Abstract
Most pleurodont lizard families (anoles, iguanas and their relatives), with the exception of the basilisks and casquehead lizards (family Corytophanidae), share homologous XX/XY sex chromosomes, syntenic with chicken chromosome 15. Here, we used a suite of methods (i.e. RADseq, RNAseq and qPCR) to identify corytophanid sex chromosomes for the first time. We reveal that all examined corytophanid species have partially degenerated XX/XY sex chromosomes, syntenic with chicken chromosome 17. Transcriptomic analyses showed that the expression of X-linked genes in the corytophanid, Basiliscus vittatus, is not balanced between the sexes, which is rather exceptional under male heterogamety, and unlike the dosage-balanced sex chromosomes in other well-studied XX/XY systems, including the green anole, Anolis carolinensis. Corytophanid sex chromosomes may represent a rare example of a turnover away from stable, differentiated sex chromosomes. However, because of poor phylogenetic resolution among pleurodont families, we cannot reject the alternative hypothesis that corytophanid sex chromosomes evolved independently from an unknown ancestral system.
Keywords: dosage compensation, RADseq, reptiles, RNAseq, sex chromosomes, sex determination
1. Introduction
Transitions between sex-determining systems have occurred repeatedly during the course of animal evolution [1,2]. Some of these transitions involve new sex chromosomes evolving from autosomes after acquisition of a sex-determining gene and the subsequent halting of recombination between the X and Y (or Z and W) [3–6]. Newly evolved sex chromosomes typically share a large amount of sequence similarity. However, over time, reduced recombination between the X and Y (or Z and W) results in degeneration of the Y (or W), largely owing to the accumulation of deleterious mutations and genetic hitchhiking, which may lead to differentiated—often heteromorphic—sex chromosomes. Degenerated Y (or W) chromosomes are typically gene-poor, resulting in a difference in copy number of the X- (or Z)-linked genes between males and females. In some but not all species, this may lead to the subsequent evolution of dosage balance to correct the changes to male–female gene expression [5,7]. These chromosomal specializations and long-term stability of differentiated sex chromosomes in many lineages have led to a hypothesis that heteromorphic sex chromosomes may serve as an evolutionary trap, preventing transition to new sex-determining system(s) [1,2,8–10]. While trap-like, differentiated sex chromosomes are thought to be common, identifying escapees can be important to understanding sex chromosome evolution [11].
The lizard clade Pleurodonta (iguanas, anoles and their relatives) has all the hallmarks of an evolutionary trap. Eleven of the twelve recognized families share the same, ancient XX/XY sex chromosome system with gene content homologous to chicken chromosome 15 (GGA15; [12,13]). Their X and Y chromosomes are highly divergent and at least one species, Anolis carolinensis, has complete dosage balance by upregulating expression of male X-linked genes [14,15]. The pleurodont family Corytophanidae (basilisks and casque-headed lizards), however, is an exception. Previous karyotypic work found no evidence of heteromorphic sex chromosomes (albeit using a limited number of taxa; [16–17]). More recent efforts failed to find homologous sex chromosomes between corytophanids and other pleurodonts; rather genes specific to X chromosomes in other iguana families are pseudoautosomal or autosomal in corytophanids [12,18]. Thus, the sex-determining system of corytophanids remains unknown.
Here, we used restriction site-associated DNA sequencing (RADseq) to identify the XX/XY sex chromosomes in the brown basilisk (Basiliscus vittatus) and PCR and quantitative real-time PCR (qPCR) to confirm that other corytophanid species share this same XX/XY system. We also conducted mRNA sequencing (RNAseq) to identify sex-specific genes in the brown basilisk and explore the presence of dosage balance in transcripts from X-specific genes. Lastly, we tested the hypothesis that corytophanid sex chromosomes represent a rare escape from an evolutionary trap. At present, the phylogenetic relationships among pleurodont families are poorly resolved (using both molecular and morphological data) although the Corytophanidae are typically resolved as nested within other pleurodonts [19–22]. This phylogenetic uncertainty makes it unclear whether corytophanids have a derived sex-determining system that evolved via a transition from an ancestral pleurodont XX/XY syntenic with GGA15, representing an escape from the evolutionary trap, or whether corytophanids are the sister lineage to all other pleurodonts, and each group evolved sex chromosomes independently. Furthermore, we employed topology tests, using data from two previous phylogenetic studies, to ascertain whether corytophanids are sister to the remaining pleurodonts.
2. Material and methods
We identified and PCR-validated sex-specific RADseq markers from multiple male and female B. vittatus (electronic supplementary material, table S1) using previously described methods [23–25]. We assessed synteny between the newly identified sex-specific B. vitattus RAD markers and chicken (Gallus gallus) using BLAST [26] and confirmed synteny results using two methods. First, we validated one of the RAD markers with BLAST hits to chicken genes using PCR with one or both primer pairs anchored in the gene's coding region (electronic supplementary material, tables S2 and S3). Second, we used qPCR to measure sex differences in the number of gene copies for putative X-specific genes. In species with degenerated Y chromosomes, females (XX) have twice as many X copies as males (XY), whereas genes in autosomal, pseudoautosomal, or poorly differentiated sex-specific regions have equal copy number in both sexes. Sex-specific variation in gene copy number is detectable by qPCR (for detailed methodology see [27,28]). We further tested both PCR and qPCR primers in four additional corytophanid species, representing all corytophanid genera, and A. carolinensis (electronic supplementary material, table S1) to investigate whether corytophanids share homologous sex chromosomes (electronic supplementary material, table S4).
Total RNA was extracted from the eyes of three male and four female B. vittatus. Paired-end RNAseq (80 bp reads) was conducted using Illumina NextSeq500 by GeneCore (EMBL, Heidelberg, Germany), resulting in 61–95 million reads per specimen after quality filtering. RNAseq data from the male basilisks were used to identify putative X-linked hemizygous genes based on polymorphism analysis using previously described methods [9,29]—loci on the non-recombining portion of the X should be hemizygous and lack single nucleotide polymorphisms (SNPs) in all male individuals. RNAseq data from both sexes were used to investigate dosage balance (using previously described methodology [9,29]). Briefly, a male reference transcriptome was assembled using Trinity [30]. We mapped RNAseq reads from each individual independently to the reference and calculated FPKM (fragments per kilobase million) expression values in Geneious R7.1 (https://www.geneious.com). Next, we assigned as many transcripts as possible to putative syntenic blocks according to the chromosomal position of their chicken (Gallus gallus) orthologs (GRCg6a; http://www.ensembl.org). We removed transcripts less than 300 bp and all gene duplicates (electronic supplementary material, table S5). Differences in the expression between sexes and among particular putative syntenic blocks were tested using analysis of variance (ANOVA) of log2-transformed ratios of male to female average gene-specific FPKM values.
Finally, we tested whether we could reject a phylogenetic hypothesis of Corytophanidae as sister to a clade composed of the remaining pleurodont families using two datasets: (1) a multi-locus molecular genetic dataset [21] using the maximum likelihood (ML)-based AU test with 10 000 RELL bootstraps, implemented in IQtree [31,32] and (2) a morphological dataset [19] using the parsimony-based Wilcoxon signed-ranks test in PAUP* [v. 4.0a165] [33,34].
3. Results and discussion
(a). RADseq and identification of sex chromosome genomic content in Basiliscus vittatus
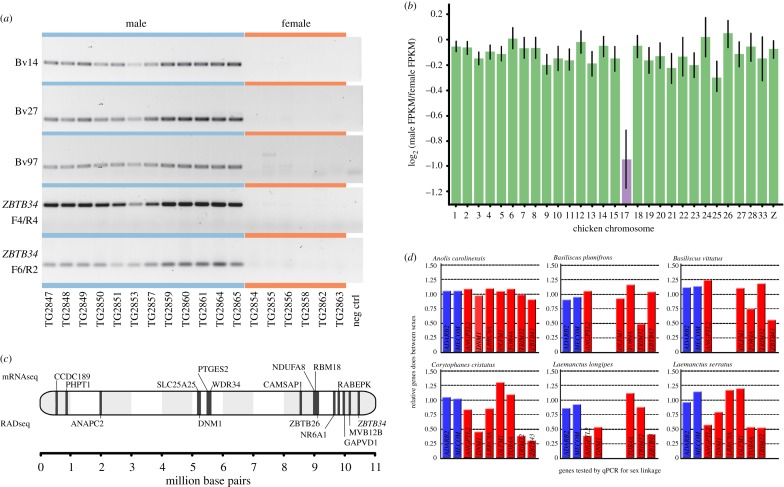
We identified 160 confirmed male-specific RAD tags and four confirmed female-specific RAD tags, considering RAD loci with either 1 or 2 alleles. Because sex-specific RAD markers are presumed to be on the Y (or W) chromosome, the excess of male-specific RAD markers indicates an XX/XY system [23,35] (figure 1; electronic supplementary material, figure S1). BLAST of male-specific RAD markers to chicken genes had twelve matches, eight of which were on chicken chromosome 17 (GGA17; electronic supplementary material, table S2). PCR validation of a subset of the male-specific RAD markers, including the GGA17 gene ZBTB34, showed male-specific amplification in B. vittatus (figure 1; electronic supplementary material table S3). qPCR of seven GGA17 genes, as well as three autosomal genes for positive control and qPCR normalization (electronic supplementary material, table S4), confirmed that the sex chromosomes in B. vittatus are syntenic with GGA17.
Figure 1.
(a) Male-specific PCR amplification of RADseq markers and two fragments of the sex-linked ZBTB34 gene in 12 male and six female B. vittatus. (b) Comparison of sex-specific gene expression from B. vittatus, genes clustered by chicken chromosome. Genes on GGA17 (in purple, the corytophanid X chromosome) lack dosage balance. (c) Location of B. vittatus hemizygous transcripts (top) and RADseq BLAST hits (bottom) on GGA17. (d) Relative male/female gene dose for seven GGA17 (in red) and two autosomal genes (in blue) in five corytophanid species and Anolis carolinensis, as measured by qPCR. Empty columns did not successfully amplify. X-linked hemizygous genes should have approximately half the gene dose of autosomal or pseudoautosomal genes (see electronic supplementary material, table S4 for details). (Online version in colour.)
(b). Sex chromosome homology in Corytophanidae
Comparative qPCR confirmed that five out of the seven GGA17 genes are sex linked in other corytophanid species but appeared autosomal or pseudoautosomal in A. carolinensis (figure 1; electronic supplementary material, table S4). Notably, these five genes did not give the pattern expected for X-specific genes in all corytophanid species, but instead exhibited an autosomal or pseudoautosomal pattern in some species (figure 1d). Many qPCR values from GGA17 genes show male:female ratios of 1, but we did not observe a clear phylogenetic pattern. Therefore, we assume that the corytophanid X is either highly rearranged, and/or exhibits a high degree of lineage-specific Y chromosome degeneration, with some species maintaining more extensive pseudoautosomal regions, or poorly differentiated gametologs in both sex chromosomes. Despite this variability, all corytophanids appear to have homologous sex chromosomes (syntenic with GGA17), which can be dated to 15–50 Mya (i.e. the crown age of corytophanids, corresponding to the minimum age of this sex chromosome system) [19,21,22,36,37]. One set of B. vittatus ZBTB34 primers also amplified in a sex-specific manner in B. plumifrons (electronic supplementary material figure S2). Sex-specific RADtags are on the Y chromosome and thus not directly comparable to the X-linked alleles, which we tested for X-specificity by qPCR. Owing to limitations on primer design, including sub-optimal thermodynamics, restrictions on the amplicon size (150–200 bp) and non-specific amplification (e.g. owing to presence of paralogs), we were not able to test the X-specificity of additional genes from our RAD-seq and RNA-seq datasets by qPCR.
(c). SNPs analysis of RNAseq data
Nine out of 40 B. vittatus transcripts with homologs on GGA17 lacked SNPs and were expressed from hemizygous X-specific loci. GGA17 is significantly enriched for these hemizygous loci compared with transcripts mapping to other chicken chromosomes (Fisher's post hoc test: p < 0.00001). Thus, the 31 remaining GGA17 transcripts have SNPs and are likely pseudoautosomal, suggesting the non-recombining portion of the corytophanid X corresponds to a rather small fraction of GGA17, consistent with our qPCR result.
We also identified a lack of dosage balance in B. vittatus, and ANOVA showed log2-transformed ratios of male to female average FPKM of GGA17 genes differed significantly from genes located on other chicken chromosomes (F28,2098 = 4.26, p < 0.00001). Dosage balance appears more common in XX/XY species than ZZ/ZW species in animals [7]. Thus, comparing B. vittatus to other pleurodonts with dosage balance, e.g. anoles [14,15], may be a fruitful way to investigate the evolution and maintenance of dosage balance.
(d). Phylogenetic topology tests
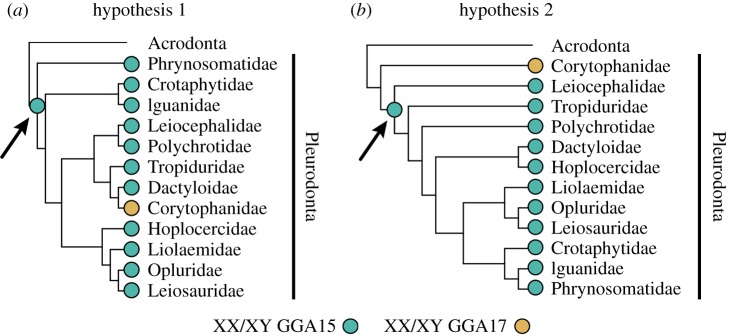
A defining characteristic of clades caught in a sex chromosome trap is that no taxa within that clade have transitioned to a different sex chromosome system [10,23,38]. Transitions among sex chromosomes can be identified by interpreting sex chromosome evolution in a phylogenetic context. Thus, uncertainty in the underlying phylogenetic relationships can extend into uncertainty of the timing and directionality of sex chromosome evolution. In cases where the underlying phylogeny is poorly resolved, phylogenetic topology tests offer one way of investigating alternative hypotheses. This involves testing whether a topology that constrains the clade with the ‘new’ sex chromosome system as sister to a clade of the remaining taxa with the trap-like sex chromosomes is a significantly worse fit to the data than the optimal tree (figure 2). Phylogenetic relationships among pleurodont families are poorly resolved, characterized by short internal branch lengths and low nodal support, e.g. bootstrap [19–22]. These conflicting topologies make it difficult to interpret our results and lead to two conflicting hypotheses (figure 2). Hypothesis 1 posits an XX/XY syntenic with GGA15 evolving in the most recent common ancestor to all pleurodonts and subsequent transition away in corytophanids, thus an escape from the evolutionary trap. Hypothesis 2, where corytophanids are the sister clade to the remaining pleurodonts, has XX/XY systems evolving on homologs of GGA15 and GGA17 independently from an ancestor with some unknown sex-determining system. We could not reject Corytophanidae as sister to remaining pleurodonts (hypothesis 2) using either molecular [21] or morphological data [19] (electronic supplementary material, figures S3–S6): AU test of molecular genetic data (tree loglikelihood: best tree = −356338.318; alternative topology = −356366.256; difference in loglikelihood = 27.938; p = 0.879) and Wilcoxon signed-ranks test of morphological data (tree length: best tree = 2105; alternative topology = 2108; N = 59; z = −0.367; p = 0.714). Therefore, we cannot differentiate whether corytophanids represent a rare case of the turnover of differentiated sex chromosomes, or whether non-homologous XX/XY sex chromosomes evolved independently in corytophanids and other pleurodont families.
Figure 2.
Two phylogenetic hypotheses for investigating pleurodont sex chromosome evolution. (a) Hypothesis 1, modified from [19], shows corytophanids nested among other pleurodonts and implies the XX/XY syntenic with GGA15 evolved in the most recent common ancestor to all pleurodonts (arrow) with a subsequent transition to GGA17 in corytophanids. (b) Hypothesis 2 shows corytophanids as sister to the remaining pleurodonts with sex chromosomes evolving independently in each clade. The XX/XY syntenic with GGA15 evolved after the split with Corytophanidae (arrow). (Online version in colour.)
(e). Sex chromosome evolution
There is an ongoing debate whether some chromosomes are more likely to become sex chromosomes than others [29,39,40]. For example, the chromosome containing the testis-determining gene DMRT1 has become a sex chromosome multiple times in amniotes [41–45]. Chromosomes homologous to GGA17 are parts of sex chromosomes not only in corytophanids, but also lacertid lizards, Paroedura geckos, the agamid Pogona vitticeps and monotremes [29,45,46]. GGA17 contains at least one gene, NR5A1 (also known as steroidogenic factor 1 or Sf1), which is critical for gonadal development [47,48]. While our data did not identify a candidate B. vittatus sex-determining gene, NR5A1 may be a promising nominee.
4. Conclusion
Our combination of RADseq, RNAseq and qPCR methods revealed that B. vittatus and all other corytophanid genera share an XX/XY sex-determination system, syntenic with GGA17. Corytophanid sex chromosomes are not homologous to the XX/XY sex chromosomes syntenic with GGA15 shared by all other pleurodont families. The green anole, with typical pleurodont XX/XY sex chromosomes, possesses global dosage compensation [14,15]. Male and female B. vittatus, on the other hand, differ in the expression of X-specific genes representing a rare exception of a lack of dosage balance under male heterogamety. Future investigations should focus on resolving the pleurodont phylogeny. This may solve the current uncertainty of whether corytophanids and other Pleurodonta sex chromosomes evolved independently from an unknown ancestral sex-determination system, or whether corytophanids are nested among other pleurodonts and escaped from an evolutionary trap.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank laboratory members and Petr Kodym for their support and assistance throughout this study. We also thank the editors and anonymous reviewers for insights that improved the manuscript.
Ethics
USA: Fieldwork conducted under Miami-Dade County Parks and Recreation Scientific Research Permit no. 263-2016 and MU IACUC AR-288 (T.G. and S.V.N.). C.Z.: Ethical committee permission MSMT- 8604/2019-7 (M.R.). All methods were carried out in accordance with relevant guidelines and regulations, by researchers accredited for animal experimental design.
Data accessibility
Sequencing reads are deposited at NCBI (SAMN12231009-23), under the BioProject PRJNA553192. Custom python script adapted from [21] (https://github.com/DrPintoThe2nd/Nielsen-et-al.-python-script).
Authors' contributions
Conceived project (S.V.N., T.G., M.R. and L.K.), fieldwork (T.G. and S.V.N.), laboratory work (S.V.N., I.A.G.M., B.J.P. and M.R.), bioinformatics (M.B., M.R., T.G., B.J.P. and I.A.G.M.), statistics (L.K.) and wrote the first draft of the manuscript (T.G., S.V.N., M.R. and L.K.). All authors contributed to the final form, read and approved the final manuscript, and agree to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
M.B. was supported by NSF-DBI1659595 to A. Abbott and E. Blumenthal; B.J.P. was supported by NSF-DEB1657662 [to T.G.]; I.A.G.M. was supported by CONACYT 2018-000022-01EXTV-00437; M.R. was supported by GAČR 19-19672S, Charles University PRIMUS/SCI/46 and Research Centre program (204069).
References
- 1.Bachtrog D, et al. 2014. Sex determination: why so many ways of doing it? PLoS Biol. 12, e1001899 ( 10.1371/journal.pbio.1001899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bull JJ. 1983. Evolution of sex determining mechanisms. Menlo Park, CA: Benjamin Cummings Publishing Company, Inc. [Google Scholar]
- 3.Bachtrog D. 2013. Y chromosome evolution: emerging insights into processes of Y chromosome degeneration. Nat. Rev. Genet. 14, 113–124. ( 10.1038/nrg3366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graves JAM. 2008. Weird animal genomes and the evolution of vertebrate sex and sex chromosomes. Annu. Rev. Genet. 42, 565–586. ( 10.1146/annurev.genet.42.110807.091714) [DOI] [PubMed] [Google Scholar]
- 5.Ohno S. 1967. Sex chromosomes and sex-linked genes. Berlin, Germany: Springer. [Google Scholar]
- 6.Wright AE, Dean R, Zimmer F, Mank JE. 2016. How to make a sex chromosome. Nat. Commun. 7, 12087 ( 10.1038/ncomms12087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mank JE. 2013. Sex chromosome dosage compensation: definitely not for everyone. Trends Genet. 29, 677–683. ( 10.1016/j.tig.2013.07.005) [DOI] [PubMed] [Google Scholar]
- 8.Bull J, Charnov E. 1985. On irreversible evolution. Evolution 39, 1149–1155. ( 10.1111/j.1558-5646.1985.tb00455.x) [DOI] [PubMed] [Google Scholar]
- 9.Rovatsos M, Vukić J, Kratochvíl L. 2016. Mammalian X homolog acts as sex chromosome in lacertid lizards. Heredity 117, 8–13. ( 10.1038/hdy.2016.18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pokorná M, Kratochvíl L. 2009. Phylogeny of sex-determining mechanisms in squamate reptiles: are sex chromosomes an evolutionary trap? Zool. J. Linn. Soc. 156, 168–183. ( 10.1111/j.1096-3642.2008.00481.x) [DOI] [Google Scholar]
- 11.Vicoso B, Bachtrog D. 2013. Reversal of an ancient sex chromosome to an autosome in Drosophila. Nature 499, 332–335. ( 10.1038/nature12235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altmanová M, Rovatsos M, Pokorná MJ, Veselý M, Wagner F, Kratochvíl L. 2018. All iguana families with the exception of basilisks share sex chromosomes. Zoology 126, 98–102. ( 10.1016/j.zool.2017.11.007) [DOI] [PubMed] [Google Scholar]
- 13.Alföldi J, et al. 2011. The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 477, 587–591. ( 10.1038/nature10390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marin R, et al. 2017. Convergent origination of a Drosophila-like dosage compensation mechanism in a reptile lineage. Genome Res. 27, 1974–1987. ( 10.1101/gr.223727.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rupp SM, Webster TH, Olney KC, Hutchins ED, Kusumi K, Sayres MAW. 2017. Evolution of dosage compensation in Anolis carolinensis, a reptile with XX/XY chromosomal sex determination. Genome Biol. Evol. 9, 231–240. ( 10.1093/gbe/evw263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorman GC, Atkins L, Holzinger T. 1967. New karyotypic data on 15 genera of lizards in the family Iguanidae with a discussion of taxonomic and cytological implications. Cytogenetics 6, 286–299. ( 10.1159/000129949) [DOI] [PubMed] [Google Scholar]
- 17.Schwenk K, Sessions SK, Peccinini SDM. 1982. Karyotypes of the basiliscine lizards Corytophanes cristatus and Corytophanes hernandesii, with comments on the relationship between chromosomal and morphological evolution in lizards. Herpetologica 38, 493–501. [Google Scholar]
- 18.Rovatsos M, Pokorná M, Altmanová M, Kratochvíl L. 2014. Cretaceous park of sex determination: sex chromosomes are conserved across iguanas. Biol. Lett. 10, 20131093 ( 10.1098/rsbl.2013.1093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conrad JL. 2015. A new Eocene casquehead lizard (Reptilia, Corytophanidae) from North America. PLoS ONE 10, e0127900 ( 10.1371/journal.pone.0127900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streicher JW, Schulte JA II, Wiens JJ. 2015. How should genes and taxa be sampled for phylogenomic analyses with missing data? An empirical study in iguanian lizards. Syst. Biol. 65, 128–145. ( 10.1093/sysbio/syv058) [DOI] [PubMed] [Google Scholar]
- 21.Townsend TM, Mulcahy DG, Noonan BP, Sites JW Jr, Kuczynski CA, Wiens JJ, Reeder TW. 2011. Phylogeny of iguanian lizards inferred from 29 nuclear loci, and a comparison of concatenated and species-tree approaches for an ancient, rapid radiation Mol. Phylogenet. Evol. 61, 363–380. ( 10.1016/j.ympev.2011.07.008) [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, Wiens JJ. 2016. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol. Phylogenet. Evol. 94, 537–547. ( 10.1016/j.ympev.2015.10.009) [DOI] [PubMed] [Google Scholar]
- 23.Gamble T, Coryell J, Ezaz T, Lynch J, Scantlebury DP, Zarkower D. 2015. Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining mechanisms. Mol. Biol. Evol. 32, 1296–1309. ( 10.1093/molbev/msv023) [DOI] [PubMed] [Google Scholar]
- 24.Baxter SW, Davey JW, Johnston JS, Shelton AM, Heckel DG, Jiggins CD, Blaxter ML. 2011. Linkage mapping and comparative genomics using next-generation RAD sequencing of a non-model organism. PLoS ONE 6, e19315 ( 10.1371/journal.pone.0019315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH. 2011. Stacks: building and genotyping loci de novo from short-read sequences. G3: Genes, Genomes, Genetics 1, 171–182. ( 10.1534/g3.111.000240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403–410. ( 10.1016/S0022-2836(05)80360-2) [DOI] [PubMed] [Google Scholar]
- 27.Rovatsos M, Altmanová M, Pokorná MJ, Kratochvíl L. 2014. Novel X-linked genes revealed by quantitative polymerase chain reaction in the green anole, Anolis carolinensis. G3: Genes, Genomes, Genetics 4, 2107–2113. ( 10.1534/g3.114.014084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gamble T, Geneva AJ, Glor RE, Zarkower D. 2014. Anolis sex chromosomes are derived from a single ancestral pair. Evolution 68, 1027–1041. ( 10.1111/evo.12328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rovatsos M, Farkačová K, Altmanová M, Johnson Pokorná M, Kratochvíl L. 2019. The rise and fall of differentiated sex chromosomes in geckos. Mol. Ecol. 28, 3042–3052. ( 10.1111/mec.15126) [DOI] [PubMed] [Google Scholar]
- 30.Grabherr MG, et al. 2011. Trinity: reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 29, 644–652. ( 10.1038/nbt.1883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chernomor O, von Haeseler A, Minh BQ.. 2016. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 65, 997–1008. ( 10.1093/sysbio/syw037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimodaira H. 2002. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 51, 492–508. ( 10.1080/10635150290069913) [DOI] [PubMed] [Google Scholar]
- 33.Swofford D. 2002. PAUP*. phylogenetic analysis using parsimony (*and other methods) (v. 4.0a165). Sunderland, MA: Sinauer. [Google Scholar]
- 34.Templeton AR. 1983. Phylogenetic inference from restriction endonuclease cleavage site maps with particular reference to the evolution of humans and the apes. Evolution 37, 221–244. ( 10.1111/j.1558-5646.1983.tb05533.x) [DOI] [PubMed] [Google Scholar]
- 35.Gamble T, Zarkower D. 2014. Identification of sex-specific molecular markers using restriction site associated DNA sequencing. Mol. Ecol. Resour. 14, 902–913. ( 10.1111/1755-0998.12237) [DOI] [PubMed] [Google Scholar]
- 36.Prates I, Rodrigues MT, Melo-Sampaio PR, Carnaval AC. 2015. Phylogenetic relationships of Amazonian anole lizards (Dactyloa): taxonomic implications, new insights about phenotypic evolution and the timing of diversification. Mol. Phylogenet. Evol. 82, 258–268. ( 10.1016/j.ympev.2014.10.005) [DOI] [PubMed] [Google Scholar]
- 37.Taylor GW, Santos JC, Perrault BJ, Morando M, Vásquez Almazán CR, Sites JW Jr. 2017. Sexual dimorphism, phenotypic integration, and the evolution of head structure in casque-headed lizards. Ecol. Evol. 7, 8989–8998. ( 10.1002/ece3.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pennell MW, Mank JE, Peichel CL. 2018. Transitions in sex determination and sex chromosomes across vertebrate species. Mol. Ecol. 27, 3950–3963. ( 10.1111/mec.14540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graves JA.M, Peichel CL. 2010. Are homologies in vertebrate sex determination due to shared ancestry or to limited options? Genome Biol. 11, 205 ( 10.1186/gb-2010-11-4-205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Meally D, Ezaz T, Georges A, Sarre SD, Graves JAM. 2012. Are some chromosomes particularly good at sex? Insights from amniotes. Chromosome Res. 20, 7–19. ( 10.1007/s10577-011-9266-8) [DOI] [PubMed] [Google Scholar]
- 41.Nielsen SV, Daza JD, Pinto BJ, Gamble T. 2019. ZZ/ZW sex chromosomes in the endemic Puerto Rican leaf-toed gecko (Phyllodactylus wirshingi). Cytogenet Genome Res. 157, 89–97. ( 10.1159/000496379) [DOI] [PubMed] [Google Scholar]
- 42.Kawai A, Ishijima J, Nishids C, Kosaka A, Ota H, Kohno S, Matsuda Y. 2009. The ZW sex chromosomes of Gekko hokouensis (Gekkonidae, Squamata) represent highly conserved homology with those of avian species. Chromosoma 118, 43–51. ( 10.1007/s00412-008-0176-2) [DOI] [PubMed] [Google Scholar]
- 43.Nanda I, et al. 1999. 300 million years of conserved synteny between chicken Z and human chromosome 9. Nat. Genet. 21, 258–259. ( 10.1038/6769) [DOI] [PubMed] [Google Scholar]
- 44.Kawagoshi T, Uno Y, Nishida C, Matsuda Y. 2014. The Staurotypus turtles and aves share the same origin of sex chromosomes but evolved different types of heterogametic sex determination. PLoS ONE 9, e105315 ( 10.1371/journal.pone.0105315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veyrunes F, et al. 2008. Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes. Genome Res. 18, 965–973. ( 10.1101/gr.7101908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deakin JE, et al. 2016. Anchoring genome sequence to chromosomes of the central bearded dragon (Pogona vitticeps) enables reconstruction of ancestral squamate macrochromosomes and identifies sequence content of the Z chromosome. BMC Genomics 17, 447 ( 10.1186/s12864-016-2774-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekido R, Lovell-Badge R. 2008. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453, 930–934. ( 10.1038/nature06944) [DOI] [PubMed] [Google Scholar]
- 48.Bashamboo A, et al. 2016. A recurrent p.Arg92Trp variant in steroidogenic factor-1 (NR5A1) can act as a molecular switch in human sex development. Hum. Mol. Genet. 25, 3446–3453. ( 10.1093/hmg/ddw186) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing reads are deposited at NCBI (SAMN12231009-23), under the BioProject PRJNA553192. Custom python script adapted from [21] (https://github.com/DrPintoThe2nd/Nielsen-et-al.-python-script).