ABSTRACT
A 23-year-old female, who had undergone a sleeve gastrectomy two weeks earlier, presented with abdominal complaints. A CT scan showed portal vein thrombosis, bowel ischemia, and intra-abdominal sepsis. Anastomosis and antibiotic therapy were not successful, and the patient went into multi-organ failure and died. Multiple cultures revealed a yeast fungus confirmed asPichia kudriavzevii using rRNA gene sequencing. We report the first case of peritonitis in association with P kudriavzevii. In addition to the abdominal complications and surgical interventions, the yeast was found to have significantly contributed to the patient's death.
SIMILAR CASES PUBLISHED:
None.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Mycotic peritonitis due toCandida species with critical complications is found frequently in surgically ill patients and can be fatal if untreated. Death occurs following dissemination, fungemia, and candida sepsis.1 Pichia kudriavzevii (synonymous with Candida krusei; Saccharomyces krusei and Issatchenkia orientalis) is a yeast fungus involved in beverage and food fermentations, which is also of clinical importance, as indicated by many case reports.2 Various isolates from humans and animals have been detected. In fact,C kruzei is the 5th most common cause of candidemia and occurs secondary to duodenal perforation.3 C krusei is a common fungal isolate in patients undergoing biliary surgery4 and has been encountered with gastropericardial fistula and pericarditis following laparoscopic Nissen fundoplication (gastropericardial fistula).5
CASE
A 23-year-old woman presented to the emergency department in March 2017, complaining of generalized abdominal pain with bouts of repeated vomiting and low-grade fever. Apart from still being morbidly obese following a sleeve gastrectomy two weeks earlier (BMI 43.2 kg/m2), she had been healthy with no history of steroid or other immunosuppressive medication use and no prior diagnosis of any hematological malignancy. She was febrile (39°C) and had tachycardia of 120 beats per minute, although she initially maintained a normal blood pressure. Her laboratory test showed a total leukocyte count of 18×109/L, 80% neutrophils, normal hemoglobin, and a normal platelet count. A CT scan with contrast of the abdomen showed portal vein thrombosis and bowel ischemia (Figure 1). The patient was intubated, mechanically ventilated, and managed with intravenous fluid along with a therapeutic heparin infusion targeting an APTT ratio of 1.5–2.5, along with ICU supportive care measures. She was started on piperacellin/tazobacam and tegacyclin. Three days later, exploratory laparotomy revealed small bowel ischemia 30 cm from the duodenojejunal junction, thick mottled mesentery and 500 cc of hemorrhagic fluid. We did a resection of the ischemic segment and side-to-side anastomosis. Her condition showed initial improvement because her white cell count normalized, and she was weaned from a mechanical ventilator. On day 10 following the initial laparotomy, she became acidotic and hypotensive and was thus explored again; there was extension of her bowel ischemia and another set of resection anastomosis was performed. Three days after the second operation, she underwent a third laparotomy also with resection and anastomosis, but showed manifestations of sepsis postoperatively and was treated with antibiotics without improvement. Multiple cultures from the peritoneal fluid specimens revealed the same yeast organism in pure form. These mycological findings prompted the escalation of treatment to include antifungal therapy (anidulafungin). The patient's condition worsened, with heavy discharge from the abdominal wound, and she developed multiorgan failure and died after three weeks in the ICU.
Figure 1.
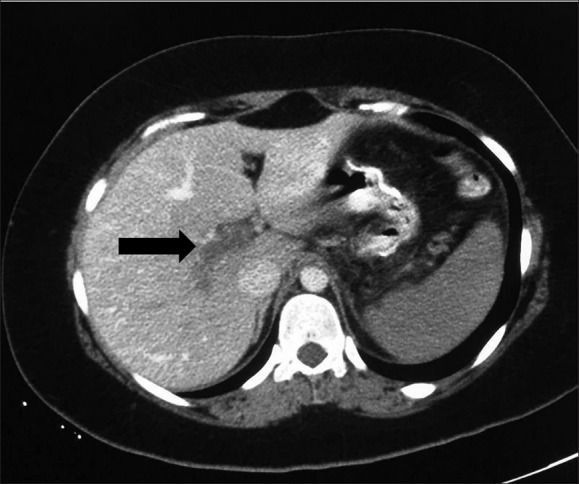
CT scan of abdomen of a 23- year old female showing evidence of portal vein thrombosis (arrow).
Culture from the peritoneal fluid revealed the growth of creamy yellow, irregular, wrinkled yeast-like colonies (Figure 2A). Culture was done on Sabouraud dextrose agar (SDA), which was incubated aerobically for five days at 30°C. The organism was labeled V49-4 and identified on the basis of colony morphology on SDA and on the basis of microscopic features following recommended guidelines.6 The culture plate showed a yeast type of growth, with a distinctive yeast smell, smooth, glabrous in texture and creamy yellow. It was initially identified as a non-albicans Candida sp. Microscopically, it demonstrated pseudohyphae, elongated cylindrical- and spheroidal-shaped blastoconidia in a diverse arrangement (Figure 2B). The strain V49-4 was highly sensitive to voriconazole, itraconazole, caspinofungin, nystatin, fluconazole, and ketoconazole, and less sensitive to amphotericin but resistant to metronidazole. The antifungal, concentrations and inhibition zones were as follows: amphotericin B 100 mg/mL (Sigma, Missouri, USA), 7 mm inhibition zones; caspinofungin 5 mg/mL (caspofungin acetate injection, Merck Sharp & Dohme Corp.), 32 mm; fluconazole 2 mg/mL (Diflucan I.V. Roerig/Pfizer Inc., France), 18 mm; itraconazole 10 mg/mL Sporanox I.V, Janssen Biotech N.V, Belgium), 33 mm; ketoconazole (Diflucan I.V. Roerig/Pfizer Inc., France), 23 mm; metronidazole 5 mg/mL (PSI Pharmaceutical Co., Jeddah, Saudi Arabia), 0 mm; nystatin 200 mg/mL (Sigma-Aldrich, Romania), 22 mm and voriconazole 2 mg/mL (Vfend, Amgen technology, Ireland), 50 mm.
Figure 2.
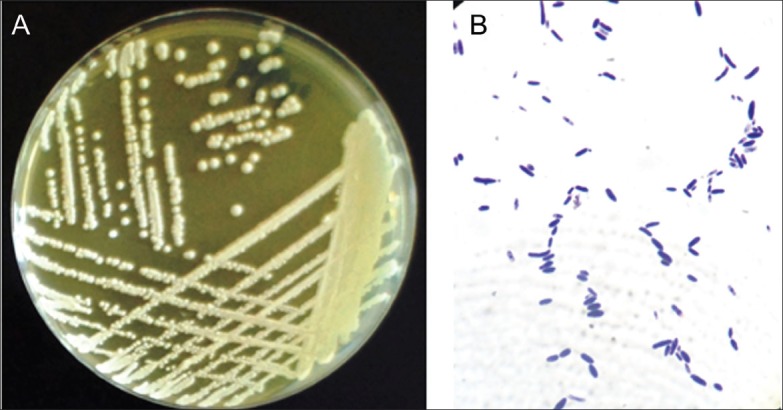
Growth of a 5-day old culture of Pichia kudriavzevii grown on Sabouraud's dextrose agar at 30°C showing yeast-like colonies, smooth, glabrous in texture and creamy yellow (A) and microscopic appearance (B) of the yeast showing gram positive mixtures of elongated cylindrical and spheroidal shaped blastoconidia. Pseudohyphae can be seen and multilateral budding lined up in a miscellaneous arrangement.
Confirmation of the identity of this infrequent organism was completed by the analysis of regions from a large subunit of ribosomal RNA, amplified using primer: 5.8SR (5'-TCGATGAAGAACGCAGCG) and LR7 (5'-TACTACCACCAAGATCT). A neighbor-joining phylogenetic tree was constructed based on sequences from 26S rRNA gene sequence-based analysis. The evolutionary analyses were conducted with MEGA v6 software.7 The analysis showed that the isolate V49-4 (GenBank accession number MG583716; https://www.ncbi.nlm.nih.gov/nuccore/MG583716) is a member of the genus Pichia. The strain V49-9 falls in the branch of the phylogenetic tree that accommodates members of the genus Pichia (Figure 3). The strain V49-4 is within a subclade together with the type strains of P kudriavzevii (similarity 100%) along withCandida krusei ATCC14243 (identical to Pichia kudriavzevii) (similarity 100%) and P cecembensis (similarity 100%). Although strain V49-4 shared 100% similarity with both P kudriavzevii andP cecembensis, it was identified as P kudriavzevii, given its phenotypic characteristics and clinical relevance.
Figure 3.
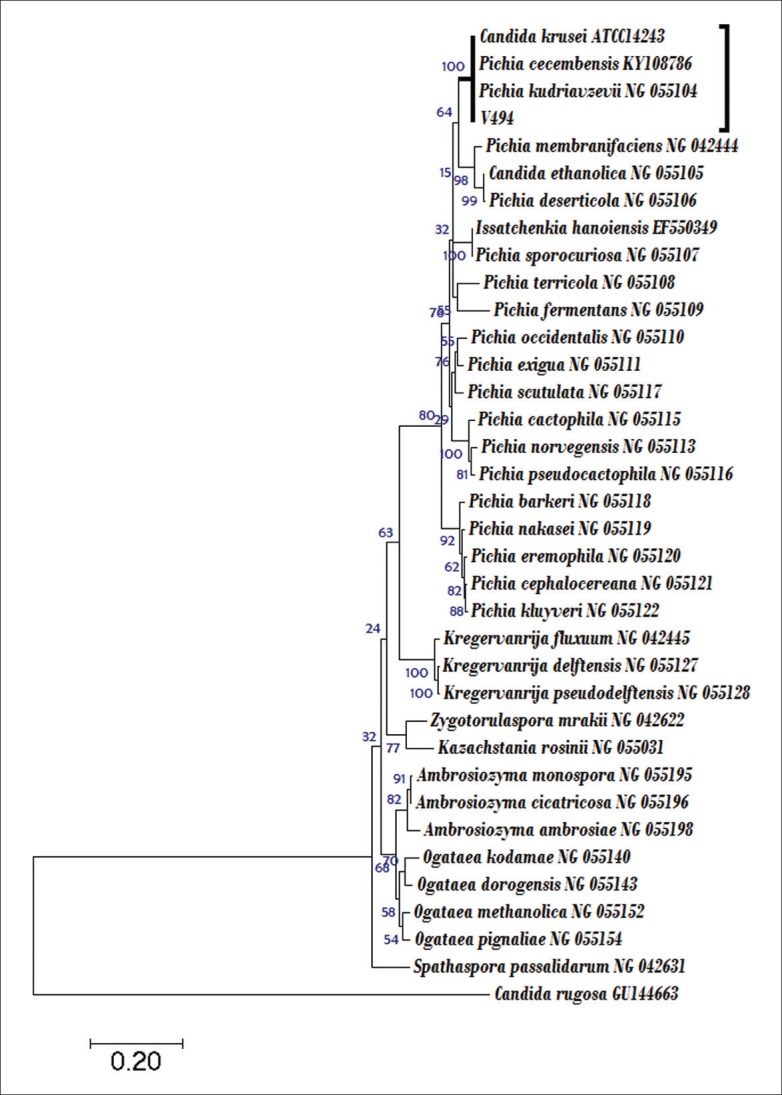
A phylogenetic tree based on the analysis of regions from large subunit rRNA showing the position of the strain V49-4 (accession MG583716) with representative closely related yeast species. The evolutionary history was inferred using the Neighbor-Joining method. Bar, base substitution rate.
DISCUSSION
Identification of yeasts, notably Candida, to the species level is becoming critical and has new clinical significance with the worldwide emergence of multidrug-resistant species Candida auris. Species should be identified for invasive candidiasis isolates, and species-level identification can be considered for selected noninvasive isolates to improve detection of C auris.8 P kudriavzevii is regularly associated with some forms of infant diarrhea and occasionally with systemic disease as well as with animals and the environment, and such species would not be easily recognized without meticulous attention to phenotypic and genotypic traits.6
Candida albicans in the presence of bacteria has been encountered in cases of peritonitis. The interaction between fungi and bacteria in peritonitis pathogenesis is not fully understood and may result in a synergistic, competitive, or simply a neutral interaction. A mixture of C albicans, Escherichia coli, and Bacteroides fragilis in a peritonitis case has been considered non-synergistic but also an interaction of partial competition. This symbiosis-like setting permits the colonization and survival of the two bacterial species and theCandida yeast, with all three leading to the development of abscess formation.9 Candida spp is a component of the endogenous flora of the digestive tract and can as a result form part of the etiology of many forms of abdominal infection. Candida spp is for that reason commonly isolated in polymicrobial secondary peritonitis due to intestinal suture dehiscence. Most ICU and intensivists decide to start antifungal treatment when positive cultures are associated with signs of sepsis and the patient's condition appears critical.8 While intra-abdominal candidiasis is widespread in seriously sick surgical patients, punctual antifungal therapy and sufficient control measures can result in a good outcome. The fatal outcome of the present case is closely related to the conclusions from literature generalization.
The gut mycobiome seems not as fully stable and is liable to be affected by environmental factors. How the normal fungal flora of the human gut might be linked to disease is not fully understood. Fungi able to colonize the gut are limited to a small number of species, mostly Candida yeasts and yeasts in the family Dipodascaceae, Malassezia, and the filamentous fungus Cladosporium.10 In this report and from repeated cultures on SDA from the patient's peritoneal fluids, similar results (i.e. the isolation of P kudriavzevii) were obtained, which caused us to initiate antifungal therapy. Nevertheless, the antifungal agent was not adequate to save the patient's life in view of the fact that her condition had deteriorated rapidly, and manifestation of multiple-organ failure was evident.
In summary, we report an intra-abdominal candidiasis or Pichia infection in a critically ill post-sleeve gastrectomy patient. We believe that the yeast infection significantly contributed to the death of the patient. Fungal infections, particularly yeast of the Candida types (Pichia spp is a related yeast), should be considered for laboratory analyses and confirmation because it demands prompt antifungal therapy. Delay may further complicate the patient's health and result in a poor outcome.
ACKNOWLEDGMENTS
The authors declare that they have no conflicts of interest and confirm that each has made considerable contribution to the ideas, materials, or data submitted for publication.
Funding Statement
None.
REFERENCES
- 1.Sandven P, Qvist H, Skovlund E, Giercksky KE, Group N, and the Norwegian Yeast Study G, Significance of Candida recovered from intraoperative specimens in patients with intra-abdominal perforations. Crit Care Med. 2002. 30: 541–547. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzman CP, Fell JW, and Boekhout T, The Yeasts, a Taxonomic Study. Volume 1 Fifth edition. 2011: Elsevier. [Google Scholar]
- 3.Sridhar AV, Nichani S, Luyt D, and Nour S, Candida peritonitis: a rare complication following early dislodgement of percutaneous endoscopic gastrostomy tube. J Paediatr Child Health. 2006. 42: 145–146. [DOI] [PubMed] [Google Scholar]
- 4.Gupta BK, Kumar R, Kaur S, and Khurana S, Incidence of fungal infection in extra hepatic biliary stone disease. Indian J Pathol Microbiol. 1993. 36: 110–112. [PubMed] [Google Scholar]
- 5.Farjah F, Komanapalli CB, Shen I, and Sukumar MS, Gastropericardial fistula and Candida kruzei pericarditis following laparoscopic Nissen fundoplication (gastropericardial fistula). Thorac Cardiovasc Surg. 2005. 53: 365–367. [DOI] [PubMed] [Google Scholar]
- 6.Ellis D, Mycology Online. Identification and Antifungal Susceptibility of Medically Important Fungi. 2016, School of Molecular & Biomedical Science, The University of Adelaide; Australia 5005: http://www.mycology.adelaide.edu.au. Accessed Nov. 2017. [Google Scholar]
- 7.Tamura K, Stecher G, Peterson D, Filipski A, and Kumar S, MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013. 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lockhart SR, Jackson BR, Vallabhaneni S, Ostrosky-Zeichner L, Pappas PG, and Chiller T, Thinking beyond the Common Candida Species: Need for Species-Level Identification of Candida Due to the Emergence of Multidrug-Resistant Candida auris. J Clin Microbiol. 2017. 55: 3324–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawyer RG, Adams RB, Rosenlof LK, May AK, and Pruett TL, The role of Candida albicans in the pathogenesis of experimental fungal/bacterial peritonitis and abscess formation. Am Surg. 1995. 61: 726–731. [PubMed] [Google Scholar]
- 10.Hallen-Adams HE and Suhr MJ, Fungi in the healthy human gastrointestinal tract. Virulence. 2017. 8: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]


