ABSTRACT
BACKGROUND:
Adiposity is associated with high serum levels of adipokines and chemokines which are possibly implicated in a co-existence of obesity and asthma.
OBJECTIVES:
Elucidate the possible roles of leptin, interleukin (IL)-4, IL-5 and IL-21 in linking obesity with childhood asthma.
DESIGN:
Cross-sectional, analytical.
SETTING:
Population of schoolchildren in a small Saudi city.
SUBJECTS AND METHODS:
The study included a representative sample of Saudi schoolchildren grouped as obese asthmatics, non-obese asthmatics, or obese nonasthmatics, with nonobese nonasthmatics as a control group. An asthma control test was done for the asthmatic groups.
MAIN OUTCOME MEASURES:
Serum levels of leptin, IL-4, IL-5, and IL-21.
SAMPLE SIZE:
345 male schoolchildren with a mean (SD) age of 13.0 (2.3) years.
RESULTS:
Median serum leptin concentrations in obese asthmatics were significantly higher than in nonobese asthmatics (P<.001). Uncontrolled asthmatics also had significantly higher leptin levels than controlled asthmatic children (P<.002). Leptin levels were weakly but significantly correlated with the cytokines IL-4, IL-5, and IL-21.
CONCLUSIONS:
Leptin may contribute to a link between obesity and childhood asthma. Differences in IL-21 levels between nonobese and obese asthmatics suggest that the co-existence of asthma and obesity increased IL-21 levels. Leptin plus some proinflammatory cytokines especially IL-21 may be potential predictors for asthma control in children.
LIMITATIONS:
Blood sampling at different stages of asthma might influence cytokine expression.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Bronchial asthma (BA) is a common and potentially serious inflammatory disease of the respiratory tract characterized by reversible obstruction and hyper-responsiveness of the tracheobronchial system.1 The disease imposes a fundamental role in patients' interactivity with the community. Asthma is occasionally fatal; flare-ups can sometimes entail urgent health care.2 About 2 million Saudis have asthma and the prevalence is rising.3 The overall prevalence of asthma in Saudi children ranges from 8% to 25% based on studies conducted over the past three decades.4
Obesity and morbid obesity are serious health problems reaching epidemic proportions in many countries.5 Adipose tissue produces leptin, the so-called satiety hormone, which aids in regulating energy balance by hindering the appetitle. This hormone is counteracted by another hormone called ghrelin, the “hunger hormone”. The arcuate nucleus of the hypothalamus contains receptors for leptin and ghrelin to control appetite and maintain energy homeostasis.6 Sensitivity to leptin is diminished in obese individuals, leading to a failure of to recognize satiety despite increased fuel reservoirs.7
Leptin is a versatile hormone that counteracts many adipokines and cytokines. A recent suggestion proposed that leptin acts as a key risk factor in the emergence of allergic asthma in obese individuals via inducement of the unfolded protein response factor XBP1s that stimulates pro-allergic lymphocyte survival and cytokine production.8 Asthma and obesity in children may cause some sort of chronic low grade inflammation that induces adipose tissue to produce proinflammatory cytokines. Hence, many chemokines and cytokines, including IL-4 which mediates important proinflammatory functions in asthma, IL-5 which is associated with eosinophil development and its expression declines with corticosteroids, and IL-21 which enhances neutrophil production during asthma, may have roles in the severity of pediatric asthma.9-13 Nevertheless, deciphering their pulmonary impacts is a challenging point of research. This study aimed to show the possible roles of leptin, IL-4, IL-5 and IL-21 in a link between obesity and asthma in children.
SUBJECTS AND METHODS
This cross-sectional study was carried out from January 2016 to January 2018. Official letters were directed to the headmasters of the selected schools to clarify the objectives and methodology, and the active cooperation of school staff was ensured. A clear informative letter that included a consent statement was given to each student. Written informed consent was obtained from the parents or legal guardians of the students who agreed to participate in the study. This work was carried out under the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human subjects. The Ethics and Research Committee of the College of Medicine, Najran University approved the study protocol. Formal approval by active communication with Najran General Directorate of Education was obtained
Using multistage random sampling, we selected a representative sample of Saudi schoolchildren in Najran, Southwestern Saudi Arabia. Sample size was calculated from the formula n=Z2 P (1-P)/d2 where n is the sample size, Z is the statistic corresponding to level of confidence, P is the expected prevalence of asthma (estimated as 4.05 % in Saudi Arabia [95 % confidence interval: 3.54–4.62%) and d is the proportion of sampling error.14 Hence, the sample size for asthmatic children was chosen as 100 and the same sample size was roughly chosen for obese asthmatics, obese nonasthmatics, and control (nonobese nonasthmatics). Children with diabetes or any other endocrine diseases were excluded from the study. Grouping of children into obese and nonobese was based on their body mass index (BMI) percentile, since obesity is defined as a BMI greater than the 95th percentile for age and gender. The BMI was calculated according to the formula: weight (kg)/height2 (m2). Because logistical problems prevented the enrollment of enough females to provide meaningful leptin and other values, the study includes only male children.
In accordance with the guidelines of The Saudi Initiative for Asthma and the Global Initiative for Asthma,15 asthma was diagnosed based on a history of recurrent or chronic chest symptoms such as cough, wheezing, difficulty breathing, and chest tightness that demonstrated clinical reversibility with short-acting bronchodilator treatment. Symptom scores in children with asthma were assessed according to a six-domain asthma symptom score that includes dyspnea, tightness in the chest, wheezing during the day, wheezing during the night, and daily performance.
Children with asthma were classified into controlled and uncontrolled groups according to the revised guidelines from the Global Initiative for Asthma (GINA).15 There were no partly controlled cases. Children with any other acute or chronic disease, including acute upper or lower respiratory tract infection, were excluded along with children who had received corticosteroid treatment for asthma within the previous 4 weeks.
For the measurement of leptin and interleukins 4, 5 and 21, venous blood (2 mL) was obtained at 9:00 a.m. after an overnight fast. Blood samples were allowed to clot at room temperature for 60 minutes. The sera were separated by centrifugation at 1200 μg for 10 minutes and stored at -80°C. Sera were thawed at room temperature before measurements. The serum leptin concentrations were measured using the double antibody sandwich ELISA method with an antibody specific for human leptin (MyBiosource Leptin ELISA kit, USA). IL-4 and IL-5 were measured using Quantikine ELISA (R&D SYSTEMS, USA) kits whereas IL-21 was measured using LifeSpan BioSciences (LS Bio, USA) ELISA kits.
Data were analyzed using IBM SPSS version 23 software package (IBM<Armonk, NY). Means or medians, or frequencies and percentages were used to express the data. Since leptin levels were not normally distributed, nonparametric tests including the Kruskal Wallis tests were used for statistical comparisons. Nonparametric tests were also used for comparisons of levels of inter-leukins because the data were skewed. Linear regression was used for correlations of continuous variables.
RESULTS
The mean age (SD) of the 345 male schoolchildren was 13.3 (1.8, 14.0) years, and ages ranged from 7 to 16 years (Table 1). There were no statistically significant differences in mean age between nonobese nonasthmatic and nonobese asthmatics, nor between obese nonasthmatic and obese asthmatics. Differences in BMI between the nonobese nonasthmatics and non-obese children with asthma were statistically significant (P=.036, Tukey post-hoc test). Differences in BMI betweeen the obese nonasthmatics and obese children with asthma were not statistically significant (P=.482, Tukey post-hoc test).
Table 1.
Distribution of age and body mass index of study groups.
| n (%) | Age (y) | BMI (kg/m2) | |
|---|---|---|---|
| Nonobese, nonasthmatic | 66 (19.1) | 12.9 (2.0) | 17.8 (2.6), 17.1 |
| Nonobese, asthmatic | 100 (29.0) | 13.7 (1.5) | 19.5 (3.2), 18.6 |
| Obese, nonasthmatic | 83 (24.1) | 13.1 (1.7) | 28.6 (4.7), 27.7 |
| Obese, asthmatic | 96 (27.8) | 13.2 (1.8) | 27.7 (4.6), 28.3 |
Data are mean (standard deviation) for age and mean (standard deviation, median for BMI.
Leptin values values ranged from 172 to 7200 pg/mL with a median (IQR) of 1070 (520-2560) pg/mL. Median leptin values for the study groups are shown in Figure 1. Leptin values in obese children were higher than in nonobese children (P<.001, Mann-Whitney U test). Leptin levels among children with asthma (obese and nonobese) were higher than that of nonasthmatic children, but the difference was not statistically significant (P=.413, Mann-Whitney U test). Leptin levels in obese asthmatics were higher than in nonobese asthmatics (P<.001, Tukey post-hoc test). The leptin values in uncontrolled asthmatic children were significantly higher than in controlled asthmatics (median 1190, range 210-7200 vs. median 540, range 200-5560 pg/mL, P<.001, Mann-Whitney U test).
Figure 1.
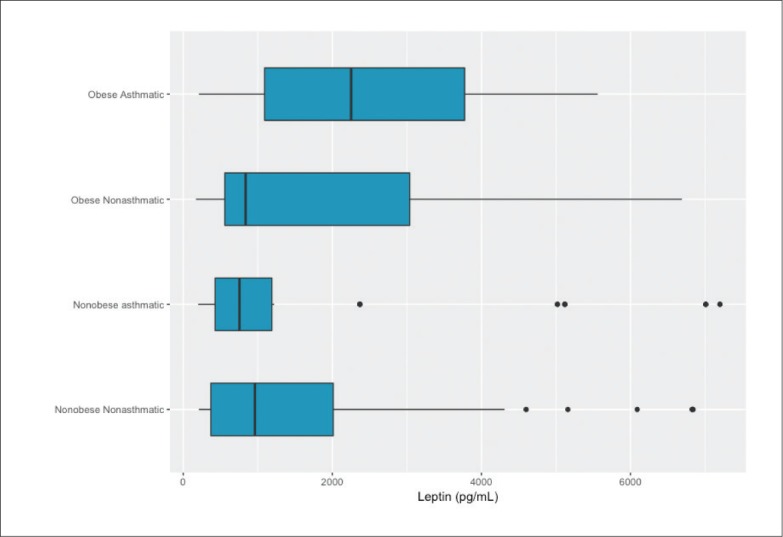
Median (interquartile range) leptin (pg/nL) for males by study group (n=345) (P<.001 by Kruskal-Wallis test; P<.001 for obese asthmatics vs nonobese asthmatics).
There was a significant positive but weak correlation between serum levels of leptin and BMI in all children (r=0.118, P=.029) (Figure 2). The regression equation showed that BMI predicted leptin levels according to the formula: 996.21+32.45×BMI.
Figure 2.
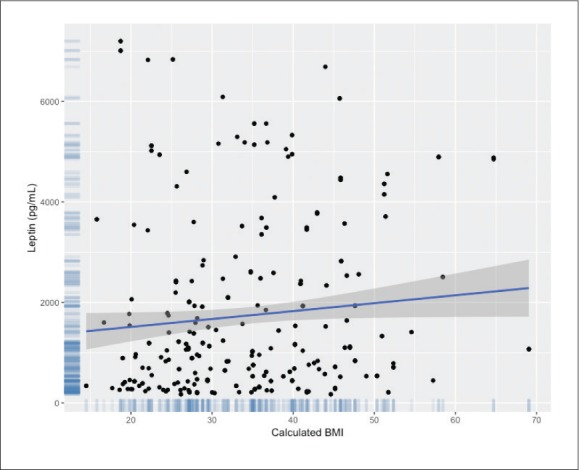
Correlation between leptin levels and body mass index (n=345).
Mean concentrations of IL-4, IL-5, and IL-21 differed significantly across the study groups (Figure 3). Children with asthma had significantly higher mean IL-4, IL-5, and IL-21 levels than nonasthmatics (P<.001, P=.028, P=.004, respectively). Differences in IL-5 and IL-21 levels differed significantly between controlled and uncontrolled asthmatic children (Figure 4). The high levels of cytokines, especially among uncontrolled asthmatics, suggest their role in the pathogenesis of asthma.
Figure 3.
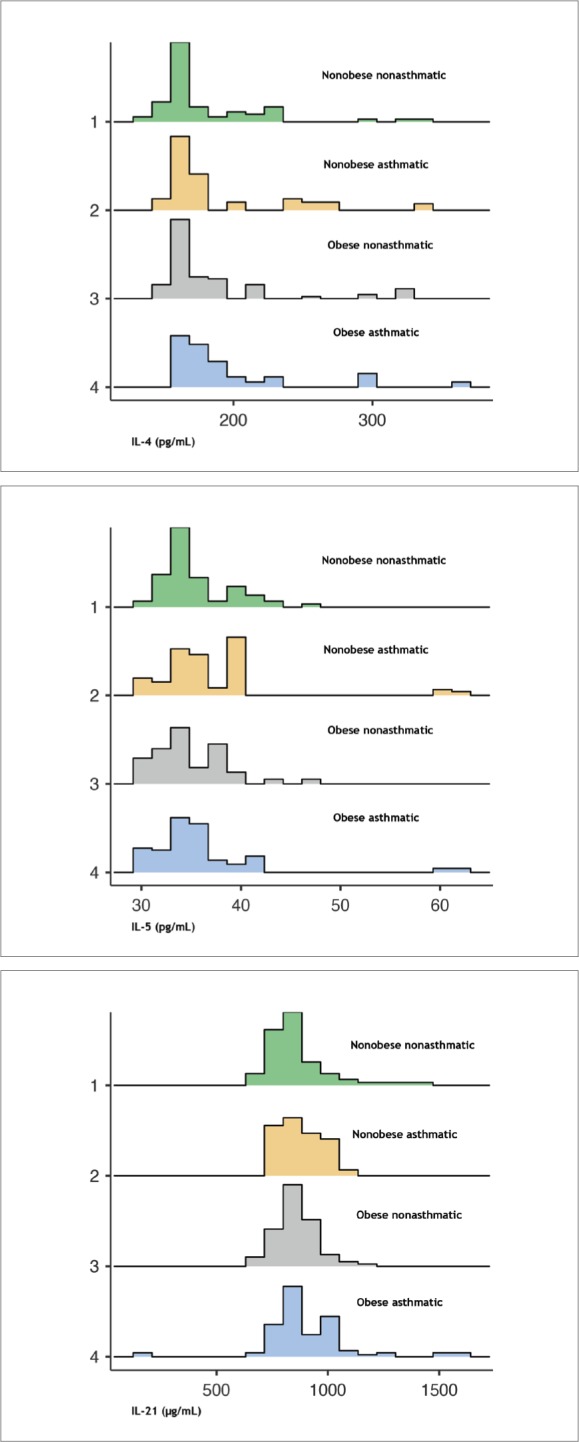
Interleukin-4 (top), interleukin-5 (middle) and interleukin-21 (bottom) by study group (n=345) (P<.001 for IL-4, .021 for IL-5, .029 for IL-21, Kruskall-Wallace test).
Figure 4.
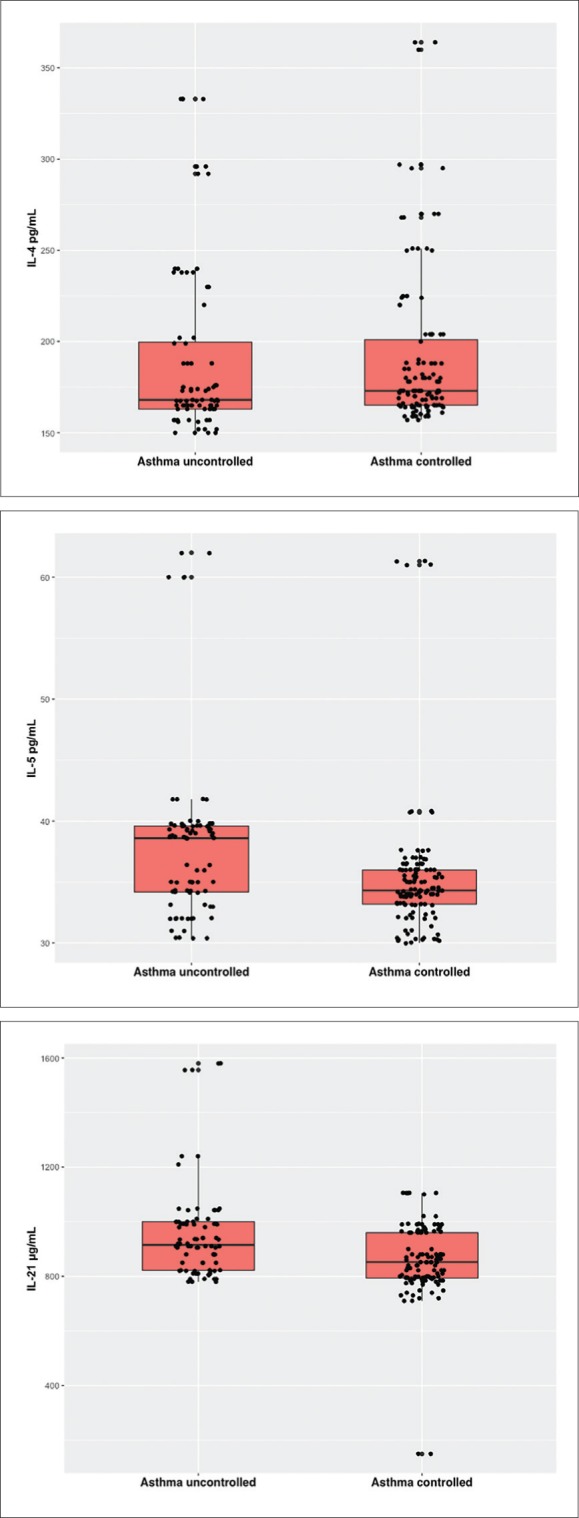
Interleukin-4 (top), Interleukin-5 (middle), and Interleukin-21 (bottom) by asthma control (P=.118 for IL-4, P<.001 for IL-5, P<.001 for IL-21 by Mann-Whitney U test (n=196).
None of the differences in IL-4, IL-5 and IL-21 levels between overweight/obese vs normal/underweight were statistically significant (Kruskall-Wallace with Tukey post-hoc tests). Our results indicate that the presence of obesity was not associated with cytokine levels, but obesity was significantly associated with the level of leptin.
Although correlations between leptin levels and interleukins 4, 5 and 21, were weakly positive, these correlations were statistically significant (r values were 0.204 (P<.001), 0.109 (P<.029), and 0.115 (P<.021, respectively). There were statistically significant positive correlations between IL-4 and IL-5 with IL-21 (r=0.224, P=.00 and r=0.27, P=.001 respectively).
DISCUSSION
Asthma is a difficult diagnosis in children due to multiple known and unknown causes and triggers.16 There has been an increasing interest in the possible role of adipocytes in the emergence and pathogenesis of asthma in obese individuals.17 Asthma is an important clinical and public health issue representing the most prevalent chronic illness in children.18 Bronchial asthma in conjunction with overweight (rather than asthma alone) has increasingly been identified as negatively impacting pulmonary function in children and adults.19 Our results show that BMI can predict the leptin level (but not the reverse). In a previous large case-control study investigating the association between obesity and asthma in 1264 Saudi schoolchildren, Nahass et al20 found that BMI was associated with asthma in boys (adjusted OR=1.11, 95% CI, 1.03-1.19) and girls (adjusted OR=1.38, 95% CI, 1.23-1.56). Obese asthmatic patients usually declare indigent asthma control despite traditional asthma therapy. The results of our study support a link between asthma and obesity in children. The possible role of leptin in this link is likely strong as well. Leptin values among obese children were significantly higher than in nonobese children (P<.001). Moreover, leptin values among uncontrolled asthmatics were significantly higher than the corresponding values among controlled-asthmatic children (P<.001). This may be explained by the association of obesity with airway dysanapsis in children which in turn is associated with increased morbidity among obese children with asthma and may partly explain their reduced response to treatment since obese asthmatic children are less responsive to corticosteroid treatments.21
Zheng et al suggested that leptin acts as a key risk factor in the emergence of allergic asthma in obese individuals via inducement of the unfolded protein response factor XBP1s that hikes pro-allergic lymphocytes survival and their cytokine production. Hence, their findings may point out a novel therapeutic approach for the treatment of obesity-linked allergic asthma.8 Chen et al carried out a cohort study on clinically diagnosed asthmatic children. They found that the risk of developing obesity is more or less 50% higher in asthmatics when compared with non-asthmatic children and medications used for asthma control are likely diminishing obesity risk regardless of physical activity.22 In this context, Mohammed et al showed that serum leptin concentrations in all patients with asthma were much greater than that in controls and this was statistically significant and increased sharply during acute exacerbation and decreased after controlling the attack. Moreover, leptin levels in obese were higher than in nonobese asthmatic patients.23 This indicates that leptin may be a substantial player in asthma pathogenesis since serum leptin and leptin/adiponectin levels are negatively correlated with lung function tests in asthmatics.24 Therefore, leptin may be regarded as a simple marker in children for non-specific airway inflammation.25 Similarly, Haynes et al26 concluded that leptin could pre-dispose to asthma through its effect on immune function mediated by IL-6 and tumor necrosis factor (TNF)-α and its effect on the sympathetic nervous system. There is a report that leptin is implicated in provoked T-helper inflammation as well as in inflammatory cell enhancement and accumulation in obese children with atopy.27
A better perception of the mechanisms for how BMI affects asthma risk may be elucidated from epidemio-logical studies. It has been proposed that the link between obesity and asthma may be attributed to the reciprocal correlation of bronchial asthma and obesity with related etiological factors28 including lifestyle. Obesity is associated with high-calorie diets and unhealthy dietary habits that may be associated with an increased rate of incidence of asthma in children.29 Consequently, one of the guises of the lifestyle-related obesity, such as frequent indoor living, maybe the etiologically significant factor in some communities. The adipose tissue in obese individuals results in a state of systemic inflammation which generates an elevation in the serum levels of diversified proinflammatory chemokines, adipokines, and cytokines such as adiponectin as an anti-inflammatory mediator and leptin as a proinflammatory one.30 More leptin is secreted as body weight increases.15 This is evident in our study which revealed that BMI can predict the leptin level (but not the reverse). However, the tendency to experience asthma in obese children cannot purely be explained by leptin-mediated immunomodulatory effects of obesity since not all obese children develop bronchial asthma.31
In previous studies, reports on concentrations of adipokines in asthma patients, including children, have been discordant. Leptin has been reported as elevated,32 or unchanged33 in asthmatic individuals. These conflicting findings are likely explained by differences in patient selection. In addition, there are patient-related contributing factors like distribution of adipocytes, age, and gender.18
Gender is a significant factor in BMI-related asthma risk.34 Some studies have demonstrated a link between adipokines including leptin and asthma in pediatric and adult populations, with a stronger correlation seen in females rather than males.35,36 One study which showed that asthma is associated with a high BMI and is more pronounced in boys than in girls.37 Gilliland and co-authors29 concluded that overweight is associated with an elevated risk of newly recognized asthma in male and non-atopic children. Thus, leptin serum levels could be regarded as a significant gender-dependent marker for the degree of obesity in prepubertal children prone to asthma.
Adipocytes are the provenance of proinflammatory chemokines and cytokines such as leptin, TNF-α, IL-6, and IL-18. Pulmonary inflammation is enhanced by elevation of proinflammatory cytokines, which is a clue for asthma pathophysiology. The consolidated influence of increased proinflammatory ambiance and bronchial hyperresponsiveness in obese individuals may determine the phase for asthma onset.38 Activated eosinophils induced by the allergic airway inflammation release an eosinophilic cationic protein which causes airway hyper-reactivity and tissue damage. Interleukin-37-interceded regulation of inflammatory mechanism, linking the activation of eosinophils and infection, ensures the in vivo anti-inflammatory efficiency of IL-37b on allergic asthma.39
Obesity may result in asthmatic symptoms in susceptible subjects through such inflammatory mechanisms or lifestyle changes.40 Moreover, serum values of IL-21 were significantly higher in asthmatics when compared with nonasthmatics and controlled asthmatics had significantly reduced IL-21 values than uncontrolled-asthmatic children. Furthermore, leptin levels were significantly correlated with IL-4, IL-5, and IL-21.
Eosinophil-mediated inflammation may be a result of chemotactic IL-5 action derived from tissues after exposure to allergens. It increases χ2 integrin-mediated adhesion to endothelial cells and hence eosinophil transmigration that promotes inflammation and tissue modeling along with many enzymes, growth factors, cytokines, and chemokines encompassing IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, TNF-α and interferon (INF)-α.41 Moreover, there is some sturdy evidence strengthening the role for IL-4, IL-5, and IL-13 derived from T-innate lymphoid cells and T helper (h)-2 cells as precursors for eosinophil-mediated inflammatory reactions in approximately half of asthmatic individuals.42,43 Consequently, recent evidence has correlated eosinophil-mediated inflammation of airways with poor response to bronchodilator drugs.44 These findings induced the emergence of IL-5 inhibitors like benralizumab as a unique inhibitor of severe asthma targeting the IL-5 receptor, as well as other cytokines contributing to eosinophilic inflammation.45
Some experimental evidence, in animal models, has supported the hypothesis that Th2 cytokines are linked with allergic airway inflammation.46 IL-21 is formed substantially by natural killer cells and Th17 cells. It alters immunoglobulin isotype switching and is implicated in the hindering of IgE output in human beings.47,48 IL-21 is preferentially expressed by Vα14 NKT cells with concomitant production of IFN-α.49 This may explain the contributory role of IL-21 in asthma via its important contribution to the coordination of IgE-mediated reactions in the human immune system. The proinflammatory action of leptin may hence explain the statistically significant correlation between leptin and interleukins 4, 5 and 21 on a variety of CD4+ T cells and its role in the induction of T lymphocytes proliferation50 and the production of these cytokines. A limitation of this study is that blood samples were taken from asthmatic children at various stages of asthma, perhaps leading to varying cytokine expressions.
Leptin contributed a link between obesity and childhood asthma. Leptin plus some proinflammatory cytokines especially IL-21 have been suggested as potential predictors for asthma control in children. Because not all obese children develop asthma, the immunomodulatory impacts of leptin may play a role in a higher predilection to asthma in obese children. Further large-sized studies are recommended to explore other implicated proinflammatory chemokines and cytokines.
Funding Statement
King Abdulaziz City for Science and Technology (KACST). Grant number # A.T 34/116.
REFERENCES
- 1.Boulet LP, Boulay ME.. Asthma-related comorbidities. Expert Rev Respir Med 2011; 59(3):377–393. [DOI] [PubMed] [Google Scholar]
- 2.Pocket guide for asthma management and prevention for adults and children older than 5 years. Global initiative for asthma (updated 2016). Available at www.ginasthma.org.
- 3.Hussain SM,Farhana SA, Alnasser SM.. Time trends and regional variation in prevalence of asthma and associated factors in Saudi Arabia-A systematic review and Meta-Analysis. BioMed Research International 2018; Article ID 8102527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Frayh AR, Shakoor Z, Gad El Rab MO, Hasnain SM.. Increased prevalence of asthma in Saudi Arabia. Ann Allergy Asthma Immunol. 2001. March;86(3):292-6. PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 5.Alsareii SA, Elbashir AM, Shalayel MHF.. Obesity and Bariatric Surgery: Ultimate Need for Vitamin D Supplementation. Biomed Pharmacol J 2017; 10(3). [Google Scholar]
- 6.Brennan AM, Mantzoros CS.. Drug Insight: the role of leptin in human physiology and pathophysiology–emerging clinical applications. Nat Clin Pract Endocrinol Metab 2006; 2(6): 318–27. [DOI] [PubMed] [Google Scholar]
- 7.Pan H, Guo J, Su Z.. Advances in understanding the interrelations between leptin resistance and obesity. Physiology & Behavior 2014; 130: 157–69. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H, Wu D, Wu X, Zhang X, Zhou Q, Luo Y, et al. Leptin Promotes Allergic Airway Inflammation through Targeting the Unfolded Protein Response Pathway. Nature (Scientific Reports) 2018; 8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krasteva N, Shentov B, Elkina S.. Cytokines in asthma and obesity in children. European Respiratory Journal 2018; 52 (suppl) 62: PA 1303. [Google Scholar]
- 10.Hatami H, Ghaffari N, Ghaffari J, Rafatpanah H.. Role of Cytokines and Chemokines in the Outcome of Children With Severe Asthma. J Ped Rev 2019; 7(1): 17-28. [Google Scholar]
- 11.Steinke JW, Borish L.. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2001;2:66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halwani R, Sultana A, Vazquez-Tello A, Jamhawi A, Al-Masri AA, Al-Muhsen S.. Th-17 regulatory cytokines IL-21, IL-23, and IL-6 enhance neutrophil production of IL-17 cytokines during asthma. J Asthma 2017; 54:893-904. [DOI] [PubMed] [Google Scholar]
- 13.Gregory B, Kirchem A, Phipps S, Gevaert P, Pridgeon C, Rankin SM, et al. Differential regulation of human eosinophil IL-3, IL-5, and GM-CSF receptor alpha-chain expression by cytokines: IL-3, IL-5, and GM-CSF down-regulate IL-5 receptor alpha expression with loss of IL-5 responsiveness, but up-regulate IL-3 receptor alpha expression. J Immunol. 2003;170:5359-66. [DOI] [PubMed] [Google Scholar]
- 14.Moradi-Lakeh M, El Bcheraoui C, Daoud F, Tuffaha M, Kravitz H, Al Saeedi M, et al. Prevalence of asthma in Saudi adults: findings from a national household survey, 2013. BMC Pulm Med 2015; 15(1): 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Jahdali HH, Al-Hajjaj MS, Alanezi MO, Zeitoni MO, Al-Tasan TH.. Asthma control assessment using asthma control test among patients attending 5 tertiary care hospitals in Saudi Arabia. Saudi Med J 2008; 29(5):714-7. [PubMed] [Google Scholar]
- 16.Kleinert S, Horton R.. After asthma: airways diseases need a new name and a revolution. The Lancet. 2018; 391(10118): 292–4. [DOI] [PubMed] [Google Scholar]
- 17.Abdul Wahab A, Maarafiya MM, Soliman A, Younes NB, Chandra P.. Serum Leptin and Adiponectin Levels in Obese and Nonobese Asthmatic School Children in relation to Asthma Control. J Allergy. 2013(654104): 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umetsu DT. Mechanisms by which obesity impacts asthma. Thorax 2017; 72(2): 174-7. [DOI] [PubMed] [Google Scholar]
- 19.Jones MH, Roncada C, Fernandes MTC, Heinzmann-Filho JP, Sarria Icaza EE, Mattiello R, et al. Asthma and Obesity in Children Are Independently Associated with Airway Dysanapsis. Front Pediatr 2017; 5:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nahhas M, Bhopal R, Anandan C, Elton R, Sheikh A.. Investigating the association between obesity and asthma in 6- to 8-year-old Saudi children: a matched case-control study. NPJ Prim Care Respir Med 2014; 24: 14004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forno E, Weiner DJ, Mullen J, Sawicki G, Kurland G, Han YY.. Obesity and airway dysanapsis in children with and without asthma. Am J Respir Crit Care Med 2017; 195(3):314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K, et al. Effects of Childhood Asthma on the Development of Obesity among School-aged Children. Am J Respir Crit Care Med 2017; 195(9): 1181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammed EA, Omar MM, Hibah NAA, Essa HA.. Study of serum leptin level in obese and nonobese asthmatic patients. Egypt J Bronchol 2015; 9(2):118-24. [Google Scholar]
- 24.Nasiri Kalmarzi R, Ataee P, Mansori M, Moradi G, Ahmadi S, Kaviani Z, et al. Serum levels of adiponectin and leptin in asthmatic patients and its relation with asthma severity, lung function and BMI. Allergol Immunopathol (Madr) 2017; 45(3): 258-64. [DOI] [PubMed] [Google Scholar]
- 25.Bodini A, Tenero L, Sandri M, Maffeis C, Piazza M, Zanoni L, et al. Serum and exhaled breath condensate leptin levels in asthmatic and obesity children: a pilot study. Journal of Breath Research 2017; 11(4): 046005. [DOI] [PubMed] [Google Scholar]
- 26.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL.. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 1999; 33 (1 Pt 2):542-7. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Zeng Q, Zhou L, Luo R, Dong H.. Association of leptin with disease severity and inflammation indicators in Chinese obese children with allergic rhinitis. Pediatr Allergy Immunol 2018; 29(2): 186-93. [DOI] [PubMed] [Google Scholar]
- 28.Grad R. Risk of asthma in children with exposure to mite and cat allergens. Lancet 2000; 356(9239): 1369–70. [DOI] [PubMed] [Google Scholar]
- 29.Gilliland FD, Berhane K, Islam T, McConnell R, Gauderman WJ, Gilliland SS et al. Obesity and the Risk of Newly Diagnosed Asthma in School-age Children. American Journal of Epidemiology. 2003; 158(5): 406–15. [DOI] [PubMed] [Google Scholar]
- 30.Magrone T, Simone M, Altamura M, Munno I.. Characterization of the Immune Inflammatory Profile in Obese Asthmatic Children. Endocr Metab Immune Disord Drug Targets. 2014; 14: 187-95. [DOI] [PubMed] [Google Scholar]
- 31.Vijayakanthi N, Greally JM, Rastogi D.. Pediatric Obesity-Related Asthma: The Role of Metabolic Dysregulation. Pediatrics 2016; 137(5): e20150812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sood A, Ford ES, Camargo CA Jr.. Association between leptin and asthma in adults. Thorax 2006; 61(4):300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jartti T, Saarikoski L, Jartti L, Lisinen I, Jula A, Huupponen R, et al. Obesity, adipokines and asthma. European Journal of Allergy and Clinical Immunology. 2009; 64(5):770–7. [DOI] [PubMed] [Google Scholar]
- 34.Ho W, Lin Y, Caffrey JL, Lin M, Hsu H, Myers L, et al. Higher body mass index may induce asthma among adolescents with preasthmatic symptoms: a prospective cohort study. BMC Public Health 2011; 11:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muc M, Mota-Pinto A, Padez C.. Association between obesity and asthma–epidemiology, pathophysiology and clinical profile. Nutrition Research Reviews. 2016; 29(2): 194–201. [DOI] [PubMed] [Google Scholar]
- 36.Tsaroucha A, Daniil Z, Malli F, Georgoulias P, Minas M, Kostikas K, et al. Leptin, adiponectin, and ghrelin levels in female patients with asthma during stable and exacerbation periods. J Asthma 2013; 50(2): 188–97. [DOI] [PubMed] [Google Scholar]
- 37.Chen YC, Huang YL, Ho WC, Wang YC, Yu YH.. Gender differences in effects of obesity and asthma on adolescent lung function: Results from a population-based study. Journal of Asthma 2017; 54(3): 279-85. [DOI] [PubMed] [Google Scholar]
- 38.Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST.. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: The Normative Aging Study. Thorax 2002; 57(7): 581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Dong J, Ji L, Jiang P, Leung TF, Liu D et al. Anti-Allergic Inflammatory Activity of Interleukin-37 Is Mediated by Novel Signaling Cascades in Human Eosinophils. Front Immunol 2018; 9: 1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castro-Rodríguez JA. Relationship Between Obesity and Asthma. Arch Bronconeumol 2007; 43(3): 86-184. [DOI] [PubMed] [Google Scholar]
- 41.Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ.. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy 2016; 9: 71-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung KF. Targeting the interleukin pathway in the treatment of asthma. The Lancet 2015; 336(9998): 1086–96. [DOI] [PubMed] [Google Scholar]
- 43.Coleman M. Teaching corner: Adult asthma in Malawi. Malawi Medical Journal 2011; 23(1): 22-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellido-Casado J, Plaza V, Perpina M, Picado C, Bardagí S, Martínez-Brúet C, et al. Inflammatory response of rapid onset asthma exacerbation. Arch Bronconeumol 2010; 46(11):587–93. [DOI] [PubMed] [Google Scholar]
- 45.Nixon J, Newbold P, Mustelin T, Anderson GP, Kolbeck R.. Monoclonal antibody therapy for the treatment of asthma and chronic obstructive pulmonary disease with eosinophilic inflammation. Pharmacology & Therapeutics 2017; 169: 57-77. [DOI] [PubMed] [Google Scholar]
- 46.Bhakta NR, Woodruff PG.. Human asthma phenotypes: from the clinic, to cytokines, and back again. Imm Rev 2011; 242(1): 220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engelberg R, Martin M, Wrotniak BH, Hicar MD.. Observational study of Interleukin-21 (IL-21) does not distinguish Kawasaki disease from other causes of fever in children. Pediatr Rheumatol 2017; 15(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood N, Bourque K, Donaldson DD, Collins M, Vercelli D, Goldman SJ, et al. IL-21 effects on human IgE production in response to IL-4 or IL-13. Cell Immunol 2004; 231(1-2):133–45. [DOI] [PubMed] [Google Scholar]
- 49.Harada M, Magara-Koyanagi K, Watarai H, Nagata Y, Ishii Y, Kojo S, et al. IL-21–induced B? cell apoptosis mediated by natural killer T cells suppresses IgE responses. J Exp Med (JEM) 2006; 203(13): 2929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraszula L, Eusebio M-O, Jasinska A, Kupczyk M, Kuna P, Pietruczuk M.. The Relationship between Leptin, Adiponectin, Resistin and FoxP3+ Treg cells in Patients with Severe Asthma. J Clin Cell Immunol 2016; 7(3): 426. [Google Scholar]