Abstract
Spatial learning is an ecologically important trait well studied in vertebrates and a few invertebrates yet poorly understood in crustaceans. We investigated the ability of European shore crabs, Carcinus maenas, to learn a complex maze over four consecutive weeks using food as a motivator. Crabs showed steady improvement during this conditioning period in both the time taken to find the food and in the number of wrong turns taken. Crabs also clearly remembered the maze as when returned two weeks later but without any food, they all returned to the end of the maze in under 8 min. Crabs that had not been conditioned to the maze (naive animals) took far longer to reach the end, and many (42%) did not venture to the end of the maze at all during the 1 h study period. This study provides an initial description of spatial learning in a benthic decapod; a better appreciation of this adaptive trait in these animals will develop our understanding of resource exploitation by benthic crustaceans and their ecological roles.
Keywords: crab, Carcinus maenas, spatial learning, maze
1. Background
Some forms of learning, for instance habituation and sensitization, are evident throughout the animal kingdom [1]. More complex forms of learning, such as spatial learning, have so far been demonstrated in only vertebrates and a select number of invertebrate species [2–7]. Insects, for example, display an extensive repertoire of learned behaviours and some impressive cognitive abilities [6,8], but aquatic arthropods, such as crustaceans, are poorly studied despite their key roles in marine and freshwater ecosystems. The substantial differences between crustacean and insectan brains [9], especially the much lower neuronal counts in crustaceans (for example, ca 90 000 neurons in a crayfish brain [10], cf. with ca 1 million in a honeybee brain [11]), might predict a diminished level of behavioural complexity in Crustacea, but the relationship between brain size (measured by either volume or the number of neurons) and behavioural complexity is far from consistent [8]. Decapod crustaceans, for example, show a variety of sophisticated navigational behaviours, including homing [12], path integration [13] and true navigation [14].
Decapod crustaceans often live in complex, three-dimensional, benthic habitats. Learning the location of, and routes to, resources should, therefore, be an adaptive trait that we can investigate using mazes. Mazes provide a quantifiable measure of an animal's performance, and while investigations into spatial learning in insects have used some quite complex maze configurations [7,15,16], crustacean studies have used much simpler arrangements (cross-, Y- or T-shaped mazes [17–20]), and the ability of crustaceans to solve more complex mazes has not been explored since some very limited studies in the early twentieth century [21,22]. We, therefore, used a more complex, multiple-turn maze, resembling those used in classic mouse studies (reviewed in [3]), to investigate spatial learning in the European shore crab, Carcinus maenas; an important generalist predator and scavenger in intertidal and shallow sea ecosystems. Our experimental design differed from many spatial learning studies in that animals were tested weekly, rather than several times a day, to investigate the formation of memory over longer timescales. A better appreciation of spatial learning in decapods will develop our understanding of resource exploitation by benthic crustaceans and their ecological roles, as well as leading to potential comparative studies with other animals, especially their insectan allies.
2. Material and methods
(a). Animals
Twelve C. maenas (mean carapace width, CW, ±1 s.d. = 54 ± 16 mm, range = 32–82 mm; mean weight ± 1 s.d. = 28.7 ± 13.0 g, range = 6.2–43.3 g) were collected from two locations in South Wales: Oxwich Bay (51°32′48.04″ N, 4°8′38.41″ W) and Swansea Docks (51°36′59.26″ N, 3°55′6.38″ W) and kept individually in 30 l tanks connected to a recirculating 40 000 l seawater system. All crabs were healthy with intact appendages and identified by the tank they were kept in (1–12). Animals acclimated to this system for four weeks under an illumination cycle of 13 L : 11 D and were fed half a blue mussel, Mytilus edulis, twice a week before commencement of the study. No crabs died or moulted during the study.
(b). Maze design
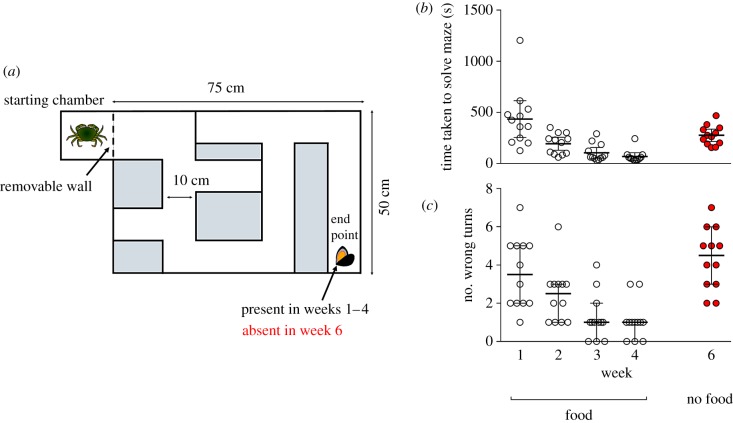
A maze with external dimensions 75 cm × 50 cm × 12.5 cm high was constructed from 8 mm opaque black Perspex (figure 1a). A starting chamber (15 cm × 15 cm × 12.5 cm high) was positioned adjacent to the entrance and separated from the main maze with a removable piece of black 8 mm Perspex. The maze had a single correct path to the endpoint, requiring five changes of direction and included three dead ends. All passages were 10 cm wide and a direct route from the starting box to the endpoint required the crabs to traverse ca 2 m.
Figure 1.
(a) Scale schematic of the experimental maze showing an individual C. maenas present in the starting chamber and a single, crushed M. edulis (present in weeks 1–4, absent in week 6) at the endpoint. (b) Time taken to reach the endpoint of the maze (latency; s) by C. maenas individuals in weeks 1–6. Lines = mean ± 95% confidence intervals (CIs), n = 12. (c) The number of wrong turns taken by individual C. maenas in weeks 1–6. Lines = median ± 95% CIs, n = 12. Carcinus maenas clipart courtesy of Tanya L. Rogers. (Online version in colour.)
(c). Conditioning study
Crabs were tested weekly on the same day for four weeks; all crabs were fasted for a minimum of 3 days before they were tested, with some fasted for 5 days. The maze was placed in a large raceway tank (1.5 m × 1 m) in the same room as the holding tanks and both the maze and raceway were filled with still system water to a depth of 10 cm. Individual crabs were placed in the starting chamber and a single crushed mussel was placed at the maze endpoint. After a 60 s acclimation period, the wall between the starting chamber and maze was removed. Movements of the crab were recorded using a Praktica DVC5.1 high definition video camera mounted on a tripod without additional lighting. The trial stopped when the crab located the food and started to feed, or after 60 min had elapsed. Nobody was present in the laboratory during the trial, with the maze checked after 30 min and then every 15 min until the end of the trial. The maze and raceway were emptied, cleaned and refilled between each trial. The video was used to calculate latency (defined as the time elapsed) and the number of wrong turns taken while trying to reach the end of the maze.
(d). Trials without food
Crabs from the conditioning study (hereafter ‘conditioned’) were tested again after six weeks (two weeks after the last conditioning trial) in the absence of food. The trials were identical to the conditioning study but with no mussel at the endpoint. The maze was thoroughly cleaned with EtOH in week 5 to remove any scent from the maze. To investigate whether another factor might attract the crabs to the endpoint, 12 new (naive) C. maenas (mean CW ± 1 s.d. = 51 ± 19 mm, range = 34–89 mm; mean weight ± 1 s.d. = 26.1 ± 14.6 g, range = 7.7–50.0 g) were collected from Oxwich Bay and maintained in individual tanks in the system for four weeks as before, then tested in the maze in the absence of food. There was no significant difference in the mean CW (unpaired t-test, td.f.=22 = 0.522, p = 0.607) or weight (unpaired t-test, td.f.=22 = 0.474, p = 0.640) between the naive and conditioned crabs.
(e). Data analysis
Latency and the number of wrong turns were analysed using separate generalized linear mixed-effects models. Latency was natural logarithm-transformed and modelled as a Gaussian process. The number of wrong turns was modelled as a Poisson process. The week was initially treated as a categorical variable and crab weight as a continuous variable; both as main effects and interacting. Data were grouped by individual crab, fitted as random intercepts. The significance of fixed effects was tested using likelihood ratios tests. Pairwise comparisons between weeks were assessed using post hoc Tukey tests. Subsequently, week 6 was dropped from the model, and week was refitted as a linear response, interacting with weight. Here, week was modelled with random intercepts and slopes, by crab. The degree to which individuals deviated from population-average model predictions was quantified using concordance correlation coefficients (ρc) [23]. The latency of conditioned and naive crabs in the absence of food was compared using a Mann–Whitney U-test. Statistical analyses were performed using R v. 3.6.0 [24] and GraphPad Prism 7; mixed-effects modelling was undertaken using the R package, lme4 [25].
3. Results
(a). Conditioning study
All crabs completed the maze within 25 min when food was present. Crab weight did not significantly affect latency (weight × week: , p = 0.95, weight: , p = 0.83) or the number of wrong turns (weight × week: , p = 0.20, weight: , p = 0.92). Latency showed a significant log-linear trend over time (slope = −0.634, s.e. = 0.079, td.f.=11 = 7.98, p < 0.001), decreasing from 435 ± 283 s (mean ± 1 s.d.) in week 1 to 68 ± 58 s by week 4 (figure 1b). Crabs also took fewer wrong turns in successive weeks; there was a significant, negative log-linear trend in the number of wrong turns over time (slope = −0.455, s.e. = 0.107, z = 4.24, p < 0.001), with the median number of wrong turns decreasing from 3.5 (interquartile range, IQR 2–5) in week 1 to one (IQR 0.25–1) in week 4 (figure 1c).
Concordance correlation between individual crab performance and population-average predictions ranged between ρc = 0.686–0.977 (median = 0.923) for latency and ρc = 0.623–0.925 (median = 0.896) for the number of wrong turns, differences between slopes (latency: cvslopes = 24.6%; wrong turns: cvslopes = 20.8%) dominated rather than intercepts (latency: cvintercepts = 5.75%; wrong turns: cvintercepts = 2.28%). There was little rank correlation among individuals between concordance correlation coefficients for latency and wrong turns (Kendall's τ = 0.091, p = 0.74), nor between individual response intercepts (Kendall's τ = −0.382, p = 0.09) or individual slopes over time (Kendall's τ = 0.030, p = 0.95) for latency and wrong turns.
(b). Trials without food
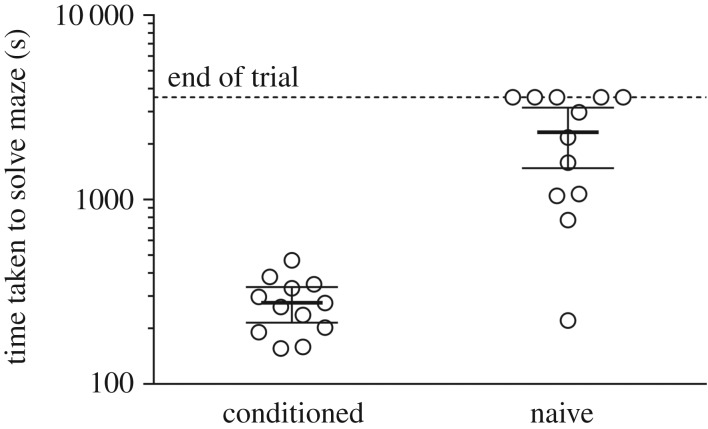
All conditioned (week 6) crabs moved to the endpoint within 8 min in the absence of food; mean (±1 s.d.) latency for these animals was 276 ± 95 s, which was significantly greater than in weeks 3 and 4 in the presence of food (Tukey's multiple comparisons: week 3 versus 6, mean difference = 181 s, p < 0.001, week 4 versus 6, mean difference = 204 s, p < 0.001) but not significantly different from crabs in weeks 1 or 2 (Tukey's multiple comparisons: week 1 versus 6, mean difference = −108s, p = 0.458, week 2 versus 6, mean difference = 94.5 s, p = 0.193). There was a significant difference in latency between naive and conditioned (week 6) crabs (Mann–Whitney U = 8, p < 0.0001; figure 2) with only seven naive crabs reaching the endpoint within the 60 min trial and a mean (±1 s.d.) latency for all 12 naive crabs of 2321 ± 1320 s.
Figure 2.
Time taken to reach the endpoint of the maze (latency; s) for conditioned (n = 12) and naive (n = 12) C. maenas individuals in week 6 (food absent). Lines show mean values ± 95% CIs. The study was stopped after 1 h (3600 s) with animals that did not reach the end awarded this time.
4. Discussion
Crabs showed a strong capacity for spatial learning over the timescale of this work. Navigation in invertebrates is known to rely on several principles: compass directions, landmarks, path integration and magnetic maps [6,12,14,26]. The crabs did not complete the maze without error until week 3, suggesting either adoption of a search strategy or memory of approximate distance travelled and sequential turn direction. Carcinus maenas shows a strong thigmotactic behaviour in natural and tank conditions [27] which could manifest in our study as wall-hugging. Consistently following a wall on either the right or left would result in one or two wrong turns, respectively, however, and we, therefore, propose the crabs displayed a degree of spatial learning. Similarly, while habituation to the maze may have resulted in lower latencies in progressive weeks, it would not produce the observed concurrent decrease in the number of wrong turns. Visual and tactile cues were minimized, so a response strategy based on sequential learning (in this case, right turn, ignore two openings, left turn, left turn, right turn, right turn) is possible. The potential for allocentric (the use of landmarks) learning cannot be entirely discarded; however, as crabs may have used the position of the camera or other overhead features. Future work using other experimental designs, including placing food in more than one location, and maze configurations, such as consecutive T-mazes, might further elaborate spatial learning in these animals.
Learning ability was consistent across all animals, with individuals highly correlated against population-average predictions. Consistency in behaviour, including exploratory behaviour, has been demonstrated in C. maenas before [28–30] but not in learning, and studies investigating invertebrate learning often record high levels of behavioural variability [2,18], which could be attributed to either behavioural plasticity or consistent individual differences (sometimes referred to as personality). We used concordance correlation coefficients to quantify individual differences [23,31], then compared rank concordance among individuals for consistent (intercepts) and plastic (slopes) changes over time [32,33]. There was a very weak correlation between individual differences in latency and wrong turns, and this was dominated by idiosyncracies in plasticity rather than consistent differences between individuals—an individual that habituates to its environment strongly is not necessarily a faster learner. Caution is needed in ascribing behavioural mechanisms to observed responses, but these findings suggest maze learning in crabs is not simply accounted for by boldness or habituation to their environment.
Decapod crustaceans display anxiety-like behaviour mediated by serotonin [19], so the maze conditions were as close to those in the husbandry tanks as possible (i.e. same system water, no additional lighting), and the experimental design included a substantial acclimation period to captivity. We believe these accommodations contributed substantially to our results. The much longer latencies displayed by naive crabs compared to conditioned (week 6) animals (figure 2) may be partially attributable to increased anxiety-like behaviour in these crabs, although both groups experienced the same four-week acclimation period. The inclusion of naive animals demonstrates that no factor other than food was attracting animals to the endpoint, however, and while the olfactory cues from this food were undoubtedly important in navigating the maze, all conditioned crabs showed some memory of the maze in their absence with no significant difference in latency between week 6 (food absent) and weeks 1 and 2 when food was present (figure 1b). The increase in latency and the number of wrong turns from week 4 to week 6 might simply show the importance of these olfactory cues but could also suggest some loss of memory during the intervening two weeks. The discovery that decapod crustaceans are able to learn mazes has important ecological implications but will also allow the development of a model system to investigate the effects of waterborne contaminants, or changes in water chemistry, on a sophisticated behaviour in ecologically and economically important invertebrates.
Acknowledgements
Thanks to Keith Naylor and Hilary Williams for logistical support and Julian Kivell for building the maze.
Ethics
Handling of animals and the use of this equipment was approved by the Swansea University College of Science Ethics Committee, ref.: STU_BIOL_66191_120618193745_1.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h2cp37f [34].
Authors' contributions
R.D., M.H.G. and E.C.P. conceived the experiments, R.D. performed the study, R.D. extracted information from videos, R.D., E.C.P. and J.C.B. analysed the data. E.C.P. led the writing of the paper with the input from all authors, who read and approved the final manuscript. All authors are also agreed to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
R.D. was supported by the Swansea University College of Science and the Swansea University Science for Schools Scheme.
References
- 1.Perry CJ, Barron AB, Cheng K. 2013. Invertebrate learning and cognition: relating phenomena to neural substrate. WIREs Cogn. Sci. 4, 561–582. ( 10.1002/wcs.1248) [DOI] [PubMed] [Google Scholar]
- 2.Boal JG, Dunham AW, Williams KT, Hanlon RT. 2000. Experimental evidence for spatial learning in octopuses (Octopus bimaculoides). J. Comp. Psychol. 114, 246–252. ( 10.1037/0735-7036.114.3.246) [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, Rakoczy S, Brown-Borg H. 2010. Assessment of spatial memory in mice. Life Sci. 87, 521–536. ( 10.1016/j.lfs.2010.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karson MA, Boal JG, Hanlon RT. 2003. Experimental evidence for spatial learning in cuttlefish (Sepia officinalis). J. Comp. Psychol. 117, 149–155. ( 10.1037/07357036.117.2.149) [DOI] [PubMed] [Google Scholar]
- 5.Dale RH. 1988. Spatial memory in pigeons on a four-arm radial maze. Can. J. Psychol. 42, 78–83. ( 10.1037/h0084177) [DOI] [PubMed] [Google Scholar]
- 6.Collett M. 2009. Spatial memories in insects. Curr. Biol. 19, 1103–1108. ( 10.1016/j.cub.2009.10.004) [DOI] [PubMed] [Google Scholar]
- 7.Zhang SW, Bartsch K, Srinivasan MV. 1996. Maze learning by honeybees. Neurobiol. Learn. Mem. 66, 267–282. ( 10.1006/nlme.1996.0069) [DOI] [PubMed] [Google Scholar]
- 8.Chittka L, Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008. ( 10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 9.Strausfeld NJ. 1998. Crustacean–insect relationships: the use of brain characters to derive phylogeny amongst segmented invertebrates. Brain. Behav. Evol. 52, 186–206. ( 10.1159/000006563) [DOI] [PubMed] [Google Scholar]
- 10.Wiersma CAG. 1957. On the number of nerve cells in a crustacean central nervous system. Acta Physiol. Pharmacol. Neerl. 6, 135–142. [PubMed] [Google Scholar]
- 11.Menzel R, Giurfa M. 2001. Cognitive architecture of a mini-brain: the honeybee. Trends Cogn. Sci. 5, 62–71. ( 10.1001/jama.1969.03160200053027) [DOI] [PubMed] [Google Scholar]
- 12.Vannini M, Cannicci S. 1995. Homing behaviour and possible cognitive maps in crustacean decapods. J. Exp. Mar. Bio. Ecol. 193, 67–91. ( 10.1016/0022-0981(95)00111-5) [DOI] [Google Scholar]
- 13.Zeil J. 1998. Homing in fiddler crabs (Uca lactea annulipes and Uca vomeris: Ocypodidae). J. Comp. Physiol. A 183, 367–377. ( 10.1007/s003590050263) [DOI] [Google Scholar]
- 14.Boles LC, Lohmann KJ. 2003. True navigation and magnetic map in spiny lobsters. Nature 421, 60–63. ( 10.1038/nature01333.1) [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Mizutani A, Srinivasan MV. 2000. Maze navigation by honeybees: learning path regularity. Learn. Mem. 7, 363–374. ( 10.1101/lm.32900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirwan HB, Kevan PG. 2015. Maze navigation and route memorization by worker bumblebees (Bombus impatiens (Cresson) (Hymenoptera: Apidae)). J. Insect Behav. 28, 345–357. ( 10.1007/s10905-015-9507-3) [DOI] [Google Scholar]
- 17.Tierney AJ, Lee J. 2011. Spatial learning in a T-maze by the crayfish Orconectes rusticus. J. Comp. Psychol. 125, 31–39. ( 10.1037/a0020866) [DOI] [PubMed] [Google Scholar]
- 18.Tierney AJ, Andrews K. 2013. Spatial behavior in male and female crayfish (Orconectes rusticus): learning strategies and memory duration. Anim. Cogn. 16, 23–34. ( 10.1007/s10071-012-0547-1) [DOI] [PubMed] [Google Scholar]
- 19.Fossat P, Bacque-Cazenave J, Du Deurwaerdere P, Delbecque J-P, Cattaert D.. 2014. Anxiety-like behavior in crayfish is controlled by serotonin. Science 344, 1293–1298. ( 10.1126/science.1248811) [DOI] [PubMed] [Google Scholar]
- 20.Shuranova Z, Burmistrov Y, Abramson CI. 2005. Habituation to a novel environment in the crayfish Procambrus cubensis. J. Crustac. Biol. 25, 488–494. ( 10.1651/C-2556) [DOI] [Google Scholar]
- 21.Yerkes R. 1902. Habit formation in the green crab, Carcinus granulatus. Biol. Bull. 3, 241–244. ( 10.2307/1535878) [DOI] [Google Scholar]
- 22.van der Heyde A. 1920. Uber die Lernfahigheit der Strankrabbe Carcinus maenas. Biol. Zentr. 40, 503–514. [Google Scholar]
- 23.Lin LI-K. 1989. A concordance correlation coefficient to evaluate reproducibility. Biometrics 45, 255–268. ( 10.2307/2532051) [DOI] [PubMed] [Google Scholar]
- 24.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- 25.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01). [DOI] [Google Scholar]
- 26.Cannicci S, Barelli C, Vannini M. 2000. Homing in the swimming crab Thalamita crenata: a mechanism based on underwater landmark memory. Anim. Behav. 60, 203–210. ( 10.1006/anbe.2000.1458) [DOI] [PubMed] [Google Scholar]
- 27.Burrows MT, Kawai K, Hughes RN. 1999. Foraging by mobile predators on a rocky shore: underwater TV observations of movements of blennies Lipophrys pholis and crabs Carcinus maenas. Mar. Ecol. Prog. Ser. 187, 237–250. ( 10.3354/meps187237) [DOI] [Google Scholar]
- 28.Tanner CJ, Jackson AL. 2012. Social structure emerges via the interaction between local ecology and individual behaviour. J. Anim. Ecol. 81, 260–267. ( 10.1111/j.13652656.2011.01879.x) [DOI] [PubMed] [Google Scholar]
- 29.Fürtbauer I. 2015. Consistent individual differences in haemolymph density reflect risk propensity in a marine invertebrate. R. Soc. open sci. 2 140482 ( 10.1098/rsos.140482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fürtbauer I, Fry A. 2018. Social conformity in solitary crabs, Carcinus maenas, is driven by individual differences in behavioural plasticity. Anim. Behav. 135, 131–137. ( 10.1016/j.anbehav.2017.11.010) [DOI] [Google Scholar]
- 31.Huang S, Meng SX, Yang Y. 2009. Assessing the goodness of fit of forest models estimated by nonlinear mixed-model methods. Can. J. For. Res. 39 2418–2436. ( 10.1139/X09-140) [DOI] [Google Scholar]
- 32.Carrasco J, Jover L. 2003. The concordance correlation coefficient estimated through variance components. Biometrics 59, 849–858. ( 10.1111/j.0006-341X.2003.00099.x) [DOI] [PubMed] [Google Scholar]
- 33.Briffa M, Rundle SD, Fryer A. 2008. Comparing the strength of behavioural plasticity and consistency across situations: animal personalities in the hermit crab Pagurus bernhardus. Proc. R. Soc. B 275, 1305–1311. ( 10.1098/rspb.2008.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies R, Gagen MH, Bull JC, Pope EC. 2019. Data from: Maze learning and memory in a decapod crustacean Dryad Digital Repository. ( 10.5061/dryad.h2cp37f) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Davies R, Gagen MH, Bull JC, Pope EC. 2019. Data from: Maze learning and memory in a decapod crustacean Dryad Digital Repository. ( 10.5061/dryad.h2cp37f) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h2cp37f [34].