Abstract
Sepsis is commonly associated with acute kidney injury (AKI), particularly in those requiring dialysis (AKI-D). To date, Sepsis-3 criteria have not been applied to AKI-D patients. We investigated sepsis prevalence defined by Sepsis-3 criteria and evaluated the outcomes of septic-associated AKI-D among critically ill patients. Using the data collected from a prospective multi-center observational study, we applied the Sepsis-3 criteria to critically ill AKI-D patients treated in intensive care units (ICUs) in 30 hospitals between September 2014 and December 2015. We described the prevalence, outcomes, and characteristics of sepsis as defined by the screening Sepsis-3 criteria among AKI-D patients, and compared the outcomes of AKI-D patients with or without sepsis using the Sepsis-3 criteria. A total of 1078 patients (median 70 years; 673 (62.4%) men) with AKI-D were analyzed. The main etiology of AKI was sepsis (71.43%) and the most frequent indication for acute dialysis was oliguria (64.4%). A total of 577 (53.3% of 1078 patients) met the Sepsis-3 criteria, and 206 among the 577 patients (19.1%) had septic shock. Having sepsis and septic shock were independently associated with 90-day mortality among these ICU AKI-D patients (hazard ratio (HR) 1.23 (p = 0.027) and 1.39 (p = 0.004), respectively). Taking mortality as a competing risk factor, AKI-D patients with septic shock had a significantly reduced chance of weaning from dialysis at 90 days than those without sepsis (HR 0.65, p = 0.026). The combination of the Sepsis-3 criteria with the AKI risk score led to better performance in forecasting 90-day mortality. Sepsis affects more than 50% of ICU AKI patients requiring dialysis, and one-fifth of these patients had septic shock. In AKI-D patients, coexistent with or induced by sepsis (as screened by the Sepsis-3 criteria), there is a significantly higher mortality and reduced chance of recovering sufficient renal function, when compared to those without sepsis.
Keywords: Sepsis-3, acute dialysis, qSOFA, acute kidney injury
1. Introduction
Sepsis is considered the most frequent cause of acute kidney injury (AKI) in critically ill patients in the intensive care unit (ICU) [1]. It is a heterogeneous syndrome caused by an unbalanced host response to an infection, often resulting in variable clinical signs and symptoms. Until the early 1990s, sepsis was not formally defined, and numerous different criteria were used in research and clinical practice. In 2016, the ‘Sepsis-3’ consensus definition was published. Accordingly, sepsis constitutes life-threatening organ dysfunction that is caused by a dysregulated host response to infection and defined by an acute change in total Sequential Organ Failure Assessment (SOFA) score by ≥2 points (delta SOFA). In addition, the concept of the quick SOFA (qSOFA) was introduced as a possible tool to identify patients with sepsis outside the ICU [2]. The qSOFA describes the presence of any two of the following three factors: Respiratory rate ≥ 22/min, altered mentation or systolic blood pressure ≤ 100 mmHg. Septic shock is recognized as a subset of sepsis with profound circulatory, cellular, and metabolic abnormalities as evidenced by a serum lactate concentration >2 mmol/L and vasopressor requirement to maintain a mean arterial pressure (MAP) of at least 65 mmHg in the absence of hypovolemia. Of note, the terms systemic inflammatory response syndrome (SIRS) and severe sepsis were removed.
An analysis of data from a large cohort of patients admitted to 409 hospitals in the USA in 2004–2009 revealed that more than 40% of patients with sepsis, as defined by the Sepsis-3 criteria, also had AKI [3]. In patients with AKI, mortality and long-term outcomes are worst in those treated with renal replacement (RRT), also known as ‘AKI with dialysis’ (AKI-D) [2,4]. To date, the prevalence and outcomes of sepsis defined by the Sepsis-3 criteria among AKI-D patients has not been well reported. It is unclear whether the two criteria of the Sepsis-3 definition are equally predictive when used in association with AKI-D patients.
The aims of our project were: (i) To describe the incidence, outcomes, and characteristics of sepsis, as defined by the screening Sepsis-3 criteria, among AKI-D patients; and (ii) to show the outcomes of AKI-D patients without or with sepsis.
2. Methods
2.1. Study Population
We analyzed data of the Taiwan Consortium for Acute Kidney Injury and Renal Diseases (CAKs) study. The CAKS study was approved by the institutional review boards of the participating institutions. The need for informed consent was waived because all personal data was fully de-identified and only data that were routinely collected for clinical purposes were analyzed (approval number NRPB2014050014). The CAKs study has been extensively described previously [5,6]. In brief, it is a prospective observational study of ICU patients with AKI-D admitted to one of 30 hospitals in Taiwan. The hospitals are distributed widely through the various geographical regions of Taiwan (north, middle, south, and east) and there is a 1:1 ratio of tertiary medical centers to regional hospitals in each region. We analyzed patients who had been enrolled in four distinct months (October 2014, January 2015, April 2015 and July 2015) and were followed-up for at least three months after hospital discharge. Patients receiving chronic dialysis before the index hospitalization were excluded.
2.2. Dialysis Initiation
The pre-determined indications for RRT protocol or algorithm or simply clinician judgement were: (1) Presence of azotemia (blood urea nitrogen (BUN) > 80 mg/dL and serum creatinine (sCr) > 2 mg/dL) and uremic symptoms (encephalopathy, pericarditis or pleurisy); (2) oliguria (urine output < 400 mL/24 h) or anuria refractory to fluid challenges and diuretics; (3) fluid overload refractory to diuretics with a central venous pressure (CVP) > 12 mmHg or pulmonary edema with a PaO2/FiO2 < 300 mmHg; (4) hyperkalemia (serum potassium > 5.5 mmol/L) refractory to medical treatment, and/or (5) metabolic acidosis (arterial pH < 7.2) [7,8,9].
2.3. Infection and Sepsis
The medical records of all patients were independently reviewed by two investigators to identify AKI-D patients who met the Sepsis-3 criteria at initiation of RRT. In case of any discordance, a third investigator (VCW) acted as an adjudicator.
To be classified as having sepsis, patients with a suspected or confirmed infection had to have at least two qSOFA criteria [10] or an acute increase in total SOFA score by ≥2 within the 24 h period before acute RRT was started (distribution as Figure S1). Infection was defined as body fluids with positive culture or antibiotics started as a criterion of suspected infection. In patients with a nosocomial infection in whom a pre-admission SOFA score was not available, the first SOFA value at hospital admission qualified as baseline score (1). Patients with hepatic dysfunction who received acute RRT within 24 h of ICU admission, were assigned a baseline SOFA score of four, and in case of chronic respiratory impairment, a baseline score of two was assigned [11]. In all other cases, the baseline SOFA score was considered to be zero [2]. Consistent with previous studies, missing values were considered to be normal [10].
2.4. Outcomes
The primary study outcome was 90-day mortality after hospital discharge. Secondary outcomes were inability to wean from acute RRT and/or a composite outcome of mortality or RRT dependence at 90 days after hospital discharge [12].
2.5. Clinical Data Collection
In patients with AKI-D at initiation of RRT, we extracted the following data: Baseline characteristics and demographics, severity of illness scores including SOFA score, acute physiology and chronic health evaluation II (APACHE II) score and multiple organ dysfunction syndrome (MODS) score, comorbidities and the presumed etiology of AKI. AKI was defined by the serum creatinine criteria of the Kidney Disease Improving Global Outcome classification [13]. We also calculated the AKI risk prediction score as proposed by Demirjian et al. [14] (Table S1). The worst physiological and biochemical values during the initial 24-h period before RRT were recorded, together with the severity of illness scores and vasopressor administration at initiation of RRT [15].
The pre-determined indications for RRT were mentioned above and as in supplemental methods.
2.6. Statistical Analyses
Continuous data were expressed as mean ± standard deviation (SD) and group comparisons were conducted using χ 2 tests for equal proportions, t tests for normally distributed data, and Wilcoxon rank sum tests otherwise.
We performed multivariable analyses of all factors that were significant in univariate analyses, including age, sex, baseline comorbidities, indication for RRT, etiology of AKI, renal parameters and SOFA score at initiation of RRT and modality of RRT. The significance levels for entry (SLE) and for stay (SLS) were set at a conservative level of 0.15. The best candidate final logistic regression model was identified manually by dropping the covariates with a p value > 0.05 one at a time until all regression coefficients were significantly different from zero.
Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) analysis were used to examine the role of the Sepsis-3 criteria to stratify individuals into higher or lower risk categories (reclassification) [16,17]. Focusing on 90-day mortality, an increase in NRI was calculated in a model containing the Sepsis-3 criteria in combination with the AKI risk prediction or SOFA score. The results were compared with the individual criteria of the Sepsis-3 definition. We distinguished between three risk categories (0%–40%, 40%–80%, and >80%) and reclassified the patients who died from all-causes or were still dialysis dependent at 90 days after hospital discharge (according to decision curve analysis, Figure S2).
All analyses were performed with R software, version 3.2.2 (Free Software Foundation, Inc., Boston, MA, USA), MedCalc Statistical Software, version 15.11.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2015) and Stata version 12 (StataCorp LP, College Station, TX, USA) for competing-risk analysis. A p-value < 0.05 was considered significant.
3. Results
3.1. Patient Cohort
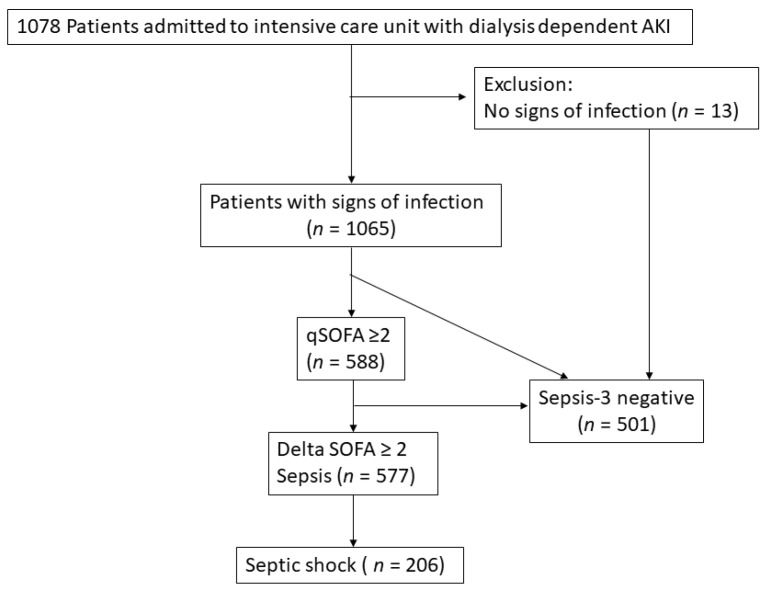
We enrolled a total of 1078 critically ill patients with AKI-D (median age 70 years; 673 (62.4%) male). At initiation of RRT, their median SOFA score was 12, APACHE II score 24 and MODS score was 11. A total of 577 (53.5%) patients had sepsis, of whom 206 (19.1%) met the criteria for septic shock (Figure 1). Supplementary Figure S1 shows the distribution of the qSOFA and SOFA scores. The main source of infection was the respiratory tract (53.6%), followed by the genitourinary tract (31.4%). The missing data were mainly come from bilirubin (n = 4, 3.7%) and coagulation INR (5, 4.6%).
Figure 1.
Algorithm of enrollee.
Based on judgment by the clinicians treating the patients, the main etiologies of AKI were sepsis (71.4%), shock (57.1%) and rhabdomyolysis (14.4%). The most frequent indication for acute RRT was oliguria (64.4%), followed by fluid overload (56.0%), azotemia (54.4%) and metabolic acidosis (49.6%).
3.2. Impact of Sepsis
Patients with AKI-D who had sepsis (53.5%) or septic shock (19.1%) at initiation of RRT were significantly older, had better baseline renal function and a higher serum lactate result on admission to ICU compared to AKI-D patients without sepsis (Table 1).
Table 1.
Clinical characteristics of patients with and without sepsis.
| Non-Sepsis | Sepsis | Septic Shock | p Value | |
|---|---|---|---|---|
| (n = 501) | (n = 371) | (n = 206) | ||
| Patient characteristics | ||||
| Age, median (range) | 71.8 (60.6–80.3) | 69.3 (57.6–79.7) | 65.8 (54.3–76.3) | 0.011 |
| Male gender, n (%) | 299 (59.68%) | 228 (61.46%) | 146 (70.87%) | 0.018 |
| BMI, median (range) | 23.9 (21.4–27.2) | 24 (21–27.2) | 23.9 (21–26.9) | 0.579 |
| Charlson comorbidity index | 7 (5–9) | 7 (5–9) | 6 (5–8) | 0.020 |
| Baseline sCr (mg/dL), median (range) | 1.7 (1–3.3) | 1.4 (0.9–2.4) | 1 (0.8–1.7) | <0.001 |
| eGFR (ml/min/1.73 m2), median (range) | 32.5 (15.5–63.3) | 44 (21.9–73.8) | 63.9 (35.2–88) | <0.001 |
| Comorbidities, n (%) | ||||
| Diabetes mellitus, n (%) | 276 (55.09%) | 189 (50.94%) | 97 (47.09%) | 0.131 |
| Liver cirrhosis, n (%) | 56 (11.18%) | 58 (15.63%) | 40 (19.42%) | 0.011 |
| COPD, n (%) | 43 (8.58%) | 29 (7.82%) | 12 (5.83%) | 0.462 |
| CAD, n (%) | 156 (31.14%) | 102 (27.49%) | 52 (25.24%) | 0.233 |
| CVA, n (%) | 78 (15.57%) | 59 (15.90%) | 22 (10.68%) | 0.185 |
| Hemiplegia, n (%) | 22 (4.39%) | 20 (5.39%) | 6 (2.91%) | 0.383 |
| GI bleeding, n (%) | 129 (25.75%) | 109 (29.38%) | 58 (28.16%) | 0.479 |
| Dementia, n (%) | 11 (2.20%) | 12 (3.23%) | 9 (4.37%) | 0.282 |
| Cancer, n (%) | 86 (17.17%) | 79 (21.29%) | 63 (30.58%) | <0.001 |
| Congestive heart failure, n (%) | 194 (48.7%) | 159 (58.49%) | 68 (44.66%) | <0.001 |
| Laboratory data at ICU admission | ||||
| BUN (mg/dL), median (range) | 61 (34.5–95.9) | 57.7 (27–92) | 42 (23.2–68.8) | <0.001 |
| sCr (mg/dL), median (range) | 3.7 (2–6.4) | 2.7 (1.4–5) | 2.3 (1.3–3.8) | <0.001 |
| Lactate (mmol/L), median (range) | 2.5 (1.4–5.6) | 2.4 (1.3–4.8) | 6.3 (2.9–10) | <0.001 |
| Etiology of AKI (except sepsis), n (%) | ||||
| Shock | 225 (44.91%) | 203 (54.72%) | 188 (91.26%) | <0.001 |
| Cardiorenal syndrome | 206 (41.12%) | 134 (36.12%) | 53 (25.73%) | <0.001 |
| Drug nephrotoxicity | 26 (5.19%) | 18 (4.85%) | 10 (4.85%) | 0.969 |
| Rhabdomyolysis | 34 (6.79%) | 24 (6.47%) | 23 (11.17%) | 0.086 |
| Intravascular hemolysis | 16 (3.19%) | 10 (2.70%) | 8 (3.88%) | 0.735 |
| Hepatorenal | 26 (5.19%) | 22 (5.93%) | 21 (10.19%) | 0.043 |
| ATIN | 4 (0.80%) | 5 (1.35%) | 0 (0%) | 0.276 |
| Contrast | 38 (7.58%) | 24 (6.47%) | 13 (6.31%) | 0.750 |
| Obstructive | 8 (1.60%) | 3 (0.81%) | 1 (0.49%) | 0.472 |
| Others | 117 (23.35%) | 56 (15.09%) | 24 (11.65%) | <0.001 |
| At initiation of RRT | ||||
| Urine output (mL/24 h), median (range) | 450 (150–1095) | 250 (70–620) | 130 (50–418) | <0.001 |
| AKI risk prediction score, median (range) | 22 (17–28) | 27 (21–33) | 33.5 (26–40) | <0.001 |
| Lactate (mmol/L), median (range) | 2.2 (1.3–5.2) | 1.6 (1–3.1) | 6.6 (3.4–10.7) | <0.001 |
| SOFA score, median (range) | 10 (7–13) | 12 (10–15) | 15 (13–17) | <0.001 |
| qSOFA, median (range) | 1 (1–1) | 2 (2-3) | 2 (2–3) | <0.001 |
| APACHE II score, median (range) | 20 (16–25) | 25 (21–30) | 27 (22.8–33) | <0.001 |
| MODS score, median (range) | 9 (7–11) | 12 (10–14) | 12 (10–15) | <0.001 |
| Site of infection, n (%) | ||||
| Respiratory | 227 (45.31%) | 237 (63.88%) | 114 (55.34%) | <0.001 |
| GU | 156 (31.14%) | 129 (34.77%) | 53 (25.73%) | 0.080 |
| Bacteremia | 96 (19.16%) | 84 (22.64%) | 57 (27.67%) | 0.043 |
| Abdomen | 41 (8.18%) | 40 (10.78%) | 33 (16.02%) | 0.009 |
| Others | 56 (11.18%) | 38 (10.24%) | 21 (10.19%) | 0.880 |
| Indication for dialysis, n (%) | ||||
| Azotemia | 291 (58.08%) | 225 (60.65%) | 70 (33.98%) | <0.001 |
| Fluid overload | 245 (48.90%) | 225 (60.65%) | 134 (65.05%) | <0.001 |
| Electrolyte imbalance | 190 (37.92%) | 148 (39.89%) | 79 (38.35%) | 0.835 |
| Metabolic acidosis | 210 (41.92%) | 192 (51.75%) | 133 (64.56%) | <0.001 |
| Oliguria | 275 (54.89%) | 255 (68.73%) | 164 (79.61%) | <0.001 |
| Uremic encephalopathy | 46 (9.18%) | 26 (7.01%) | 6 (2.91%) | 0.014 |
| Dialysis modality, n (%) | <0.001 | |||
| CVVH | 128 (25.55%) | 110 (29.65%) | 133 (64.56%) | |
| IHD | 334 (66.67%) | 252 (67.92%) | 58 (28.16%) | |
| SLEDD | 39 (7.78%) | 9 (2.43%) | 15 (7.28%) | |
| Outcomes of interest | ||||
| Dialysis days in hospital, median (range) | 12 (4–26) | 10 (4–27) | 6 (3–15) | 0.012 |
| Hospital mortality, n (%) | 221 (44.11%) | 228 (61.46%) | 167 (81.07%) | <0.001 |
| 90-day ICU free days | 63 (0–85) | 1 (0–78) | 0 (0–0) | <0.001 |
| 90-day hospital free days | 30 (0–70) | 0 (0–53) | 0 (0–0) | <0.001 |
| 90-day mortality, n (%) | 246 (49.10%) | 253 (68.19%) | 172 (83.49%) | <0.001 |
| 90-day composite outcome, n (%) | 352 (70.26%) | 296 (79.78%) | 175 (84.95%) | <0.001 |
Paired comparison between the groups. Abbreviations: AKI, acute kidney injury; APACHE; acute physiology and chronic health evaluation; ATIN, acute tubule-interstitial nephritis; BMI, body mass index; BUN, blood urea nitrogen; CAD, coronary artery disease; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; CVA, cerebrovascular accident; CVVH, continuous veno-venous hemofiltration; CPR, cardio-pulmonary resuscitation; eGFR, estimated glomerular filtration rate; GSC, Glasgow Coma Scale; GI, gastrointestinal; GU, genitourinary; IABP, intra-aortic balloon pump; ICU, intensive care unit; IHD, intermittent hemodialysis; IQR, interquartile range; MODS, multiple organ dysfunction; RRT, renal replacement therapy; sCr, serum creatinine; SLEDD, sustained low efficiency daily dialysis; SOFA, Sequential Organ Failure Assessment.
3.3. Comparison between 90-Day Survivors and Non-Survivors
Mortality and composite outcome (mortality or RRT dependence) at 90 days were 62.3%, and 76.4% in the 1078 AKI-D patients, respectively (Table 2). Sepsis and septic shock were more common among non-survivors compared to survivors (63.2% and 25.6% versus 37.4% and 8.4%, respectively). Other significant differences between 90-day survivors and non-survivors were older age, a higher comorbidity score, lower baseline serum creatinine and a higher prevalence of liver cirrhosis and cancer in non-survivors.
Table 2.
Clinical characteristics of survivors and non-survivors.
| All | 90 Day Survivors | 90 Day Mortality | p Value | No Dialysis Dependence or Mortality at 90 Days | Dialysis Dependence or Mortality at 90 Days | p Value | |
|---|---|---|---|---|---|---|---|
| (n = 1078) | (n = 406) | (n = 672) | (n = 254) | (n = 824) | |||
| Baseline characteristics | |||||||
| Age, median (range) | 70 (57.8–79.5) | 69 (56.7–77.4) | 71 (58.9–81) | 0.014 | 67.7 (53.9–76.8) | 70.9 (59.8–80.4) | <0.001 |
| Male gender, n (%) | 673 (62.43%) | 247 (60.84%) | 426 (63.39%) | 0.401 | 158 (62.20%) | 515 (62.50%) | 0.932 |
| BMI, median (range) | 23.95 (21.2–27.1) | 24.2 (21.5–27.6) | 23.7 (21–26.8) | 0.870 | 24.6 (22–27.9) | 23.7 (21–26.8) | 0.598 |
| Charlson comorbidity index, median (range) | 7 (5–9) | 7 (5–8.3) | 7 (5–9) | 0.001 | 6 (4–8) | 7 (5–9) | <0.001 |
| Baseline sCr (mg/dL), median (range) | 1.4 (0.9–2.7) | 1.8 (1–3.6) | 1.3 (0.9–2.3) | <0.001 | 1.2 (0.9–2.1) | 1.5 (0.9–2.8) | <0.001 |
| eGFR (mL/min/1.73 m2), median (range) | 41.79 (20.4–73.6) | 31.9 (14.6-64.3) | 48.2 (24.4-77.1) | <0.001 | 49.9 (25.3-78) | 40.5 (18.2-71.7) | 0.040 |
| Comorbidities | |||||||
| Diabetes mellitus, n (%) | 562 (52.13%) | 228 (56.16%) | 334 (49.70%) | 0.040 | 139 (54.72%) | 423 (51.33%) | 0.344 |
| Liver cirrhosis, n (%) | 154 (14.29%) | 26 (6.40%) | 128 (19.05%) | <0.001 | 19 (7.48%) | 135 (16.38%) | <0.001 |
| COPD, n (%) | 84 (7.79%) | 31 (7.64%) | 53 (7.89%) | 0.881 | 19 (7.48%) | 65 (7.89%) | 0.832 |
| CAD, n (%) | 310 (28.76%) | 130 (32.02%) | 180 (26.79%) | 0.066 | 77 (30.31%) | 233 (28.28%) | 0.530 |
| CVA, n (%) | 159 (14.75%) | 61 (15.02%) | 98 (14.58%) | 0.843 | 30 (11.81%) | 129 (15.66%) | 0.131 |
| Hemiplegia, n (%) | 48 (4.45%) | 19 (4.68%) | 29 (4.32%) | 0.779 | 11 (4.33%) | 37 (4.49%) | 0.914 |
| GI bleeding, n (%) | 296 (27.46%) | 89 (21.92%) | 207 (30.80%) | 0.002 | 53 (20.87%) | 243 (29.49%) | 0.007 |
| Dementia, n (%) | 32 (2.97%) | 7 (1.72%) | 25 (3.72%) | 0.061 | 6 (2.36%) | 26 (3.16%) | 0.515 |
| Cancer, n (%) | 228 (21.15%) | 60 (14.78%) | 168 (25.00%) | <0.001 | 40 (15.75%) | 188 (22.82%) | 0.016 |
| Congestive heart failure, n (%) | 553 (51.30%) | 205 (50.49%) | 348 (50.79%) | 0.787 | 122 (48.41%) | 431 (52.31%) | 0.019 |
| Parameters at ICU admission | |||||||
| BUN (mg/dL), median (range) | 56 (29.2–89) | 63 (35–91) | 50.5 (26.4–88) | 0.001 | 51 (26–80.5) | 57.7 (30–91.6) | 0.192 |
| sCr (mg/dL), median (range) | 3 (1.7–5.5) | 4.1 (2.2–6.9) | 2.6 (1.4–4.5) | <0.001 | 3 (1.9–5.3) | 3 (1.6–5.6) | 0.984 |
| Lactate (mmol/L), median (range) | 3.1 (1.7–7) | 2.6 (1.3–5.2) | 3.7 (2–8.6) | <0.001 | 3 (1.6–6.1) | 3.2 (1.7–7.3) | 0.030 |
| Etiology of AKI, n (%) | |||||||
| Shock, n (%) | 616 (57.14%) | 165 (40.64%) | 451 (67.11%) | <0.001 | 132 (51.97%) | 484 (58.74%) | 0.057 |
| Sepsis, n (%) | 770 (71.43%) | 242 (59.61%) | 528 (78.57%) | <0.001 | 153 (60.24%) | 617 (74.88%) | <0.001 |
| Cardiorenal syndrome, n (%) | 393 (36.46%) | 170 (41.87%) | 223 (33.18%) | 0.010 | 93 (36.61%) | 300 (36.41%) | 0.952 |
| Nephrotoxic drugs, n (%) | 54 (5.01%) | 27 (6.65%) | 27 (4.02%) | 0.055 | 22 (8.66%) | 32 (3.88%) | 0.002 |
| Rhabdomyolysis, n (%) | 81 (7.51%) | 33 (8.13%) | 48 (7.14%) | 0.552 | 28 (11.02%) | 53 (6.43%) | 0.015 |
| Intravascular hemolysis, n (%) | 34 (3.15%) | 14 (3.45%) | 20 (2.98%) | 0.667 | 11 (4.33%) | 23 (2.79%) | 0.220 |
| Hepatorenal syndrome, n (%) | 69 (6.40%) | 4 (0.99%) | 65 (9.67%) | <0.001 | 4 (1.57%) | 65 (7.89%) | <0.001 |
| ATIN, n (%) | 9 (0.83%) | 5 (1.23%) | 4 (0.60%) | 0.309 | 2 (0.79%) | 7 (0.85%) | 0.999 |
| Contrast exposure, n (%) | 75 (6.96%) | 33 (8.13%) | 42 (6.25%) | 0.240 | 22 (8.66%) | 53 (6.43%) | 0.222 |
| Obstruction, n (%) | 12 (1.11%) | 6 (1.48%) | 6 (0.89%) | 0.375 | 3 (1.18%) | 9 (1.09%) | 1.000 |
| Others, n (%) | 197 (18.27%) | 103 (25.37%) | 94 (13.99%) | <0.001 | 61 (24.02%) | 136 (16.50%) | 0.007 |
| Parameters at RRT initiation | |||||||
| Urine output (mL/24 h), median (range) | 300 (90–822.5) | 490 (160–1223) | 204 (70–595) | <0.001 | 520 (180–1305) | 250 (75–670) | <0.001 |
| AKI risk prediction score | 25 (19–33) | 21 (16–28.3) | 27.5 (22–35) | <0.001 | 22 (17–29.3) | 26 (21–34) | <0.001 |
| Lactate (mmol/L), median (range) | 3.2 (1.6–7.6) | 2.3 (1.2–5.4) | 3.9 (1.9–9.1) | <0.001 | 2.8 (1.4–6.5) | 3.3 (1.6–8.2) | 0.090 |
| SOFA score, median (range) | 12 (8–15) | 9 (7–12) | 13 (10–16) | <0.001 | 10 (7–13) | 12 (9–15) | <0.001 |
| qSOFA, median (range) | 2 (1–2) | 1 (1–2) | 2 (1–2) | <0.001 | 1 (1–2) | 2 (1–2) | <0.001 |
| qSOFA ≥ 2, n (%) | 582 (53.99%) | 153 (37.66%) | 429 (63.84%) | <0.001 | 107 (42.13%) | 475 (57.65%) | <0.001 |
| APACHE II score, median (range) | 24 (19–28) | 20 (16–25) | 25 (21–30) | <0.001 | 21 (16–26) | 24 (20–29) | <0.001 |
| MODS score, median (range) | 11 (9–13) | 10 (7–12) | 11 (9–14) | <0.001 | 10 (7.5–13) | 11 (9–13) | 0.008 |
| Sepsis 3 criteria | |||||||
| Sepsis, n (%) | 577 (53.53%) | 152 (37.44%) | 425 (63.24%) | <0.001 | 106 (41.73%) | 471 (57.16%) | <0.001 |
| Septic shock, n (%) | 206 (19.11%) | 34 (8.37%) | 172 (25.60%) | <0.001 | 31 (12.20%) | 175 (21.24%) | 0.001 |
| Site of infection, n (%) | |||||||
| Respiratory | 578 (53.62%) | 188 (46.31%) | 390 (58.04%) | <0.001 | 1113 (44.49%) | 465 (56.43%) | 0.001 |
| GU | 338 (31.35%) | 134 (33.00%) | 204 (30.36%) | 0.364 | 83 (32.68%) | 255 (30.95%) | 0.603 |
| Bacteremia | 237 (21.99%) | 60 (14.78%) | 177 (26.34%) | <0.001 | 43 (16.93%) | 194 (23.54%) | 0.026 |
| Abdomen | 114 (10.58%) | 39 (9.61%) | 75 (11.16%) | 0.421 | 32 (12.60%) | 82 (9.95%) | 0.230 |
| Others | 115 (10.67%) | 37 (9.11%) | 78 (11.61%) | 0.199 | 25 (9.84%) | 90 (10.92%) | 0.626 |
| Indication for RRT | |||||||
| Azotemia, n (%) | 586 (54.36%) | 220 (54.19%) | 366 (54.46%) | 0.929 | 113 (44.49%) | 473 (57.40%) | <0.001 |
| Fluid overload, n (%) | 604 (56.03%) | 200 (49.26%) | 404 (60.12%) | 0.001 | 132 (51.97%) | 472 (57.28%) | 0.136 |
| Electrolyte imbalance, n (%) | 417 (38.68%) | 160 (39.41%) | 257 (38.24%) | 0.704 | 108 (42.52%) | 309 (37.50%) | 0.151 |
| Metabolic acidosis, n (%) | 535 (49.63%) | 180 (44.33%) | 355 (52.83%) | 0.007 | 114 (44.88%) | 421 (51.09%) | 0.084 |
| Oliguria, n (%) | 694 (64.38%) | 205 (50.49%) | 489 (72.77%) | <0.001 | 122 (48.03%) | 572 (69.42%) | <0.001 |
| Uremic encephalopathy, n (%) | 78 (7.24%) | 38 (9.36%) | 40 (5.95%) | 0.036 | 16 (6.30%) | 62 (7.52%) | 0.510 |
| First Dialysis modality, n (%) | <0.001 | 0.239 | |||||
| CVVH | 371 (34.42%) | 97 (23.89%) | 274 (40.77%) | 79 (31.10%) | 292 (35.44%) | ||
| IHD | 644 (59.74%) | 289 (71.18%) | 355 (52.83%) | 163 (64.17%) | 481 (58.37%) | ||
| SLEDD | 63 (5.84%) | 20 (4.93%) | 43 (6.40%) | 12 (4.72%) | 51 (6.19%) | ||
| Outcomes of interest | |||||||
| 90-day ICU free days | 7 (0–81) | 81 (69–86) | 0(0–1) | <0.001 | 80 (68–86) | 0 (0–54.5) | <0.001 |
| 90-day hospital free days | 0 (0–59.25) | 63 (44.5–76) | 0(0–0) | <0.001 | 63 (42–76) | 0 (0–2.5) | <0.001 |
| Days of RRT in hospital, median (range) | 10 (4–24) | 11 (3–26) | 9.5 (4–22.8) | 0.461 | 5.5 (2–13.3) | 12 (4–27.8) | <0.001 |
Abbreviations: AKI, acute kidney injury; APACHE; acute physiology and chronic health evaluation; ATIN, acute tubule-interstitial nephritis; BMI, body mass index; BUN, blood urea nitrogen; CAD, coronary artery disease; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; CVA, cerebrovascular accident; CVVH, continuous veno-venous hemofiltration; CPR, cardio-pulmonary resuscitation; eGFR, estimated glomerular filtration rate; GSC, Glasgow Coma Scale; GI, gastrointestinal; GU, genitourinary; IABP, intra-aortic balloon pump; ICU, intensive care unit; IHD, intermittent hemodialysis; IQR, interquartile range; MODS, multiple organ dysfunction; RRT, renal replacement therapy; sCr, serum creatinine; SLEDD, sustained low efficiency daily dialysis; SOFA, Sequential Organ Failure Assessment.
3.4. Sepsis-3 Criteria versus 90-Day Outcomes
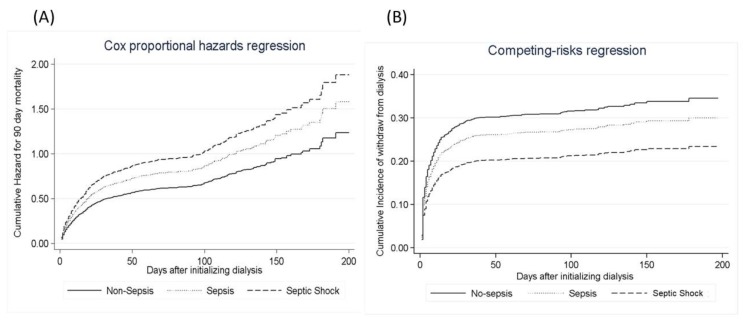
Multivariable analysis showed that AKI-D patients with sepsis or septic shock at initiation of RRT had a significantly higher risk of mortality at 90 days compared to AKI-D patients without sepsis (Table 3 and Figure 2a). There was a positive correlation between the Sepsis-3 criteria and APACHE II score (r = 0.385, p < 0.001), SOFA score (r = 0.391, p < 0.001) and AKI risk prediction score (r = 0.359, p < 0.001). AKI-D patients with septic shock had a greater incremental increase in 90-day mortality across all deciles of APACHE II at initiation of RRT compared to AKI-D patients with qSOFA ≥ 2 (Figure S4, Table S2).
Table 3.
Multivariable risk model for hospital mortality or composite outcome at discharge.
| Sepsis | Non-Shock Sepsis vs Non-Sepsis | Septic Shock vs. Non-Sepsis | ||||
|---|---|---|---|---|---|---|
| Outcome of interests | Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p |
| Hospital mortality | 1.12 | 0.91–1.37 | 0.276 | 1.48 | 1.17–1.88 | 0.001 |
| Hospital composite outcomes | 0.97 | 0.80–1.17 | 0.732 | 1.24 | 1.08–1.47 | 0.047 |
| For 90-day mortality | 1.23 | 1.02–1.47 | 0.027 | 1.39 | 1.11–1.75 | 0.004 |
| For 90-day composite outcome | 1.26 | 1.03–1.53 | 0.022 | 1.45 | 1.15–1.83 | 0.002 |
| For 90-day weaning from dialysis | 0.96 | 0.76–1.22 | 0.760 | 0.65 | 0.45–0.95 | 0.026 |
p; paired comparison between the groups. All relevant covariates included in the multi-variable analysis, including age, sex, baseline comorbidities, indication for dialysis, etiology of AKI, kidney profile and SOFA score at dialysis initiation, dialysis modality, and some of their interactions. Abbreviations: AKI, acute kidney injury; CI, confidence interval.
Figure 2.
Cox proportional hazards models. (A) Cox proportional hazards models are plotted to model the probability of free from 90 days mortality, stratified by Sepsis-3 status. (B) Model the risk of chronic dialysis, taking mortality as a competing risk.
3.5. Sepsis per Sepsis-3 Criteria versus the Risk of Dialysis Dependence
Multivariable analysis showed that after controlling for mortality, AKI-D patients with septic shock who survived had a significantly lower likelihood of weaning from dialysis, when compared to AKI-D patients without sepsis (hazard ratio (HR), 0.65, p = 0.026) (Table 3). There was no significant difference in likelihood of weaning from dialysis at 90 days between AKI-D patients without sepsis and AKI-D patients with sepsis but without septic shock (HR 0.96, p = 0.760) (Table 3, Figure 2b).
3.6. Evaluation of Sepsis-3 Criteria in Combination with AKI Risk Prediction Score and SOFA Score versus the 90 Days Mortality
Combining the Sepsis-3 criteria with the AKI risk prediction score at initiation of RRT led to a significant increase in risk stratification (total NRI = 0.07; 95% CI, 0.02–0.11; p < 0.01). The majority of this effect came from those without death (NRI event = 0.04; 95% CI, 0.01–0.07; p = 0.039). Likewise, the total IDI was significant (0.02, 95% CI, 0.01–0.02; p < 0.001).
In case of sequential diagnosis of sepsis according to the Sepsis-3 criteria, we added Sepsis-3 to the qSOFA criteria in estimating the risk of 90-day mortality after initiation of dialysis. This led to a significant increase in risk stratification (total NRI = 0.11; 95% CI, 0.04–0.19; p = 0.004). The majority of this effect came from those without death (NRI event = 0.07; p = 0.03), whereas the NRI with death was 0.05 (NRI non-event = 0.04, p = 0.049) (Figure S3). Similarly, the total integrated discrimination improvement (IDI) was significant at 0.06 (95% CI, 0.03 to 0.05; p < 0.001).
4. Discussion
To our best knowledge, this is the first study that applied the most recent Sepsis-3 screening criteria to the patients with AKI-D. The key findings of this large multi-center prospective study are that sepsis affects 53.5% of patients with AKI-D, and that at time of RRT 19.1% of patients had septic shock. The chances of renal recovery at 90 days were significantly lower in AKI-D patients with septic shock compared to those without sepsis. Presence of sepsis per Sepsis-3 criteria in AKI-D patients is associated with higher mortality rate and composite outcome at 90 days. Combining the Sepsis-3 criteria with the AKI risk score or SOFA criteria led to a further improvement in risk identification.
A 53.5% prevalence of sepsis among AKI-D patients is slightly higher than previously reported [18]. This may be a reflection of our specific patient cohort of critically ill ICU patients or a result of using the Sepsis-3 criteria. Similarly, in-hospital mortality rate of this cohort was high, including its non-septic controls (44.1%), which again may be explained by the characteristics of our patient population (older age, high comorbidity and acute severity of illness scores) and the criteria used to identify patients.
4.1. Association of Mortality and Non-Recovery from Dialysis
Patients with AKI requiring RRT constitute a high-risk group. An accurate prognostic assessment is crucial for clinical management and planning of future care. The Sepsis-3 criteria identified AKI-D patients with a suspected or confirmed infection who were at increased risk of mortality, and a combo endpoint of mortality or dialysis dependence at 90 days. Our data also showed that the criteria to define septic shock (i.e., a raised serum lactate level and the need for inotropic support) indeed identified the subgroup of patients with the highest mortality (>80%).
The Sepsis-3 criteria correlated with SOFA and APACHE II scores at initiation of dialysis which underpins the potential use of Sepsis-3 in a critical care setting. It can be hypothesized that a higher Sepsis-3 score also reflects a higher degree of systemic inflammation.
Data on the risk of long-term dialysis dependence in AKI-D patients with sepsis are conflicting [18,19], similar to a French multicentric study [19]. We found a statistically significant trend towards reduced likelihood of recovery from dialysis in AKI-D patients with septic shock compared to those without sepsis. In contrast, Bagshaw and colleagues analyzed data from 2000/2001 and reported improved renal recovery in patients with septic AKI compared to patients without sepsis [18]. It is important to note that there were differences in patient characteristics, criteria to define sepsis and clinical care. Moreover, our analysis has extra strength by including mortality as a competing outcome for analysis of dialysis dependence.
4.2. Sepsis-3 Criteria and Outcome
It is important to emphasize that most AKI patients already had a SOFA score of two or more at the time when RRT was initiated simply as a result of AKI and oliguria. It is possible that different delta SOFA cut-off points are necessary for this patient cohort to differentiate sepsis from non-sepsis (Figure S6). As such, our results provide confirmation that AKI-D patients with sepsis, as defined by Sepsis-3 criteria, had higher mortality and less withdraw from dialysis in AKI-D patients with septic shock.
Combining the Sepsis-3 criteria with a clinical AKI risk prediction score resulted in greater IDI and NRI, and improved the ability associating subsequent death. Given the lack of appropriate risk stratification tools for septic AKI patients requiring RRT, the new Sepsis-3 criteria may associate patients outcome (Figures S3 and S5).
4.3. Strengths and Limitations
This is the first study that applied the new consensus criteria for sepsis to AKI-D patients, a cohort of patients that is known to have a high risk of sepsis and also a high risk of poor outcomes. Using a large multi-center national database with prospectively collected representative data from 30 ICUs, we showed for the first time that the Sepsis-3 criteria identified a group of patients that were at higher risk of dying or remaining dialysis dependent after discharge. With complete follow-up for 90 days after discharge from hospital, we focused on patient-centered outcomes (mortality and long-term dialysis dependence) and provide important data for a group of patients that is commonly seen in ICUs worldwide. Further in-depth studies are mandatory before we can make any positive comment on this issue.
The limitations of our study are related to any observational cohort study and include the possibility of both unmeasured and residual confounding factors. We could not identify how many patients received early goal-directed therapy in our cohort, however the nationwide education program instituted in Taiwan is able to positively change critical-care physician behavior in sepsis care following the Surviving Sepsis Campaign guidelines. We also acknowledge that we recorded the worst value of SOFA criteria within 24 h before initiation of dialysis. Although this approach is consistent with clinical practice, the daily SOFA score or qSOFA value may not reflect the value immediately before initiation of RRT.
5. Conclusions
More than half of the critically ill AKI patients treated with dialysis had sepsis, as defined by the Sepsis-3 criteria, at dialysis initiation, and one-fifth of AKI-D patients had septic shock screened by Sepsis-3 criteria. Having sepsis and septic shock were independently associated with 90-day mortality among these ICU AKI-D patients. Among survivors, AKI-D patients with septic shock had a significantly reduced chance of recovering sufficient renal function to wean-off dialysis, when compared to those without sepsis. These findings provide support for the use of Sepsis-3 criteria in the AKI-D patients.
Acknowledgments
We would like to thank the National Taiwan University Hospital, Taiwan’s National Health Research Institutes and Taiwan’s Ministry of Science and Technology. And we would like to express our sincere gratitude to all staff of the Taiwan Clinical Trial Consortium, TR15, TCTC. We also express our sincere gratitude to all the staff of the Taiwan Clinical Trial Consortium, TCTC. The authors appreciate and thank all the members of the CAKS and NSARF, which are listed in alphabetical order of their affiliation names: (Fu Jen Catholic University Hospital): Kuo-Cheng Lu. (Chi-Mei Medical Center, Liouying): Jian-Jhong Wang. (Chi-Mei Medical Center, Yongkang): Wei-Chih Kan. (China Medical University Hospital): Chiu-Ching Huang, Che-Yi Chou, Ya-Fei Yang. (Dalin Tzu-Chi Hospital): Jen-Pi Tsai. (International-Harvard Statistical Consulting Company): Fu-Chang Hu. (Kaohsiung Chang Gung Memorial Hospital): Chien-Te Lee, Jin-Bor Chen, Chih-Hsiung Lee, Wen-Chin Lee, Lung-Chih Li, Te-Chuan Chen. (Kaohsiung Municipal Ta-Tung Hospital): Hugo You-Hsien Lin. (Keelung Chang Gung Memorial Hospital): Yung-Chang Chen, Chin-Chan Lee, Chiao-Yin Sun, Heng-Chih Pan. (Linkou Chang Gung Memorial Hospital): Ming-Yang Chang, Chang-Chyi Jenq, Chan-Yu Lin, Chih-Hsiang Chang, Tsung-Yu Tsai. (Lin-Shin Hospital): Cheng-Min Chen. (Lotung Poh-Ai Hospital): En-Tzu Lin. (Saint Mary’s Hospital Luodong): Chih-Chung Shiao. (Mackay Memorial Hospital): Chih-Jen Wu, Cheng-Jua Lin, Pei-Chen Wu. (Mackay Memorial Hospital Taitung Branch): Feng-Chi Kuo. (Min-Sheng General Hospital): Chih-Jen Weng. (National Health Research Institutes): Li-Kwang Chen. (National Taiwan University Hospital): Kwan-Dun Wu, Tzong-Shinn Chu, Yung-Ming Chen, Shuei-Liong Lin, Vin-Cent Wu, Tao-Min Huang, Yu-Feng Lin, Chun-Fu Lai. Tai-Shuan Lai. (National Taiwan University Hospital Hsin Chu Branch): Wei-Shun Yang. (New Taipei City Hospital Sanchong Branch): Wen-Ding Hsu. (Shin-Kong Wo Ho-Su Memorial Hospital): Jyh-Gang Leu, Jui-Ting Chang. (Sin-Ren Hospital): Hung-Hsiang Liou. (Taichung Veteran General Hospital): Kuo-Hsiung Hsu, Ming-Ju Wu, Chun-Te Huang. (Taichung Veteran General Hospital Chiayi Branch): Zi-hong You. (Taipei City Hospital Heping Branch): Chao-Fu Chang. (Taipei Medical University Hospital): Tzen-Wen Chen, Hsi-Hsien Chen, Fan-Chi Chang, Yen- Chung Lin, Mai-Szu Wu, Chih-Chin Kao. (Taipei Tzu Chi Hospital): Szu-Chun Hung, Ko-Lin Kuo, Che-Hsiung Wu. (Taipei Veterans General Hospital): Der-Cherng Tarng, Jinn-Yang Chen, Chih-Yu Yang, Kuo-Hua Lee. (Taoyuan General Hospital, Ministry of Health and Welfare): Wei-Jie Wang, Sheng-Wen Ko, Jui-Hsiang Lin. The authors thank the English editing by Eric B. Chueh of Case Western University, Cleveland, Ohio, USA.
Abbreviations
| APACHEII | Acute Physiology and Chronic Health Evaluation II |
| AKI | acute kidney injury |
| AKI-D | acute kidney injury with dialysis |
| MODS | multiple organ dysfunction syndrome |
| qSOFA | quick Sequential Organ Failure Assessment |
| RRT | renal replacement therapy |
| SOFA | Sequential Organ Failure Assessment |
| ICU | intensive care units |
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/8/10/1731/s1, Figure S1: Distribution of Patients by SOFA Score, and qSOFA Score at initiation of acute dialysis (n = 1,078), X axis is %, Figure S2: Prognostic Accuracy of Sepsis-3 components among AKI-D patients with suspected or confirmed infection at dialysis initiation. a) 90 day mortality Receiver-operator characteristic curves discriminate (denoted area under the receiver operating characteristic curve): Sepsis-3, (0.650), qSOFA score ≥ 2 (0.631), AKI risk prediction score (0.688) and increased SOFA ≥ 2 (0.520). b) 90 day composite outcome. Receiver-operator characteristic curves discriminate (denoted area under the receiver operating characteristic curve): Sepsis-3, (0.587), qSOFA score ≥ 2 (0.578), AKI risk prediction score (0.596) and increased SOFA ≥ 2 (0.501), Figure S3: Decision curve analysis (DCA) plot to assess the clinical consequences of screening AKI-D patients for risk of 90 day mortality using sepsis-3 score in addition to AKI risk prediction score. Y-axis is the net benefit of the decision strategy. Net benefit is the net proportion of patients with 90 day mortality in whom a prediction model would provide benefit without applying a prediction model to patients with good outcomes. For AKI patients initiated on dialysis, forecasting with the AKI predicting model and Sepsis-3 criteria in combination would yield no net benefit. For risk thresholds between 30 and 80% the superior strategy, forecasting with the AKI risk prediction score and Sepsis-3 is beneficial. For moderate to high-risk thresholds (80 to 100%), there is no net benefit from using the AKI risk prediction score together with the Sepsis-3 model, Figure S4. Hazard Ratios for 90-day Mortality comparing different criteria of Sepsis-3 definition, Figure S5: Scatter plot of AKI risk prediction forecasted probabilities without and with the Sepsis-3 score. Note that some AKI-D patients have higher predicted risks in the model with Sepsis-3 values than in the model without Sepsis-3 (dots in right lower corner of the graph), Figure S6. Generalized additive model (GAM) plot for the probability of 90-day mortality against delta SOFA, in term of the difference of SOFA at initializing dialysis and ICU admission, initiating the subject-specific (longitudinal) random effects expressed as the logarithm of the odds (logit). The relationship of delta SOFA with these variables was further illustrated by GAM analysis, adjusted for Acute Physiology and Chronic Health Evaluation (APACHEII) at ICU admission, sex and age, showing that qSOFA levels at dialysis initializing could predict risk of mortality. GAM results showed the best cut-off points predicting 90 day mortality were a change of SOFA score by more than 10, Table S1: Integer risk score for prediction of 60-day mortality in critically ill patients with AKI requiring dialysis, Table S2. Diagnostic performance of Sepsis-3 criteria in prediction of 90-day hospital mortality.
Author Contributions
Study selection and analysis, writing up of the first draft of the paper, critical revision, and final approval of the article: V.-C.W. Data curation and investigation: S.-C.J.C. Study selection and analysis, critical revision, and final approval of the article: J.-S.C., J.-T.C. and B.-G.H. Data interpretation, critical revisions, and final approval of the article: M.O. and T.-S.C.
Funding
This study was supported by Taiwan National Science Council (grants NSC 101-2314-B-002-132-MY3, NSC100-2314-B-002-119, NSC 101-2314-B-002-085-MY3, MOST 104-2314-B-002 -125 -MY3) and NTUH 100-N1776, 101-M1953, 102-S2097. We also received support from the Ministry of Science and Technology (MOST) of the Re-public of China (Taiwan) (grant number, MOST 106-2321-B-182-002).
Conflicts of Interest
The authors declare no conflict of interest. There was no role of the funding body in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
References
- 1.Hoste E.A., Bagshaw S.M., Bellomo R., Cely C.M., Colman R., Cruz D.N., Edipidis K., Forni L.G., Gomersall C.D., Govil D., et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 2.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhee C., Dantes R., Epstein L., Murphy D.J., Seymour C.W., Iwashyna T.J., Kadri S.S., Angus D.C., Danner R.L., Fiore A.E., et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009–2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu C.Y., McCulloch C.E., Fan D., Ordonez J.D., Chertow G.M., Go A.S. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiao C.C., Wu P.C., Wu V.C., Lin J.H., Pan H.C., Yang Y.F., Lai T.S., Huang T.M., Wu C.H., Yang W.S., et al. Nationwide epidemiology and prognosis of dialysis-requiring acute kidney injury (NEP-AKI-D) study: Design and methods. Nephrology (Carlton) 2016;21:758–764. doi: 10.1111/nep.12670. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y.Y., Wu V.C., Huang W.C., Yeh Y.C., Wu M.S., Huang C.C., Wu K.D., Fang J.T., Wu C.J., CAKS Group Norepinephrine Administration Is Associated with Higher Mortality in Dialysis Requiring Acute Kidney Injury Patients with Septic Shock. J. Clin. Med. 2018;7:274. doi: 10.3390/jcm7090274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu V.C., Ko W.J., Chang H.W., Chen Y.S., Chen Y.W., Chen Y.M., Hu F.C., Lin Y.H., Tsai P.R., Wu K.D. Early renal replacement therapy in patients with postoperative acute liver failure associated with acute renal failure: Effect on postoperative outcomes. J. Am. Coll. Surg. 2007;205:266–276. doi: 10.1016/j.jamcollsurg.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y.F., Ko W.J., Wu V.C., Chen Y.S., Chen Y.M., Hu F.C., Shiao C.C., Wu M.S., Chen Y.W., Li W.Y., et al. A modified sequential organ failure assessment score to predict hospital mortality of postoperative acute renal failure patients requiring renal replacement therapy. Blood Purif. 2008;26:547–554. doi: 10.1159/000178771. [DOI] [PubMed] [Google Scholar]
- 9.Shiao C.C., Ko W.J., Wu V.C., Huang T.M., Lai C.F., Lin Y.F., Chao C.T., Chu T.S., Tsai H.B., Wu P.C., et al. U-curve association between timing of renal replacement therapy initiation and in-hospital mortality in postoperative acute kidney injury. PLoS ONE. 2012;7:e42952. doi: 10.1371/journal.pone.0042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seymour C.W., Liu V.X., Iwashyna T.J., Brunkhorst F.M., Rea T.D., Scherag A., Rubenfeld G., Kahn J.M., Shankar-Hari M., Singer M., et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raith E.P., Udy A.A., Bailey M., McGloughlin S., MacIsaac C., Bellomo R., Pilcher D.V. Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA. 2017;317:290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 12.Wu V.C., Ko W.J., Chang H.W., Chen Y.W., Lin Y.F., Shiao C.C., Chen Y.M., Chen Y.S., Tsai P.R., Hu F.C., et al. Risk factors of early redialysis after weaning from postoperative acute renal replacement therapy. Intensive Care Med. 2008;34:101–108. doi: 10.1007/s00134-007-0813-x. [DOI] [PubMed] [Google Scholar]
- 13.Bellomo R., Kellum J.A., Ronco C., Wald R., Martensson J., Maiden M., Bagshaw S.M., Glassford N.J., Lankadeva Y., Vaara S.T., et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 14.Demirjian S., Chertow G.M., Zhang J.H., O’Connor T.Z., Vitale J., Paganini E.P., Palevsky P.M., VA/NIH Acute Renal Failure Trial Network Model to predict mortality in critically ill adults with acute kidney injury. Clin. J. Am. Soc. Nephrol. 2011;6:2114–2120. doi: 10.2215/CJN.02900311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y.S., Ko W.J., Lin F.Y., Huang S.C., Chou T.F., Chou N.K., Hsu R.B., Wang S.S., Chu S.H. Preliminary result of an algorithm to select proper ventricular assist devices for high-risk patients with extracorporeal membrane oxygenation support. J. Heart Lung Transplant. 2001;20:850–857. doi: 10.1016/S1053-2498(01)00267-4. [DOI] [PubMed] [Google Scholar]
- 16.Shu K.H., Wang C.H., Wu C.H., Huang T.M., Wu P.C., Lai C.H., Tseng L.J., Tsai P.R., Connolly R., Wu V.C. Urinary pi-glutathione S-transferase Predicts Advanced Acute Kidney Injury Following Cardiovascular Surgery. Sci. Rep. 2016;6:26335. doi: 10.1038/srep26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J.J., Chi N.H., Huang T.M., Connolly R., Chen L.W., Chueh S.J., Kan W.C., Lai C.C., Wu V.C., Fang J.T., et al. Urinary biomarkers predict advanced acute kidney injury after cardiovascular surgery. Crit. Care. 2018;22:108. doi: 10.1186/s13054-018-2035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagshaw S.M., Uchino S., Bellomo R., Morimatsu H., Morgera S., Schetz M., Tan I., Bouman C., Macedo E., Gibney N., et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 19.Venot M., Weis L., Clec’h C., Darmon M., Allaouchiche B., Goldgran-Toledano D., Garrouste-Orgeas M., Adrie C., Timsit J.F., Azoulay E. Acute Kidney Injury in Severe Sepsis and Septic Shock in Patients with and without Diabetes Mellitus: A Multicenter Study. PLoS ONE. 2015;10:e0127411. doi: 10.1371/journal.pone.0127411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.