Abstract
Yellow fever virus (YFV) represents a re-emerging zoonotic pathogen, transmitted by mosquito vectors to humans from primate reservoirs. Sporadic outbreaks of YFV occur in endemic tropical regions, causing a viral hemorrhagic fever (VHF) associated with high mortality rates. Despite a highly effective vaccine, no antiviral treatments currently exist. Therefore, YFV represents a neglected tropical disease and is chronically understudied, with many aspects of YFV biology incompletely defined including host range, host–virus interactions and correlates of host immunity and pathogenicity. In this article, we review the current state of YFV research, focusing on the viral lifecycle, host responses to infection, species tropism and the success and associated limitations of the YFV-17D vaccine. In addition, we highlight the current lack of available treatments and use publicly available sequence and structural data to assess global patterns of YFV sequence diversity and identify potential drug targets. Finally, we discuss how technological advances, including real-time epidemiological monitoring of outbreaks using next-generation sequencing and CRISPR/Cas9 modification of vector species, could be utilized in future battles against this re-emerging pathogen which continues to cause devastating disease.
Keywords: yellow fever virus, flavivirus entry factor, global diversity, yellow fever virus tropism, animal models, transmission and vector control, vaccine, host immune response, E protein structure, re-emerging virus
1. General Introduction
Yellow fever was historically the first described viral hemorrhagic fever (VHF) and its causative agent, the yellow fever virus (YFV), is the prototypic member of the family Flaviviridae. Pioneering experimental work in the 19th century identified the Aedes aegypti mosquitos as the vector responsible for transmission to humans, facilitating subsequent eradication from Europe and North America [1,2,3,4]. However, today, YFV remains prevalent in tropical regions of Africa and South America [4,5], with over 900 million people currently living in endemic areas, 500 million of whom reside in Africa [6]. YFV represents a zoonotic pathogen and complete eradication in tropical regions is not achievable due to rainforest circulation in primate reservoirs [4,6,7]. A highly effective live-attenuated vaccine, the 17D strain, was developed in the 1930s and still in use today, over 80 years later [3,6,8]. Indeed, vaccination represents one of the core strategies employed to stop YFV outbreaks or minimize their impact when they do occur [9].
The Flaviviridae is a diverse group of small enveloped viruses representing over 100 species [10]. Viral genomes from Flaviviridae members are non-segmented positive-stranded RNAs ranging from ~9 to ~13 kb in length, and phylogenetic analyses of 125 family members revealed four well-supported genera: Pestivirus, Hepacivirus, Pegivirus and Flavivirus (Figure 1 and [10]). Members of the Flaviviridae which cause a significant disease burden in humans are highlighted on the tree presented in Figure 1. Genome organization and cytoplasmic replication on endoplasmic reticulum (ER) membranes are conserved between divergent Flaviviridae members. However, host-range, tissue-tropism, pathogenicity and modes of transmission can differ greatly [10]. YFV is a member of the Flavivirus genus, which is composed of arboviruses exhibiting broad host ranges which can be transmitted to humans from animal reservoirs via insect vectors. This genus contains a number of important human pathogens which can cause acute infections, including dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV) and Zika virus (ZIKV) (Figure 1). Transmission is mediated via mosquito vectors and infection of humans can cause a range of clinical outcomes, ranging from asymptomatic to encephalitis (WNV, JEV and ZIKV) or fatal hemorrhagic fever (YFV and DENV).
Figure 1.
Phylogenetic relationships within the Flaviviridae. The evolutionary tree presented was generated using MEGA7 [11] from a conserved region of the viral RNA dependant RNA polymerase (RdRp: NS5 or NS5B) and represents n = 125 members of the Flaviviridae. The in-frame nucleotide alignment was downloaded from https://talk.ictvonline.org/, adjusted manually, and all positions containing gaps or missing data were eliminated prior to analyses (734 sites in the final dataset). The resulting phylogeny was inferred via the Maximum Likelihood Composite method under the GTR+I+Γ model of nucleotide substitution to account for evolutionary rate differences among sites. The genera Pegivirus, Hepacivirus, Pestivirus and Flavivirus are color-coded, in addition to distinct lineages within the genus Flavivirus. Statistical robustness of highlighted groupings was determined using the bootstrap approach and values assigned to these branches are percentages derived from 500 replications. Branch lengths are proportional to the scale bar (units: substitutions per site).
YFV continues to pose a very real threat to public health, which has been starkly illustrated by recent outbreaks in Angola in 2015–2016 [1,12,13] and Brazil in 2016–2017 [14,15,16], and associated imports into non-endemic regions [15,17]. This is compounded by a limited vaccine production, which can easily be overwhelmed by a large YFV outbreak, and the collapse of vector control programs [1,14,15], coupled with large populations of unvaccinated individuals in endemic regions and a lack of effective antivirals for treatment of infected patients [18]. Indeed, despite long-standing knowledge of YFVs genomic architecture, favorable growth kinetics in tissue culture and the availability of reverse genetic systems [19,20,21,22,23,24], YFV remains a neglected tropical disease attracting relatively little research attention [25]. This is, in part, due to vaccine availability, which has led to research complacency [3], and recent commentaries have highlighted the need for intensification of YFV research, as many facets of the virus life cycle remain poorly defined [26,27]. These include determination of vertebrate and arthropod host-ranges, identification of co-opted host factors which are essential for YFV propagation in humans and insects, and identification of genetic markers and associated mechanisms underlying differential pathogenesis and observed clinical outcomes in infected humans.
2. Clinical Outcomes and Potential Therapeutic Options
Infection with YFV has a broad spectrum of clinical manifestations ranging from entirely asymptomatic to hemorrhagic fever associated with high mortality rates. Indeed, estimates indicate only one symptomatic case for every 7–12 cases that remain clinically silent [5]. In the majority of symptomatic cases, YFV infection is a self-limiting disease with abrupt onset of fever and pain (predominantly myalgia, lumbosacral pain and headache with associated conjunctival injection) that occurs after an incubation period of 3–6 days. In this phase, YFV infection is difficult to diagnose, as symptoms are unspecific. The duration of fever is approximately three days, during which the patient is usually viremic [28]. Laboratory abnormalities during this “period of infection” include elevated liver enzymes, leukopenia and proteinuria. A phase of improvement, lasting up to two days, can be interposed but does not occur in all cases. In around 15% of patients, the disease progresses to a “toxic phase”, which is characterized by the return of fever, nausea, vomiting, epigastric pain and renal insufficiency [18]. This is characterized by systemic viscerotropic disease and liver injury is a hallmark of this phase [29], leading to jaundice and contributing to bleeding diathesis, which may lead to more severe hemorrhaging. It is not well understood why this phase occurs in some patients although host adaptive immune responses are believed to contribute to the severity [5], in addition to underlying genetic factors which predispose Caucasians to a higher mortality rate [30]. The case fatality rate of hospitalized patients ranges between 25% and 50% and is correspondingly higher in South America compared to Africa [31]. Mortality rates may depend on the virulence of the infecting strain, as well as the underlying intrinsic immunity and genetic factors of the exposed population. In addition, cross-immunity to other flaviviruses, such as DENV, is believed to reduce the severity of YFV infections [5,32]. Patients who survive the disease may suffer from fatigue and may have elevated liver enzymes for extended periods of time [29].
Vaccination campaigns represent the only current approach to control and prevent the spread of YFV in endemic regions (see Section 6). Indeed, despite the long history of humankind being afflicted by YFV and the large populations in tropical Africa and Central and South America at risk of potentially catastrophic epidemics, today, treatment options for infected individuals are limited to relief of symptoms and intensive care support of patients with severe disease. Presently, no specific licensed treatment options for YFV-associated severe disease are available. As development of new drugs is expensive and time-consuming, one potential option includes repurposing drugs already licensed for other diseases. This includes drugs targeted at other members of the family Flaviviridae, most prominently hepatitis C virus (HCV) and DENV. Ribavirin, a nucleoside analog, in combination with pegylated interferon-α (PEG IFN-α), is effective for the treatment of HCV, a related member of the family Flaviviridae (see Figure 1), as well as a variety of other RNA and DNA viruses. In vitro studies and mouse models yielded promising results against YFV, but these results could not be replicated in non-human primates [33]. More recently, considerable efforts have been invested in the development of direct-acting antivirals (DAAs) against HCV and DENV. Sofosbuvir, a DAA licensed for use against HCV, is a nucleotide analog inhibitor of the viral RdRp NS5B. It also has described antiviral activity against the related flaviviruses, ZIKV [34] and DENV [35]. Computer modelling and in vitro experiments in hepatoma cell lines demonstrated sofosbuvir’s inhibitory effects on YFV replication: in a mouse model, sofosbuvir was able to decrease mortality and weight loss associated with liver injury, however, only in the case of pre-exposure treatment [36]. A varying efficacy against YFV has also been demonstrated for other compounds targeting the NS5 RdRp [37], the NS3 helicase [38] and capsid protein [39]. Screening compound libraries via high-throughput in vitro approaches were successful at identifying additional compounds with in-vitro activity against YFV. Patkar et al. (2009) identified two compounds with proposed activity against the NS4B protein [40] and Guo et al. (2016) were able to successfully apply a benzodiazepine compound (BDAA) in a hamster model of YFV infection, where it protected 90% of animals from death via targeting of NS4B [41]. Using RNA interference (RNAi) technology, shRNAs (short hairpin RNAs) targeted at the YFV E and NS1 regions were effective in inhibiting YFV replication in cell lines as well as improving survival in mice when injected intracerebrally prior to exposure to the YFV-17D strain [42]. However, for all the approaches outlined above, demonstration of efficacy in humans or non-human primates remains outstanding.
The host immune response is a believed to contribute to the severity of the “toxic phase”, but approaches targeting the host’s immune response in severe YFV disease are lacking. When patients present with severe symptoms, the therapeutic window of IFNs and other immune modulators may have already passed [43]. The potential benefits of corticosteroids [18] or the therapeutic application of anti-YFV antibodies [44] (see Section 3.1) have not been evaluated but represent promising avenues for further investigation. In summary, many potential candidates for treatment of YFV-induced symptoms have been identified, although none are currently licensed. The toxicity and efficacy of promising anti-YFV therapeutics should be further investigated in animal studies and clinical trials.
3. The Replication Cycle of YFV
The replication cycle of YFV is defined by multiple distinct stages, which are shared between divergent members of the Flaviviridae. These include (a) attachment of the viral particle to target cells, (b) interaction with surface receptors for internalization, (c) fusion of viral and endosomal membranes and release of the nucleocapsid into cytoplasm, (d) disintegration of the nucleocapsid, (e) translation and replication of the viral genome, (f) assembly and maturation of viral particles and (g) release of nascent virions [45]. Currently, many of these stages are poorly defined for YFV, especially with respect to identification of co-opted host factors, which are essential at every stage of the life cycle. Additionally, how these processes differ between mosquitos and humans requires further investigation.
3.1. YFV Virion Composition and E Protein Functions
The YFV virion is spherical in shape, with a 40–60 nm diameter [10,46]. The virion is composed of host-hijacked lipid membranes associated with three virally encoded structural proteins. These include the highly basic core protein (C) which binds and encapsidates the YFV RNA genome [47] and two envelope glycoproteins: prM/M (membrane) and E (envelope). The transmembrane domains of prM and E act as membrane anchors and also represent ER retention signals [48]. The E protein of YFV represents a class II fusion protein, which possesses structural homology with the ectodomains of the envelope proteins from divergent arboviruses (related flaviviruses and unrelated alphaviruses), despite no obvious amino acid conservation [49]. The E protein facilitates YFV entry into permissive cells, mediating the interaction between the virus and cell surface receptors, and also elicits host neutralizing antibody responses. YFV E consists of three domains: DI, DII and DIII (see Figure 2A, upper panel). In the pre-fusion state, the E protein forms dimeric structures. DII contains a highly conserved 16 amino acid fusion peptide motif (DRGWGNGCGLFGKGSI) (Figure 2, highlighted pink) [50] and can be found in a parallel orientation to the virion’s lipid envelope in the pre-fusion state (See Figure 2A, lower panel). DIII harbors epitopes essential for neutralization of YFV [51,52]. However, antibody repertoire cloning from YFV patients also identified key neutralizing epitope residues in domains I (residue 71) and II (residues 153-5), which likely combine to form a conformational epitope [53].
Figure 2.
YFV E protein structures with 17D attenuating mutations and fusion peptide highlighted. (A) The structure of the YFV-17D E protein pre-fusion monomer is presented with DI, DII and DIII highlighted in different colors. YFV-17D-attenuating mutations are highlighted on the E structure in orange while the fusion peptide is highlighted in pink. Pre-fusion E dimers are presented directly below. (B) The structure of the YFV-17D E protein post-fusion monomer is presented with DI, DII and DIII, highlighted in different colors. Again, attenuating mutations are highlighted on the structure in orange while the fusion peptide is highlighted in pink. Post-fusion E trimers are positioned directly below with both top (left) and side (right) views presented. The YFV E structures presented were previously described in [54] (PDB IDs: 6IW1 and 6IW4) and the presented figures were generated in UCSF Chimera [55].
The potent anti-E monoclonal antibody 5A identified in [53] was able to neutralize divergent YFV strains (YFV-17D and Angola lineage) [54,56]. The mAb 5A recognizes a conserved region within DII, which is accessible in both the pre- and post-fusion states of the virus. It is suggested that mAb 5A interferes at three stages during YFV infection: (A) by inhibition of virus attachment due to modifying the spatial distance between the E protein and host receptors; (B) by entering early endosomes and inhibiting the “dimer-to-trimer transition” of the E protein by blocking membrane fusion and therefore, blocking the release of nucleocapsid into the cytoplasm and; (C) by binding the E protein trimers in their post-fusion state [54]. Structural and functional studies have suggested conformational change from a dimeric to a trimeric state, which is induced by acidification of endosomes (see Figure 2B) and leads to a release of the nucleocapsid into the cytoplasm via E protein-mediated fusion of virus and endosomal membranes [57,58]. In the post-fusion state, the fusion peptide is located at the apex of the trimer (see Figure 2B, lower panels).
To date, only the structure of the immature YFV particle has been resolved, with the architecture of mature virions containing a processed (pr)M protein still outstanding [59]. By facilitating virus entry into target cells and determining the global structure of the virion, the YFV E is essential for infection. Thus, it represents an ideal target for an intervention at an early state of infection, e.g., using a structure-based drug design or E-targeted monoclonal antibody (mAb) therapies [44]. Antiviral mAbs represent viable therapeutic options, including palivizumab (marketed as Synagis), which is licensed for use against RSV in humans [60], and a number of anti-HIV mAbs in clinical trials [61,62]. In most cases, therapeutic mAbs interfere with virus entry via targeting the envelope glycoprotein(s), thus preventing further dissemination. However, currently, while mAb based therapies have proven effective, they are also expensive, which limits their widespread application in the resource constrained settings where YFV epidemics often occur.
3.2. YFV Entry: Implicated Host Factors
Out of the three domains of the YFV E protein, DIII is suspected to mediate interaction with cellular receptors during infection [45]. While flavivirus entry into target cells is incompletely defined, many host molecules have been implicated in the process. These include CD209 and the mannose receptor for DENV entry [63,64] and G protein-coupled receptor kinase 6 (GRK6) for WNV entry [65]. The related GRK2 was reported to both promote YFV entry and enhance viral RNA synthesis, properties which were conserved across key members of the Flaviviridae, including DENV and HCV [66]. The transmembrane immunoglobulin and mucin domain (TIM) and TAM (Tyro3, Axl and Mer) families of phosphatidylserine receptors have been shown to mediate DENV infection, with TIM-1 and TIM-4 also enhancing infection rates for WNV and YFV-17D to differing degrees, while herpes simplex virus-1 (HSV-1) infection rates were unaffected [67]. Therefore, these cellular factors represent general enhancers of flavivirus infection. Additionally, candidates implicated in YFV host-cell attachment include heparin sulfate, a member of the glycosaminoglycan (GAG) family, which also facilitates attachment for the related flaviviruses DENV [68] and tick-borne encephalitis virus (TBEV) [69], as well as unrelated viruses, including HSV and human immunodeficiency virus (HIV) [70,71]. Indeed, the antiviral effect of PI-88, a heparin sulfate mimetic, was demonstrated against DENV and neurotropic flaviviruses [72]. Despite reports of multiple factors which are potentially involved in YFV entry, it is still unknown whether a ubiquitous cellular receptor is responsible for facilitating flavivirus infection of permissive cells [73]. Similarly to DENV, YFV is able to infect a range of cell-types from diverse host species, indicating either binding to a ubiquitous cell-surface receptor, or the ability to engage multiple distinct receptors [74]. RNAi screening has revealed many potential host factors influencing WNV or DENV infection of human or drosophila cell-lines [65,75]. Analogous to mammalian CD209, an Aedes aegypti C-type lectin, mosGCTL-7, has been described as an entry factor for JEV [76]. Previous studies already identified mosGCTL-1 and mosPTP-1 as cascade receptors facilitating flaviviral entry in mosquitoes [77]. Thus, distinct flaviviruses may use conserved entry factor(s) for entry into permissive cells in mosquito vector species.
Divergent entry mechanisms have been observed for even closely related YFV strains. The parental Asibi strain and the derived YFV-17D vaccine strain both infect permissive cells by a pH-dependent fusion mechanism, although these related viruses enter via distinct routes [78]. Both strains enter in a dynamin-2 dependent fashion and traffic through Rab5- and Rab11-positive early and recycling endosomes. However, the wild-type Asibi strain enters via clathrin-mediated endocytosis, while YFV-17D utilizes a clathrin-independent route. YFV-17D also exhibits enhanced binding and entering capabilities in vitro, and initiates inflated antiviral responses when compared to its wild-type progenitor [78]. These divergent properties can be pin-pointed to eleven mutations within the E protein’s primary amino acid sequence (see Figure 2), which occurred during long-term passaging of the Asibi strain in mouse brains and chicken embryos to generate the attenuated YFV-17D derivate (see Section 6) [20]. Of note, a T380R mutation in the present E-DIII vaccine strains creates a RGD motif, a known integrin binding site [78,79,80], and is part of a heparin sulfate binding domain: increased heparin sulfate affinity is known to be important for viral spread and attenuation [68,78]. In summary, mechanistic insights into the YFV infection process, in addition to many host factors implicated in YFV entry, have been described. Nevertheless, identification of bona fide receptor(s) integral for YFV entry remains outstanding. The entry cascade of YFV is comprised of multiple steps which could be targeted by therapeutic intervention to inhibit further virus spreading within infected patients. For example, targeting the HCV entry factor CLDN1 via a mAb inhibited viral entry and promoted viral clearance in a humanized mouse model [81].
3.3. Polyprotein Processing, RNA Replication and Virion Maturation
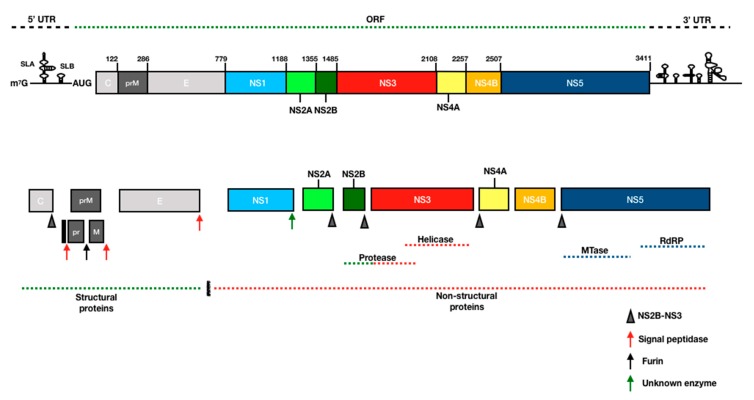
After entry and uncoating, viral RNA is transported to the endoplasmic reticulum (ER) and translated as a single polyprotein precursor. Host factors RPLP1 and RPLP2, phosphoproteins that bind to ribosomes, are essential for efficient ribosomal translation of the YFV polyprotein and have panflaviviral activity [82]. The YFV polyprotein is post-translationally modified by cellular glycosyltransferases, and the liberation of ten mature viral proteins is mediated by both host and viral proteases [83,84]. The processed polyprotein produces three structural proteins which comprise the virion (see above) and seven nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [85] which are involved in viral RNA replication on ER membranes (Figure 3 and Table S1). After translation of the genome, a domain of the RdRp NS5 binds to a promotor sequence on the stem-loop A (SLA) at 5’ end of 5’ untranslated region (UTR) to promote the synthesis of uncapped negative-sense RNA, which is produced at low levels from the positive-sense RNA template [86,87]. Flavivirus positive-stranded RNA genomes are similar to vertebrate mRNAs, although they differ in critical aspects: they possess a methylated type 1 cap structure at the 5′-terminus (me7-GpppA-me2) and lack a polyadenylation signal at the 3′-terminus. The type 1 cap is synthesized by virally encoded proteins: an NS3-encoded RNA triphosphatase [88] and the two NS5-encoded enzymes: methyltransferase [89,90] and guanylyltransferase [91]. NS3 and NS5 possess additional functions and constitute the core of the flaviviral RNA replication complex [85] which occurs on ER membranes [92]. Indeed, DENV NS3 and NS5 were identified on the virus-induced membrane vesicles of the ER, which are open to the cytoplasm [93]. A serine protease encoded at the N-terminus of NS3 is involved in cleavage of multiple NS protein junctions [94] and requires the NS2B cofactor for its activity [95]. NS3 also possesses a C-terminally encoded NTPase/RNA helicase to remove viral RNA secondary structures and double-strand RNA (dsRNA) intermediates, generated by NS5 during virus replication [96]. The flavivirus NS5 protein encodes the viral RdRp, synthesizing negative-stranded RNA, which forms a double-stranded complex with the genomic template [97]. Experiments with DENV demonstrated that the phosphorylation of NS5 controls the interaction with NS3 [98]. Furthermore, mutation of the phosphorylation site on YFV NS5 (S56) disrupted the capping process via inhibition of the methyltransferase activity and inhibited viral replication [99]. NS1, NS2A, NS4A and NS4B represent hydrophobic membrane proteins which modify the structure of the ER, creating a scaffold that anchors the replicase complex during virus assembly, although their precise functions remain unclear [100,101,102,103,104,105,106].
Figure 3.
Schematic representation of the YFV genome and encoded polyprotein. The genome of YFV is a single-stranded positive-stranded RNA of approximately 11kb encoding a single ORF containing three structural proteins (C, prM, and E) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). The genome has methylated type 1 cap at the 5′ end and is not polyadenylated. Secondary structures (stem-loop A and B; SLA/SLB) in the 5′ and 3′ UTR are required for translation and RNA synthesis. The polyprotein is post-translationally modified by viral and host proteases into 10 mature proteins which are incorporated into the ER membrane.
Flaviviral replication is coupled with the process of assembly [107]. Open pores on the lumen of virus-induced ER membrane vesicles allow spatial coordination between the replication and assembly processes [108], facilitating association of newly synthesized viral RNA with structural proteins to produce immature virions during virion assembly [109,110]. The viral lipid-envelope is acquired from the host cell by budding from the lumen of the ER [111] and virion maturation occurs through trans-Golgi network trafficking to the cellular surface, where furin cleavage of the pr peptide from the M protein produces a rearrangement of the E protein [112], generating infectious virions that are fusogenic under low pH conditions [27]. Viral replication, assembly, and maturation, as well as host factors essential for viral propagation, are potential targets for the development of anti-YFV drugs. However, a better understanding of determinants of viral replication and the virus-host interactome are required for YFV: many of the critical steps of the flavivirus replication cycle are described for DENV or WNV, but confirmation with YFV is lacking.
4. Host Responses to YFV Infection
4.1. Cytokine Induction by YFV
Using paraffin-embedded liver tissue from patients who succumbed to fatal YFV disease, it was demonstrated that CD4+ T cells represent the main infiltrating cell population into liver tissue, with a high proportion of cells expressing TGF-β and, to a lesser extent, TNF-α and IFN-γ [113]. YFV-induced apoptosis markers were prevalent in infected hepatocytes, indicating that apoptosis, rather than necrosis, is the primary cell-death mechanism in YFV-infected livers [113]. With respect to serum markers, higher concentrations of IL-6, MCP-1, IP-10, TNF-α, and IL-1RA were detected in human sera from YFV-infected patients with fatal outcomes when compared to sera from non-fatal outcomes [114]. Vaccination with YFV-17D also increased the production of TNF-α, IL-1RA and IL-6 in plasma at 2 and 7 days post-vaccination [115]. Together, these results indicate that induction of pro- and anti-inflammatory cytokines is associated with the immunopathology of YFV infection and responses to YFV-17D vaccination.
4.2. Cellular Responses to YFV
Dendritic cells (DCs) are essential antigen-presenting cells, recognizing pathogens by toll-like receptors (TLRs) to trigger maturation and the induction of adaptive immunity and immunological memory. The activation of DCs by YFV-17D through TLR 2, 7, 8 and 9 was shown to induce a broad range of innate and adaptive responses [116]. Activated plasmacytoid dendritic cells (pDC) rapidly secrete large amounts of type I-IFNs to modulate effector molecules and cell populations of the innate and adaptive immune system. YFV also was shown to induce the production of IFN-γ and type III-IFNs from pDCs via interaction with TLR7 or cell contact, and immature virions were more efficient at stimulating pDCs than mature virions [117]. However, YFV also possesses the ability to antagonize the IFN system. YFV NS5 interacts with STAT2 when STAT1 is phosphorylated during type I IFN signaling: the E3 ubiquitin ligase TRIM23 polyubiquinates NS5, mediating STAT2 binding and subsequent inhibition of IFN signaling [118]. Human and rhesus macaque monocyte-derived dendritic cells (MoDC) and macrophages (MDM) were also shown to be susceptible to infection with YFV wild-type Asibi and 17D vaccine strains, with differences in cytokine responses to infection observed between strains. Additionally, only MoDCs infected with YFV-17D could initiate IFN-γ and IL-2 expression in CD4+ T cells, confirming differential patterns of immune induction between attenuated and wild-type YFV [119]. Natural killer (NK) cells represent an essential component of innate immune responses, shaping adaptive and antiviral immunity [120]. NK cells were shown to be the highest producers of IFN-γ, which protects against viscerotropic disease in YFV-17D infection but not for wild-type virus, indicating that YFV vaccine attenuation results in increased sensitivity to IFN-γ [121]. Replication of YFV-17D in NK cells was enhanced by STAT1 knockout, although NK cells do not appear to be a major reservoir for YFV replication [122]. After YFV-17D vaccination, NK expression of TLR3, TLR9 and CD69 increases, concomitant with increased circulating IFN-γ [123] and 17D-YFV infection stimulated vigorous early NK cell responses in vivo primed by type I and III IFNs [124].
4.3. Pattern Recognition Receptors and YFV
In addition to TLRs, the pattern recognition receptors (PRRs) RIG-I, MDA5 and LGP2, which form part of the RIG-I like receptor (RLR) family, are critical for initiating the IFN response to infection via recognition of intracellular viral RNA [125]. While RIG-I recognizes dsRNA, which possesses a triphosphate and U or A-rich motifs, MDA5 preferentially recognizes long dsRNAs, [126]. Both RIG-1 and MDA5 have important roles in restricting flavivirus infections ([127] as reported for DENV [128], WNV [129] and JEV [130]), interacting with both MAVS and STING to mediate their antiviral effects [131]. However, viral genome modifications can prevent RIG-I recognition, with cap1 2′O-methylation of YFV RNA constituting an RIG-I evasion mechanism [132]. Cyclic GMP-AMP synthetase (cGAS) is a cytoplasmic DNA sensor, synthesizing, 2′3′-cGAMP from ATP and GTP to mediate the activation of STING. After TBK1-mediated phosphorylation of IRF3, nuclear translocation of IRF3 and NF-κB induces type I-IFN and cytokine expression [133]. In addition to cytoplasmic DNA, cGAS has also been implicated in the recognition of RNA viruses, including flaviviruses: cGAS knockout mice were more susceptible to lethal WNV infection outcomes when compared to wild-type mice [134], and DENV is able to target cGAS for degradation via NS2B, inhibiting IFN production [135,136]. YFV NS4B exhibits strong homology with the C-terminus of STING and can inhibit its activity [137]. However, while human STING can be targeted for cleavage by ZIKV, DENV, WNV and JEV proteases, it is not susceptible to cleavage by either YFV-17D or YFV-Asibi proteases [138]. Finally, nucleotide-binding and oligomerization domain (NOD) like receptors (NLRs), are cytoplasmic receptors that trigger the engagement of inflammasomes and signalosomes after the detection of viral danger signals. This occurs via activation of caspase-1, which mediates maturation of pro-inflammatory cytokines (e.g., IL-1β and IL-18) and secretion of the alarmin HMGB1 [139]. While inflammasome activation has been reported for WNV and DENV infection [126], the exact danger signals detected and its significance in YFV infection remains unclear. Together these studies implicate RLRs and the cGAS-STING axis as important modulators of YFV infection, although further investigation is required. Additionally, whether inflammasome activation is mediated by YFV replication warrants further study.
4.4. Anti-YFV Effectors
The mammalian chaperone protein DNAJC14 was demonstrated to confer protection from YFV-mediated cell-death via inhibition of YFV replication and possesses broad activity against diverse Flaviviridae members [140]. This factor is constitutively expressed and not IFN-inducible. The breadth and antiviral potency of over 380 human interferon-stimulated genes (ISGs) were tested against a panel of important pathogenic viruses, including YFV, using lentiviral overexpression systems in different cellular backgrounds [141]. This comprehensive study identified antiviral effectors which inhibited YFV replication, including IRF1, TREX1, IFITM3, RTP4, IFI6 and HPSE: in contrast, the IFN regulated genes APOBEC3A, FAM46C, IDO1, LY6E, MCOLN2 and ADAR genes enhanced YFV replication [141]. More recently, CRISPR/Cas9 knockout genetic screening confirmed IFI6 as an IFN-induced gene with anti-YFV activity and provided mechanistic insight into its mode of action [142]. IFI6 has broad activity against Flaviviridae members but minimal activity against other viruses, interfering with flaviviral proteins when they invaginate ER membranes through interaction with the ER-resident heat shock protein 70 chaperone BiP [142]. Together, these results point to the complexity of the IFN system: expression of both pro- and anti-YFV effector genes are modulated by the type I IFN response. In addition, some genes have panflaviviral potency, while others are virus specific. The contribution of patient and tissue-specific differences in ISG expression to viral clearance and the correlation between ISG induction and differential disease outcomes is currently unclear.
5. Tropism of YFV
In humans, after transcutaneous inoculation, YFV exhibits broad patterns of tissue tropism and is disseminated systemically via infection of multiple white cell subsets. With respect to organ tropism, the liver is the primary target, although the heart, kidneys, liver, spleen, and less frequently, the brain tissue, can support YFV replication [18]. The natural host range of YFV includes humans, non-human primates and mosquitos. Additionally, wild animals from the diverse orders Artiodactyla, Carnivora and the superorder Xenarthra have been reported to be serologically positive for anti-YFV antibodies, and the elicitation of antibody responses against YFV indicates a potentially productive infection within these species, indicating that diverse mammalian species could represent YFV potential reservoirs [143] (see Section 8.3).
Mouse Models for YFV
YFV research is currently limited as it remains challenging and expensive to establish workflows with non-human primates. While YFV has a limited host species-tropism, artificial infection of laboratory mice and hamsters is possible, although the course of disease differs from that observed in humans [144]. Nevertheless, a number of studies in genetically modified or humanized mice have yielded insights into YFV infection and dissemination in vivo. Single cell-tracking of YFV-17D dissemination in different mouse models identified novel YFV replication reservoirs in secondary lymphoid compartments, with hematopoietic STAT1 knockout increasing virus-induced pathogenicity [122]. Double-humanized mice have been developed, possessing both human immune systems and hepatocytes [145,146], and these animals represent ideal tools for monitoring systemic YFV infection in vivo as both compartments are permissive for YFV. In addition to hepatotropism, YFV can also be neurotropic in rodents, primates and humans [147,148]. In contrast to polio virus and reovirus, which infect the brain via the CNS, YFV-17D infects brain tissue by crossing the blood-brain barrier [149]. A mouse-neurovirulent strain (SPYF) based on the Porterfield 17D (PYF17D) strain was derived by additional passaging in SCID mice, exhibiting enhanced neurovirulence and neuroinvasiveness. This enhanced neurotopism was correlated with 13 amino acid substitutions, five of which were located in the E protein [80]. Arboviruses can encounter dissemination barriers in mosquitos, restricting the spreading of the virus within the vector species. These include innate immunity related pathways as well as physical tissue barriers associated with the midgut and salivary glands [150,151]. However, while patterns of YFV-17D dissemination in immunocompetent mice were restricted, infection of IFNAR knockout mice resulted in broad dissemination preceding viral clearance, indicating that no barriers to dissemination exist in the absence of type I IFN [152]. Furthermore, while I and II type IFNs contribute to control of YFV-17D infection, type III IFN was demonstrated to have immunomodulatory functions and was protective against YFV-17D neuroinvasion in mouse models [153]. YFD-17D infection of IFN-α/β receptor and STAT1 signaling molecule deficient mice resulted in systemic viscerotropic infection: a course of disease which resembles that observed in humans rather than the typical encephalitic disease course commonly reported for mice [154]. Finally, while many flaviviruses share the common ability to antagonize IFN signaling and therefore enhance viral replication, the YFV NS5 is unable to bind murine STAT2 and in contrast to ZIKV and DENV, is unable to engage human STAT2 in murine cells. Murine STAT2 was demonstrated to repress YFV replication in murine cells, which was correlated with an absence of IFN-α/β-mediated NS5 ubiquitination [155]. Together, these mouse studies enhance our understanding of YFV dissemination and host control in vivo. These findings are useful for the development of the next generation of small animal disease models for YFV, which should endeavor to recapitulate the disease course seen in humans. Thus, the generation of an immunocompetent mouse model would be beneficial, which could be realized by knocking out downstream specific anti-YFV effectors that directly inhibit differential aspects of the YFV lifecycle, rather than upstream master-regulators of the antiviral response. Screening approaches could identify such factors.
6. YFV Vaccine: Development and Limitations
Immunization is one of the most successful and cost-effective health interventions. Over the past decades, immunization has achieved eradication of smallpox, lowered the global incidence of polio by 99% and reduced illness, disability and death from vaccine-preventable diseases [156]. At present, there are eleven live attenuated vaccines (LAV) and among them, the YFV vaccine is the oldest and the most potent. As there are no specific treatments for YFV infection, vaccination represents the only current protection against the disease.
6.1. History and Development of the YFV Vaccine
In the 1920s, two parallel approaches were taken to develop a live attenuated vaccine against YFV. In 1927, YFV was isolated from a Ghanaian patient suffering from mild yellow fever and inoculated into rhesus macaques by direct blood/serum transfer [157]. Max Theiler and colleagues discovered that this “Asibi” strain, which was refractory for growth in other small laboratory animals, was able to replicate in the brains of mice following intracranial injection. Following sequential passage in mouse brains, reduced viscerotropic virulence was observed, coupled with enhanced neurotropic properties. Therefore, the Asibi strain subsequently underwent serial passage in chicken embryo tissue, after head and spinal column removal. After 176 passages, the attenuated YFV-17D strain had lost its viscerotropism, neurotropism and mosquito competence, while retaining its immunogenicity [8]. Concurrently, French researchers isolated YFV from a patient in Dakar, followed by serial passage in mouse brains. This attenuated strain, termed the French neurotropic vaccine (FNV), was isolated at passage 260 and was widely used in the francophone countries of Africa, virtually eradicating the disease. However, serial passaging in mouse brain increased FNV’s neurotropism, leading to high levels of post-vaccinal encephalitis in children. Consequently, the use of FNV was discontinued in the 1980s [158]. The 17D subculture is the seed strain for all modern-day YFV vaccines. There are three sub-strains obtained from the original 17D vaccine: the 17DD (Passage 195), 17D-204 (Passage 204) and the 17D-213, derived from 17D-204 and used by the Robert Koch Institute, Germany. These strains have slightly different genomic sequences: in particular, glycosylation sites on the envelope protein vary, although no differences in attenuation or immunogenicity have been observed [159]. The original passage 176 virus no longer exists, and comparisons are mainly made between the three strains outlined above [160].
The Centre for Disease Control and Prevention (CDC) recommends YF vaccination for travelers to- and residents of- endemic areas aged 9 months and older. Vaccination is also recommended for people who work in research laboratories and may be exposed through needle stick injury or inhalation of aerosolized viral droplets [161]. The YFV vaccine is a freeze-dried live-attenuated virus delivered subcutaneously. It is available as a single dose or multi-dose vials to be stored at 2–8 °C. It is reconstituted with normal saline and should be used within an hour of reconstitution [162]. Usually, a single dose confers life-long immunity, but a booster is recommended every 10 years for people at risk of exposure or people who were infected with HIV when they received their last dose. Additional doses can also be administered to people who receive hematopoietic stem cell transplants after receiving the vaccine and who are immunocompetent [163].
6.2. Immune Responses Post Vaccination
When humans are vaccinated with YFV-17D, anti-yellow fever serum is detected as early as 2 days post immunization [164,165]. It induces low-grade viremia in half of the vaccines and elicits protective neutralizing antibody (nabs) levels in 99% patients [166]. Nabs represent the main correlates of protection induced by YFV-17D. These are quantified using plaque reduction neutralization tests (PRNT) demonstrating robust responses as early as 6 days following vaccination and peaking in titer after 30 days, suggesting a functional memory response [166,167]. While the humoral arm of vaccine-induced immunity has been studied in detail, a role for T cells is expected in the development of nabs [26,168], and strong initial activation of innate immunity primes subsequent adaptive responses [169,170,171]. The role of other cell types, including NK cells and monocytes, has been inferred in generating protective responses post YFV-17D vaccination. A role for TLRs in NK cell activation was identified in YFV-17D-induced immune responses and may contribute to protective immunological memory [172]. Indeed, a systems biology approach indicates a coordinated induction of transcription factors precedes a broad persistent immune response that incorporates all effector cells of the immune system [173].
6.3. Success Story
The 17D vaccine has been in use for over 75 years. Over 600 million doses have been administered to date, although rare cases of serious adverse reactions are described [162]. Remarkably, very few cases of vaccine failure have been documented due to its near-global sero-conversion rate in human vaccinees and induction of persistent immunity [174]. Due to its success, the YF vector backbone is currently being trialed as an antigen delivery platform for other experimental vaccines including HIV-1, Lassa fever, malaria and DENV [175,176,177,178,179,180]. After early successes in outbreak prevention and control in the mid-20th century, largely due to mass vaccination campaigns and routine child immunization programs in endemic countries, a reduction of YFV cases was observed globally [181]. However, when Angola was hit by urban outbreak which spread to neighboring countries, including in the Democratic Republic of the Congo’s capital Kinshasa, the epidemic created an urgent need for more than 28 million doses of the YFV vaccine, exhausting the existing global vaccine supply [182,183,184]. Furthermore, an unusually large outbreak of the disease in Brazil in early 2017, including areas that previously were not considered endemic [185,186], confirmed the need to review the previous strategies [185]. In order to maximize the impact of limited vaccine supplies in a context of depleted global stockpiles, fractional doses of YFV vaccine were administered. Because promising data were available on the immunogenicity of fractional dosing, 10 million individuals were immunized with 2 million doses of 17DD [183,186].
6.4. Limitations: Production and Adverse Reactions
The disease has re-emerged as a public health threat in recent decades, triggered by different factors, such as climate change, increasing population movement and expanding ranges of mosquito vectors. It is estimated that vaccine manufactures can produce a combined 80 million doses per year [160]. However, as YFV-17D-based vaccines are still produced in chicken eggs, this results in issues with supply and demand during outbreaks due to limited production capabilities [187]. Another limiting issue during current production is the stability of live-attenuated vaccines. Antigen instability is an inherent attribute of vaccines, compounded by a requirement for conformationally correct three-dimensional structures that elicit protective host responses to these complex bio-therapeutics. Therefore, more stable vaccines with reduced dependency on the cold chain represent an area for future vaccine development [188]. Thermal stability is important for the manufacture, distribution and administration of vaccines, especially in tropical developing countries where adverse field conditions exist. Current live-attenuated vaccines exhibit relatively poor liquid stability in clinical settings, and clinicians are instructed to discard the YFV-17D vaccine one hour after reconstitution [189]. This issue can be best addressed by stabilization. Indeed, poliovirus has been experimentally stabilized by the addition of pirodavir and deuterium oxide, with viral RNA and viral capsid resisting a 10-h incubation at 42 °C [190].
Despite the protective nature of the YFV-17D vaccine against potentially fatal hemorrhagic fever, there are certain contraindications, including children aged less than 9 months or 6 months during an epidemic, pregnant women, lactating women, people with severe immunodeficiency due to symptomatic HIV or the presence of thymus disorder, and hypersensitivity to chicken eggs [161,187]. In addition, there are common side effects of the vaccine, ranging from muscle aches, chills, flu-like symptoms, joint pain, mild fever, mild rash, muscle weakness and swelling at the injection site. Using transcriptomic and metabolic profiling of 17D-204 vaccinees, it has recently been demonstrated that symptomatic outcomes to vaccination were associated with baseline endoplasmic reticulum stress and reduced tricarboxcylic acid cycle activity, initiating downstream proinflammatory responses [191]. The use of a live attenuated vaccine can prevent yellow fever, but vaccine-associated neurologic disease has been reported and is a safety concern [192,193,194]. In severe cases, memory loss, problems with breathing, behavioral changes, seizures, viscerotropic disease, encephalitis and death can occur. In addition, the vaccine is known to interact with about 42 drugs, but overall, the risk of developing adverse effects from vaccination is low. The causes of serious adverse events to vaccination were previously unknown. However, recent studies implicated abnormal immune responses and identified patient-specific polymorphisms in immune-associated gene loci, including CCR5, RANTES and IFNAR1 [193,195,196,197]. Finally, the risk of a large-scale epidemic in Asia remains daunting and YFV is the only infectious disease where an international vaccination certificate is required [160]. However, not all countries enforce international health regulations with respect to requiring a vaccination certificate for entry, and there has been evidence of fake/forged certificates [198].
6.5. Future Directions
Yellow fever is a re-emerging infectious disease, as vector control and routine immunization strategies have dwindled in recent years. To combat this, the WHO has initiated a global strategy to eliminate yellow fever epidemics (EYE) by 2026, focusing on management of global vaccine supply, including emergency stockpiling, improving surveillance and outbreak control [199]. Accordingly, 1.3 billion individuals will be vaccinated in the next five years, focusing on endemic areas and areas where YF is sporadic [199]. To guide responses to future outbreaks, mathematical modelling studies are being used to evaluate the efficacy and effectiveness of full and fractional dosing regimens. The model explores actual and hypothetical vaccination strategies and the impacts of possible human reactive behaviors [200,201,202].
To overcome production limitations and reduce adverse reactions, alternative vaccination approaches should be explored, including vaccinia vector-based systems, DNA vaccines, inactivated 17D vaccines and recombinant subunit vaccines produced in both mammalian cells and plants [203,204,205,206,207,208]. In line with this, current efforts to develop a new inactivated YF vaccine to reduce serious adverse events have shown good immunogenicity in preclinical and clinical studies [209,210,211]. In addition, DNA-launched vaccine technology has the advantages of both traditional DNA and live-attenuated vaccines. This platform minimizes the potential for reversions or adverse effects of traditional live-attenuated vaccine, ensures genetic stability, and results in efficient immunization in animal models [212]. The use of live attenuated viruses can lead to reversion, generation of new biological properties and recombination [213,214]. Therefore, as an alternate to conventional attenuation approaches, codon re-encoding was developed with its basic principal to introduce synonymous mutations into the viral genome [215] that would impose the least detrimental effects on viral fitness [216]. This approach has been applied to other RNA viruses, including chikungunya (CHIKV) and TBEV [217,218].
In summary, in addition to vaccination, vector control is imperative: one should also consider therapeutic options and not just rely on prophylactic vaccines. Fractional dosing for other strains needs to be validated for future outbreaks, and there is a need to manage the potential risk of YFV introduction to Asia. In the coming decade, vaccine manufacturers are expected to meet the global demand of 1.38 billion doses needed to eliminate the risk of YF epidemics [199]. Hence, novel manufacturing techniques to produce large quantities of yellow fever vaccine at short notice are required which also eliminate the chicken embryo proteins that lead to allergic reactions. The mechanism by which YFV-17D mediates protection against the virus is not well defined. This is due, in part, to the limited animal models available to evaluate vaccine-induced immune responses [168] and the difficulty accessing samples from fatal YF patients. Together, this highlights the need for further research and refining of this effective vaccine [183].
7. Global Diversity of YFV
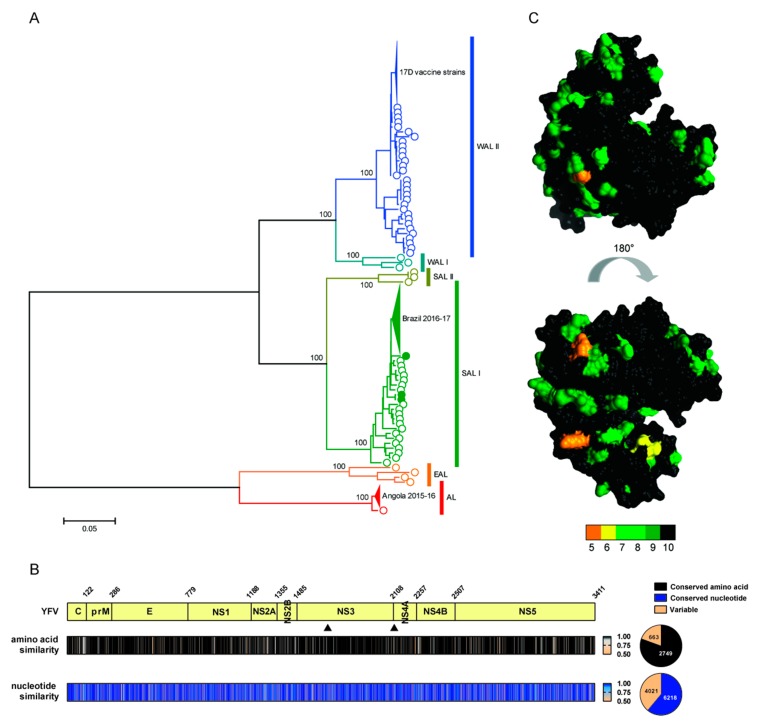
At the time of writing this review, n = 1112 YFV sequences were deposited on the NCBI GenBank sequence database. To assess patterns of globally sampled YFV diversity, we performed phylogenetic analyses of all available YFV full-length ORF sequences (n = 178) (Figure 4a). Collapsed clades contain closely related isolates from recent Brazilian and Angolan outbreaks, in addition to 17D vaccine strains. Based on this analysis, global YFV sequences can be divided into six well-supported lineages: West African lineages I and II (WAL I and WAL II), South American lineages I and II (SAL I and SAL II), the East African lineage (EAL) and the Angola lineage (AL). In agreement with a previous phylogenetic analysis of partial [219] and full-length ORFs [16,27], our expanded analysis illustrates that South American lineages are monophyletic, sharing a common ancestor with the West African lineages, and confirms a deep-divergence between these lineages and the more distantly related East African and Angolan strains, indicating an ancient separation. This observation is consistent with an African origin for YFV, with South American lineages representing historical import of West African YFV strains linked to European colonial expansions and the slave trade [2,6,18,219].
Figure 4.
YFV genomic and amino acid diversity. (A) Phylogenetic analysis of YFV genomes. The presented tree was generated using PhyML [220] from full-length ORF sequences. Nucleotide sequence alignment (10239 nucleotides) was generated in MEGA7 [11] and adjusted manually to ensure maintenance of the polyprotein ORF. Collapsed clades in the YFV phylogeny contain closely related isolates from the 2016–2017 Brazilian outbreak (n = 52, green triangle), the 2015–2016 Angolan outbreak (n = 16, red triangle) and 17D vaccine strains (n = 46, blue triangle). The green filled circles in SAL I (n = 3) represent YFV sequences isolated from non-human primates during the 2016–2017 Brazilian epidemic, which co-cluster with previously sampled non-outbreak sequences and are antecedent to the main outbreak grouping (green triangle). The statistical robustness of YFV lineages were calculated using the bootstrap approach in MEGA7 [11] and values assigned to the branches are percentages derived from 500 replications. Branch lengths are proportional to the scale bar (units: substitutions per site). (B) Conservation and variability in YFV genomes. Amino acid and underlying nucleotide similarity plots for globally sampled YFV genomes are presented. For the purpose of positional referencing, a cartoon of the YFV reference strain polyprotein is provided directly above, with coordinates of polyprotein cleavage sites for the liberation of mature proteins highlighted. The triangles positioned below indicate the position of the NS3-encoded RNA helicase. Degrees of amino acid or underlying nucleotide or similarity at individual sites/residues in global alignments were calculated in CLC Genomics Workbench (Qiagen Aarhaus) and relative similarities are color-coded according to the scale bars positioned to the right. The associated pie charts indicate the proportion of conserved or variable sites in each dataset. The numbers inset indicate the actual numbers of variable and conserved amino acids or nucleotides in the alignment. (C) Conservation and variability in the YFV RNA helicase. Amino acid conservation and variability estimated in (B) were mapped onto the YFV NS3 RNA helicase structure. Relative conservation of amino acids on the helicase structure are color-coded according to the scale bar positioned below. Completely conserved residues are colored black while variable residues highlighted dark green through to orange. The YFV helicase structure presented was described in [221] (PDB ID: 1YKS; 440 amino acids) and the conservation and variability were visualized on the structure using UCSF Chimera [222].
While clinical YFV isolates can be readily propagated in tissue culture, the majority of studies utilize the YFV-17D vaccine strain which has atypical properties [78], or its wild-type progenitor, the Asibi strain, representing only a small fraction of the global YFV diversity (Figure 4a). Consequently, the phenotypic correlates of YFV global sequence diversity are incompletely defined and future studies should address this discrepancy: it is currently unknown whether infection with isolates from different YFV lineages contributes to differential pathogenicity or mortality rates in humans. However, in spite of these deep-divergences apparent in the YFV phylogeny, the 17D vaccine provides robust protection against strains from all known YFV lineages.
YFV Genome and Polyprotein Conservation
To visualize variability across YFV genomes and encoded polyproteins, similarity plots were generated from YFV nucleotide and amino acid alignments. Inspection of genetic and amino acid variability in globally sampled YFV isolates highlights conserved and variable regions in the genome and encoded polyprotein (Figure 4b). Of note, YFV nucleotide variability is much greater than amino acid variability, indicating strong purifying selection shapes global patterns of YFV evolution. This is likely a reflection of the selective constraints imposed by the viral transmission cycle: YFV experiences sequential bottlenecks upon continued cycling through an insect vector, coupled with requirements for host factor compatibility in both vertebrates and arthropods. Furthermore, the YFV RdRp has a relatively high fidelity for an RNA virus, with an estimated mutation rate per site of 1.9–2.3 × 10−7 [223]. Together, these analyses indicate that both the structural and non-structural proteins of YFV exhibit extensive conserved regions which are therefore viable targets for pharmacological intervention. Consequently, the development or repurposing of direct-acting antiviral compounds targeting YFV proteins should represent an achievable goal. Indeed, it was previously noted that the RNA helicase domain of NS3, integral for RNA duplex unwinding during viral replication, has a conserved function in the Flaviviridae and represents a potential drug target [224]. Mapping of global amino acid diversity on to the resolved helicase structures for YFV [221] is presented in Figure 4c, highlighting the conserved regions of this essential virtual protein. Together these analyses, conducted using sequence and structural data available in public databases, can inform rational development of antivirals targeting different components of the YFV virion or replication machinery. Currently, structural data is only available for YFV E, NS3 and NS5 proteins. The elucidation of structural information for the outstanding YFV proteins will expand the number of potential viral targets for future structure-based antiviral drug design studies.
8. Monitoring YFV Outbreaks, Disease-Course and Reservoir Species with NGS
8.1. Diagnostic Utility of NGS
The use of NGS technologies allows recovery of complete viral genomic sequences directly from infected patients’ material, and these metagenomic approaches have revolutionized infectious disease outbreak diagnostics and surveillance in recent years [225]. In Africa and South America, YFV co-circulates with multiple endemic VHFs which can emerge in humans sporadically, complicating initial diagnosis. These include Ebola viruses (EBOV), Marburg virus (MARV), Rift Valley fever virus (RVFV), Junin virus (JUNV) and Crimean-Congo hemorrhagic fever (CCHFV). The diagnostic capacity of NGS to discriminate between hemorrhagic fever viruses from diverse families and orders, including the Arenaviridae, Bunyavirales, Filoviridae and Flaviviridae, has been reported [226]. Indeed, using 454 sequencing (Roche), YFV was identified as the causative agent of an outbreak of VHF in Northern Uganda in 2010 [227], validating the diagnostic utility of NGS technologies for discriminating YFV outbreaks from unrelated hemorrhagic fever viruses.
8.2. Outbreak Monitoring in Real-Time
More recently, as NGS technology has advanced, hand-held nanopore sequencing devices (MinION, Oxford Nanopore) have been deployed to monitor viral outbreaks, including the West African 2013-16 EBOV outbreak [228] and the Lassa fever virus (LASV) outbreak in Nigeria in 2018 [229]. This technology has multiple advantages for pathogen surveillance in real-time when compared to conventional NGS platforms including low cost, portability, easy library preparation, and rapid generation of data in just a few hours [230]. The re-emergence of YFV as a global health burden has been demonstrated by multiple recent outbreaks [15]. In combination with the ION Torrent and Illumina platforms, MinION sequencing was utilized to provide insights into the molecular epidemiology of the 2016–2017 YFV outbreak in Brazil, generating data spatially correlating infections of humans with infection of non-human primates [16]. These data indicate that vector-mediated transmission in the Brazilian outbreak was dominated by continued primate-to-human transmission rather than a human-to-human transfer often observed in explosive African outbreaks. While future outbreaks are inevitable, rapid identification of YFV as the causative agent and subsequent real-time tracking of epidemics can be facilitated by recent advances in NGS technologies and phylogenetics [231], enhancing effective outbreak management, including coordinating vaccination programs and vector eradication campaigns. Additionally, in future outbreaks, viral sampling could be coupled with serological markers of infection (anti-YFV antibodies) and patient DNA bio-banking to enable stratification of host-genetic correlates of YFV infection outcomes, and correlation with host single nucleotide polymorphisms (SNPs). It is likely that host genetics underlie the range of clinical symptoms observed in YFV infection, which include subclinical, symptomatic, severe and fatal outcomes. However, significant challenges to wide-spread implementation of the strategies outlined above should not be underestimated. High costs associated with NGS, coupled with underdeveloped infrastructure in the majority of YFV-endemic countries remain logistical obstacles to overcome.
In addition to diagnosis and outbreak surveillance, the application of NGS to longitudinal and cross-sectional monitoring of infected patient cohorts can facilitate advances in our understanding of within-host viral evolution. Despite near-perfect environmental conditions, including the presence of replication-competent Aedes vector species, YFV outbreaks in Asia remain conspicuously absent. However, import of YFV from the Angolan outbreak to China was recorded for the first time in 2016 [15,232]. Using Illumina sequencing to follow virus evolution in 12 imported YFV cases revealed the infecting strains were closely related to each other and possessed limited divergence from the Angola 1971 strain (three amino acid substitutions) despite a 45-year period between samplings [12,233]. The action of strong purifying selection was detected throughout viral genomes, with intra-host variation dominated by synonymous substitutions [233]. In agreement with these observations, the evolutionary rate of YFV has been shown to be lower than that of DENV [234] and the YFV RdRp possesses high fidelity [223]. In addition, sequential bottlenecks associated with vector-host switching could potentially select for variants with an enhanced capacity for transmission, reducing variation. Constraints on YFV evolution could also be mediated by the requirement for interactions with host-dependency factors essential for replication and dissemination in both mammals and arthropods, coupled with a limited duration of infection in human hosts.
8.3. Identification of Novel Animal Reservoirs
YFV represents a zoonotic pathogen: while many zoonotic viruses cause severe pathology in humans, tolerance and absence of disease is often observed in reservoir species [235]. After long periods of absence, sporadic and often explosive YFV outbreaks occur in endemic regions, initiated by vector-mediated spillover from jungle primates when environmental conditions are favorable. This pattern of emergence poses the question of whether, in addition to described host- and vector-species, other as yet unidentified animal or arthropod species can act as reservoirs for YFV. Vertical transmission of YFV between mosquitos has been reported [236,237]. While transmission rates are low (~1%), this capacity could maintain YFV reservoirs during dry periods which are not favorable for transmission [4]. It has been suggested that primate density is often not sufficient to maintain stable sylvatic transmission, and YFV reservoirs in non-primate species could maintain inter-epidemic YFV [4]. Indeed, serum neutralizing antibodies for YFV were detected in ten forest mammal species from French Guiana, including members of the divergent orders Rodentia and Carnivora, the superorder Xenarthra, as well as multiple primate species [143]. Thus, the breadth of YFV species-specificity remains to be determined. To further investigate this, extensive sampling and NGS screening of jungle species in YFV endemic regions could be undertaken, potentially identifying novel animal reservoirs which silently maintain YFV transmission between intermittent outbreaks. In summary, future YFV outbreaks can be rapidly identified and epidemics monitored at high resolution using NGS to inform containment strategies. This technology also lends itself to tracking intra-host evolution and the identification of novel animal reservoirs.
9. Modes of YFV Transmission and Future Vector Control Strategies
YFV is transmitted by mosquito vectors between primate hosts and transmission to humans in three distinct cycles is possible. 1. The sylvatic or jungle cycle—where epizootic transmission between rainforest primates by jungle-resident mosquito species occurs, with sporadic spillover to forestry workers. This is mediated by multiple Aedes species in Africa and Haemogogus and Sabethes species in South America; 2. The intermediate or savannah cycle—occurs in isolated rural communities on the rainforest periphery where infected mosquito species feed simultaneously on both primate and human hosts. In Africa, Aedes africanus preferentially feeds on primates while Aedes simpsonii feeds indiscriminately on nonhuman primates and humans; 3. The urban cycle—where invasive domesticated mosquitoes (Aedes aegypti) facilitate YFV transmission from human-to-human in areas of high human and vector population density [3,5,6]. The 2015–2016 outbreak in Angola is considered an urban outbreak, with human mobility and population density identified as important factors in the spatial expansion of the epidemic [13]. Contrastingly, detailed analyses of the 2016–2017 Brazilian YFV outbreak suggests the sylvatic cycle represents the dominant mode of transmission [16].
Urban YFV outbreaks are associated with Ae. aegypti transmission, although the Asian tiger mosquito, Aedes albopictus, is also competent for YFV transmission [238]. Both species are invasive, are able to breed in water containers and have drought-resistant eggs. Indeed, the geographical distribution of these important vector species continues to increase, fueled by human population growth, globalization, increasing urbanization and ongoing climate change [239], expanding the risk of human exposure to YFV infection. The expansion of the 2016–2017 outbreak in Brazil to an area not previously considered endemic for YFV serves to underline this point [15,16]. However, during YFV outbreaks, while isolated cases can be imported from endemic to non-endemic regions [17], continued host-to-host transmission of YFV observed in outbreaks is only possible in geographic regions where competent vector species reside: the disease is not contagious in their absence. This facet of the viral life cycle has been exploited in the past to control and even eradicate YFV from specific regions. While YFV has never been controlled in Africa, vector control programs coupled with the absence of a sylvatic cycle enabled its complete eradication from Europe and North America, where devastating outbreaks were previously common [2,3,4,6]. Vector control programs in South America all but eradicated Ae. aegypti by the 1960s [4], although this vector species has re-populated the continent due to the cessation of such programs and remains endemic in 13 countries [240]. In addition, vector resistance to traditional insecticides has been detected in the major invasive Aedes vectors and is becoming an increasing problem [7,241,242]. Thus, in combination with traditional chemical spraying of vector breeding sites and vaccination campaigns targeted to at-risk people in endemic regions, innovative new strategies for vector control are required to help to manage and reduce the likelihood of future arbovirus outbreaks, including YFV [243].
Mosquito population suppression strategies which harness the power of genetic modification include the release of insects carrying a dominant lethal gene (RIDL). In trials performed on the Cayman Islands, engineered male Ae. aegypti RIDL mosquitos (strain OX513A) carrying a dominant lethal transgene were released, resulting in the suppression of wild mosquito numbers [244]. In a complementary approach using bacteria which are known to render mosquitos sterile, researchers were recently able to virtually eliminate Ae. albopictus from two test-release sites in China, combining population suppression and population replacement strategies [245]. Male mosquitoes artificially infected with Wolbachia wPip strains were unable to produce viable offspring after mating with non-wPip infected females, and wPip infected mosquitos of both sexes were less susceptible to infection with the pathogenic flaviviruses DENV and ZIKV [246]. Pupal irradiation boosted viable mosquito numbers for use in the trial ten-fold compared to previous conventional manual inspection techniques, and this combined approach was able to substantially reduce both wild mosquito numbers and biting rates at test-sites [245,246]. While the current limitations of approaches outlined above include associated costs, relatively small trial sizes and isolated trial sites, these exiting results pave the way for analogous trials with Ae. aegypti in YFV endemic regions.
Arboviruses often replicate in mosquito mid-gut and targeting this site of viral replication via genetic modification represents another possible strategy for ablating vector-mediated transmission of YFV. Indeed, DENV dissemination and replication were restricted in transgenic Ae. aegypti mosquitos ectopically expressing Loqs2, which interacts with intrinsically expressed Loquacious and r2d2 to restore efficient siRNA suppression of viral replication in the mosquito midgut [247]. Furthermore, attenuated YFV-17D is able to replicate in mosquito mid-gut. However, in contrast to wild-type strains, the basal membrane of the midgut blocks YFV-17D dissemination to other tissues [153], indicating a loss of interaction with a co-opted mid-gut host factor during 17D attenuation. In the future, genetic modification of YFV Aedes vector species using CRISPR/Cas9 [222] could preferentially target co-opted factors in the mosquito midgut or other tissues which are essential YFV propagation and dissemination. The ability of researchers to identify such factors, which are currently unknown, is enhanced by an improved annotated genome for Ae. aegypti [248]. Indeed, these data identified gene loci potentially associated with DENV competence and insecticide resistance. In addition, quantification and comparison of gene expression profiles for distinct Ae. aegypti tissues, from both male and female mosquitos at different stages of the reproductive cycle are described [248,249]. Together, these data highlight tissue- and sex-specific gene products, which could be further investigated to identify essential YFV replication cofactors in the mosquito mid-gut via iterative screening approaches. Genetically modified mosquitos with CRISPR/Cas9 targeted deletion of such factors could then be generated for replacement studies. In summary, targeting vector species, which have become resistant to traditional insecticides, will be key in reducing future YFV outbreaks. The future of eliminating YFV from endemic regions may involve the suppression or replacement of wild-type mosquitos with modified insects which are not competent for YFV replication. Furthermore, sequencing efforts to expand the number of annotated genomes from jungle-resident mosquito species should commence.
10. Concluding Remarks
YFV is the prototypic flavivirus—a zoonotic pathogen which is responsible for continuing outbreaks of VHF in tropical regions. Recent epidemics in Angola and Brazil serve to underline that the threat from this re-emerging pathogen remains high despite the long-standing availability of a protective vaccine. Scaling back of vector control programs, low vaccine coverage and limitations on vaccine production impedes effective management of outbreaks. Furthermore, rapid global warming, coupled with increasing urbanization and globalization, is expanding permissive mosquito ranges and increasing the potential for future outbreaks. Therefore, intensification of basic YFV research is imperative to expand current knowledge of the complex viral life cycle and stimulate the development of novel anti-YFV therapeutics targeted at both the virus and the host. Additionally, disentangling immune correlates of YFV-induced pathogenesis versus protection, addressing bottlenecks associated with vaccine production, real-time surveillance of outbreaks and genetic modification of vector species to reduce YFV permissiveness can all contribute to future efforts aimed at reducing mortality rates associated with YFV epidemics.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/11/10/960/s1, Table S1: Summary of structural and functional properties of YFV proteins.
Author Contributions
Writing—original draft preparation: R.D.V.K., E.M.-D., T.K., S.H., S.C., R.J.P.B.; writing—review & editing: R.D.V.K., R.J.P.B.; data analysis and visualization: K.W., B.T. and R.J.P.B. All authors gave their final approval.
Funding
This work was funded by BMG grant 1-2516-FSB-416.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Butler D. Fears rise over yellow fever’s next move. Nature. 2016;532:155–156. doi: 10.1038/532155a. [DOI] [PubMed] [Google Scholar]
- 2.Tolle M.A. Mosquito-borne diseases. Curr. Probl. Pediatr. Adolesc. Health Care. 2009;39:97–140. doi: 10.1016/j.cppeds.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Brès P.L.J. A century of progress in combating yellow fever*. Bull. World Health Organ. 1986;64:775–786. [PMC free article] [PubMed] [Google Scholar]
- 4.Monath T.P. Yellow fever and dengue—the interactions of virus, vector and host in the re-emergence of epidemic disease. Semin. Virol. 1994;5:133–145. doi: 10.1006/smvy.1994.1014. [DOI] [Google Scholar]
- 5.Monath T.P., Vasconcelos P.F.C. Yellow fever. J. Clin. Virol. 2015;64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 6.Gardner C.L., Ryman K.D. Yellow fever: A reemerging threat. Clin. Lab. Med. 2010;30:237–260. doi: 10.1016/j.cll.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downs W.G. History of Epidemiological Aspects of Yellow Fever. Yale J. Biol. Med. 1982;55:179–185. [PMC free article] [PubMed] [Google Scholar]
- 8.Theiler M., Smith H.H. The Use of Yellow Fever Virus Modified by in Vitro Cultivation For Human Immunization. J. Exp. Med. 1937;65:787–800. doi: 10.1084/jem.65.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garske T., van Kerkhove M.D., Yactayo S., Ronveaux O., Lewis R.F., Staples J.E., Perea W., Ferguson N.M. Yellow Fever in Africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014;11:e1001638. doi: 10.1371/journal.pmed.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmonds P., Becher P., Bukh J., Gould E.A., Meyers G., Monath T., Muerhoff S., Pletnev A., Rico-Hesse R., Smith D.B., et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017;98:2–3. doi: 10.1099/jgv.0.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grobbelaar A.A., Weyer J., Moolla N., van Jansen Vuren P., Moises F., Paweska J.T. Resurgence of Yellow Fever in Angola, 2015–2016. Emerg. Infect. Dis. 2016;22:1854–1855. doi: 10.3201/eid2210.160818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraemer M.U.G., Faria N.R., Reiner R.C., Golding N., Nikolay B., Stasse S., Johansson M.A., Salje H., Faye O., Wint G.R.W., et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–16: A modelling study. Lancet Infect. Dis. 2017;17:330–338. doi: 10.1016/S1473-3099(16)30513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paules C.I., Fauci A.S. Yellow Fever—Once Again on the Radar Screen in the Americas. N. Engl. J. Med. 2017;376:1397–1399. doi: 10.1056/NEJMp1702172. [DOI] [PubMed] [Google Scholar]
- 15.Barrett A.D.T. The reemergence of yellow fever. Science. 2018;361:847–848. doi: 10.1126/science.aau8225. [DOI] [PubMed] [Google Scholar]
- 16.Faria N.R., Kraemer M.U.G., Hill S.C., Goes de Jesus J., Aguiar R.S., Iani F.C.M., Xavier J., Quick J., Du Plessis L., Dellicour S., et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science. 2018;361:894–899. doi: 10.1126/science.aat7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorigatti I., Hamlet A., Aguas R., Cattarino L., Cori A., Donnelly C.A., Garske T., Imai N., Ferguson N.M. International risk of yellow fever spread from the ongoing outbreak in Brazil, December 2016 to May 2017. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.28.30572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monath T.P. Yellow fever: An update. Lancet Infect. Dis. 2001;1:11–20. doi: 10.1016/S1473-3099(01)00016-0. [DOI] [PubMed] [Google Scholar]
- 19.Rice C.M., Grakoui A., Galler R., Chambers T.J. Transcription of infectious yellow fever RNA from full-length cDNA templates produced by in vitro ligation. New Biol. 1989;1:285–296. [PubMed] [Google Scholar]
- 20.Hahn C.S., Dalrymple J.M., Strauss J.H., Rice C.M. Comparison of the virulent Asibi strain of yellow fever virus with the 17D vaccine strain derived from it. Proc. Natl. Acad. Sci. USA. 1987;84:2019–2023. doi: 10.1073/pnas.84.7.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bredenbeek P.J., Kooi E.A., Lindenbach B., Huijkman N., Rice C.M., Spaan W.J.M. A stable full-length yellow fever virus cDNA clone and the role of conserved RNA elements in flavivirus replication. J. Gen. Virol. 2003;84:1261–1268. doi: 10.1099/vir.0.18860-0. [DOI] [PubMed] [Google Scholar]
- 22.Rice C.M., Lenches E.M., Eddy S.R., Shin S.J., Sheets R.L., Strauss J.H. Nucleotide sequence of yellow fever virus: Implications for flavivirus gene expression and evolution. Science. 1985;229:726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- 23.McElroy K.L., Tsetsarkin K.A., Vanlandingham D.L., Higgs S. Characterization of an infectious clone of the wild-type yellow fever virus Asibi strain that is able to infect and disseminate in mosquitoes. J. Gen. Virol. 2005;86:1747–1751. doi: 10.1099/vir.0.80746-0. [DOI] [PubMed] [Google Scholar]
- 24.Aubry F., Nougairède A., Gould E.A., de Lamballerie X. Flavivirus reverse genetic systems, construction techniques and applications: A historical perspective. Antivir. Res. 2015;114:67–85. doi: 10.1016/j.antiviral.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuse Y. Analysis of research intensity on infectious disease by disease burden reveals which infectious diseases are neglected by researchers. Proc. Natl. Acad. Sci. USA. 2019;116:478–483. doi: 10.1073/pnas.1814484116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douam F., Ploss A. Yellow Fever Virus: Knowledge Gaps Impeding the Fight Against an Old Foe. Trends Microbiol. 2018;26:913–928. doi: 10.1016/j.tim.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klitting R., Fischer C., Drexler J.F., Gould E.A., Roiz D., Paupy C., de Lamballerie X. What Does the Future Hold for Yellow Fever Virus? (II) Genes. 2018;9:425. doi: 10.3390/genes9090425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Yellow Fever—Clinical and Laboratory Evaluation. [(accessed on 12 August 2019)];2015 Available online: https://www.cdc.gov/yellowfever/healthcareproviders/healthcareproviders-clinlabeval.html.
- 29.Quaresma J.A., Barros V.L., Pagliari C., Fernandes E.R., Andrade H.F., Jr., Vasconcelos P.F., Duarte M.I. Hepatocyte lesions and cellular immune response in yellow fever infection. Trans. R. Soc. Trop. Med. Hyg. 2007;101:161–168. doi: 10.1016/j.trstmh.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Blake L.E., Garcia-Blanco M.A. Human genetic variation and yellow fever mortality during 19th century U.S. epidemics. MBio. 2014;5:e01253-14. doi: 10.1128/mBio.01253-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuboi S.H., Costa Z.G., da Costa Vasconcelos P.F., Hatch D. Clinical and epidemiological characteristics of yellow fever in Brazil: Analysis of reported cases 1998-2002. Trans. R. Soc. Trop. Med. Hyg. 2007;101:169–175. doi: 10.1016/j.trstmh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira R.A., de Oliveira-Filho E.F., Fernandes I.V.A., Brito C.A., Marques E.T., Tenório M.C., Gil L.H. Previous dengue or Zika virus exposure can drive to infection enhancement or neutralisation of other flaviviruses. Mem. Inst. Oswaldo Cruz. 2019;114:e190098. doi: 10.1590/0074-02760190098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monath T.P. Treatment of yellow fever. Antivir. Res. 2008;78:116–124. doi: 10.1016/j.antiviral.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Sacramento C.Q., de Melo G.R., de Freitas C.S., Rocha N., Hoelz L.V.B., Miranda M., Fintelman-Rodrigues N., Marttorelli A., Ferreira A.C., Barbosa-Lima G., et al. The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication. Sci. Rep. 2017;7:40920. doi: 10.1038/srep40920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H.-T., Colby-Germinario S.P., Hassounah S.A., Fogarty C., Osman N., Palanisamy N., Han Y., Oliveira M., Quan Y., Wainberg M.A. Evaluation of Sofosbuvir (β-D-2’-deoxy-2’-α-fluoro-2’-β-C-methyluridine) as an inhibitor of Dengue virus replication. Sci. Rep. 2017;7:6345. doi: 10.1038/s41598-017-06612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Freitas C.S., Higa L.M., Sacramento C.Q., Ferreira A.C., Reis P.A., Delvecchio R., Monteiro F.L., Barbosa-Lima G., James Westgarth H., Vieira Y.R., et al. Yellow fever virus is susceptible to sofosbuvir both in vitro and in vivo. PLoS Negl. Trop. Dis. 2019;13:e0007072. doi: 10.1371/journal.pntd.0007072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin Z., Chen Y.-L., Schul W., Wang Q.-Y., Gu F., Duraiswamy J., Kondreddi R.R., Niyomrattanakit P., Lakshminarayana S.B., Goh A., et al. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. USA. 2009;106:20435–20439. doi: 10.1073/pnas.0907010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrd C.M., Grosenbach D.W., Berhanu A., Dai D., Jones K.F., Cardwell K.B., Schneider C., Yang G., Tyavanagimatt S., Harver C., et al. Novel benzoxazole inhibitor of dengue virus replication that targets the NS3 helicase. Antimicrob. Agents Chemother. 2013;57:1902–1912. doi: 10.1128/AAC.02251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrd C.M., Dai D., Grosenbach D.W., Berhanu A., Jones K.F., Cardwell K.B., Schneider C., Wineinger K.A., Page J.M., Harver C., et al. A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob. Agents Chemother. 2013;57:15–25. doi: 10.1128/AAC.01429-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patkar C.G., Larsen M., Owston M., Smith J.L., Kuhn R.J. Identification of inhibitors of yellow fever virus replication using a replicon-based high-throughput assay. Antimicrob. Agents Chemother. 2009;53:4103–4114. doi: 10.1128/AAC.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo F., Wu S., Julander J., Ma J., Zhang X., Kulp J., Cuconati A., Block T.M., Du Y., Guo J.-T., et al. A Novel Benzodiazepine Compound Inhibits Yellow Fever Virus Infection by Specifically Targeting NS4B Protein. J. Virol. 2016;90:10774–10788. doi: 10.1128/JVI.01253-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pacca C.C., Severino A.A., Mondini A., Rahal P., D’Avila S G., Cordeiro J.A., Nogueira M.C., Bronzoni R.V., Nogueira M.L. RNA interference inhibits yellow fever virus replication in vitro and in vivo. Virus Genes. 2009;38:224–231. doi: 10.1007/s11262-009-0328-3. [DOI] [PubMed] [Google Scholar]
- 43.Julander J.G. Experimental therapies for yellow fever. Antivir. Res. 2013;97:169–179. doi: 10.1016/j.antiviral.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierson T.C., Diamond M.S. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert Rev. Mol. Med. 2008;10:e12. doi: 10.1017/S1462399408000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukhopadhyay S., Kuhn R.J., Rossmann M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 46.Pierson T.C., Kielian M. Flaviviruses: Braking the entering. Curr. Opin. Virol. 2013;3:3–12. doi: 10.1016/j.coviro.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patkar C.G., Jones C.T., Chang Y.-H., Warrier R., Kuhn R.J. Functional requirements of the yellow fever virus capsid protein. J. Virol. 2007;81:6471–6481. doi: 10.1128/JVI.02120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Op de Beeck A., Rouillé Y., Caron M., Duvet S., Dubuisson J. The transmembrane domains of the prM and E proteins of yellow fever virus are endoplasmic reticulum localization signals. J. Virol. 2004;78:12591–12602. doi: 10.1128/JVI.78.22.12591-12602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kielian M. Class II virus membrane fusion proteins. Virology. 2006;344:38–47. doi: 10.1016/j.virol.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 50.Seligman S.J. Constancy and diversity in the flavivirus fusion peptide. Virol. J. 2008;5:27. doi: 10.1186/1743-422X-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volk D.E., May F.J., Gandham S.H.A., Anderson A., von Lindern J.J., Beasley D.W.C., Barrett A.D.T., Gorenstein D.G. Structure of yellow fever virus envelope protein domain III. Virology. 2009;394:12–18. doi: 10.1016/j.virol.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryman K.D., Ledger T.N., Campbell G.A., Watowich S.J., Barrett A.D. Mutation in a 17D-204 vaccine substrain-specific envelope protein epitope alters the pathogenesis of yellow fever virus in mice. Virology. 1998;244:59–65. doi: 10.1006/viro.1998.9057. [DOI] [PubMed] [Google Scholar]
- 53.Daffis S., Kontermann R.E., Korimbocus J., Zeller H., Klenk H.-D., Ter Meulen J. Antibody responses against wild-type yellow fever virus and the 17D vaccine strain: Characterization with human monoclonal antibody fragments and neutralization escape variants. Virology. 2005;337:262–272. doi: 10.1016/j.virol.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 54.Lu X., Xiao H., Li S., Pang X., Song J., Liu S., Cheng H., Li Y., Wang X., Huang C., et al. Double Lock of a Human Neutralizing and Protective Monoclonal Antibody Targeting the Yellow Fever Virus Envelope. Cell Rep. 2019;26:438–446. doi: 10.1016/j.celrep.2018.12.065. [DOI] [PubMed] [Google Scholar]
- 55.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z., Liu L., Lv Y., Zhang W., Li J., Zhang Y., Di T., Zhang S., Liu J., Li J., et al. A fatal yellow fever virus infection in China: Description and lessons. Emerg. Microbes Infect. 2016;5:e69. doi: 10.1038/emi.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinz F.X., Allison S.L. Flavivirus structure and membrane fusion. Adv. Virus Res. 2003;59:63–97. doi: 10.1016/s0065-3527(03)59003-0. [DOI] [PubMed] [Google Scholar]
- 58.Monath T.P., Barrett A.D. Pathogenesis and pathophysiology of yellow fever. Adv. Virus Res. 2003;60:343–395. doi: 10.1016/s0065-3527(03)60009-6. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y., Corver J., Chipman P.R., Zhang W., Pletnev S.V., Sedlak D., Baker T.S., Strauss J.H., Kuhn R.J., Rossmann M.G. Structures of immature flavivirus particles. EMBO J. 2003;22:2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Resch B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum. Vaccin. Immunother. 2017;13:2138–2149. doi: 10.1080/21645515.2017.1337614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar R., Qureshi H., Deshpande S., Bhattacharya J. Broadly neutralizing antibodies in HIV-1 treatment and prevention. Ther. Adv. Vaccines Immunother. 2018;6:61–68. doi: 10.1177/2515135518800689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Margolis D.M., Koup R.A., Ferrari G. HIV antibodies for treatment of HIV infection. Immunol. Rev. 2017;275:313–323. doi: 10.1111/imr.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller J.L., de Wet B.J.M., Martinez-Pomares L., Radcliffe C.M., Dwek R.A., Rudd P.M., Gordon S. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008;4:e17. doi: 10.1371/annotation/98b92fca-fa6e-4bf3-9b39-13b66b640476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tassaneetrithep B., Burgess T.H., Granelli-Piperno A., Trumpfheller C., Finke J., Sun W., Eller M.A., Pattanapanyasat K., Sarasombath S., Birx D.L., et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krishnan M.N., Ng A., Sukumaran B., Gilfoy F.D., Uchil P.D., Sultana H., Brass A.L., Adametz R., Tsui M., Qian F., et al. RNA interference screen for human genes associated with West Nile virus infection. Nature. 2008;455:242–245. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Sommer C., Barrows N.J., Bradrick S.S., Pearson J.L., Garcia-Blanco M.A. G protein-coupled receptor kinase 2 promotes flaviviridae entry and replication. PLoS Negl. Trop. Dis. 2012;6:e1820. doi: 10.1371/journal.pntd.0001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meertens L., Carnec X., Lecoin M.P., Ramdasi R., Guivel-Benhassine F., Lew E., Lemke G., Schwartz O., Amara A. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Germi R., Crance J.-M., Garin D., Guimet J., Lortat-Jacob H., Ruigrok R.W.H., Zarski J.-P., Drouet E. Heparan sulfate-mediated binding of infectious dengue virus type 2 and yellow fever virus. Virology. 2002;292:162–168. doi: 10.1006/viro.2001.1232. [DOI] [PubMed] [Google Scholar]
- 69.Kroschewski H., Allison S.L., Heinz F.X., Mandl C.W. Role of heparan sulfate for attachment and entry of tick-borne encephalitis virus. Virology. 2003;308:92–100. doi: 10.1016/S0042-6822(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 70.Shukla D., Liu J., Blaiklock P., Shworak N.W., Bai X., Esko J.D., Cohen G.H., Eisenberg R.J., Rosenberg R.D., Spear P.G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/S0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 71.Patel M., Yanagishita M., Roderiquez G., Bou-Habib D.C., Oravecz T., Hascall V.C., Norcross M.A. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retrovir. 1993;9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 72.Lee E., Pavy M., Young N., Freeman C., Lobigs M. Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic flaviviruses. Antivir. Res. 2006;69:31–38. doi: 10.1016/j.antiviral.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 73.Perera-Lecoin M., Meertens L., Carnec X., Amara A. Flavivirus entry receptors: An update. Viruses. 2013;6:69–88. doi: 10.3390/v6010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodenhuis-Zybert I.A., Wilschut J., Smit J.M. Dengue virus life cycle: Viral and host factors modulating infectivity. Cell. Mol. Life Sci. 2010;67:2773–2786. doi: 10.1007/s00018-010-0357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sessions O.M., Barrows N.J., Souza-Neto J.A., Robinson T.J., Hershey C.L., Rodgers M.A., Ramirez J.L., Dimopoulos G., Yang P.L., Pearson J.L., et al. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu K., Qian Y., Jung Y.-S., Zhou B., Cao R., Shen T., Shao D., Wei J., Ma Z., Chen P., et al. mosGCTL-7, a C-Type Lectin Protein, Mediates Japanese Encephalitis Virus Infection in Mosquitoes. J. Virol. 2017;91:e01348-16. doi: 10.1128/JVI.01348-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng G., Cox J., Wang P., Krishnan M.N., Dai J., Qian F., Anderson J.F., Fikrig E. A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell. 2010;142:714–725. doi: 10.1016/j.cell.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernandez-Garcia M.D., Meertens L., Chazal M., Hafirassou M.L., Dejarnac O., Zamborlini A., Despres P., Sauvonnet N., Arenzana-Seisdedos F., Jouvenet N., et al. Vaccine and Wild-Type Strains of Yellow Fever Virus Engage Distinct Entry Mechanisms and Differentially Stimulate Antiviral Immune Responses. MBio. 2016;7 doi: 10.1128/mBio.01956-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plow E.F., Haas T.A., Zhang L., Loftus J., Smith J.W. Ligand binding to integrins. J. Biol. Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 80.Chambers T.J., Nickells M. Neuroadapted Yellow Fever Virus 17D: Genetic and Biological Characterization of a Highly Mouse-Neurovirulent Virus and Its Infectious Molecular Clone. J. Virol. 2001;75:10912. doi: 10.1128/JVI.75.22.10912-10922.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mailly L., Xiao F., Lupberger J., Wilson G.K., Aubert P., Duong F.H.T., Calabrese D., Leboeuf C., Fofana I., Thumann C., et al. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat. Biotechnol. 2015;33:549–554. doi: 10.1038/nbt.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Campos R.K., Wong B., Xie X., Lu Y.-F., Shi P.-Y., Pompon J., Garcia-Blanco M.A., Bradrick S.S. RPLP1 and RPLP2 Are Essential Flavivirus Host Factors That Promote Early Viral Protein Accumulation. J. Virol. 2017:91. doi: 10.1128/JVI.01706-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chambers T.J., Grakoui A., Rice C.M. Processing of the yellow fever virus nonstructural polyprotein: A catalytically active NS3 proteinase domain and NS2B are required for cleavages at dibasic sites. J. Virol. 1991;65:6042–6050. doi: 10.1128/jvi.65.11.6042-6050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chambers T.J., Droll D.A., Tang Y., Liang Y., Ganesh V.K., Murthy K.H.M., Nickells M. Yellow fever virus NS2B-NS3 protease: Characterization of charged-to-alanine mutant and revertant viruses and analysis of polyprotein-cleavage activities. J. Gen. Virol. 2005;86:1403–1413. doi: 10.1099/vir.0.80427-0. [DOI] [PubMed] [Google Scholar]
- 85.Lindenbach B.D., Rice C.M. Molecular Biology of Flaviviruses. Elsevier; Amsterdam, The Netherlands: 2003. pp. 23–61. [DOI] [PubMed] [Google Scholar]
- 86.Filomatori C.V., Lodeiro M.F., Alvarez D.E., Samsa M.M., Pietrasanta L., Gamarnik A.V. A 5′ RNA element promotes dengue virus RNA synthesis on a circular genome. Genes Dev. 2006;20:2238–2249. doi: 10.1101/gad.1444206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu L., Nomaguchi M., Padmanabhan R., Markoff L. Specific requirements for elements of the 5′ and 3′ terminal regions in flavivirus RNA synthesis and viral replication. Virology. 2008;374:170–185. doi: 10.1016/j.virol.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wengler G. The NS3 nonstructural protein of flaviviruses contains an RNA triphosphatase activity. Virology. 1993;197:265–273. doi: 10.1006/viro.1993.1587. [DOI] [PubMed] [Google Scholar]
- 89.Egloff M.-P., Benarroch D., Selisko B., Romette J.-L., Canard B. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: Crystal structure and functional characterization. EMBO J. 2002;21:2757–2768. doi: 10.1093/emboj/21.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Y., Ray D., Zhao Y., Dong H., Ren S., Li Z., Guo Y., Bernard K.A., Shi P.-Y., Li H. Structure and function of flavivirus NS5 methyltransferase. J. Virol. 2007;81:3891–3903. doi: 10.1128/JVI.02704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Issur M., Geiss B.J., Bougie I., Picard-Jean F., Despins S., Mayette J., Hobdey S.E., Bisaillon M. The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA. 2009;15:2340–2350. doi: 10.1261/rna.1609709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Welsch S., Miller S., Romero-Brey I., Merz A., Bleck C.K.E., Walther P., Fuller S.D., Antony C., Krijnse-Locker J., Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Junjhon J., Pennington J.G., Edwards T.J., Perera R., Lanman J., Kuhn R.J. Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. J. Virol. 2014;88:4687–4697. doi: 10.1128/JVI.00118-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chambers T.J., Weir R.C., Grakoui A., McCourt D.W., Bazan J.F., Fletterick R.J., Rice C.M. Evidence that the N-terminal domain of nonstructural protein NS3 from yellow fever virus is a serine protease responsible for site-specific cleavages in the viral polyprotein. Proc. Natl. Acad. Sci. USA. 1990;87:8898–8902. doi: 10.1073/pnas.87.22.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Westaway E.G., Mackenzie J.M., Kenney M.T., Jones M.K., Khromykh A.A. Ultrastructure of Kunjin virus-infected cells: Colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luo D., Vasudevan S.G., Lescar J. The flavivirus NS2B-NS3 protease-helicase as a target for antiviral drug development. Antivir. Res. 2015;118:148–158. doi: 10.1016/j.antiviral.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 97.Tan B.-H., Fu J., Sugrue R.J., Yap E.-H., Chan Y.-C., Tan Y.H. Recombinant Dengue Type 1 Virus NS5 Protein Expressed inEscherichia coliExhibits RNA-Dependent RNA Polymerase Activity. Virology. 1996;216:317–325. doi: 10.1006/viro.1996.0067. [DOI] [PubMed] [Google Scholar]
- 98.Kapoor M., Zhang L., Ramachandra M., Kusukawa J., Ebner K.E., Padmanabhan R. Association between NS3 and NS5 proteins of dengue virus type 2 in the putative RNA replicase is linked to differential phosphorylation of NS5. J. Biol. Chem. 1995;270:19100–19106. doi: 10.1074/jbc.270.32.19100. [DOI] [PubMed] [Google Scholar]
- 99.Bhattacharya D., Hoover S., Falk S.P., Weisblum B., Vestling M., Striker R. Phosphorylation of yellow fever virus NS5 alters methyltransferase activity. Virology. 2008;380:276–284. doi: 10.1016/j.virol.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kümmerer B.M., Rice C.M. Mutations in the yellow fever virus nonstructural protein NS2A selectively block production of infectious particles. J. Virol. 2002;76:4773–4784. doi: 10.1128/JVI.76.10.4773-4784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller S., Kastner S., Krijnse-Locker J., Bühler S., Bartenschlager R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J. Biol. Chem. 2007;282:8873–8882. doi: 10.1074/jbc.M609919200. [DOI] [PubMed] [Google Scholar]
- 102.Leung J.Y., Pijlman G.P., Kondratieva N., Hyde J., Mackenzie J.M., Khromykh A.A. Role of nonstructural protein NS2A in flavivirus assembly. J. Virol. 2008;82:4731–4741. doi: 10.1128/JVI.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stern O., Hung Y.-F., Valdau O., Yaffe Y., Harris E., Hoffmann S., Willbold D., Sklan E.H. An N-terminal amphipathic helix in dengue virus nonstructural protein 4A mediates oligomerization and is essential for replication. J. Virol. 2013;87:4080–4085. doi: 10.1128/JVI.01900-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mackenzie J.M., Khromykh A.A., Jones M.K., Westaway E.G. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology. 1998;245:203–215. doi: 10.1006/viro.1998.9156. [DOI] [PubMed] [Google Scholar]
- 105.Kaufusi P.H., Kelley J.F., Yanagihara R., Nerurkar V.R. Induction of endoplasmic reticulum-derived replication-competent membrane structures by West Nile virus non-structural protein 4B. PLoS ONE. 2014;9:e84040. doi: 10.1371/journal.pone.0084040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Desprès P., Girard M., Bouloy M. Characterization of yellow fever virus proteins E and NS1 expressed in Vero and Spodoptera frugiperda cells. J. Gen. Virol. 1991;72:1331–1342. doi: 10.1099/0022-1317-72-6-1331. [DOI] [PubMed] [Google Scholar]
- 107.Apte-Sengupta S., Sirohi D., Kuhn R.J. Coupling of replication and assembly in flaviviruses. Curr. Opin. Virol. 2014;9:134–142. doi: 10.1016/j.coviro.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khromykh A.A., Varnavski A.N., Sedlak P.L., Westaway E.G. Coupling between replication and packaging of flavivirus RNA: Evidence derived from the use of DNA-based full-length cDNA clones of Kunjin virus. J. Virol. 2001;75:4633–4640. doi: 10.1128/JVI.75.10.4633-4640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saeedi B.J., Geiss B.J. Regulation of flavivirus RNA synthesis and capping. Wiley Interdiscip. Rev. RNA. 2013;4:723–735. doi: 10.1002/wrna.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pong W.-L., Huang Z.-S., Teoh P.-G., Wang C.-C., Wu H.-N. RNA binding property and RNA chaperone activity of dengue virus core protein and other viral RNA-interacting proteins. FEBS Lett. 2011;585:2575–2581. doi: 10.1016/j.febslet.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elshuber S., Allison S.L., Heinz F.X., Mandl C.W. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J. Gen. Virol. 2003;84:183–191. doi: 10.1099/vir.0.18723-0. [DOI] [PubMed] [Google Scholar]
- 112.Stadler K., Allison S.L., Schalich J., Heinz F.X. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Quaresma J.A.S., Barros V.L.R.S., Pagliari C., Fernandes E.R., Guedes F., Takakura C.F.H., Andrade H.F., Vasconcelos P.F.C., Duarte M.I.S. Revisiting the liver in human yellow fever: Virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity. Virology. 2006;345:22–30. doi: 10.1016/j.virol.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 114.Ter Meulen J., Sakho M., Koulemou K., Magassouba N.F., Bah A., Preiser W., Daffis S., Klewitz C., Bae H.-G., Niedrig M., et al. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J. Infect. Dis. 2004;190:1821–1827. doi: 10.1086/425016. [DOI] [PubMed] [Google Scholar]
- 115.Hacker U.T., Jelinek T., Erhardt S., Eigler A., Hartmann G., Nothdurft H.D., Endres S. In Vivo synthesis of tumor necrosis factor-alpha in healthy humans after live yellow fever vaccination. J. Infect. Dis. 1998;3:774–778. doi: 10.1086/517806. [DOI] [PubMed] [Google Scholar]
- 116.Querec T., Bennouna S., Alkan S., Laouar Y., Gorden K., Flavell R., Akira S., Ahmed R., Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sinigaglia L., Gracias S., Décembre E., Fritz M., Bruni D., Smith N., Herbeuval J.-P., Martin A., Dreux M., Tangy F., et al. Immature particles and capsid-free viral RNA produced by Yellow fever virus-infected cells stimulate plasmacytoid dendritic cells to secrete interferons. Sci. Rep. 2018;8:10889. doi: 10.1038/s41598-018-29235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laurent-Rolle M., Morrison J., Rajsbaum R., Macleod J.M.L., Pisanelli G., Pham A., Ayllon J., Miorin L., Martinez C., tenOever B.R., et al. The interferon signaling antagonist function of yellow fever virus NS5 protein is activated by type I interferon. Cell Host Microbe. 2014;16:314–327. doi: 10.1016/j.chom.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cong Y., McArthur M.A., Cohen M., Jahrling P.B., Janosko K.B., Josleyn N., Kang K., Zhang T., Holbrook M.R. Characterization of Yellow Fever Virus Infection of Human and Non-human Primate Antigen Presenting Cells and Their Interaction with CD4+ T Cells. PLoS Negl. Trop. Dis. 2016;10:e0004709. doi: 10.1371/journal.pntd.0004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hamerman J.A., Ogasawara K., Lanier L.L. NK cells in innate immunity. Curr. Opin. Immunol. 2005;17:29–35. doi: 10.1016/j.coi.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 121.Lam L.K.M., Watson A.M., Ryman K.D., Klimstra W.B. Gamma-interferon exerts a critical early restriction on replication and dissemination of yellow fever virus vaccine strain 17D-204. NPJ Vaccines. 2018;3:5. doi: 10.1038/s41541-017-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Douam F., Hrebikova G., Albrecht Y.E.S., Sellau J., Sharon Y., Ding Q., Ploss A. Single-cell tracking of flavivirus RNA uncovers species-specific interactions with the immune system dictating disease outcome. Nat. Commun. 2017;8:14781. doi: 10.1038/ncomms14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Neves P.C.C., Santos J.R., Tubarão L.N., Bonaldo M.C., Galler R. Early IFN-gamma production after YF 17D vaccine virus immunization in mice and its association with adaptive immune responses. PLoS ONE. 2013;8:e81953. doi: 10.1371/journal.pone.0081953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marquardt N., Ivarsson M.A., Blom K., Gonzalez V.D., Braun M., Falconer K., Gustafsson R., Fogdell-Hahn A., Sandberg J.K., Michaëlsson J. The Human NK Cell Response to Yellow Fever Virus 17D Is Primarily Governed by NK Cell Differentiation Independently of NK Cell Education. J. Immunol. 2015;195:3262–3272. doi: 10.4049/jimmunol.1401811. [DOI] [PubMed] [Google Scholar]
- 125.Kawai T., Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suthar M.S., Aguirre S., Fernandez-Sesma A. Innate immune sensing of flaviviruses. PLoS Pathog. 2013;9:e1003541. doi: 10.1371/journal.ppat.1003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo H.-Y., Zhang X.-C., Jia R.-Y. Toll-Like Receptors and RIG-I-Like Receptors Play Important Roles in Resisting Flavivirus. J. Immunol. Res. 2018;2018:6106582. doi: 10.1155/2018/6106582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olagnier D., Scholte F.E.M., Chiang C., Albulescu I.C., Nichols C., He Z., Lin R., Snijder E.J., van Hemert M.J., Hiscott J. Inhibition of dengue and chikungunya virus infections by RIG-I-mediated type I interferon-independent stimulation of the innate antiviral response. J. Virol. 2014;88:4180–4194. doi: 10.1128/JVI.03114-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fredericksen B.L., Keller B.C., Fornek J., Katze M.G., Gale M. Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J. Virol. 2008;82:609–616. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nazmi A., Mukhopadhyay R., Dutta K., Basu A. STING mediates neuronal innate immune response following Japanese encephalitis virus infection. Sci. Rep. 2012;2:347. doi: 10.1038/srep00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schuberth-Wagner C., Ludwig J., Bruder A.K., Herzner A.-M., Zillinger T., Goldeck M., Schmidt T., Schmid-Burgk J.L., Kerber R., Wolter S., et al. A Conserved Histidine in the RNA Sensor RIG-I Controls Immune Tolerance to N1-2’O-Methylated Self RNA. Immunity. 2015;43:41–51. doi: 10.1016/j.immuni.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cai X., Chiu Y.-H., Chen Z.J. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol. Cell. 2014;54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 134.Schoggins J.W., MacDuff D.A., Imanaka N., Gainey M.D., Shrestha B., Eitson J.L., Mar K.B., Richardson R.B., Ratushny A.V., Litvak V., et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van Gent M., Gack M.U. Viral pathogenesis: Dengue virus takes on cGAS. Nat. Microbiol. 2017;2:17050. doi: 10.1038/nmicrobiol.2017.50. [DOI] [PubMed] [Google Scholar]
- 136.Aguirre S., Luthra P., Sanchez-Aparicio M.T., Maestre A.M., Patel J., Lamothe F., Fredericks A.C., Tripathi S., Zhu T., Pintado-Silva J., et al. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat. Microbiol. 2017;2:17037. doi: 10.1038/nmicrobiol.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ding Q., Gaska J.M., Douam F., Wei L., Kim D., Balev M., Heller B., Ploss A. Species-specific disruption of STING-dependent antiviral cellular defenses by the Zika virus NS2B3 protease. Proc. Natl. Acad. Sci. USA. 2018;115:E6310–E6318. doi: 10.1073/pnas.1803406115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lamkanfi M., Dixit V.M. Modulation of inflammasome pathways by bacterial and viral pathogens. J. Immunol. 2011;187:597–602. doi: 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]
- 140.Yi Z., Sperzel L., Nürnberger C., Bredenbeek P.J., Lubick K.J., Best S.M., Stoyanov C.T., Law L.M.J., Yuan Z., Rice C.M., et al. Identification and characterization of the host protein DNAJC14 as a broadly active flavivirus replication modulator. PLoS Pathog. 2011;7:e1001255. doi: 10.1371/journal.ppat.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schoggins J.W., Wilson S.J., Panis M., Murphy M.Y., Jones C.T., Bieniasz P., Rice C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Richardson R.B., Ohlson M.B., Eitson J.L., Kumar A., McDougal M.B., Boys I.N., Mar K.B., de La Cruz-Rivera P.C., Douglas C., Konopka G., et al. A CRISPR screen identifies IFI6 as an ER-resident interferon effector that blocks flavivirus replication. Nat. Microbiol. 2018;3:1214–1223. doi: 10.1038/s41564-018-0244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.de Thoisy B., Dussart P., Kazanji M. Wild terrestrial rainforest mammals as potential reservoirs for flaviviruses (yellow fever, dengue 2 and St Louis encephalitis viruses) in French Guiana. Trans. R. Soc. Trop. Med. Hyg. 2004;98:409–412. doi: 10.1016/j.trstmh.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 144.Julander J.G. Animal models of yellow fever and their application in clinical research. Curr. Opin. Virol. 2016;18:64–69. doi: 10.1016/j.coviro.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 145.Wilson E.M., Bial J., Tarlow B., Bial G., Jensen B., Greiner D.L., Brehm M.A., Grompe M. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res. 2014;13:404–412. doi: 10.1016/j.scr.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Strick-Marchand H., Dusseaux M., Darche S., Huntington N.D., Legrand N., Masse-Ranson G., Corcuff E., Ahodantin J., Weijer K., Spits H., et al. A novel mouse model for stable engraftment of a human immune system and human hepatocytes. PLoS ONE. 2015;10:e0119820. doi: 10.1371/journal.pone.0119820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barrett A.D., Gould E.A. Comparison of neurovirulence of different strains of yellow fever virus in mice. J. Gen. Virol. 1986;67:631–637. doi: 10.1099/0022-1317-67-4-631. [DOI] [PubMed] [Google Scholar]
- 148.Almeida M.A.B., Cardoso J.d.C., Dos Santos E., da Fonseca D.F., Cruz L.L., Faraco F.J.C., Bercini M.A., Vettorello K.C., Porto M.A., Mohrdieck R., et al. Surveillance for yellow Fever virus in non-human primates in southern Brazil, 2001–2011: A tool for prioritizing human populations for vaccination. PLoS Negl. Trop. Dis. 2014;8:e2741. doi: 10.1371/journal.pntd.0002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luethy L.N., Erickson A.K., Jesudhasan P.R., Ikizler M., Dermody T.S., Pfeiffer J.K. Comparison of three neurotropic viruses reveals differences in viral dissemination to the central nervous system. Virology. 2016;487:1–10. doi: 10.1016/j.virol.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Franz A.W.E., Kantor A.M., Passarelli A.L., Clem R.J. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses. 2015;7:3741–3767. doi: 10.3390/v7072795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Danet L., Beauclair G., Berthet M., Moratorio G., Gracias S., Tangy F., Choumet V., Jouvenet N. Midgut barriers prevent the replication and dissemination of the yellow fever vaccine in Aedes aegypti. PLoS Negl. Trop. Dis. 2019:577213. doi: 10.1371/journal.pntd.0007299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Erickson A.K., Pfeiffer J.K. Dynamic viral dissemination in mice infected with yellow fever virus strain 17D. J. Virol. 2013;87:12392–12397. doi: 10.1128/JVI.02149-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Douam F., Soto Albrecht Y.E., Hrebikova G., Sadimin E., Davidson C., Kotenko S.V., Ploss A. Type III Interferon-Mediated Signaling Is Critical for Controlling Live Attenuated Yellow Fever Virus Infection In Vivo. MBio. 2017;8:e00819-17. doi: 10.1128/mBio.00819-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meier K.C., Gardner C.L., Khoretonenko M.V., Klimstra W.B., Ryman K.D. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. PLoS Pathog. 2009;5:e1000614. doi: 10.1371/journal.ppat.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miorin L., Laurent-Rolle M., Pisanelli G., Co P.H., Albrecht R.A., Garcia-Sastre A., Morrison J. Host-Specific NS5 Ubiquitination Determines Yellow Fever Virus Tropism. J. Virol. 2019:93. doi: 10.1128/JVI.00151-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Minor P. The polio endgame. Hum. Vaccin. Immunother. 2014;10:2106–2108. doi: 10.4161/21645515.2014.981115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stokes A., Bauer J.H., Hudson N.P. The transmission of yellow fever to Macacus rhesus. Rev. Med. Virol. 2001;11:141–148. doi: 10.1002/rmv.311. [DOI] [PubMed] [Google Scholar]
- 158.Roukens A.H., Visser L.G. Yellow fever vaccine: Past, present and future. Expert Opin. Biol. Ther. 2008;8:1787–1795. doi: 10.1517/14712598.8.11.1787. [DOI] [PubMed] [Google Scholar]
- 159.Ferreira C.C., Campi-Azevedo A.C., Peruhype-Magalhaes V., Costa-Pereira C., Albuquerque C.P., Muniz L.F., Yokoy de Souza T., Oliveira A.C.V., Martins-Filho O.A., da Mota L.M.H. The 17D-204 and 17DD yellow fever vaccines: An overview of major similarities and subtle differences. Expert Rev. Vaccines. 2018;17:79–90. doi: 10.1080/14760584.2018.1406800. [DOI] [PubMed] [Google Scholar]
- 160.Barrett A.D.T. Yellow fever live attenuated vaccine: A very successful live attenuated vaccine but still we have problems controlling the disease. Vaccine. 2017;35:5951–5955. doi: 10.1016/j.vaccine.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 161.Verma R., Khanna P., Chawla S. Yellow fever vaccine: An effective vaccine for travelers. Hum. Vaccin. Immunother. 2014;10:126–128. doi: 10.4161/hv.26549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cetron M.S., Marfin A.A., Julian K.G., Gubler D.J., Sharp D.J., Barwick R.S., Weld L.H., Chen R., Clover R.D., Deseda-Tous J., et al. Yellow fever vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2002. MMWR Recomm. Rep. 2002;51:1–11. [PubMed] [Google Scholar]
- 163.Collaborative Group for Studies on Yellow Fever Vaccines Duration of post-vaccination immunity against yellow fever in adults. Vaccine. 2014;32:4977–4984. doi: 10.1016/j.vaccine.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 164.Gotuzzo E., Yactayo S., Córdova E. Efficacy and duration of immunity after yellow fever vaccination: Systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 2013;89:434–444. doi: 10.4269/ajtmh.13-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reinhardt B., Jaspert R., Niedrig M., Kostner C., L’age-Stehr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: A model of human flavivirus infection. J. Med. Virol. 1998;56:159–167. doi: 10.1002/(SICI)1096-9071(199810)56:2<159::AID-JMV10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 166.Wieten R.W., Jonker E.F.F., van Leeuwen E.M.M., Remmerswaal E.B.M., ten Berge I.J.M., de Visser A.W., van Genderen P.J.J., Goorhuis A., Visser L.G., Grobusch M.P., et al. A Single 17D Yellow Fever Vaccination Provides Lifelong Immunity; Characterization of Yellow-Fever-Specific Neutralizing Antibody and T-Cell Responses after Vaccination. PLoS ONE. 2016;11:e0149871. doi: 10.1371/journal.pone.0149871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Niedrig M., Lademann M., Emmerich P., Lafrenz M. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: Neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop. Med. Int. Health. 1999;4:867–871. doi: 10.1046/j.1365-3156.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- 168.Watson A.M., Klimstra W.B. T Cell-Mediated Immunity towards Yellow Fever Virus and Useful Animal Models. Viruses. 2017;9:77. doi: 10.3390/v9040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Querec T.D., Pulendran B. Understanding the role of innate immunity in the mechanism of action of the live attenuated Yellow Fever Vaccine 17D. Adv. Exp. Med. Biol. 2007;590:43–53. doi: 10.1007/978-0-387-34814-8_3. [DOI] [PubMed] [Google Scholar]
- 170.Querec T.D., Akondy R.S., Lee E.K., Cao W., Nakaya H.I., Teuwen D., Pirani A., Gernert K., Deng J., Marzolf B., et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kasturi S.P., Skountzou I., Albrecht R.A., Koutsonanos D., Hua T., Nakaya H.I., Ravindran R., Stewart S., Alam M., Kwissa M., et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Da Costa Neves P.C., Matos D.C.d.S., Marcovistz R., Galler R. TLR expression and NK cell activation after human yellow fever vaccination. Vaccine. 2009;27:5543–5549. doi: 10.1016/j.vaccine.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 173.Gaucher D., Therrien R., Kettaf N., Angermann B.R., Boucher G., Filali-Mouhim A., Moser J.M., Mehta R.S., Drake D.R., Castro E., et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Monath T.P. 17D Yellow Fever Virus Vaccine. Am. J. Trop. Med. Hyg. 2013;89:1225. doi: 10.4269/ajtmh.13-0443a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tao D., Barba-Spaeth G., Rai U., Nussenzweig V., Rice C.M., Nussenzweig R.S. Yellow fever 17D as a vaccine vector for microbial CTL epitopes: Protection in a rodent malaria model. J. Exp. Med. 2005;201:201–209. doi: 10.1084/jem.20041526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bredenbeek P.J., Molenkamp R., Spaan W.J., Deubel V., Marianneau P., Salvato M.S., Moshkoff D., Zapata J., Tikhonov I., Patterson J., et al. A recombinant Yellow Fever 17D vaccine expressing Lassa virus glycoproteins. Virology. 2006;345:299–304. doi: 10.1016/j.virol.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Franco D., Li W., Qing F., Stoyanov C.T., Moran T., Rice C.M., Ho D.D. Evaluation of yellow fever virus 17D strain as a new vector for HIV-1 vaccine development. Vaccine. 2010;28:5676–5685. doi: 10.1016/j.vaccine.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 178.Guy B., Guirakhoo F., Barban V., Higgs S., Monath T.P., Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28:632–649. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 179.Stoyanov C.T., Boscardin S.B., Deroubaix S., Barba-Spaeth G., Franco D., Nussenzweig R.S., Nussenzweig M., Rice C.M. Immunogenicity and protective efficacy of a recombinant yellow fever vaccine against the murine malarial parasite Plasmodium yoelii. Vaccine. 2010;28:4644–4652. doi: 10.1016/j.vaccine.2010.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bonaldo M.C., Sequeira P.C., Galler R. The yellow fever 17D virus as a platform for new live attenuated vaccines. Hum. Vaccin. Immunother. 2014;10:1256–1265. doi: 10.4161/hv.28117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Beck A.S., Barrett A.D.T. Current status and future prospects of yellow fever vaccines. Expert Rev. Vaccines. 2015;14:1479–1492. doi: 10.1586/14760584.2015.1083430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Otshudiema J.O., Ndakala N.G., Mawanda E.-T.K., Tshapenda G.P., Kimfuta J.M., Nsibu L.-R.N., Gueye A.S., Dee J., Philen R.M., Giese C., et al. Yellow Fever Outbreak - Kongo Central Province, Democratic Republic of the Congo, August 2016. MMWR Morb. Mortal. Wkly. Rep. 2017;66:335–338. doi: 10.15585/mmwr.mm6612a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vannice K., Wilder-Smith A., Hombach J. Fractional-Dose Yellow Fever Vaccination—Advancing the Evidence Base. N. Engl. J. Med. 2018;379:603–605. doi: 10.1056/NEJMp1803433. [DOI] [PubMed] [Google Scholar]
- 184.Barrett A.D.T. Yellow Fever in Angola and Beyond--The Problem of Vaccine Supply and Demand. N. Engl. J. Med. 2016;375:301–303. doi: 10.1056/NEJMp1606997. [DOI] [PubMed] [Google Scholar]
- 185.Goldani L.Z. Yellow fever outbreak in Brazil, 2017. Braz. J. Infect. Dis. 2017;21:123–124. doi: 10.1016/j.bjid.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Possas C., Lourenco-de-Oliveira R., Tauil P.L., Pinheiro F.P., Pissinatti A., Cunha R.V.D., Freire M., Martins R.M., Homma A. Yellow fever outbreak in Brazil: The puzzle of rapid viral spread and challenges for immunisation. Mem. Inst. Oswaldo Cruz. 2018;113:e180278. doi: 10.1590/0074-02760180278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jonker E.F., Visser L.G., Roukens A.H. Advances and controversies in yellow fever vaccination. Ther. Adv. Vaccines. 2013;1:144–152. doi: 10.1177/2051013613498954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kartoglu U., Milstien J. Tools and approaches to ensure quality of vaccines throughout the cold chain. Expert Rev. Vaccines. 2014;13:843–854. doi: 10.1586/14760584.2014.923761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wiggan O., Livengood J.A., Silengo S.J., Kinney R.M., Osorio J.E., Huang C.Y., Stinchcomb D.T. Novel formulations enhance the thermal stability of live-attenuated flavivirus vaccines. Vaccine. 2011;29:7456–7462. doi: 10.1016/j.vaccine.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schotte L., Strauss M., Thys B., Halewyck H., Filman D.J., Bostina M., Hogle J.M., Rombaut B. Mechanism of action and capsid-stabilizing properties of VHHs with an in vitro antipolioviral activity. J. Virol. 2014;88:4403–4413. doi: 10.1128/JVI.03402-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chan K.R., Gan E.S., Chan C.Y.Y., Liang C., Low J.Z.H., Zhang S.L.-X., Ong E.Z., Bhatta A., Wijaya L., Lee Y.H., et al. Metabolic perturbations and cellular stress underpin susceptibility to symptomatic live-attenuated yellow fever infection. Nat. Med. 2019;25:1218–1224. doi: 10.1038/s41591-019-0510-7. [DOI] [PubMed] [Google Scholar]
- 192.McMahon A.W., Eidex R.B., Marfin A.A., Russell M., Sejvar J.J., Markoff L., Hayes E.B., Chen R.T., Ball R., Braun M.M., et al. Neurologic disease associated with 17D-204 yellow fever vaccination: A report of 15 cases. Vaccine. 2007;25:1727–1734. doi: 10.1016/j.vaccine.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 193.Thomas R.E., Lorenzetti D.L., Spragins W., Jackson D., Williamson T. Active and passive surveillance of yellow fever vaccine 17D or 17DD-associated serious adverse events: Systematic review. Vaccine. 2011;29:4544–4555. doi: 10.1016/j.vaccine.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 194.Porudominsky R., Gotuzzo E.H. Yellow fever vaccine and risk of developing serious adverse events: A systematic review. Rev. Panam Salud Publica. 2018;42:e75. doi: 10.26633/RPSP.2018.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Belsher J.L., Gay P., Brinton M., DellaValla J., Ridenour R., Lanciotti R., Perelygin A., Zaki S., Paddock C., Querec T., et al. Fatal multiorgan failure due to yellow fever vaccine-associated viscerotropic disease. Vaccine. 2007;25:8480–8485. doi: 10.1016/j.vaccine.2007.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Conesa-Botella A., Colebunders R. Response to “Case of yellow fever vaccine-associated viscerotropic disease with prolonged viremia, robust adaptive immune responses, and polymorphisms in CCR5 and RANTES genes. J. Infect. Dis. 2009;199:601. doi: 10.1086/596209. [DOI] [PubMed] [Google Scholar]
- 197.Hernandez N., Bucciol G., Moens L., Le Pen J., Shahrooei M., Goudouris E., Shirkani A., Changi-Ashtiani M., Rokni-Zadeh H., Sayar E.H., et al. Inherited IFNAR1 deficiency in otherwise healthy patients with adverse reaction to measles and yellow fever live vaccines. J. Exp. Med. 2019 doi: 10.1084/jem.20182295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Calisher C.H., Woodall J.P. Yellow Fever-More a Policy and Planning Problem than a Biological One. Emerg. Infect. Dis. 2016;22:1859–1860. doi: 10.3201/eid2210.160875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.World Health Organization . A Global Strategy to Eliminate Yellow Fever Epidemics 2017–2026. World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
- 200.Jean K., Donnelly C.A., Ferguson N.M., Garske T. A Meta-Analysis of Serological Response Associated with Yellow Fever Vaccination. Am. J. Trop. Med. Hyg. 2016;95:1435–1439. doi: 10.4269/ajtmh.16-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao S., Stone L., Gao D., He D. Modelling the large-scale yellow fever outbreak in Luanda, Angola, and the impact of vaccination. PLoS Negl. Trop. Dis. 2018;12:e0006158. doi: 10.1371/journal.pntd.0006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hamlet A., Jean K., Yactayo S., Benzler J., Cibrelus L., Ferguson N., Garske T. POLICI: A web application for visualising and extracting yellow fever vaccination coverage in Africa. Vaccine. 2019;37:1384–1388. doi: 10.1016/j.vaccine.2019.01.074. [DOI] [PubMed] [Google Scholar]
- 203.Schafer B., Holzer G.W., Joachimsthaler A., Coulibaly S., Schwendinger M., Crowe B.A., Kreil T.R., Barrett P.N., Falkner F.G. Pre-clinical efficacy and safety of experimental vaccines based on non-replicating vaccinia vectors against yellow fever. PLoS ONE. 2011;6:e24505. doi: 10.1371/journal.pone.0024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Maciel M., Jr., Cruz Fda S., Cordeiro M.T., da Motta M.A., Cassemiro K.M., Maia Rde C., de Figueiredo R.C., Galler R., Freire Mda S., August J.T., et al. A DNA vaccine against yellow fever virus: Development and evaluation. PLoS Negl. Trop. Dis. 2015;9:e0003693. doi: 10.1371/journal.pntd.0003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tottey S., Shoji Y., Jones R.M., Chichester J.A., Green B.J., Musiychuk K., Si H., Manceva S.D., Rhee A., Shamloul M., et al. Plant-Produced Subunit Vaccine Candidates against Yellow Fever Induce Virus Neutralizing Antibodies and Confer Protection against Viral Challenge in Animal Models. Am. J. Trop. Med. Hyg. 2018;98:420–431. doi: 10.4269/ajtmh.16-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tripathi N.K., Shrivastava A. Recent Developments in Recombinant Protein-Based Dengue Vaccines. Front. Immunol. 2018;9:1919. doi: 10.3389/fimmu.2018.01919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Khera T., Behrendt P., Bankwitz D., Brown R.J.P., Todt D., Doepke M., Khan A.G., Schulze K., Law J., Logan M., et al. Functional and immunogenic characterization of diverse HCV glycoprotein E2 variants. J. Hepatol. 2019;70:593–602. doi: 10.1016/j.jhep.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 208.Wong G. Progress in Elucidating Potential Markers and Mechanisms of Rapid Protection Conferred by the VSV-Vectored Ebola Virus Vaccine. MBio. 2019;10 doi: 10.1128/mBio.01597-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Monath T.P., Lee C.K., Julander J.G., Brown A., Beasley D.W., Watts D.M., Hayman E., Guertin P., Makowiecki J., Crowell J., et al. Inactivated yellow fever 17D vaccine: Development and nonclinical safety, immunogenicity and protective activity. Vaccine. 2010;28:3827–3840. doi: 10.1016/j.vaccine.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 210.Monath T.P., Fowler E., Johnson C.T., Balser J., Morin M.J., Sisti M., Trent D.W. An inactivated cell-culture vaccine against yellow fever. N. Engl. J. Med. 2011;364:1326–1333. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]
- 211.Chen L.H., Hamer D.H. Vaccination Challenges in Confronting the Resurgent Threat From Yellow Fever. JAMA. 2017;318:1651–1652. doi: 10.1001/jama.2017.14258. [DOI] [PubMed] [Google Scholar]
- 212.Pushko P., Lukashevich I.S., Weaver S.C., Tretyakova I. DNA-launched live-attenuated vaccines for biodefense applications. Expert Rev. Vaccines. 2016;15:1223–1234. doi: 10.1080/14760584.2016.1175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lukashev A.N., Lashkevich V.A., Ivanova O.E., Koroleva G.A., Hinkkanen A.E., Ilonen J. Recombination in circulating enteroviruses. J. Virol. 2003;77:10423–10431. doi: 10.1128/JVI.77.19.10423-10431.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bandyopadhyay A.S., Garon J., Seib K., Orenstein W.A. Polio vaccination: Past, present and future. Future Microbiol. 2015;10:791–808. doi: 10.2217/fmb.15.19. [DOI] [PubMed] [Google Scholar]
- 215.Klitting R., Riziki T., Moureau G., Piorkowski G., Gould E.A., de Lamballerie X. Exploratory re-encoding of yellow fever virus genome: New insights for the design of live-attenuated viruses. Virus Evol. 2018;4:vey021. doi: 10.1093/ve/vey021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Martinez M.A., Jordan-Paiz A., Franco S., Nevot M. Synonymous Virus Genome Recoding as a Tool to Impact Viral Fitness. Trends Microbiol. 2016;24:134–147. doi: 10.1016/j.tim.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 217.Nougairede A., de Fabritus L., Aubry F., Gould E.A., Holmes E.C., de Lamballerie X. Random codon re-encoding induces stable reduction of replicative fitness of Chikungunya virus in primate and mosquito cells. PLoS Pathog. 2013;9:e1003172. doi: 10.1371/journal.ppat.1003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.de Fabritus L., Nougairede A., Aubry F., Gould E.A., de Lamballerie X. Attenuation of tick-borne encephalitis virus using large-scale random codon re-encoding. PLoS Pathog. 2015;11:e1004738. doi: 10.1371/journal.ppat.1004738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Carrington C.V.F., Auguste A.J. Evolutionary and ecological factors underlying the tempo and distribution of yellow fever virus activity. Infect. Genet. Evol. 2013;13:198–210. doi: 10.1016/j.meegid.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 220.Lemoine F., Correia D., Lefort V., Doppelt-Azeroual O., Mareuil F., Cohen-Boulakia S., Gascuel O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019;47:W260–W265. doi: 10.1093/nar/gkz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu J., Bera A.K., Kuhn R.J., Smith J.L. Structure of the Flavivirus helicase: Implications for catalytic activity, protein interactions, and proteolytic processing. J. Virol. 2005;79:10268–10277. doi: 10.1128/JVI.79.16.10268-10277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Doudna J.A., Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 223.Pugachev K.V., Guirakhoo F., Ocran S.W., Mitchell F., Parsons M., Penal C., Girakhoo S., Pougatcheva S.O., Arroyo J., Trent D.W., et al. High fidelity of yellow fever virus RNA polymerase. J. Virol. 2004;78:1032–1038. doi: 10.1128/JVI.78.2.1032-1038.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vlachakis D., Koumandou V.L., Kossida S. A holistic evolutionary and structural study of flaviviridae provides insights into the function and inhibition of HCV helicase. PeerJ. 2013;1:e74. doi: 10.7717/peerj.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gardy J.L., Loman N.J. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat. Rev. Genet. 2018;19:9–20. doi: 10.1038/nrg.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brinkmann A., Ergünay K., Radonić A., Kocak Tufan Z., Domingo C., Nitsche A. Development and preliminary evaluation of a multiplexed amplification and next generation sequencing method for viral hemorrhagic fever diagnostics. PLoS Negl. Trop. Dis. 2017;11:e0006075. doi: 10.1371/journal.pntd.0006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.McMullan L.K., Frace M., Sammons S.A., Shoemaker T., Balinandi S., Wamala J.F., Lutwama J.J., Downing R.G., Stroeher U., MacNeil A., et al. Using next generation sequencing to identify yellow fever virus in Uganda. Virology. 2012;422:1–5. doi: 10.1016/j.virol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 228.Quick J., Loman N.J., Duraffour S., Simpson J.T., Severi E., Cowley L., Bore J.A., Koundouno R., Dudas G., Mikhail A., et al. Real-time, portable genome sequencing for Ebola surveillance. Nature. 2016;530:228–232. doi: 10.1038/nature16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kafetzopoulou L.E., Pullan S.T., Lemey P., Suchard M.A., Ehichioya D.U., Pahlmann M., Thielebein A., Hinzmann J., Oestereich L., Wozniak D.M., et al. Metagenomic sequencing at the epicenter of the Nigeria 2018 Lassa fever outbreak. Science. 2019;363:74–77. doi: 10.1126/science.aau9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jain M., Olsen H.E., Paten B., Akeson M. The Oxford Nanopore MinION: Delivery of nanopore sequencing to the genomics community. Genome Biol. 2016;17:239. doi: 10.1186/s13059-016-1103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Grubaugh N.D., Ladner J.T., Lemey P., Pybus O.G., Rambaut A., Holmes E.C., Andersen K.G. Tracking virus outbreaks in the twenty-first century. Nat. Microbiol. 2019;4:10–19. doi: 10.1038/s41564-018-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wasserman S., Tambyah P.A., Lim P.L. Yellow fever cases in Asia: Primed for an epidemic. Int. J. Infect. Dis. 2016;48:98–103. doi: 10.1016/j.ijid.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 233.Chen C., Jiang D., Ni M., Li J., Chen Z., Liu J., Ye H., Wong G., Li W., Zhang Y., et al. Phylogenomic analysis unravels evolution of yellow fever virus within hosts. PLoS Negl. Trop. Dis. 2018;12:e0006738. doi: 10.1371/journal.pntd.0006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sall A.A., Faye O., Diallo M., Firth C., Kitchen A., Holmes E.C. Yellow fever virus exhibits slower evolutionary dynamics than dengue virus. J. Virol. 2010;84:765–772. doi: 10.1128/JVI.01738-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mandl J.N., Ahmed R., Barreiro L.B., Daszak P., Epstein J.H., Virgin H.W., Feinberg M.B. Reservoir host immune responses to emerging zoonotic viruses. Cell. 2015;160:20–35. doi: 10.1016/j.cell.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lequime S., Paul R.E., Lambrechts L. Determinants of Arbovirus Vertical Transmission in Mosquitoes. PLoS Pathog. 2016;12:e1005548. doi: 10.1371/journal.ppat.1005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Diallo M., Thonnon J., Fontenille D. Vertical transmission of the yellow fever virus by Aedes aegypti (Diptera, Culicidae): Dynamics of infection in F1 adult progeny of orally infected females. Am. J. Trop. Med. Hyg. 2000;62:151–156. doi: 10.4269/ajtmh.2000.62.151. [DOI] [PubMed] [Google Scholar]
- 238.Amraoui F., Vazeille M., Failloux A.B. French Aedes albopictus are able to transmit yellow fever virus. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.39.30361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kraemer M.U.G., Reiner R.C., Jr., Brady O.J., Messina J.P., Gilbert M., Pigott D.M., Yi D., Johnson K., Earl L., Marczak L.B., et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019;4:854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hamrick P.N., Aldighieri S., Machado G., Leonel D.G., Vilca L.M., Uriona S., Schneider M.C. Geographic patterns and environmental factors associated with human yellow fever presence in the Americas. PLoS Negl. Trop. Dis. 2017;11:e0005897. doi: 10.1371/journal.pntd.0005897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kupferschmidt K. After 40 years, the most important weapon against mosquitoes may be failing. Science. 2016 doi: 10.1126/science.aal0254. [DOI] [Google Scholar]
- 242.Moyes C.L., Vontas J., Martins A.J., Ng L.C., Koou S.Y., Dusfour I., Raghavendra K., Pinto J., Corbel V., David J.-P., et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017;11:e0005625. doi: 10.1371/journal.pntd.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Flores H.A., O’Neill S.L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018;16:508–518. doi: 10.1038/s41579-018-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Harris A.F., McKemey A.R., Nimmo D., Curtis Z., Black I., Morgan S.A., Oviedo M.N., Lacroix R., Naish N., Morrison N.I., et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 2012;30:828–830. doi: 10.1038/nbt.2350. [DOI] [PubMed] [Google Scholar]
- 245.Armbruster P.A. Tiger mosquitoes tackled in a trial. Nature. 2019;65:395. doi: 10.1038/d41586-019-02000-0. [DOI] [PubMed] [Google Scholar]
- 246.Zheng X., Zhang D., Li Y., Yang C., Wu Y., Liang X., Liang Y., Pan X., Hu L., Sun Q., et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature. 2019 doi: 10.1038/s41586-019-1407-9. [DOI] [PubMed] [Google Scholar]
- 247.Olmo R.P., Ferreira A.G.A., Izidoro-Toledo T.C., Aguiar E.R.G.R., de Faria I.J.S., de Souza K.P.R., Osório K.P., Kuhn L., Hammann P., de Andrade E.G., et al. Control of dengue virus in the midgut of Aedes aegypti by ectopic expression of the dsRNA-binding protein Loqs2. Nat. Microbiol. 2018;3:1385–1393. doi: 10.1038/s41564-018-0268-6. [DOI] [PubMed] [Google Scholar]
- 248.Matthews B.J., Dudchenko O., Kingan S.B., Koren S., Antoshechkin I., Crawford J.E., Glassford W.J., Herre M., Redmond S.N., Rose N.H., et al. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature. 2018;563:501–507. doi: 10.1038/s41586-018-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Matthews B.J., McBride C.S., DeGennaro M., Despo O., Vosshall L.B. The neurotranscriptome of the Aedes aegypti mosquito. BMC Genomics. 2016;17:32. doi: 10.1186/s12864-015-2239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.