ABSTRACT
BACKGROUND:
Screening for congenital hypothyroidism (CH) using cord blood or heel-stick samples is considered essential for the prevention of long-term complications CH, which include intellectual disability and slow growth.
OBJECTIVE:
Compare the sensitivity and specificity of cord blood and heel-stick samples for determining thyroid-stimulating hormone (TSH) levels for the detection of CH.
DESIGN:
Comparative diagnostic accuracy.
SETTINGS:
Tertiary care center in Riyadh.
PATIENTS AND METHODS:
The study included all infants who were delivered during the period from May 2011 to May 2013. As part of routine newborn screening, both cord blood and heel-stick samples were collected from each newborn for CH screening by measuring TSH levels. A cord TSH level was considered positive if the concentration of TSH was more than 60 mIU/L and negative if less than 30 mIU/L. Any cord TSH level between 30-60 mIU/L was considered borderline, and free T4 was measured from the same cord sample. The result was considered positive if the free T4 level was below 9 pmol/L. Heel-stick TSH levels more than 20 µU/L were considered positive. All newborns with positive results were recalled and a peripheral venous sample was taken for TSH and free T4 for confirmation.
MAIN OUTCOME MEASURES:
Sensitivity and specificity, positive and negative predictive values and recall rates.
SAMPLE SIZE:
17 729 screened babies.
RESULTS:
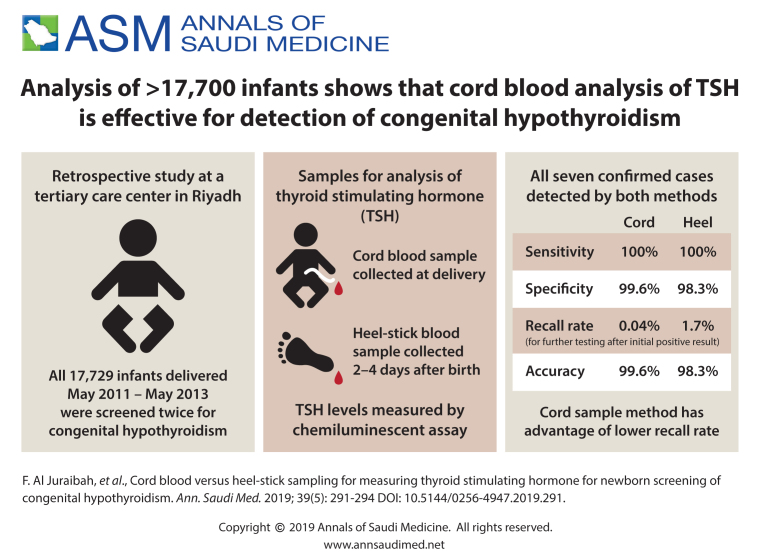
Of 17 729 neonates screened, 7 were diagnosed as having primary CH. All confirmed cases were detected by both cord and heel-stick TSH levels: 88 cord results were positive (sensitivity 100%, specificity 99.6%, with a recall rate of 0.04%) and 305 heel-stick results were positive (sensitivity 100%, specificity 98.3%, with a recall rate of 1.68%).
CONCLUSION:
Both cord and heel-stick TSH testing detected all cases of CH. Cord testing was superior to heel-stick testing as the recall rate was lower. We think cord TSH testing is preferable when heel-stick is difficult or early discharge is the practice.
LIMITATIONS:
Retrospective; the timing of newborn screening for TSH sampling was premature.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Congenital hypothyroidism (CH) screening is considered one of the best tools for the prevention of long-term devastating complications like mental retardation, which can occur in untreated cases. The global intelligence quotient (IQ) has improved after implementation of screening programs; the mean IQ in cases with congenital hypothyroidism who underwent early treatment was 107 compared to 82-88 in the prescreening era.1,2 More than half of cases are easily missed using clinical diagnosis.3 The median age for the appearance of clinical signs and symptoms of CH is 4 months.4
The first screening program for CH was established in Canada in 1974.5 Under this program, the number of cases discovered increased, with worldwide variation between 1:3500 and 1:5000 live births.6 Saudi Arabia is one of the countries with a high incidence rate—1:2279–1:2500 live births.7-9
Strategies proposed for thyroid hormone assessment are to obtain a blood sample at birth using cord blood or later in the neonatal period using a heel stick.10-12 Thyroid stimulating hormone (TSH) screening is more sensitive than primary T4 screening tests in detecting CH.13 In our center, we use cord blood TSH as a screening tool to detect CH (with a cutoff value of 30 mIU/L considered a positive result). In May 2011, heel-stick samples were initiated as part of a newborn screening program to measure TSH in neonates (with a cutoff value of 20 mIU/L).
Many studies have been published comparing heel-stick TSH testing and T4, but relatively little research has been conducted of TSH testing on cord blood samples.10-12 The effectiveness of using cord blood compared to heel-stick samples for TSH testing is rarely reported. One study showed that heel-stick sample testing was superior to cord TSH testing.12 Because of the dearth of literature on the subject and the fact that Hardy et al was based on a small sample, additional research is essential. The aim of this study was to compare the sensitivity and specificity of cord blood and heel-stick samples for determining TSH levels for the detection of CH.
PATIENTS AND METHODS
From May 2011, all infants (term and preterm) born at King Abdulaziz Medical City in Riyadh, were double screened for TSH with cord sampling on delivery and a capillary heel stick in the second to fourth day of delivery as part of the newborn screening program. All deliveries from May 2011 to May 2013 were included in this study. The study was approved by the Research and Ethics Subcommittee.
Cord blood TSH was measured by chemiluminescent microparticle immunoassay on the Abbott Architect i2000 immunoassay analyzer (https://www.corelaboratory.abbott/int/en/offerings/brands/architect/architect-i2000SR). A TSH level of more than 60 mIU/L was considered a positive result and infants were recalled for confirmation using peripheral venous samples. TSH levels below 30 mIU/L were considered normal. TSH levels between 30 and 60 mIU/L were considered borderline, and free T4 (FT4) was measured from the same cord blood sample. Infants with FT4 levels below 9 pmol/L were recalled for confirmation. A whole blood sample was obtained by heel prick between 48 to 72 hours after birth if feasible. Samples were measured using mass spectrometry. TSH levels of more than 20 μU/mL were considered positive and the newborn recalled for confirmatory peripheral venous sampling.
Cases confirmed by the peripheral venous sample were diagnosed as CH, with a full evaluation by a TC99 nuclear scan with or without thyroid ultrasound performed for determination of etiology if feasible, and to start on thyroid replacement as soon as possible. The data were analyzed using IBM SPSS, version 21 (Armonk, NY) to calculate the sensitivity, specificity and the predictive values.
RESULTS
Of 17 729 neonates screened, 7 were diagnosed as having CH for an incidence rate of 1:2532. All cases were positive by both cord sampling and heel stick (Table 1). Using heel prick TSH, 305 newborns were positive. Seven (2.3%) of the cases were true positives and a diagnosis of primary CH was confirmed. The remainder (n=298, 97.7%) were false positives as the peripheral venous test was negative. The recall rate was 1.68% (1 in 59.5 newborn). The mean age at sampling of the positive cases was 6.9 hours and the mean TSH value was 29.8 µU/mL. The heel-stick test sensitivity was 100%, the specificity was 98.3%, and the positive predictive value was 2.3%. Using cord TSH, 88 newborns were positive. The 81 false positives had a negative peripheral venous test or the free T4 from the same cord sample was normal. The recall rate was 0.04% (1 in 2532.7). The test sensitivity was 100%, the specificity was 99.55%, and the positive predictive value was 7.95% (Table 2).
Table 1.
Newborns diagnosed with congenital hypothyroidism by cord blood and heel-stick sampling for thyroid stimulating hormone levels.
| Patient No. | Gender | Cord TSH (mlU/L) | Cord free T4 (pmol/L) | Heel stick TSH (μU/mL) | Peripheral venous sample | Radiotracer uptake | Thyroid ultrasound | Etiology | |
|---|---|---|---|---|---|---|---|---|---|
| TSH | free T4 | ||||||||
| 1 | Female | 106.2 | - | 83 | 141.39 | 12.7 | RTU above the normal thyroid gland location | TG noted in the anatomical location | Ectopic |
| 2 | Female | 742.8 | - | 434 | 705.62 | 14 | RTU in the thyroid bed | Small-sized TG | Ectopic |
| 3 | Male | 52.8 | 8.7 | 36.3 | 97.48 | 12.3 | Focal increased RTU in the upper neck region | Absence of TG in the expected region | Ectopic |
| 4 | Female | 50.5 | 8.5 | 51.9 | NA | NA | - | - | Expired at 6 days of age |
| 5 | Female | 105.6 | - | 141.7 | 239.32 | 17.2 | RTU located at the tongue base | TG noted in its anatomical location | Ectopic |
| 6 | Female | 89.0 | - | 104 | 321.80 | 15.1 | RTU seen in the neck (calculate uptake 4.5%) | TG noted at the hyoid bone leve | Dyshormonogenesis |
| 7 | Male | 473.6 | - | 349 | 566.31 | 5.9 | RTU midline above the normal thyroid location | - | Ectopic |
Abbreviation: THS: thyroid stimulating hormone, NA: not available, RTU: radiotracer uptake, TG:thyroid gland
Table 2.
Indicators of the screening efficacy of the cord blood and heel-stick sampling for thyroid stimulating hormone.
| Cord sampling | Heel-stick sampling | |
|---|---|---|
| Sensitivity | 100% | 100% |
| Specificity | 99.6% | 98.3% |
| Recall rate | 0.04% | 1.7% |
| Positive predictive value | 7.95% | 2.30% |
| Diagnostic accuracy | 99.6% | 98.3% |
DISCUSSION
During the first hour after birth, there is an abrupt increase in hypothalamic thyrotropin-releasing hormone (TRH). Stimulation of pituitary TSH secretion and in turn thyroid hormone secretion is a normal physiologic adaptation to extrauterine life called physiologic TSH surge.14 TSH levels decrease progressively to normal infant levels in 3 to 5 days of life, but the serum free T4 level remains elevated for several weeks. Because of this transient physiologic increase in the TSH level, it is recommended that screening program samples be conducted at least 48 hours after birth.
To the best of our knowledge, our study is only one of a few that have compared the efficacy of cord and newborn screening heel-stick TSH samples for detecting cases of CH. We have shown that using TSH as a primary screening tool from both cord blood and capillary heel stick as a part of the newborn screening program resulted in the diagnosis of all cases of primary CH. However, the recall rate was 40 times higher for the heel-stick sample compared to the cord blood sample—1 in 59.5 and 1 in 2532.7, respectively.
Our data showed that there were 3.5 times fewer false positive cord TSH results than in the heel-stick samples. The cutoff value used for cord TSH in our screening program was 30 mIU/L, the same cut-off value used in other programs.15,16 Normal cord TSH values range from 1–39.4 mIU/L.17 Our recall rate was less than that reported in the literature,18,19 but in those studies the sample size was smaller and the cutoff value was 20 mIU/L, which is lower than in our program. The recall rate in the other studies decreased from 1.83% to 0.91% by raising the cutoff value from 20 to 30 mIU/L. Another multiregional study from Saudi Arabia, using cord TSH as a screening tool, reported a mean recall rate of 0.18% (range 0.05–0.3%),20 which is comparable to our recall rate.
The recall rate for heel stick in our screening program was about 1.7%, higher than that reported in the literature.21 We believe that the reason for the higher recall rate is because of premature sampling due to a tendency toward early discharge in our institution with the mean age of sampling being 6.7 hours. It has been shown that only 0.3% of TSH levels are greater than 15 mIU/L if the sample is taken from day 2 to 5 of life compared with 9% (30 times higher) of samples taken in the first 24 hours of life.22 If we had used 30, 40, or 50 mIU/L as a cutoff value, the recall rate would have decreased to 0.62%, 0.23%, or 0.07%, respectively. One of the major disadvantages of conducting newborn screening after 2 days is failure to get the patient to return to the hospital to do the test in some countries. In one study, the recall rate for TSH was 0.05% and the mean age at sampling was 5.3 days, but half of the newborns were not screened (the mean coverage rate was around 59%).23 Hardy et al reported the superiority of heel-stick testing, as it was more specific (recall rate of 1 in 1000 compared to 1 in 23 in cord TSH sampling).11 They used a lower cutoff value for cord TSH samples, which led to a higher recall rate. Furthermore, the cutoff value of the screening TSH level was higher than our value. Both methods had missed two separate cases with primary CH.
We think using the cord TSH blood sample as a tool for screening for CH disorders is still important, especially in countries where the earliest possible discharge is the current practice, and it is a struggle to get the newborn back to do the test in the hospital. Both cord and heel-stick testing are highly sensitive; cord TSH was superior to capillary dried blood from a heel stick in terms of a lower recall rate, which was clearly due to sampling at an early age. In countries where recall is difficult or early discharge is the practice, we recommend cord TSH testing as the best method to minimize the number of false positive samples and avoid unwanted parental anxiety as their baby needs retesting.
Our study was retrospective and did not take into account premature babies with delayed TSH surges; such cases can be easily missed by TSH screening. The timing of TSH sampling can affect the recall rate, a well-known issue. Therefore, a further prospective study is required with accurate sample procurement timing.
Funding Statement
None.
REFERENCES
- 1.Grosse SD, Van Vliet G.. Prevention of intellectual disability through screening for congenital hypothyroidism: How much and at what level? Arch Dis Child. 2011; 96:374-379. [DOI] [PubMed] [Google Scholar]
- 2.Ordooei M, Mottaghipisheh H, Fallah R, Rabiee A.. Cognitive outcomes for congenital hypothyroid and healthy children: a comparative study. Iranian journal of child neurology. 2014; 8(4):28. [PMC free article] [PubMed] [Google Scholar]
- 3.Two Alm J, Larsson A, Zetterstrom R.. Congenital hypothyroidism in Sweden. Incidence and age at diagnosis. Acta Paediatric Scand. 1978; 67:1-3. [DOI] [PubMed] [Google Scholar]
- 4.Alm J, Hagenfeldt L, Larsson A, Lundberg K.. The incidence of congenital hypothyroidism: Retrospective study of neonatal laboratory screening versus clinical symptoms as indicators leading to diagnosis. Br Med J (Clin Res Ed). 1984; 289:1171-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dussault JH. The anecdotal history of screening for congenital hypothyroidism. J Clin Endo Metab. 1999; 84:4332–4334. [DOI] [PubMed] [Google Scholar]
- 6.Toublanc J. Comparison of epidemiological data on congenital hypothyroidism in Europe with those of other parts of the world. Horm Res (Basel). 1992; 38:230–5 [DOI] [PubMed] [Google Scholar]
- 7.Tamimi W, Abokhashab M, Alhazmi Z, Alsadhan A.. Congenital hypothyroidism in Saudi children. Bri J of bio SCI. 2003; 60(1):37. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Osba YK, Mallouh A, Salamah M, Hann R, Thaliji A, Hamdan J, et al. Comprehensive newborn screening program: ARAMCO experience, the national need and recommendations. Ann Saudi MED. 1992; 12:235-240. [DOI] [PubMed] [Google Scholar]
- 9.Bacchus R, Williams S, Joyce B, Sabagh TO, MASOOD K, Paterson W.. Neonatal screening for congenital hypothyroidism in Riyadh. Saudi MED J. 1988, 9(6):588-95. [Google Scholar]
- 10.Kempers MJ, Lanting CI, Van Heijst AF, Van Trotsenburg AS, Wiedijk BM, De Vijlder JJ, et al. Neonatal screening for congenital hypothyroidism based on thyroxine, thyrotropin, and thyroxine-binding globulin measurement: potentials and pitfalls. The J of Clin Endo & Metab 2006. September; 91(9):3370-6. [DOI] [PubMed] [Google Scholar]
- 11.Ward L S, Maciel R M, Magalhães R F, Kunii I S, Kurazawa G K, Matsumura L K, et al. Comparison of two strategies for the early detection of congenital hypothyroidism. Rev Assoc Med Bras. 1998; 44:81-86. [DOI] [PubMed] [Google Scholar]
- 12.Hardy JD, Zayed R, Doss I, Dhatt GS.. Cord blood thyroxine and thyroid stimulating hormone screening for congenital hypothyroidism: How useful are they? J Pediatric Endocrinal Metab. 2008; 21:245-249. [DOI] [PubMed] [Google Scholar]
- 13.Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, Van Vliet G, et al. European Society for Pediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Hormone Research in Pediatrics 2014. January 21; 81(2):80-103. [DOI] [PubMed] [Google Scholar]
- 14.Djemli A, Van Vliet G, Belgoudi J, Lambert M, Delvin EE.. Reference intervals for free thyroxine, total triiodothyronine, thyrotropin and thyroglobulin for Quebec newborns, children and teenagers. Clinical biochemistry 2004. April 30, 37(4):328-30. [DOI] [PubMed] [Google Scholar]
- 15.Mahachoklertwattana P, Phuapradit W, Siripoonya P, Charoenpol O, Thuvasethakul P, Rajatanavin R.. Five-year thyrotropin screening for congenital hypothyroidism in Ramathibodi Hospital. J of the MED Asso of Thailand= Chotmaihet thangphaet 1999. November, 82:S27-32. [PubMed] [Google Scholar]
- 16.Al Jurayyan NA, Al Jurayyan RN.. Congenital hypothyroidism and neonatal screening in Saudi Arabia. Curr Pediatr Res. 2011; 16:31-6. [Google Scholar]
- 17.Mekonnen Y, Hawariat GW, Chamiso B, Raue F.. Thyroid Stimulating Hormone values from cord blood in neonates. Ethio J of Health Development 2003; 17(2):125-30. [Google Scholar]
- 18.Manglik AK, Chatterjee N, Ghosh G.. Umbilical cord blood TSH levels in term neonates: a screening tool for congenital hypothyroidism. Indian Pediatric 2005. October 1, 42(10):1029-32. [PubMed] [Google Scholar]
- 19.Wu LL, Sazali BS, Adeeb N, Khalid BA.. Congenital hypothyroid screening using cord blood TSH. Sing MED J. 1999. January; 40(1):23-6. [PubMed] [Google Scholar]
- 20.Al-Jurayyan NA, Al-Nuaim AA, El-Desouki MI, Al Herbish AS, Bakr AM, and Al Swailem A, et al. Neonatal screening for congenital hypothyroidism in Saudi Arabia: results of screening the first 1 million newborns. Screening. 1996. May 1, 4(4):213-20. [Google Scholar]
- 21.Rose SR, Brown RS.. American Academy of Pediatrics, American Thyroid Association. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006. June 1; 117(6):2290-303. [DOI] [PubMed] [Google Scholar]
- 22.Dussault JH, Grenier A, Morissette J.. Preliminary report on filter paper TSH levels in the first 24 h of life and the following days in a program screening for congenital hypothyroidism. Early hospital discharge: impact of newborn screening. Atlanta: Council of Regional Networks for Genetic Services, Emory University School of Medicine; 1995, 267. [Google Scholar]
- 23.Al Hosani H, Salah M, Saade D, Osman H, Al Zahid J.. United Arab Emirates National Newborn Screening Program: an evaluation 1998-2000. [PubMed] [Google Scholar]