Abstract
Herein, we report the synthesis of 5,12-dihydropyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]-diquinoline-6,14(5H,12H)diones, 2-(4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)-1,4-diphenyl- butane-1,4-diones and 4-(benzo-[d]oxazol-2-yl)-3-hydroxy-1H-[4,5]oxazolo[3,2-a]pyridine-1-one. The new candidates were synthesized and identified by different spectroscopic techniques, and X-ray crystallography.
Keywords: 6,7-Disubstituted-4-hydroxy-quinoline-2-ones; 2,3-dichloropyrazine; ANRORC process bis-pyrazinofuro-quinoline; 1,4-diphenylbutane-1,4-diones; NMR; X-ray
1. Instruction
Furoquinolones are an interesting class of 2-quinolones and representative examples of them as naturally occurring compounds are shown in compounds such as Skimmianine and γ-Fagarine (Figure 1), both compounds that have been found to have anti-cancer activity [1,2]. It was reported that furopyrazine scaffold was functionalized with an amino- and a carboxy-terminus resulting in a conformationally restricted dipeptidomimetic scaffold [3].
Figure 1.
The structures of Skimmianine, γ-Fagarine, an example of QS of PqsABCDE biosynthesis and two examples of quinolone analogues as antibacterial reagents.
Alkyl quinolones (AQs) are a species-specific class of quorum-sensing molecule that have been described in P. aeruginosa [4,5] and related bacteria including P. putida and Burkholderia spp. [6]. More than 55 distinct AQs (i.e., an example is shown in Figure 1 and assigned as PQS) are produced through the PqsABCDE (Figure 1) biosynthetic pathway in P. aeruginosa, with the majority of the diversity arising from unsaturation, different alkyl chain lengths, and modification of the ring-substituted nitrogen [6,7]. An insight into the evolutionary basis of AQ diversity has emerged from Burkholderia thailandensis where AQ analogues (i.e., two examples assigned as HHQ and HQNP and are shown in Figure 1) are shown to act synergistically to inhibit bacterial growth [8,9].
Quinolones have been also developed for clinical use in humans [10]. These antibiotics exert their effect by inhibition of two type II topoisomerase enzymes, DNA gyrase and topoisomerase IV [11]. DNA topoisomerases are found in both eukaryotic and prokaryotic cells and are a target for chemotherapeutic intervention in anti-bacterial and anti-cancer therapies [12]. In a recent publication [13], the synthesis of the Zwitter-ionic 4-hydroxycoumarin derivatives was reported, through a unique reaction of 4-hydroxycoumarins with p-benzoquinone and pyridine in aqueous acetone.
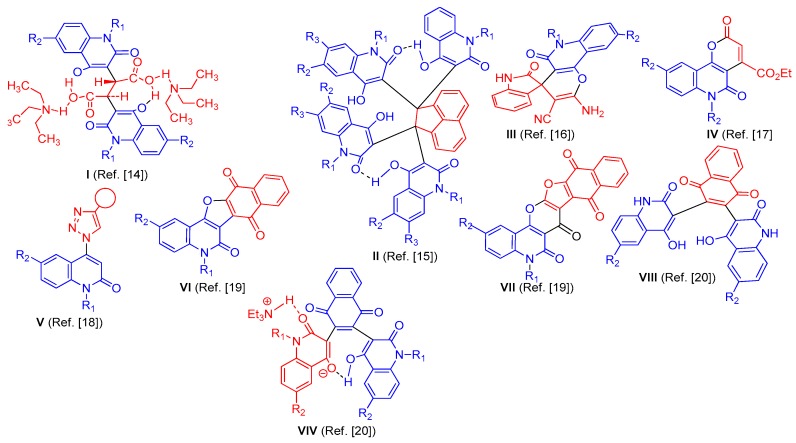
Figure 2 indicates what we previously synthesized of 4-hydroxy-2-quinolone. An example, 2,3-bis-(4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)succinic acid derivatives I, were obtained by one-pot reaction of one equivalent of aromatic amines with two equivalents of diethyl malonate in diphenyl ether and catalyzed with triethylamine [14]. On reacting four equivalents of 4-hydroxyquinolin-2(1H)-ones with one equivalent of acenaphthoquinone in absolute ethanol, containing catalytic Et3N, the reaction gave acenaphthylene-1,1,2,2-tetrayl-tetrakis- (4-hydroxyquinolin-2(1H)-ones) (II, [15]. We also reported that quinoline-2,4-diones reacted with 2-(2-oxo-1,2-dihydroindol-3-ylidene)malononitrile in pyridine to give spiro(indoline-3,4′-pyrano- [3,2-c]quinoline)-3′-carbonitriles (III, [16]. The same target materials of 2-quinolones reacted with diethyl acetylenedicarboxylate in absolute ethanol, containing catalytic triethylamine, to give pyrano [3,2-c]quinoline-4-carboxylates (IV, [17]. We have recently reported that a class of 1,2,3-triazoles derived by 2-quinolone (V, [18]) has been synthesized, via Cu-catalyzed [3 + 2]cycloadditions (Meldal–Sharpless ‘click’-reactions) of 4-azidoquinolin-2(1H)-ones with ethyl propiolate [18]. We also synthesized fused naphthofuro[3,2-c]quinoline-6,7,12-triones VI, and pyrano[3,2-c]quinoline-6,7,8,13-tetraones, VII that have shown potential as ERK inhibitors [19]. Whereas syntheses of bis(6-substituted-4-hydroxy-2-oxo-1,2-dihydro-quinolin-3-yl)-naphthalene-1,4-diones VIII and substituted N-(alkyl)bis-quinolinone triethyl-ammonium salts VIV, were explored as candidates for extracellular signal-regulated kinases 1/2 (ERK1/2) having antineoplastic activity [20].
Figure 2.
Structures of compounds that previously reported (Reproduced with permission from [14,15,16,17,18,19,20]).
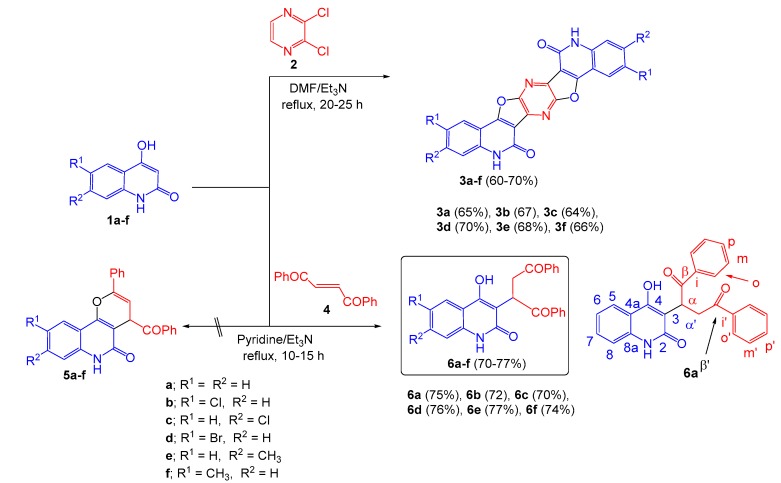
The aforementioned interesting pharmaceutical and biological activities of 4-hydroxy-2-quinolones make them valuable in drug research and development. Hence, many publications have recently dealt with their synthetic analogous and the synthesis of their heteroannelated derivatives. Consequently, we have found that it is of importance to shed new light on these interesting heterocycles. Accordingly, the reactivity of 1,6-disubstituted-4-hydroxy- quinolinones 1a–f was tested towards 2,3-dichloropyrazine (2) and (E)-dibenzoylethene (4).
2. Results and Discussion
Upon mixing equivalent amounts of 2,3-dichloropyrazine (2) and 6,7-disubstituted-4-hydroxy- quinolin-2-ones 1a–f, followed by refluxing in dimethylformamide (DMF) and catalyzed by triethylamine (Et3N), 3a–f were obtained as single products (Scheme 1). Structure elucidation of compounds 3a–f was carried out by infrared (IR), 1H-nuclear magnetic resonance (NMR), 13C-NMR and mass spectrometry, as well as elemental analyses.
Scheme 1.
Reactions of 2-quinolinones 1a–f with 2,3-dichloropyrazine (2) and 1,2-(E)-dibenzoylethene (4).
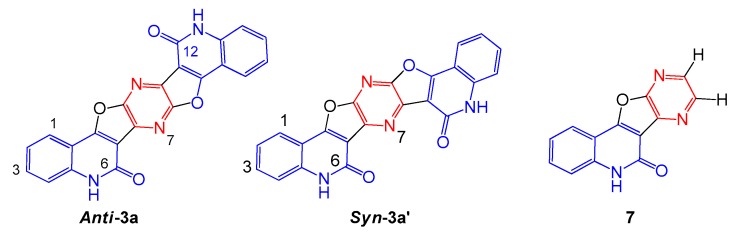
The reaction products were identified as pyrazino[2′,3′:4,5]furo[3,2-c]quinolin-6(5H)-ones 3a–f. The IR spectra showed absorption for C=N at ν = 1630–1600 cm−1. Besides the NH stretching appeared as broad peaks at ν = 3320–3260 cm−1. As for example, the elemental analysis and mass spectra proved its molecular formula of 3a as C22H10N4O4, which indicated addition of two moles of 1a to two moles of 2 accompanied with elimination of four moles of HCl. The expected structure 7 was ruled out, since 1H-NMR spectrum did not show the expected azomethine protons (Figure 3). Either the syn-structure 3a′ or the anti-form has to show symmetric carbon signals in 13C-NMR spectrum. Most assigned carbons are the two carbonyl and C=N carbons which appeared at δ = 167.1, 165.2 and 160.8, 160.2 ppm. In Spartan 18: geometries program [21] optimized at the 6-31G* level with B3LYP the energy difference between calculated Anti-3a: and Syn-3a’ shows that Anti-3a is more stable by 2.029 kcal/mol.
Figure 3.
Alternative structures of compound 3a [14,15,16,17,18,19,20] (Reproduced with permission from [14,15,16,17,18,19,20]).
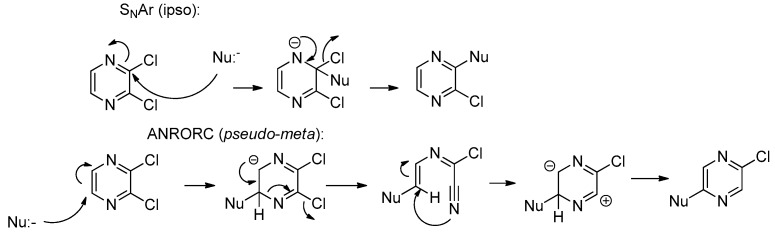
Compounds 3 are the products of reaction between one molecule of 2,3-dichloropyrazpine (2) and two molecules of a 4-hydroxy-2-quinolinone (1); substituents on 3 correspond to those on 1 in the obvious way. The reaction results in replacement of both chlorines and both hydrogens of 2, by either an α-carbon or a pseudo-phenolic oxygen of 1. Both positions involved on 1 are formally nucleophilic, although the α-carbon normally reacts first (cf. reaction of 1 with 4 to give 6). We are unaware of any direct reaction of 2 with four nucleophiles; in systems where the chlorines and hydrogens of 2 are all replaced, the reaction of C-5 and C-6 (the hydrogen-bearing carbons) involves reaction with an oxidant (e.g., a molecular halogen), sometimes followed by an organometallic coupling [22,23]. On the other hand, pyrazines bearing leaving groups readily undergo displacement of those leaving groups, by either SNAr or Addition of the Nucleophile, Ring Opening, and Ring Closure (ANRORC) mechanisms [24,25,26]. If the pyrazine is activated by an electrophile, the nucleophile can be as weak as o-phenylenediamine [27]. If 2 does not undergo four-fold nucleophilic substitution, the likeliest scenario would seem to be two-fold displacement followed by two-fold oxidative cyclization, presumably by air. If the chlorides are the leaving groups, only one can undergo ipso substitution: the other nucleophile must attack the other side of the ring. The SNAr process can give ipso substitution only, but the ANRORC process can proceed at the pseudo-meta position (Scheme 2). We are therefore led to propose the mechanism for formation of 3, shown in Scheme 3. Here “Nu:-“ is 1, attacking via its α-carbon. If one substitution gave ipso displacement and the other gave pseudo-meta displacement, one would expect the observed anti regiochemistry: the order of the two substitutions would not matter.
Scheme 2.
SNAr versus ANRORC substitutions on 2.
Scheme 3.
Proposed rationale for formation of 3.
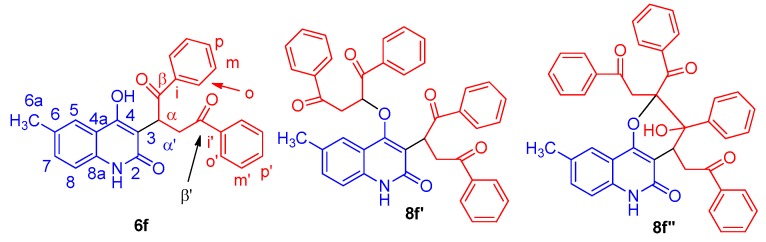
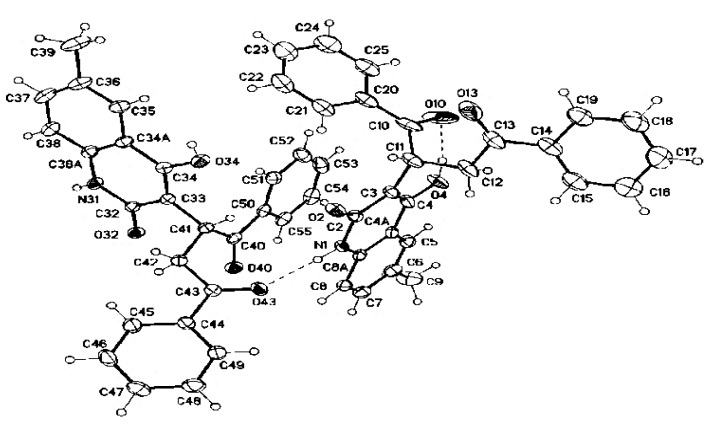
Afterward, we investigated the reactions of (E)-dibenzoylethene (4) and 1a–f under the same conditions mentioned above in pyridine/Et3N (Scheme 1). The structure elucidation depends intensively on NMR spectra. For example, the 1H spectrum of 6f consists of a 1H singlet at δH = 11.35 and a broad signal at δH = 10.77; in the aromatic region, two sets of resonances from monosubstituted phenyl rings and a three-spin system from the quinoline; and a three-spin system and a 3H methyl singlet upfield. The integrals require that there be two phenyl rings and one quinolone and, consequently, they rule out the alternative structures 8f′ and 8f′′ (Figure 4). The NMR correlations of 6f are detailed in full in Table S1 (see Supplementary Material). The structure of compound 6f was finally proved by X-ray structure analysis as shown in Figure 5.
Figure 4.
Different suggested structures of 6f, 8f’ and 8f’’.
Figure 5.
Molecular structure of the dimer of compound 6f.
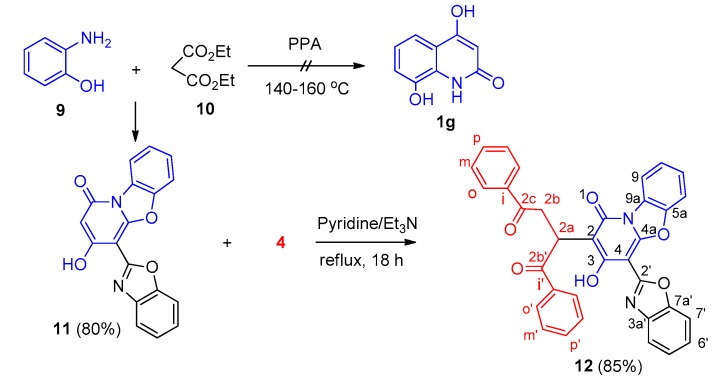
Surprisingly, on attempting to prepare 4-hydroxy-2-quinolone 1g from 2-hydroxyaniline (9) and diethyl malonate (10) in polyphosphoric acid (PPA) according to the procedure described in reference [28], compound 11 was obtained in 80% yield (Scheme 4). Similarly, reaction of 11 with 4 under the same conditions produced compound 12 in 85% yield.
Scheme 4.
Formation of compound 11 and its reaction with compound 4.
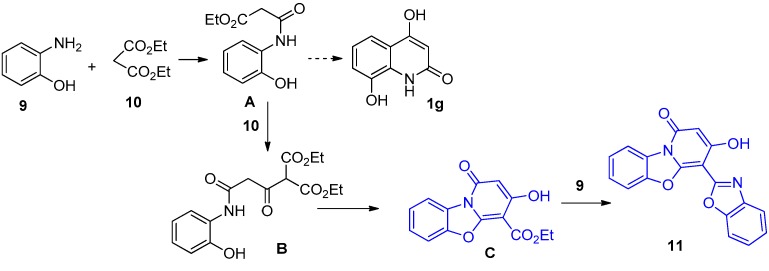
We propose that compound 11 forms as shown in Scheme 5. The reaction of 9 with 10 presumably starts with N-acylation to form intermediate A. The expected formation of 1g would then involve ring closure in the fashion of a Friedel-Crafts acylation. The pathway leading to 11, on the other hand, would require Claisen condensation between A and a second molecule of 10 to form intermediate B. One of the pendant esters of B would then react intramolecularly with the NH and OH groups, to form the benzoxazole subunit of intermediate C. Reaction of the remaining ester group of C with a second molecule of 9 would then form the other benzoxazole unit of 11.
Scheme 5.
Proposed rationale for formation of 11.
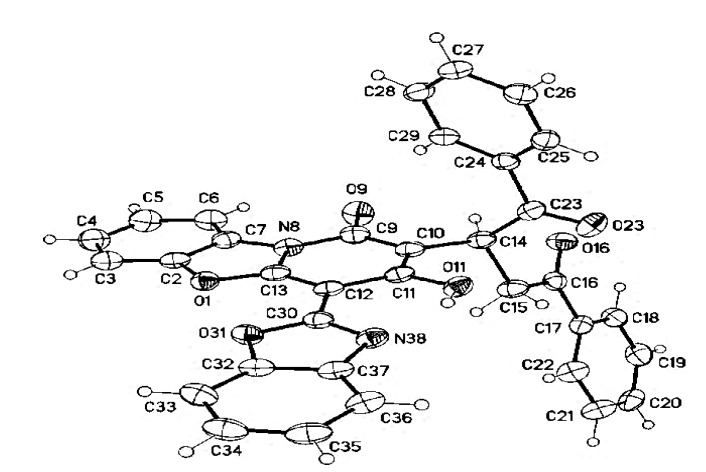
The NMR spectroscopic data of compound 12 (detailed in full in Table S2; see Supplementary Materials) show a broad OH, two sets of phenyl signals, and eight other proton signals, although most of the benzoxazole signals cannot be solved fully. The protons on sp3 carbons are distinctive at δ = 5.55, 4.36, and 2.85 ppm; the latter two are assigned as H-2b based on their geminal coupling constant of 17 Hz. The attached carbons appear at δ = 39.5 (C-2a) and 37.35 ppm (C-2b). The C-2a gives Heteronuclear Multiple Bond Correlation (HMBC) correlation with a carbon at δ = 156.92 ppm, which could be either C-1 or C-3, and is assigned based on chemical shift as C-1. Correlations within the benzoyl groups are straightforward. The structure of compound 12 was confirmed by X-ray crystallography (Figure 6).
Figure 6.
Molecular structure of compound 12 assigned as 2-(4-hydroxy-6-methyl-2-oxo-1,2-dihydroquinolin-3-yl)-1,4-diphenylbutane-1,4-dione.
3. Experimental
3.1. Material and Methods
Melting points were taken in open capillaries on a Gallenkamp melting point apparatus (Weiss-Gallenkamp, Loughborough, UK) and are uncorrected. The IR spectra were recorded from potassium bromide disks with a Fourier Transform Infrared (FT-IR) device (Mettler-Toledo GmbH, Giessen, Germany), Minia University. Elemental analyses were carried out at the Perkin-Elmer out with Elementar 306 (Perkin-Elmer, Walluf, Germany). NMR data were recorded on Bruker AM 400 or AV400 spectrometers (Bruker, Karlsruhe, Germany), at 400 MHz for 1H and 100 MHz for 13C. Chemical shifts were reported in ppm from tetramethylsilane using solvent resonance in DMSO-d6 solutions as the internal standard. Coupling constants are stated in Hz. Correlations were established using 1H-1H COSY, and 1H-13C and 1H-15N heteronuclear single quantum coherence (HSQC) and HMBC experiments. Mass spectra were recorded on a Finnigan MAT 312 instrument Fab 70 eV (Thermo Fisher, Bremen, Germany), Institute of Organic Chemistry, Karlsruhe University, Karlsruhe, Germany. Thin Layer Chromatography (TLC) was performed on analytical Merck 9385 silica aluminum sheets (Kieselgel 60) with Pf254 indicator; TLCs were viewed at λ max = 254 nm. Elemental analyses for C, H, N were carried out with Elementar 306.
3.2. Starting Materials
1,6-Disubstituted-quinoline-2,4-(1H,3H)-diones 1a–f were prepared according to the literature [28,29]. 2,3-Dichloropyrazine (2) and (E)-1,2-dibenzoylethene (4) (Aldrich, Munich, Germany) were used as received.
3.2.1. Reaction of 1a–f with 2,3-Dichloropyrazine (2)
A suspension of 1,6-disubstituted quinoline-2,4-(1H,3H)-diones 1a–f (2 mmol) in 10 mL dimethylformamide (DMF) was added to a solution of (E)-1,4-diphenylbut-2-ene-1,4-dione (2, 0.148 g, 1 mmol) in 15 mL DMF and 0.5 mL of triethylamine. The reaction mixture was gently refluxed for 20–25 h, until the reactants had disappeared (monitored by TLC). The resulting precipitates of 3a–f which were obtained cold were filtered off and dried. The precipitates were recrystallized from the stated solvents.
3.2.2. 5,12-Dihydropyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-Dione (3a)
Buff crystals (DMF/EtOH), yield: 0.256 g (65%), mp = 330–332 °C; IR (KBr): ν = 3220 (NH), 1660 (CO), 1606 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 12.11 (s, 2H, NH), 7.89–7.85 (m, 2H, Ar-H), 7.52–7.48 (m, 2H, Ar-H), 7.35–7.32 (m, 2H, Ar-H), 7.23–7.19 ppm (m, 2H, Ar-H); 13C-NMR (100 MHz, DMSO-d6): δ = 165.2 (C=O), 160.2 (C=N), 153.4, 139.4, 138.4, 130.7 (Ar-C), 125.9, 128.4, 122.8, 115.7 (Ar-CH), 109.5 ppm (Ar-C); MS (Fab, 70 eV, %): m/z = 394 (M+, 27). Anal. Calcd. for C22H10N4O4 (394.35): C, 67.01; H, 2.56; N, 14.21. Found: C, 66.90; H, 2.70; N, 14.30.
3.2.3. 2,10-Dichloro-5,12-Dihydropyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]-Diquinoline-6,14(5H,12H)-Dione (3b)
Buff crystals (DMF/EtOH), yield: 0.310 g (67%), mp = 320–322 °C, IR (KBr): ν = 3210 (NH), 1665 (CO), 1612 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 12.07 (s, 2H, 2NH), 7.98 (d, 2H, J = 0.7 Hz, Ar-2CH), 7.38–7.20 ppm (m, 4H, Ar-H); 13C-NMR (100 MHz, DMSO-d6): δ = 167.2 (C=O), 160.2 (C=N), 154.4, 139.0, 138.2 (Ar-C), 128.1, 126.9, 125.4, 120.0 (Ar-2H), 118.0, 109.4 ppm (Ar-C); MS (Fab, 70 eV,%): m/z = 464/468 (M+, 60/30), 443 (25), 371 (22), 137 (50). Anal. Calcd. for C22H8Cl2N4O4 (463.23): C, 57.04; H, 1.74; N, 12.10. Found: C, 57.20; H, 1.70; N, 12.20.
3.2.4. 3,11-Dichloro-5,12-Dihydropyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-Dione (3c)
Buff crystals (DMF), yield: 0.295 g (64%), mp = 350–352 °C; IR (KBr): ν = 3230 (NH), 1663 (CO), 1620 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 12.11 (s, 2H, 2NH), 7.95 (d, 2H, J = 1.2 Hz, Ar-2CH), 7.45–7.41 ppm (m, 4H, Ar-H); 13C-NMR (100 MHz, DMSO-d6): δ = 167.0 (C=O), 160.4 (C=N), 152.0, 138.7, 138.0, 130.6 (Ar-C), 127.0, 124.9, 122.2 (Ar-CH), 120.0, 108.2 ppm (Ar-C); MS (Fab, 70 eV,%): m/z = 464/468 (M+1, 32/64), 462 (36), 443 (32), 371 (24), 137 (54). Anal. Calcd. for C22H8Cl2N4O4 (463.23): C, 57.04; H, 1.74; N, 12.10. Found: C, 57.00; H, 1.80; N, 12.15.
3.2.5. 2,10-Dibromo-5,12-Dihydropyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-Dione (3d)
Pale yellow crystals (DMF), yield: 0.390 g (70%), mp = 310–312 °C; IR (KBr): ν = 3230 (NH), 1667 (CO), 1610 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 12.43 (s, 2H, 2NH), 8.01–7.96 (m, 2H, Ar-CH), 7.75–7.72 (m, 2H, Ar-H), 7.38–7.36 ppm (m, 2H, Ar-H); 13C-NMR (100 MHz, DMSO-d6): δ = 166.8 (C=O), 160.4 (C=N), 150.8, 141.0, 135.5, 131.4 (Ar-C), 128.6, 127.8, 124.2 (Ar-CH), 118.2, 103.0 ppm (Ar-C); MS (Fab, 70 eV, %): m/z = 552/556 (M+, 34/58), 552 (100), 371 (22), 137 (50). Anal. Calcd. for C22H8B2N4O4 (552.14): C, 47.86; H, 1.46; N, 10.15. Found: C, 47.90; H, 1.56; N, 10.25.
3.2.6. 5,12-Dihydro-3,11-Dimethylpyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-Dione (3e)
Brown crystals (DMF/CH3OH), yield: 0.280 g (66%), mp = 276–278 °C (decomp.), IR (KBr): ν = 3222 (NH), 1660 (CO), 1620 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 12.15 (s, 2H, 2NH), 7.92 (d, 2H, J = 0.8 Hz, Ar-H), 7.45–7.30 (m, 4H, Ar-H), 2.16 ppm (s, 6H, 2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 166.8 (C=O), 159.8 (C=N), 150.0 (C-O), 138.0, 137.4, 130.0, 128.2 (Ar-C), 127.8, 127.2 (Ar-CH), 125.5, 110.4 (Ar-C), 20.4 ppm (CH3); MS (Fab, 70 eV, %): m/z 422 = (M+, 28), 406 (24), 370 (20), 137 (48). Anal. Calcd. for C24H14Cl2N4O4 (422.40): C, 68.24; H, 3.34; N, 13.26. Found: C, 68.30; H, 3.45; N, 13.35.
3.2.7. 5,12-Dihydro-2,10-Dimethylpyrazino[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-Dione (3f)
Brown crystals (DMF/CH3CN), yield: 0.290 g (68%), mp 300–302 °C; IR (KBr): ν = 3225 (NH), 1665 (CO), 1630 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 12.20 (s, 1H, 2NH), 7.84 (d, 2H, J = 1.2 Hz, Ar-2CH), 7.42–7.35 (m, 4H, Ar-H), 2.18 ppm (s, 6H, 2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 166.2 (C=O), 160.0 (C=N), 152.7 (C-O), 139.6 (Ar-CH3), 135.4, 130.4, 128.6 (Ar-C), 128.0, 127.0, 126.2 (Ar-CH), 110.4 (Ar-C), 20.2 ppm (CH3); MS (Fab, 70 eV, %): m/z = 422 (M+, 28), 406 (24), 370 (20), 137 (48). Anal. Calcd. for C24H14Cl2N4O4 (422.40): C, 68.24; H, 3.34; N, 13.26. Found: C, 68.20; H, 3.50; N, 13.32.
3.3. Reaction of 1a–f with (E)-1,2-Dibenzoylethene (4)
A mixture of 1a–f (1 mmol) and (E)-1,2-dibenzoylethene (4) (0.246 g, 1 mmol) in pyridine (50 mL) and 0.5 mL of triethylamine, was gently refluxed for 10–15 h, until the reactants had disappeared (monitored by TLC). The resulting precipitates of 6a–f, which obtained on cold were filtered off and dried. The precipitates were recrystallized from the stated solvents.
3.3.1. 2-(4-Hydroxy-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6a)
Yellow crystals (DMF/EtOH), yield: 0.300 g (75%), mp = 325–327 °C, IR (KBr): ν = 3220 (NH), 3023 (Ar-CH), 3000 (Aliph-CH), 1698, 1673 (CO), 1631 (Ar-C=N), 1590 cm−1 (C=C); 1H-NMR (400 MHz, DMSO-d6): δ = 8.01 (d, J = 7.5 Hz, 2H; H-o’), 7.92 (d, J = 8.0 Hz, 1H; H-5), 7.83 (d, J = 7.5 Hz, 2H; H-o), 7.65 (t, J = 7.3 Hz, 1H; H-p’), 7.55 (“t”, J = 7.6 Hz, 2H; H-m’), 7.48 (t, J = 7.5 Hz, 1H; H-p), 7.46 (“t”, J = 7.8 Hz, 1H; H-7), 7.38 (“t”, J = 7.5 Hz, 2H; H-m), 7.23 (d, J = 8.1 Hz, 1H; H-8), 7.14 (“t”, J = 7.6 Hz, 1H; H-6), 5.47 (dd, J = 9.6, 2.4 Hz, 1H; H-α), 4.21 (dd, J = 17.3, 9.8 Hz, 1H; H-α’), 2.82 ppm (dd, J = 17.2, 2.5 Hz, 1H; H-α’). 15N-NMR: 142.95 ppm (N-1). 13C-NMR (100 MHz, DMSO-d6): δ = 198.48 (C-β), 198.31 (C-β’), 162.36 (C-2), 158.85 (C-4), 137.81, 137.01, 136.66 (C-i, i’, 8a), 132.91 (C-p’), 132.29 (C-p), 130.44 (C-7), 128.63 (C-m’), 128.21 (C-m), 127.83 (C-o’), 127.36 (C-o), 122.65 (C-5), 121.08 (C-6), 115.17, 115.06 (C-4a, 8), 111.47 (C-3), 40.41 (C-α), 37.44 ppm (C-α’). MS (Fab, 70 eV, %): m/z = 397 (M+, 100). Anal. Calcd. for C25H19NO4 (397.43): C, 75.55; H, 4.82; N, 3.52. Found: C, 75.70; H, 4.90; N, 3.62.
3.3.2. 2-(6-Chloro-4-Hydroxy-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6b)
Pale yellow crystals (DMF/CH3CN), yield: 0.310 g (72%), mp = 315–317 °C, IR (KBr): ν = 3230 (NH), 3010 (Ar-CH), 2803 (Aliph-CH), 1693, 1644 (CO), 1604 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 12.30 (s, 1H, OH), 11.50 (s, 1H, NH), 8.00 (d, 1H, J = 7.6 Hz, Ar-H), 7.90 (d, 1H, J = 7.98 Hz, Ar-H), 7.82 (d, 1H, J = 8.0 Hz, Ar-H), 7.54–7.50 (m, 5H, Ar-H), 7.30 (t, 2H, J = 7.8 Hz, Ar-H), 7.24–7.10 (m, 3H, Ar-H), 5.50 (dd, 1H, J = 10, 2.3 Hz, CH-α), 4.18 (dd, 1H, J = 17.0, 9.9 Hz, CH2-α), 2.80 (dd, 1H, J = 17.0, 2.6 Hz, CH2-α’); 13C-NMR (100 MHz, DMSO-d6): δ = 198.5, 198.3, 164.0 (CO), 158.0 (Ar-C), 136.8 (Ar-3C), 132.6, 132.1 (Ar-CH-p), 130.3 (Ar-2CH), 128.5, 128.0 (Ar-2CH-m), 127.8, 127.6 (Ar-2CH-o), 123.0 (Ar-CH-5), 120.0 (Ar-C-Cl), 115.2, 110.0 (Ar-C), 40.5 (CH2), 37.2 ppm (CH). MS (Fab, 70 eV, %): m/z = 431/433 (M+2, 23), 413 (17), 309 (14), 148 (37), 104 (100). Anal. Calcd. for C25H18ClNO4 (431.87): C, 69.53; H, 4.20; N, 3.24. Found: C, 69.62; H, 4.30; N, 3.32.
3.3.3. 2-(7-Chloro-4-Hydroxy-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6c)
White crystals (DMF/CH3CN), yield: 0.302 g (70%), mp = 340–342 °C, IR (KBr): ν = 3225 (NH), 3030 (Ar-CH), 2820 (Aliph-CH), 1690, 1650 (CO), 1612 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 12.10 (s, 1H, OH), 11.40 (s, 1H, NH), 7.98 (d, 1H, J = 7.8 Hz, Ar-H), 7.80 (d, 1H, J = 8.0 Hz, Ar-H), 7.60–7.50 (m, 6H, Ar-H), 7.20 (t, 2H, J = 7.8 Hz, Ar-H), 7.18–7.12 (m, 3H, Ar-H), 5.45 (dd, 1H, J = 10, 2.3 Hz, CH-α), 4.20 (dd, 1H, J = 17.0, 9.9 Hz, CH2-α), 2.85 ppm (dd, 1H, J = 17.0, 2.6 Hz, CH2-α’); 13C-NMR (100 MHz, DMSO-d6): δ = 198.4, 198.1, 162.4 (CO), 157.8 (Ar-C), 138.7, 137.0, 136.6 (Ar-C), 134.9, 132.9 (Ar-CH-p), 132.4 (Ar-2CH), 129.8, 128.9 (Ar-2CH-m), 128.6, 128.5 (Ar-2CH-o), 122.6 (Ar-CH-5), 119.5 (Ar-C-Cl), 121.3, 114.3 (Ar-C), 40.6 (CH2), 39.0 ppm (CH). MS (Fab, 70 eV, %): m/z = 431/433 (36/60), 413 (17), 309 (14), 148 (37), 104 (100). Anal. Calcd. for C25H18ClNO4 (431.87): C, 69.53; H, 4.20; N, 3.24. Found: C, 69.70; H, 4.15; N, 3.28.
3.3.4. 2-(6-Bromo-4-Hydroxy-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6d)
Pale yellow crystals (DMF/CH3OH), yield: 0.365 g (76%), mp = 348–350 °C, IR (KBr): ν = 3230 (NH), 3010 (Ar-CH), 2803 (Aliph-CH), 1693, 1644 (CO), 1604 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 12.30 (s, 1H, OH), 11.50 (s, 1H, NH), 8.00 (d, 1H, J = 7.6 Hz, Ar-H), 7.90 (d, 1H, J = 7.98 Hz, Ar-H), 7.82 (d, 1H, J = 8.0 Hz, Ar-H), 7.54–7.50 (m, 5H, Ar-H), 7.30 (t, 2H, J = 7.8 Hz, Ar-H), 7.24–7.10 (m, 3H, Ar-H), 5.50 (dd, 1H, J = 10, 2.3 Hz, CH-α), 4.18 (dd, 1H, J = 17.0, 9.9 Hz, CH2-α), 2.80 ppm (dd, 1H, J = 17.0, 2.6 Hz, CH2-α’); 13C-NMR (100 MHz, DMSO-d6): δ = 198.2, 197.6, 165.0 (CO), 158.2 (Ar-C), 138.0, 137.4, 133.0 (Ar-C), 132.4, 132.0 (Ar-CH-p), 130.0 (Ar-2CH), 128.8, 127.8 (Ar-2CH-m), 127.5, 127.0 (Ar-2CH-o), 122.6 (Ar-CH-5), 124.0 (Ar-C-Br), 115.0, 110.0 (Ar-C), 40.6 (CH2), 37.0 ppm (CH). MS (Fab, 70 eV, %): m/z = 475/477(32/58), 442 (18), 442 (12), 390 (6), 369 (10), 358 (12), 340 (17), 328 (24), 153 (100). Anal. Calcd. for C25H18BrNO4 (475.33): C, 63.04; H, 3.81; N, 2.94. Found: C, 62.96; H, 3.70; N, 3.08.
3.3.5. 2-(4-Hydroxy-7-Methyl-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6e)
Pale yellow crystals (DMF/EtOH), yield: 0.320 g (77%), mp 352–354 °C; IR (KBr): ν = 3230 (NH), 3010 (Ar-CH), 2890 (Aliph-CH), 1695, 1660 (CO), 1620 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 11.35 (s, 1H; NH), 10.77 (b, 1H; OH), 8.01 (dd, 2H, J = 8.5, 1.3 Hz; H-o’), 7.81 (d, 2H, J = 7.3 Hz; H-o), 7.73 (bs, 1H; H-5), 7.65 (t, 1H, J = 7.4 Hz; H-p’), 7.55 (“t”, 2H, J = 7.6 Hz; H-m’), 7.47 (t, 1H, J = 7.3 Hz; H-p), 7.38 (“t”, 2H, J = 7.6 Hz; H-m), 7.29 (dd, 1H, J = 8.4, 1.3 Hz; H-7), 7.14 (d, 1H, J = 8.3 Hz; H-8), 5.46 (dd, 1H, J = 9.7, 3.1 Hz; H-α), 4.19 (dd, 1H, J = 17.3, 9.8 Hz; H-α’), 2.82 (dd, 1H, J = 17.3, 3.1 Hz; H-α’), 2.31 ppm (s, 3H; H-6a); 13C-NMR (100 MHz, DMSO-d6): δ = 198.46, 198.31 (C-β,β’), 162.21 (C-2), 158.27 (C-4), 137.81, 137.01 (C-i,i’), 136.66 (C-8a), 132.92 (C-p’), 132.26 (C-p), 131.66 (C-7), 130.10 (C-6), 128.63 (C-m’), 128.19 (C-m), 127.83 (C-o’), 127.34 (C-o), 122.13 (C-5), 115.12 (C-8), 114.75 (C-4a), 111.68 (C-3), 40.46 (C-α), 37.45 (C-α’), 20.59 ppm (C-6a); 15N-NMR (40.55 MHz, DMSO-d6): δ = 142.1 ppm (N-1). MS (Fab, 70 eV, %): m/z = 411 = (M+, 40), 393 (16), 153 (100). Anal. Calcd. for C26H21NO4 (411.46): C, 75.90; H, 5.14; N, 3.40. Found: C, 76.10; H, 5.24; N, 3.50.
3.3.6. 2-(4-Hydroxy-6-Methyl-2-oxo-1,2-Dihydroquinolin-3-yl)-1,4-Diphenylbutane-1,4-Dione (6f)
Yellow crystals (DMF/H2O), yield: 0.320 g (77%), mp = 282–284 °C, IR (KBr): ν = 3226 (NH), 3012 (Ar-CH), 2860 (Aliph-CH), 1690, 1656 (CO), 1610 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 12.10 (s, 1H, OH), 11.70 (s, 1H, NH), 7.98 (d, 1H, J = 7.7 Hz, Ar-H), 7.86 (d, 1H, J = 8.00 Hz, Ar-H), 7.80 (d, 1H, J = 7.8 Hz, Ar-H), 7.62-7.55 (m, 5H, Ar-H), 7.28 (t, 2H, J = 8.0 Hz, Ar-H), 7.30–7.24 (m, 3H, Ar-H), 5.48 (dd, 1H, J = 10.0, 2.4 Hz, CH-α), 4.22 (dd, 1H, J = 17.0, 9.9 Hz, CH2-α), 2.82 (dd, 1H, J = 17.0, 2.6 Hz, CH2-α’), 2.20 ppm (s, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 198.0, 197.6, 163.0 (CO), 158.2 (Ar-C), 136.8, 135.4, 135.0 (Ar-C), 132.4, 132.0 (Ar-CH-p), 131.5, 130.0 (Ar-2CH-m), 128.9 (Ar-2CH), 127.8, 127.6 (Ar-2CH-o), 123.0 (Ar-CH-5), 120.0 (Ar-C-Cl), 115.2, 110.0 (Ar-C), 40.5 (CH2), 37.2 (CH), 21.2 ppm (CH3). MS (Fab, 70 eV, %): m/z = 411 (M+, 42), 393 (10), 153 (100). Anal. Calcd. for C26H21NO4 (411.46): C, 75.90; H, 5.14; N, 3.40. Found: C, 76.00; H, 5.22; N, 3.30.
3.3.7. 4-(Benzo[d]oxazol-2-yl)-3-Hydroxy-1H-Benzo[4,5]oxazolo[3,2-a]pyridine-1-one (11)
Buff crystals (DMF/CH3CN), yield: 0.255 g (80%), mp = 270–272 °C, IR (KBr): ν = 3030 (Ar-CH), 2860 (Aliph-CH), 1656 (CO), 1610 cm−1 (Ar-C=N). 1H-NMR (400 MHz, DMSO-d6): δ = 12.90 (s, 1H, OH), 8.40 (dd, 1H, J = 7.0 Hz, Ar-H), 8.00 (d, 2H, J = 7.3 Hz; Ar-H), 7.96 (m, 2H, J = 7.6 Hz; Ar-H), 7.92 (m, 1H, J = 8.2 Hz; Ar-H), 7.84 (m; 1H, Ar-H), 7.80 (m; 1H, Ar-H), 5.55 ppm (dd, 1H, J 7.5, 1.2 Hz; Ar-H). 13C-NMR (100 MHz, DMSO-d6): δ = 165.4 (CO), 160.0 (CH) 153.0, 149.8 (Ar-2C) 148.3, 147.0 (Ar-2C) 138.3 (Ar-C) 126.6, 126.3 (Ar-2CH) 124.41, 125.31, 124.24 (Ar-3CH) 123.7 (Ar-CH), 118.5, 116.2 (Ar-2C), 111.4 (Ar-CH), 110.2 (Ar-CH), 104.2 ppm (Ar-CH). MS (Fab, 70 eV, %): m/z = 318 (M+, 100), 200 (30), 118 (75). Anal. Calcd. for C18H10N2O4 (318.29): C, 67.93; H, 3.17; N, 8.80. Found: C, 68.10; H, 3.25; N, 8.90.
3.3.8. (4-(Benzo[d]oxazol-2-yl)-3-Hydroxy-1-oxo-1H-Benzo[4,5]oxazolo[3,2-a]pyridin-2-yl)-1,4-Diphenylbutane-1,4-Dione (12)
White crystals (DMF/EtOH), yield: 0.472 g (85%), mp = 278–280 °C, IR (KBr): ν = 3010 (Ar-CH), 2895 (Aliph-CH), 1665 (CO), 1620 cm−1 (Ar-C=N); 1H-NMR (400 MHz, DMSO-d6): δ = 13.16 (b, 1H; OH), 8.45 (dd, 1H, J = 7.0, 2.0 Hz; H-9)., 8.03 (d, 2H, J = 7.3 Hz; H-o/o’), 7.95 (d, 2H, J = 7.6 Hz; H-o’/o), 7.90 (bd, 1H, J = 8.2 Hz; H-6), 7.86 (m, 1H; H-7’), 7.80 (h, 1H; H-4’), 7.66 (t, 1H, J = 7.3 Hz; H-p/p’), 7.56 (“t”, 2H, J = 7.6 Hz; H-m/m’), 7.54 (m, 1H; H-7), 7.52 (m, 1H; H-8), 7.49 (t, 1H, J = 7.3 Hz; H-p’/p), 7.43 (m, 2H; H-5’,6’), 7.41 (“t”, 2H, J = 7.3 Hz; H-m’/m), 5.55 (dd, 1H, J = 10.1, 2.9 Hz; H-2a), 4.36 (dd, 1H, J = 17.4, 10.2 Hz; H-2b), 2.85 ppm (dd, 1H, J = 17.2, 2.6 Hz; H-2b); 13C-NMR (100 MHz, DMSO-d6): δ = 198.58, 197.93(C-2b’, 2c), 159.67 (C-3), 156.92 (C-1), 152.93 (C-4a), 149.58 (C-2’), 148.62 (C-7a’), 146.99 (C-5a), 138.39 (C-3a’), 136.98, 136.37 (C-i, i’), 132.94, 132.49 (C-p, p’), 128.66, 128.41 (C-m, m’), 127.85, 127.55 (C-o, o’), 126.86, 126.53 (C-7, 9a), 125.41, 125.31, 125.24 (C-5’, 6’, 8), 123.86 (C-2), 118.38 (C-4’), 115.92 (C-9), 111.24 (C-6), 110.83 (C-7’), 103.22 (C-4), 39.5 (C-2a), 37.35 ppm (C-2b). MS (Fab, 70 eV, %): m/z = 554 (M+, 40), 449 (25), 154 (100). Anal. Calcd. for C34H22N2O6 (554.15): C, 73.64; H, 4.00; N, 5.05. Found: C, 73.69; H, 4.05; N, 5.11.
3.4. Crystal Structure Determinations
The single-crystal X-ray diffraction study were carried out on a Bruker D8 Venture diffractometer (company, city, state abbr. if USA, country) with Photon100 detector at 123(2) K using Cu-Kα radiation (λ = 1.54178 Å. Direct methods (SHELXS-97 for 6f) [30] or dual space/intrinsic methods (SHELXT for 12) [31] were used for structure solution and refinement was carried out using SHELXL-2014 (full-matrix least-squares on F2; version, company, city, state abbr. if USA, country) [31]. Hydrogen atoms were localized by difference electron density determination and refined using a riding model (H(N,O)free). Semi-empirical absorption corrections were applied.
6f: colourless crystals, C26H21NO4, Mr = 411.44, crystal size 0.16 × 0.08 × 0.06 mm, triclinic, space group P-1 (No. 2), a = 11.1823(3) Å, b = 14.3827(4) Å, c = 15.3460(4) Å, α = 67.695(1)°, β = 70.666(1)°, γ = 68.389(1)°, V = 2069.77(10) Å3, Z = 4, ρ = 1.320 Mg/m−3, µ(Cu-Kα) = 0.72 mm−1, F(000) = 864, 2θmax = 144.2°, 28,579 reflections, of which 8117 were independent (Rint = 0.035), 577 parameters, 5 restraints, R1 = 0.043 (for 6749 I > 2σ(I)), wR2 = 0.112 (all data), S = 1.01, largest diff. peak/hole = 0.37/−0.50 e Å−3.
12: colourless crystals, C34H2N2O6, Mr = 554.53, crystal size 0.22 × 0.09 × 0.03 mm, triclinic, space group P-1 (No. 2), a = 8.2672(2) Å, b = 11.3140(3) Å, c = 13.7326(3) Å, α = 91.721(1)°, β = 96.833(1)°, γ = 92.198(1)°, V = 1273.64(5) Å3, Z = 2, ρ = 1.446 Mg/m−3, µ(Cu-Kα) = 0.82 mm−1, F(000) = 576, 2θmax = 144.4°, 18,981 reflections, of which 4987 were independent (Rint = 0.037), 382 parameters, 1 restraint, R1 = 0.048 (for 4212 I > 2σ(I)), wR2 = 0.127 (all data), S = 1.03, largest diff. peak/hole = 0.35/−0.25 e Å−3.CCDC 1,913,026 (6f), and 1,913,027 (12) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
4. Conclusions
Reaction of 1,6-disubstituted-4-hydroxy-quinolin-2-ones with 2,3-dichloropyrazine, catalyzed by triethylamine, furnished5:12-dihydropyrazino-[2,3-c:5,6-c′]difuro[2,3-c:4,5-c′]diquinoline-6,14(5H,12H)-diones, by an apparent SNAr/ANRORC sequence. Reaction of the same quinolones with (E)-dibenzoylethene led to conjugate addition without cyclization. Reaction of 2-hydroxyaniline with diethyl malonate led unexpectedly to 4-(benzo-[d]oxazol-2-yl)-3-hydroxy-1H-[4,5]oxazolo-[3,2-a]pyridine-1-one, which also underwent conjugate addition to dibenzoylethene.
Acknowledgments
The NMR spectrometer at Florida Institute of Technology was purchased with the assistance of the U.S. National Science Foundation (CHE 03 42251).
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/24/20/3782/s1, Table S1. NMR spectroscopic data of compound 6f and Table S2. NMR spectroscopic data of compound 12.
Author Contributions
A.A.A. (suggesting the idea, writing and revision), A.A.H. and N.K.M. review and editing), S.B. (revision), L.E.A.E.-H. (methodology), M.P. and M.N. (X-ray analysis) and A.B.B. (NMR analysis).
Funding
The authors thank Egyptian Mission, Ministry of higher education, Egypt for its financial support to Mrs Lamiaa E. Abd El-Haleem during her accommodation in Institute für Technology, Karlsruhe, Germany. The authors also thank the DFG Foundation for providing Prof Ashraf A. Aly, one-month fellowship enabling him to carry out the compounds analysis in Karlsruhe Institute of Technology, Karlsruhe, Germany in July–August 2019.
Conflicts of Interest
The authors declare no confilict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Mizuta M., Kanamori H. Mutagenic activities of dictamnine and g-fagarine from Dictamni radicis cortex (Rutaceae) Mutat. Res. Lett. 1985;144:221–225. doi: 10.1016/0165-7992(85)90054-5. [DOI] [PubMed] [Google Scholar]
- 2.Zuo Y., Pu J., Chen G., Shen W., Wang B. Study on the activity and mechanism of skimmianine against human non-small cell lung cancer. Nat. Prod. Res. 2019;33:759–762. doi: 10.1080/14786419.2017.1408096. [DOI] [PubMed] [Google Scholar]
- 3.Claerhout S., Sharma S., Skold C., Cavaluzzo C., Sandstrom A., Larhed M., Thirumal M., Parmar V.S., Van der Eycken E.V. Synthesis of functionalized furopyrazines as restricted dipeptidomimetics. Tetrahedron. 2012;68:3019–3029. doi: 10.1016/j.tet.2012.02.022. [DOI] [Google Scholar]
- 4.Pesci E.C., Milbank J.B.J., Pearson J.P., McKnight S., Kende A.S., Greenberg E.P., Iglewski B.H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrath S., Wade D.S., Pesci E.C. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS) FEMS Microbiol. Lett. 2004;230:27–34. doi: 10.1016/S0378-1097(03)00849-8. [DOI] [PubMed] [Google Scholar]
- 6.Diggle S.P., Gardner A., West S.A., Griffin A.S. Evolutionary theory of bacterial quorum sensing: When is a signal not a signal? Phil. Trans. R. Soc. B. 2007;362:1241–1249. doi: 10.1098/rstb.2007.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dulcey C.E., Dekimpe V., Fauvelle D.-A., Milot S., Groleau M.-C., Doucet N., Rahme L.G., Lépine F., Déziel E. The end of an old hypothesis: The Pseudomonas signalling molecules 4-hydroxy-2-alkylquinolines derive from fatty acids, not 3-ketofatty acids. Chem. Biol. 2013;20:1481–1491. doi: 10.1016/j.chembiol.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y., Seyedsayamdost M.R. Synergy and target promiscuity drive structural divergence in bacterial alkylquinolone biosynthesis. Cell Chem. Biol. 2017;24:1437–1444. doi: 10.1016/j.chembiol.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burden D.A., Osheroff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim. Biophys. Acta. 1998;1400:139–154. doi: 10.1016/S0167-4781(98)00132-8. [DOI] [PubMed] [Google Scholar]
- 10.Dang Z., Yang Y., Ji R., Zhang S. Synthesis and antibacterial activity of novel fluoroquinolones containing substituted piperidines. Bioorg. Med. Chem. Lett. 2007;17:4523–4526. doi: 10.1016/j.bmcl.2007.05.093. [DOI] [PubMed] [Google Scholar]
- 11.Shindikar A.V., Viswanathan C.L. Novel fluoroquinolones: Design, synthesis, and in vivo activity in mice against Mycobacterium tuberculosis H37Rv. Bioorg. Med. Chem. Lett. 2005;15:1803–1806. doi: 10.1016/j.bmcl.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 12.Andriole V.T. The quinolones: Past, present, and future. Clin. Infect. Dis. 41 Suppl. 2005;2:113–119. doi: 10.1086/428051. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S.-L., Huang Z.-S., An L.-K., Bu X.-Z., Ma L., Li Y.-M., Chan A.S.C., Gu L.-Q. Synthesis of Zwitter-ionic 4-hydroxycoumarin derivatives through a unique reaction of 4-hydroxycoumarins with p-benzoquinone and pyridine. Org. Lett. 2004;6:4853–4855. doi: 10.1021/ol048109p. [DOI] [PubMed] [Google Scholar]
- 14.Aly A.A., El-Sheref E.M., Mourad A.F.E., Bakheet M.E.M., Bräse S., Nieger M. One-pot synthesis of 2,3-bis-(4-hydroxy-2-oxo-1,2-dihydroquinolin-3-yl)succinates and arylmethylene-bis-3,3′-quinoline-2-ones. Chem. Pap. 2019;73:27–37. doi: 10.1007/s11696-018-0561-0. [DOI] [Google Scholar]
- 15.Aly A.A., Ramadan M., El-Reedy A.A.M. Reactions of 4-hydroxyquinolin-2(1H)-ones with acenaphthoquinone: Synthesis of new 1,2-dihydroacenaphthylene-spiro-tetrakis-(4-hydroxy-quinolin-2(1H)-ones) J. Heterocycl. Chem. 2019;56:642–645. doi: 10.1002/jhet.3442. [DOI] [Google Scholar]
- 16.Aly A.A., El-Sheref E.M., Mourad A.-F.E., Brown A.B., Bräse S., Bakheet M.E.M., Nieger M. Synthesis of spiro[indoline-3,4′-pyrano[3,2-c]quinolone]-3′-carbonitriles. Monatsh. Chem. 2018;149:635–644. doi: 10.1007/s00706-017-2078-6. [DOI] [Google Scholar]
- 17.El-Sheref E.M., Aly A.A., Mourad A.-F.E., Brown A.B., Bräse S., Bakheet M.E.M. Synthesis of pyrano[3,2-c]quinoline-4-carboxylates and 2-(4-oxo-1,4-dihydroquinolin-3-yl)fumarates. Chem. Pap. 2018;72:181–190. doi: 10.1007/s11696-017-0269-6. [DOI] [Google Scholar]
- 18.El-Sheref E.M., Aly A.A., Ameen M.A., Brown A.B. New 4-(1,2,3-triazolo)quinolin-2(1H)-ones via Cu-catalyzed [3+2] cycloaddition. Monatsh. Chem. 2019;150:747–756. doi: 10.1007/s00706-018-2342-4. [DOI] [Google Scholar]
- 19.Aly A.A., El-Sheref E.M., Bakheet M.E.M., Mourad M.A.E., Bräse S., Ibrahim M.A.A., Nieger M., Garvalov B.K., Dalby K.N., Kaoud T.S. Design, synthesis and biological evaluation of fused naphthofuro[3,2-c]quinoline-6,7,12-triones and pyrano[3,2-c]quinoline- 6,7,8,13-tetraones derivatives as ERK inhibitors with efficacy in BRAF-mutant melanoma. Bioorg. Chem. 2019;82:290–305. doi: 10.1016/j.bioorg.2018.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aly A.A., El-Sheref E.M., Bakheet M.E.M., Mourad M.A.E., Brown A.B., Bräse S., Nieger M., Ibrahim M.A.A. Synthesis of novel 1,2-bis-quinolinyl-1,4-naphthoquinones: ERK2 inhibition, cytotoxicity and molecular docking studies. Bioorg. Chem. 2018;81:700–712. doi: 10.1016/j.bioorg.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Spartan ’18. Wavefunction, Inc.; Irvine, CA, USA: [(accessed on 21 October 2019)]. Available online: http://downloads.wavefun.com/Spartan18Brochure.pdf. [Google Scholar]
- 22.Endres A.H., Schaffroth M., Paulus F., Reiss H., Wadepohl H., Rominger F., Krämer R., Bunz U.H.F. Coronene-containing N-heteroarenes: 13 rings in a row. J. Am. Chem. Soc. 2016;138:1792–1795. doi: 10.1021/jacs.5b12642. [DOI] [PubMed] [Google Scholar]
- 23.Bisballe N., Hedidi M., Demmer C.S., Chevallier S., Roisnel T., Dorcet V., Halauko Y.S., Ivashkevich O.A., Matulis V.E., Bentabed-Ababsa G., et al. Functionalization of oxazolo[4,5-b]pyrazines by deprotometallation. Eur. J. Org. Chem. 2018;2018:3904–3913. doi: 10.1002/ejoc.201800481. [DOI] [Google Scholar]
- 24.Lont P.J., van der Plas H.C., Verbeek A.J. Ring transformations in reactions of heterocyclic halogeno compounds with nucleophiles (XXV) Recl Trav. Chim. Pays-Bas. 1978;91:949–957. doi: 10.1002/recl.19720910810. [DOI] [Google Scholar]
- 25.Van der Plas H.C. The SN(ANRORC) mechanism: A new mechanism for nucleophilic substitution. Acc. Chem Res. 1978;11:462–468. doi: 10.1021/ar50132a005. [DOI] [Google Scholar]
- 26.Van der Plas H.C. Degenerate ring transformations of heterocyclic compounds. Adv. Heterocycl Chem. 1999;74:1–253. [Google Scholar]
- 27.Rusinov G.L., Slepukhin P.A., Charusin V.N., Chupakhin O.N. 2,3-Dichloro-1-alkyl- pyrazinium tetrafluoroborates: The synthesis and reactions with nucleophiles. Mendeleev Commun. 2001;11:78–80. doi: 10.1070/MC2001v011n02ABEH001381. [DOI] [Google Scholar]
- 28.Rao V.S., Darbarwar M. One pot synthesis of 7-[1,2-dihydro-4-hydroxy-1-methyl/phenyl- 2-oxo-3-quinolinyl]-5,7-dihydro-5-methyl/phenyl-6H-[1]-benzo-pyrano-[3,2-c]quinolin-6-ones. Synth. Commun. 1988;18:2267–2272. doi: 10.1080/00397918808082369. [DOI] [Google Scholar]
- 29.Buckle D.R., Cantello B.C.C., Smith H., Spicer B.A. 4-Hydroxy-3-nitro-2-quinolones and related compounds as inhibitors of allergic reactions. J. Med. Chem. 1975;18:726–732. doi: 10.1021/jm00241a017. [DOI] [PubMed] [Google Scholar]
- 30.Sheldrick G.M. A short history of SHELX. Acta. Crystallogr. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 31.Sheldrick G.M. SHELXT: Integrated space-group and crystal-structure determination. Acta. Crystallogr. 2015;A71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.