Abstract
Legumes are amongst the most promising crops to satisfy the increasing demand for protein-rich food and feed. Today, however, their cultivation in Europe is low, while European agriculture faces a deficit of protein-rich feed, of which the largest part is met by imported soybean. It has been suggested that some legumes can at least partially substitute for soybean in different types of feed. Despite their benefits, legumes may also remain a significant concern to human and animal health, especially regarding grain contamination with Fusaria and their mycotoxins. In this study, we determined the species composition of Fusarium field isolates recovered from diseased grains of various legumes. Our results showed that Fusarium avenaceum was mainly responsible for grain deterioration of common vetch, faba bean, and blue lupine. Besides, we found that F. equiseti also appeared to be a major pathogen of common vetch. This study is the first ever to report common vetch as a host for F. tricinctum, F. equiseti, and F. graminearum sensu stricto. Our results indicate that the composition of toxigenic Fusaria associated with grains of legumes is different than that previously observed in cereal grains.
Keywords: Fusarium, qPCR, legume plants, common vetch, blue lupine, faba bean, white lupine
1. Introduction
Legumes are amongst the most promising crops to satisfy the increasing demand for protein-rich food and feed [1]. Nowadays, they are the second most important food source after grasses and are a relatively better source of lysine and total proteins than cereals [2]. Today, however, the cultivation of legumes in Europe is low, while European agriculture faces a deficit of protein-rich feed, of which the largest part is met by imported soybean and soybean meal. It has been suggested that some legumes can at least partially substitute for soybean in different types of feed [3]. Sound evidence supports the health benefits of increasing legume intake by humans. Consumption of legumes implicated lowering risks of many diseases like heart disease, high blood pressure, stroke, and type 2 diabetes [4].
Despite their benefits, legumes may also remain a significant concern to human and animal health, especially regarding grain contamination with fungal biomass and mycotoxins. Fungi of the genus Fusarium may pose a serious problem due to their widespread occurrence and cosmopolitan range. The most common mycotoxins produced by Fusaria are trichothecenes, enniatins, zearalenone, and fumonisins, exerting various negative effects on humans and animals [5].
We searched the biomedical literature through the PubMed database with no date restrictions for case reports and outbreaks using the search terms “Fusarium”, “mycotoxins”, “faba bean”, “common vetch”, “blue lupine”, and “white lupine”. We have chosen these crops because they are suggested as the most promising alternatives for soybean protein [3]. Google Scholar was also searched with similar terms. We found that the literature reporting the incidence of toxigenic Fusaria on these alternative legume crops is scarce (Table 1). Today, most surveys characterizing seed-borne Fusaria in Europe come from studies that report the incidence of these pathogens on small-grain cereals. Although a broad range of Fusarium species may be associated with grasses, multiple surveys conducted over the last decade have provided strong evidence on the predominance of F. graminearum sensu stricto in various European localities [6].
Table 1.
Documented incidence of grain-associated Fusaria on common vetch, faba bean, and blue and white lupine.
| Fusarium Species | Host | Geographic Location | Sampling Season | References |
|---|---|---|---|---|
| F. acuminatum | Common vetch | Canada | - | [7] |
| F. acutatum | Faba bean | Sudan | - | [8] |
| F. avenaceum | Blue lupine | Poland | 2011–2013 | [9] |
| Common vetch | - | - | [10] | |
| Faba bean | Ethiopia | 1993 | [11] | |
| Faba bean | Poland | 1981–1984 | [12] | |
| Faba bean | Poland | 2001 | [13] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| White lupine | Poland | 2011 | [9] | |
| F. culmorum | Faba bean | Poland | 1981–1984 | [12] |
| Faba bean | Poland | 2001 | [13] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| F. compactum | Faba bean | Sudan | - | [8] |
| F. equiseti | Blue lupine | Poland | 2012 | [9] |
| Faba bean | Poland | 1981–1984 | [12] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| White lupine | Poland | 2011 | [9] | |
| F. graminearum | Faba bean | Poland | 2001 | [13] |
| F. nygamai | Faba bean | Sudan | - | [8] |
| F. oxysporum | Blue lupine | Poland | 2010–2012 | [15] |
| Common vetch | Canada | - | [7] | |
| Faba bean | Poland | 1981–1984 | [12] | |
| Faba bean | United Kingdom | 1973 | [16] | |
| Faba bean | Sudan | - | [8] | |
| Faba bean | Poland | 2001 | [13] | |
| Faba bean | Egypt | 2004–2005 | [17] | |
| Faba bean | Ethiopia | 2010–2011 | [18] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| Faba bean | Egypt | - | [19] | |
| F. poae | Blue lupine | Poland | 2012–2013 | [9] |
| White lupine | Poland | 2011–2012 | [9] | |
| F. proliferatum | Faba bean | Sudan | - | [8] |
| White lupine | Croatia | - | [20] | |
| F. semitectum | Faba bean | Sudan | - | [8] |
| Faba bean | Egypt | 2004–2005 | [17] | |
| F. solani | Faba bean | Poland | 1981–1984 | [12] |
| Faba bean | United Kingdom | 1973 | [16] | |
| Faba bean | Sudan | - | [8] | |
| Faba bean | Egypt | 2004–2005 | [17] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| Faba bean | Ethiopia | 2010–2011 | [18] | |
| F. sporotrichioides | Blue lupine | Poland | 2013 | [9] |
| Faba bean | Poland | 2001 | [13] | |
| Faba bean | Poland | 2010–2011 | [14] | |
| F. tricinctum | Blue lupine | Poland | 2012–2013 | [9] |
| F. verticillioides | Common vetch | Croatia | - | [20] |
| Faba bean | Egypt | 2004–2005 | [17] | |
| White lupine | Croatia | - | [20] |
(-)—data not available.
Limited data on the incidence of Fusaria on legumes prompted us to investigate the presence of Fusarium spp. in legume grains harvested in North-Eastern Poland in the 2017/2018 growing seasons. Our preliminary results showed that F. avenaceum was mainly responsible for grain deterioration of common vetch, faba bean, and blue lupine. Previous surveys from cereals indicated F. avenaceum to be the major source of enniatins in plant-derived food, posing significant risk to food and feed safety [5]. In this study, we also recovered a high number of isolates of F. equiseti from common vetch. F. equiseti belongs to the Fusarium incarnatum-equiseti species complex with the potential to produce diverse mycotoxin compounds, such as type A trichothecenes and zearalenone [5]. These results indicate that the composition of toxigenic Fusaria associated with legume grains is different from that previously observed in cereal grains. This study reports the incidence of F. tricinctum, F. equiseti, and F. graminearum sensu stricto infecting common vetch for the first time ever.
2. Results and Discussion
225 legume grains showing visual symptoms of the fungal disease were selected to obtain Fusarium isolates for analyses. Diseased grains were shriveled, discolored, and/or covered by fungal mycelia. After incubation and visual selection of fungal colonies on potato dextrose agar (PDA), we obtained forty-three Fusarium-like cultures that were further subjected to real-time polymerase chain reaction (PCR) analyses.
We used different species–specific assays to identify the isolates to the species level. Thirty-seven out of the 43 isolates gave positive results enabling their quick assignment (Table 2, Table S1).
Table 2.
List of real-time polymerase chain reaction (PCR) assays used to determine species, trichothecene genotypes, and enniatin genotypes.
| Specificity of the qPCR Assay | Primer/Probe Sequence | Reaction Reagents | Reaction Conditions | References |
|---|---|---|---|---|
| Total fungal DNA | ||||
| FungiQuant | F: GGRAAACTCACCAGGTCCAG | A | 95 °C for 20 s, (95 °C for 1 s, 60 °C for 30 s) × 40 | [21] |
| R: GSWCTATCCCCAKCACGA | ||||
| Probe: (6FAM)-TGGTGCATGGCCGTT-(MGBNFQ) | ||||
| Species | ||||
| F. avenaceum | F: CCATCGCCGTGGCTTTC R: CAAGCCCACAGACACGTTGT Probe: FAM-ACGCAATTGACTATTGC-MGB |
B | 95 °C for 20 s, (95 °C for 1 s, 60 °C for 50 s) × 40 | [22] |
| F. culmorum | F: TCGTTGACGGTGAGGGTTGT R:GACTCGAACACGTCAACCAACT Probe: FAM-CGGTTATTATTTCGAAAAGT- MGB |
A | 95 °C for 20 s, (95 °C for 1 s, 60 °C for 30 s) × 40 | [23] |
| F. equiseti | F: CACCGTCATTGGTATGTTGTCATC R: TGTTAGCATGAGAAGGTCATGAGTG |
C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| F. graminearum s.s. | F: TGGCCTGAATGAAGGATTTCTAG R: CATCGTTGTTAACTTATTGGAGATG Probe: FAM-TTAAACACTCAAACACTACA- MGB |
A | 95 °C for 20 s, (95 °C for 1 s, 60 °C for 30 s) × 40 | [25] |
| F. langsethiae | F: CAAGTCGACCACTGTGAGTACCTCT R: TGTCAAAGCATGTCAGTAAAGATGAC |
C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| F. poae | F: AAATCGGCGTATAGGGTTGAGATA R: GCTCACACAGAGTAACCGAAACCT Probe: FAM-CAAAATCACCCAACCGACCCTTTC-TAMRA |
B | 50 °C for 2 min, 95 °C for 10 min, (95 °C for 15 s, 60 °C for 60 s) × 40 | [22] |
| F. proliferatum | F: CTTCGATCGCGCGTCCT R: CACGTTTCGAATCGCAAGTG |
C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| F. sporotrichioides | F: GCAAGTCGACCACTGTGAGTACA R: CTGTCAAAGCATGTCAGTAAAAATGAT |
C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| F. subglutinans | F: TCATTGGTATGTTGTCGCTCATG R: GTGATATGTTAGTACGAATAAAGGGAGAAC |
C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| F. verticillioides | F: CGTTTCTGCCCTCTCCCA R: TGCTTGACACGTGACGATGA |
C | 95 °C for 5 min, (95 °C for 15 s, 65 °C for 60 s) × 40, dissociation curve analysis at 60–95 °C. | [24] |
| Enniatin genotype | ||||
| esyn1 | F: AGCAGTCGAGTTCGTCAACAGA R: GGCYTTTCCTGCGAACTTG Probe: FAM-CCGTCGAGTCCTCT-MGB |
B | 95 °C for 20 s, (95 °C for 3 s, 60 °C for 30 s) × 40 | [26] |
| Tri genotypes | ||||
| 3ADON | F: CATGCGGGACTTTGATCGAT | B | 95 °C for 20 s, (95 °C for 1 s, 60 °C for 50 s) × 40 | [27] |
| R: TTTGTCCGCTTTCTTTCTATCATAAA | ||||
| Probe: FAM-CTCACCGATCATGTTC-MGB | ||||
| 15ADON | F: TCCAATCATTGCCAGCCTCTA | |||
| R: TGATGCGGAACATGGTCTGT | ||||
| Probe: FAM-ATGAGGGACTTTGACCAAT-MGB | ||||
| NIV | F: TCGCCAGTCTCTGCATGAAG | |||
| R: CCTTATCCGCTTTCTTTCTATCATAAA | ||||
| Probe: FAM-CTGATCATGTCCCGCATC-MGB |
A 2 µL gDNA, 14.3 µL H2O, 6.7 µM of each primer, 1.7 µM of probe, 3.6 µL TaqMan Fast Advanced Master Mix (Applied Biosystems, Foster City, CA, USA). B 2 µL gDNA, 10.8 µL H2O, 6.7 µM of each primer, 1.7 µM of probe, 7.2 µL TaqMan Fast Advanced Master Mix (Applied Biosystems, Foster City, CA, USA). C 2 µL gDNA, 8.5 µL H2O, 1 µM of each primer, 12.5 µL 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA).
Our results showed that F. avenaceum was mainly responsible for grain deterioration of common vetch, faba bean, and blue lupine (49% of isolates) (Table 3). F. avenaceum is a common plant pathogen infecting a variety of hosts worldwide. In cereals, it is often responsible for the crown rot and head blight that affects yield and quality of grain [28]. Most research works documenting the incidence of this species on legume grains are relatively old and come mainly from Polish surveys [9,12,13]. F. avenaceum contaminates grain with enniatins [28]; however, according to our knowledge, no data is available on the contamination of legume grains with this group of mycotoxins. The ability to produce enniatins by Fusaria is governed by esyn1 gene encoding a multifunctional enzyme enniatin synthetase [29]. In this study, we showed that all examined isolates of F. avenaceum harbored esyn1 gene, which indicates their ability to produce enniatins. This highlights the need for further chemical studies to confirm the contamination of legume grains with these cyclic hexadepsipeptides. Previous Polish studies showed that besides F. avenaceum, F. culmorum was a pathogen occasionally associated with faba bean grains [12,13,14], but our results do not reveal the incidence of F. culmorum on any of the examined hosts. Recent studies on cereals have shown that F. culmorum has been displaced by F. graminearum s.s. as the major agent of Fusarium head blight (FHB) of wheat in Europe [6]. This dramatic shift has also been revealed in Poland [30]. Thus, the results obtained in this study may indicate previously undocumented loss of F. culmorum on legumes, suggesting that the reduction of F. culmorum incidence in grain-associated Fusaria may also occur in other non-cereal crops.
Table 3.
List of identified Fusarium species in different legume grains in Poland.
| Plant Host | F. avenaceum | F. equiseti | F. graminearum s.s. | F. sporotrichioides | F. tricinctum | Not Identified |
|---|---|---|---|---|---|---|
| Blue lupine | 7 | - | - | 3 | - | 1 |
| Common vetch | 7 | 9 | 1 | - | 1 | - |
| Faba bean | 7 | - | - | 1 | - | 4 |
| White lupine | - | 1 | - | - | - | 1 |
(-)—no positive results.
In our study, besides F. avenaceum, F. equiseti also appeared to be a major pathogen of common vetch. F. equiseti is a cosmopolitan soil-borne fungus that has been detected in roots and plant tissues worldwide [31]. A recent analysis conducted using genealogical concordance phylogenetic species recognition (GCPSR) has revealed that F. equiseti belongs to the Fusarium incarnatum-equiseti species complex (FIESC), consisting of at least 33 phylogenetically distinct species, grouped into two major clades: Equiseti and Incarnatum [32]. FIESC members are increasingly associated with diseases of numerous plants including Fusarium root rot in soybean [33]. They have also been associated with human and animal health problems [34]. In addition, F. equiseti has been identified in soybean grains; however, reports documenting its incidence on other legumes are mainly limited to old surveys (Table 1). According to our knowledge, this is the first report showing the incidence of F. equiseti on common vetch. Among 18 isolates recovered from this crop, a single isolate was identified as F. graminearum s.s. This phylogenetic species has been recently recognized as the major FHB member of wheat in Poland [30]. F. graminearum s.s. has been found to contaminate soybean grains worldwide, but its incidence on other legume crops has been reported only for faba bean [13]. The emergence of F. graminearum s.s. in Europe has been linked to increased production of maize, which favors ascospore formation, which survives in crop residues and may be carried over long distances [30]. Soybean residues were also found to support high levels of sporulation by F. graminearum s.s. [35]. The identified incidence of F. graminearum s.s., albeit occasional, could promote further more comprehensive studies evaluating the risk of ascospore production by this pathogen on other hosts apart from soybean legume residues. Overall, our results indicate that the composition of toxigenic Fusaria associated with grains of legumes is different from that previously observed in cereals [6]. The revealed high incidence of both F. avenaceum and F. equiseti needs to be confirmed on a larger scale by incorporating more samples from a wide geographic area. Our further work will aim at molecular characterization of the recovered isolates of F. equiseti as these strains may comprise phylogenetically distinct species having the potential to produce diverse mycotoxin compounds [32]. Our further work will also include characterization of Fusaria from soybean samples, as this crop is expected to be increasingly cultivated in the EU [36].
3. Materials and Methods
3.1. Legume Grain Samples
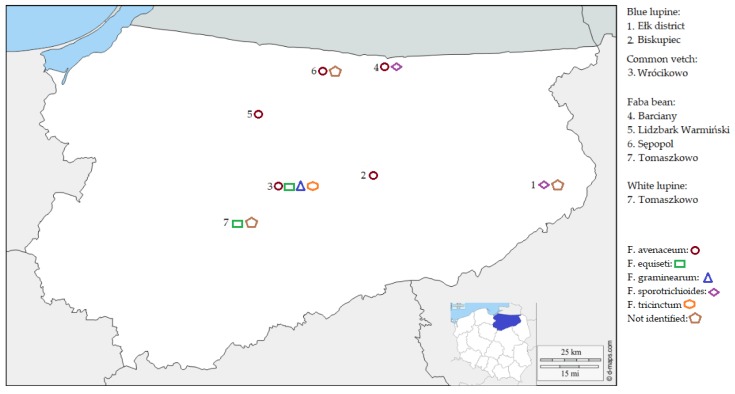
Grains with symptoms of fungal infection with purple/pink lesions and/or shriveled grains were selected from different 2017 and 2018 grain samples (0.5 kg) originating from seven different fields in the North-Eastern Poland (Figure 1). Diseased grains were placed in Petri dishes with distilled water and kept for 24 hours at room temperature. After soaking, grains were surface sterilized with 70% ethanol (EtOH) for 2 min and placed on PDA medium. Grains were incubated for 4–6 days at room temperature in darkness. Fusarium-like colonies were transferred to new PDA plates. The selection of Fusarium-like colonies was based on morphological characteristics and the color of aerial mycelium.
Figure 1.
Locations of fields in Warmia-Mazury Province in Poland, from which legume grains were sampled for analyses
For storage purposes, colonies were transferred to new PDA plates, cultured for 6 days and covered with 1.5 g of sterile soil. Fungi were cultured at room temperature for 7–14 days until mycelium had overgrown the soil. A total of 43 Fusarium isolates were assigned with unique isolate codes and are stored at –25 °C in the fungal collection of the Department of Botany and Nature Protection, University of Warmia and Mazury in Olsztyn, Poland.
3.2. DNA Isolation and Species Identification
A patch of mycelium (approximately 0.1–0.2 mg) was scraped from the PDA plate and transferred to homogenization tubes with 1 mm silica spheres (Lysing matrix C, MP Biomedicals, Santa Ana, CA, USA). DNA extraction was performed using a ChargeSwitch® gDNA Plant Kit (Invitrogen, Carlsbad, CA, USA). Homogenization was conducted using a FastPrep-24 instrument (MP Biomedicals, Santa Ana, CA, USA).
The FungiQuant assay [21] was used to check the total extracted DNA. Positive signals of amplification in all analyzed samples indicated that all extracted DNA can be examined with different real-time PCR assays (Table 2) to assign fungal species and mycotoxin genotypes.
Enniatin genotypes were determined using TaqMan assay targeting the esyn1 gene [26]. Trichothecene genotype of single F. graminearum s.s. strain was determined using TaqMan assays targeting the Tri12 gene [27]. All reactions were performed in three replicates.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/11/10/569/s1, Table S1: Species and mycotoxin genotype identification of Fusarium isolates using real-time PCR assays.
Author Contributions
Conceptualization, M.Ż. and T.K.; methodology, M.Ż., T.K. and J.O.; validation, M.Ż.; formal analysis, M.Ż.; investigation, M.Ż., T.K. and J.O.; resources, T.K. and M.Ż.; data curation, M.Ż.; writing—original draft preparation, M.Ż.; writing—review and editing, T.K.; visualization, M.Ż.; supervision, T.K.; project administration, T.K.; funding acquisition, T.K.
Funding
This research was funded by “Development Program of the University of Warmia and Mazury in Olsztyn", POWR.03.05.00-00-Z310/17, co-financed by the European Union under the European Social Fund from the Operational Program Knowledge Education Development. The first author, Maciej Żelechowski is a recipient of a scholarship from the Programme Interdisciplinary Doctoral Studies in Biology and Biotechnology (POWR.03.05.00-00-Z310/17), which is funded by the European Social Fund.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Key Contribution
Characterization of Fusarium spp. infecting legume grains in North-East Poland. Study reveals new host (common vetch) for F. tricinctum, F. equiseti, and F. graminearum sensu stricto.
References
- 1.De Ron A.M., Sparvoli F., Pueyo J.J., Bazile D. Editorial: Protein Crops: Food and Feed for the Future. Front. Plant Sci. 2017;8:105. doi: 10.3389/fpls.2017.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maphosa Y., Jideani V.A. The role of legumes in human nutrition. In: Chavarri M., editor. Functional Food: Improve Health through Adequate Food. Edition 2017. Intech Open; Rijeka, Croatia: 2017. pp. 103–109. Chapter 6. [Google Scholar]
- 3.de Visser C., Schreuder R., Stoddard F. The EU’s dependence on soya bean import for the animal feed industry and potential for EU produced alternatives. Oilseeds Fats Crop. Lipids. 2014;21:D407. [Google Scholar]
- 4.Polak R., Phillips E.M., Campbell A. Legumes: Health benefits and culinary approaches to increase intake. Clin. Diabetes. 2015;33:198–205. doi: 10.2337/diaclin.33.4.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrigo D., Raiola A., Causin R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules. 2016;21:627. doi: 10.3390/molecules21050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Lee T., Zhang H., van Diepeningen A., Waalwijk C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2015;32:453–460. doi: 10.1080/19440049.2014.984244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon W. The occurrence of Fusarium species in Canada: VI. taxonomy and geographic distribution of Fusarium species on plants, insects, and fungi. Can. J. Bot. 1959;37:257–290. doi: 10.1139/b59-021. [DOI] [Google Scholar]
- 8.Kurmut A., Nirenberg H., Bochow H., Buttner C. Fusarium nygamai, causal agent of root rot of Vicia faba L. in the Sudan. Meded. -Fac. Landbouwkd. En Toegep. Biol. Wet. 2002;67:269–274. [PubMed] [Google Scholar]
- 9.Pszczółkowska A., Okorski A., Fordoński G., Prusiński J., Faligowska A., Borowska M. Fungal colonization of seeds of three lupine species in different regions of Poland. Acta Agrobot. 2017;70 doi: 10.5586/aa.1714. [DOI] [Google Scholar]
- 10.Duke J. Handbook of Legumes of World Economic Importance. 1st ed. Plenum Press; New York, NY, USA: London, UK: 1981. pp. 281–283. [Google Scholar]
- 11.Gorfu D. Yield loss of faba bean caused by root rot (Fusarium avenaceum) [Vicia faba] Faba Bean Inf. Serv. 1993;33:24–27. [Google Scholar]
- 12.Sadowski S. Występowanie chorób bobiku (Vicia faba L.) w rejonach olsztyńsko-elbląskim i bydgoskim [Occurrence of broad bean (Vicia faba L.) diseases in Olsztyn-Elbąg and Bydgoszcz Provinces] Acta Agrobot. 1988;41:245–255. doi: 10.5586/aa.1988.014. [DOI] [Google Scholar]
- 13.Kulik T., Fordoński G., Pszczółkowska A., Płodzień K., Olszewski J. Identyfikacja wybranych gatunków grzybów z rodzaju Fusarium z nasion niektórych gatunków roślin uprawnych metodą tradycyjnąa i BIO-PCR. Acta Agrobot. 2005;58:33–54. doi: 10.5586/aa.2005.032. [DOI] [Google Scholar]
- 14.Gleń K., Boligłowa E., Gospodarek J. Grzyby zasiedlające nasiona bobu w zależności od sposobu ochrony roślin. Pol. J. Agron. 2013;12:9–16. [Google Scholar]
- 15.Podleśny J., Podleśna A., Bieniaszewski T. Occurrence of fungal diseases on blue lupine (Lupinus angustifolius L.) plants at different regions of PolandWystępowanie chorób grzybowych na roślinach łubinu wąskolistnego (Lupinus angustifolius L.) w różnych rejonach Polski. Prog. Plant Prot. 2016;56:25–33. [Google Scholar]
- 16.Clarkson J.D.S. Pathogenicity of Fusarium spp Associated with Foot-Rots of Peas and Beans. Plant Pathol. 1978;27:110–117. doi: 10.1111/j.1365-3059.1978.tb01093.x. [DOI] [Google Scholar]
- 17.Elwakil M., El-Refai I., Awadallah O., El-Metwally M., Mohammed M. Seed-borne pathogens of faba bean in Egypt: Detection and pathogencity. Plant Pathol. J. 2009;8:90–97. doi: 10.3923/ppj.2009.90.97. [DOI] [Google Scholar]
- 18.Belete E., Ayalew A., Ahmed S. Associations of biophysical factors with faba bean root rot (Fusarium solani) epidemics in the northeastern highlands of Ethiopia. Crop Prot. 2013;52:39–46. doi: 10.1016/j.cropro.2013.05.003. [DOI] [Google Scholar]
- 19.Neamat A.K., Abbas M.S., Sobhy H.M., Abou-Zeid N.M., Mahmoud N.A. Induction of Systemic Resistance in Faba Bean Plants against Fusarium oxysporum the Causal of Wilt Disease. Egypt. J. Biol. Pest Control. 2016;26:431–438. [Google Scholar]
- 20.Miličević T., Kaliterna J., Ivić D., Stričak A. Identification and occurrence of Fusarium species on seeds of common wetch, white lupine and some wild legumes. Poljoprivreda. 2013;19:25–32. [Google Scholar]
- 21.Liu C.M., Kachur S., Dwan M.G., Abraham A.G., Aziz M., Hsueh P.R., Huang Y.T., Busch J.D., Lamit L.J., Gehring C.A., et al. FungiQuant: A broad-coverage fungal quantitative real-time PCR assay. BMC Microbiol. 2012;12:255. doi: 10.1186/1471-2180-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waalwijk C., van der Heide R., de Vries I., van der Lee T., Schoen C., Costrel-de Corainville G., Hauser-Hahn I., Kastelein P., Kohl J., Lonnet P., et al. Quantitative detection of Fusarium species in wheat using TaqMan. Eur. J. Plant Pathol. 2004;110:481–494. doi: 10.1023/B:EJPP.0000032387.52385.13. [DOI] [Google Scholar]
- 23.Bilska K., Kulik T., Ostrowska-Kolodziejczak A., Busko M., Pasquali M., Beyer M., Baturo-Ciesniewska A., Juda M., Zaluski D., Treder K., et al. Development of a Highly Sensitive FcMito qPCR Assay for the Quantification of the Toxigenic Fungal Plant Pathogen Fusarium culmorum. Toxins. 2018;10:211. doi: 10.3390/toxins10050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolaisen M., Supronien S., Nielsen L.K., Lazzaro I., Spliid N.H., Justesen A.F. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Methods. 2009;76:234–240. doi: 10.1016/j.mimet.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Kulik T., Ostrowska A., Busko M., Pasquali M., Beyer M., Stenglein S., Zaluski D., Sawicki J., Treder K., Perkowski J. Development of an FgMito assay: A highly sensitive mitochondrial based qPCR assay for quantification of Fusarium graminearum sensu stricto. Int. J. Food Microbiol. 2015;210:16–23. doi: 10.1016/j.ijfoodmicro.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Kulik T., Jestoi M., Okorski A. Development of TaqMan assays for the quantitative detection of Fusarium avenaceum/Fusarium tricinctum and Fusarium poae esyn1 genotypes from cereal grain. FEMS Microbiol. Lett. 2011;314:49–56. doi: 10.1111/j.1574-6968.2010.02145.x. [DOI] [PubMed] [Google Scholar]
- 27.Kulik T. Development of TaqMan Assays for 3ADON, 15ADON and NIV Fusarium Genotypes Based on Tri12 Gene. Cereal Res. Commun. 2011;39:200–214. doi: 10.1556/CRC.39.2011.2.4. [DOI] [Google Scholar]
- 28.Kulik T., Pszczolkowska A., Lojko M. Multilocus Phylogenetics Show High Intraspecific Variability within Fusarium avenaceum. Int. J. Mol. Sci. 2011;12:5626–5640. doi: 10.3390/ijms12095626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haese A., Schubert M., Herrmann M., Zocher R. Molecular Characterization of the Enniatin Synthetase Gene Encoding a Multifunctional Enzyme Catalyzing N-Methyldepsipeptide Formation in Fusarium-Scirpi. Mol. Microbiol. 1993;7:905–914. doi: 10.1111/j.1365-2958.1993.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 30.Bilska K., Jurczak S., Kulik T., Ropelewska E., Olszewski J., Zelechowski M., Zapotoczny P. Species Composition and Trichothecene Genotype Profiling of Fusarium Field Isolates Recovered from Wheat in Poland. Toxins. 2018;10:325. doi: 10.3390/toxins10080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leslie J.F., Summerell B.A. The Fusarium Laboratory Manual. 1st ed. Blackwell Publishing; Ames, IA, USA: 2006. [Google Scholar]
- 32.Villani A., Proctor R.H., Kim H.-S., Brown D.W., Logrieco A.F., Amatulli M.T., Moretti A., Susca A. Variation in secondary metabolite production potential in the Fusarium incarnatum-equiseti species complex revealed by comparative analysis of 13 genomes. BMC Genom. 2019;20:314. doi: 10.1186/s12864-019-5567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartman G.L., McCormick S.P., O’Donnell K. Trichothecene-Producing Fusarium Species Isolated from Soybean Roots in Ethiopia and Ghana and their Pathogenicity on Soybean. Plant Dis. 2019;103:2070–2075. doi: 10.1094/PDIS-12-18-2286-RE. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs A., Mojela L., Summerell B., Venter E. Characterisation of members of the Fusarium incarnatum-equiseti species complex from undisturbed soils in South Africa. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2018;111:1999–2008. doi: 10.1007/s10482-018-1093-x. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert J., Fernando W.G.D. Epidemiology and biological control of Gibberella zeae Fusarium graminearum. Can. J. Plant Pathol. -Rev. Can. De Phytopathol. 2004;26:464–472. doi: 10.1080/07060660409507166. [DOI] [Google Scholar]
- 36.Ruralis E., Chancellor C., Attila S.B.M., Duminicioiu R. The Trouble with Soy: The Threats to Small-Scale Producers Across Europe. European Coordination Via Campesina (ECVC) and Eco Ruralis; Cluj Napoca, Romania: 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.