:10.1002/hep.30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yalamanchili K, Saadeh S, Lepe R, Davis GL. The prevalence of hepatitis C virus infection in Texas: implications for future health care. Proc (Bayl Univ Med Cent). 2005;18(1):3–6. doi:10.1080/08998280.2005.11928024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell CA, Canary L, Smith N, Teshale E, Ryerson AB, Ward JW. State HCV incidence and policies related to HCV preventive and treatment services for persons who inject drugs—United States, 2015-2016 [published erratum appears in MMWR Morb Mortal Wkly Rep. 2017;66(29):795]. MMWR Morb Mortal Wkly Rep. 2017;66(18):465–469. doi:10.15585/mmwr.mm6618a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003-2013. Clin Infect Dis. 2016;62(10):1287–1288. doi:10.1093/cid/ciw111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312(6):631–640. doi:10.1001/jama.2014.7085 [DOI] [PubMed] [Google Scholar]
- 6. Hochstatter KR, Stockman LJ, Holzmacher R, et al. The continuum of hepatitis C care for criminal justice involved adults in the DAA era: a retrospective cohort study demonstrating limited treatment uptake and inconsistent linkage to community-based care. Health Justice. 2017;5(1):10 doi:10.1186/s40352-017-0055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morris MD, Brown B, Allen SA. Universal opt-out screening for hepatitis C virus (HCV) within correctional facilities is an effective intervention to improve public health. Int J Prison Health. 2017;13(3-4):192–199. doi:10.1108/IJPH-07-2016-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varan AK, Mercer DW, Stein MS, Spaulding AC. Hepatitis C seroprevalence among prison inmates since 2001: still high but declining. Public Health Rep. 2014;129(2):187–195. doi:10.1177/0033354912900213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenberg ES, Rosenthal EM, Hall EW, et al. Prevalence of hepatitis C virus infection in US states and the District of Columbia, 2013 to 2016. JAMA Netw Open. 2018;1(8):e186371 doi:10.1001/jamanetworkopen.2018.6371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hawks L, Norton BL, Cunningham CO, Fox AD. The hepatitis C virus treatment cascade at an urban postincarceration transitions clinic. J Viral Hepat. 2016;23(6):473–478. doi:10.1111/jvh.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beckman AL, Bilinski A, Boyko R, et al. New hepatitis C drugs are very costly and unavailable to many state prisoners. Health Aff (Millwood). 2016;35(10):1893–1901. doi:10.1377/hlthaff.2016.0296 [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization. Guidelines for the Screening, Care and Treatment of Persons With Chronic Hepatitis C Infection. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 13. Federal Bureau of Prisons. Evaluation and management of chronic hepatitis C virus (HCV) infection. 2018. https://www.bop.gov/resources/pdfs/012018_hcv_infection.pdf. Accessed July 8, 2019.
- 14. Beckwith CG, Kurth AE, Bazerman L, et al. Survey of US correctional institutions for routine HCV testing. Am J Public Health. 2015;105(1):68–71. doi:10.2105/AJPH.2014.302071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhen Z. Jail Inmates in 2016. Washington, DC: Bureau of Justice Statistics, US Department of Justice; 2018. [Google Scholar]
- 16. de la Flor C, Porsa E, Nijhawan AE. Opt-out HIV and hepatitis C testing at the Dallas County Jail: uptake, prevalence, and demographic characteristics of testers. Public Health Rep. 2017;132(6):617–621. doi:10.1177/0033354917732755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stata Release [software]. Version 14.0. College Station, TX: StataCorp; 2015. [Google Scholar]
- 18. Gough E, Kempf MC, Graham L, et al. HIV and hepatitis B and C incidence rates in US correctional populations and high risk groups: a systematic review and meta-analysis. BMC Public Health. 2010;10:777 doi:10.1186/1471-2458-10-777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akiyama MJ, Kaba F, Rosner Z, Alper H, Holzman RS, MacDonald R. Hepatitis C screening of the “birth cohort” (born 1945-1965) and younger inmates of New York City jails. Am J Public Health. 2016;106(7):1276–1277. doi:10.2105/AJPH.2016.303163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345(1):41–52. doi:10.1056/NEJM200107053450107 [DOI] [PubMed] [Google Scholar]
- 21. Beckwith CG, Kurth AE, Bazerman LB, et al. A pilot study of rapid hepatitis C virus testing in the Rhode Island Department of Corrections. J Public Health (Oxf). 2016;38(1):130–137. doi:10.1093/pubmed/fdv023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schoenbachler BT, Smith BD, Seña AC, et al. Hepatitis C virus testing and linkage to care in North Carolina and South Carolina jails, 2012-2014. Public Health Rep. 2016;131(suppl 2):98–104. doi:10.1177/003333549161310S215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liang TJ, Ward JW. Hepatitis C in injection-drug users—a hidden danger of the opioid epidemic. N Engl J Med. 2018;378(13):1169–1171. doi:10.1056/NEJMp1716871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morse A, Barritt AS, 4th, Jhaveri R. Individual state hepatitis C data supports expanding screening beyond baby boomers to all adults. Gastroenterology. 2018;154(6):1850–1851. doi:10.1053/j.gastro.2018.02.035 [DOI] [PubMed] [Google Scholar]
- 25. El-Ghitany EM, Abdel Wahab MM, Abd El-Wahab EW, Hassouna S, Farghaly AG. A comprehensive hepatitis C virus risk factors meta-analysis (1989-2013): do they differ in Egypt? Liver Int. 2015;35(2):489–501. doi:10.1111/liv.12617 [DOI] [PubMed] [Google Scholar]
- 26. Fazel S, Yoon IA, Hayes AJ. Substance use disorders in prisoners: an updated systematic review and meta-regression analysis in recently incarcerated men and women. Addiction. 2017;112(10):1725–1739. doi:10.1111/add.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(suppl 2):S56–S61. doi:10.1093/cid/cit271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khodadost M, Maajani K, Arabsalmani M, Mahdavi N, Tabrizi R, Alavian SM. Is tattooing a risk factor for hepatitis C transmission? An updated systematic review and meta-analysis. Hepatitis Monthly. 2017;17(9):e14308 doi:10.5812/hepatmon.14308 [Google Scholar]
- 29. Ditah I, Al Bawardy B, Gonzalez HC, et al. Lack of health insurance limits the benefits of hepatitis C virus screening: insights from the National Health and Nutrition Examination Hepatitis C follow-up study. Am J Gastroenterol. 2015;110(8):1126–1133. doi:10.1038/ajg.2015.31 [DOI] [PubMed] [Google Scholar]
- 30. Stepanova M, Younossi ZM. Interferon-free regimens for chronic hepatitis C: barriers due to treatment candidacy and insurance coverage. Dig Dis Sci. 2015;60(11):3248–3251. doi:10.1007/s10620-015-3709-6 [DOI] [PubMed] [Google Scholar]
- 31. Berchick ER, Hood E, Barnett JC. Health Insurance Coverage in the United States: 2017. Report no. P60-264 Washington, DC: US Census Bureau; 2018. [Google Scholar]
- 32. Texas Department of Health and Human Services. Antiviral agents for hepatitis C virus initial request—standard PA addendum (Medicaid). 2018. https://hhs.texas.gov/laws-regulations/forms/1000-1999/form-1342-antiviral-agents-hepatitis-c-virus-initial-request-standard-pa-addendum-medicaid. 2018. Accessed June 18, 2019.
- 33. Wenger PJ, Rottnek F, Parker T, Crippin JS. Assessment of hepatitis C risk factors and infection prevalence in a jail population. Am J Public Health. 2014;104(9):1722–1727. doi:10.2105/AJPH.2014.301996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kapadia SN, Jeng PJ, Schackman BR, Bao Y. State Medicaid hepatitis C treatment eligibility criteria and use of direct-acting antivirals. Clin Infect Dis. 2018;66(10):1618–1620. doi:10.1093/cid/cix1062 [DOI] [PMC free article] [PubMed] [Google Scholar]