Abstract
Black chokeberry (Aronia melnocarpa) is a source of many bioactive compounds with a wide spectrum of health-promoting properties. Fresh, unprocessed chokeberry fruits are rarely consumed due to their astringent taste, but they are used in the food industry for the production of juices, nectars, syrups, jams, preserves, wines, tinctures, fruit desserts, jellies, fruit teas and dietary supplements. Polyphenols are biofactors that determine the high bioactivity of chokeberries, some of the richest sources of polyphenols, which include anthocyanins, proanthocyanidins, flavonols, flavanols, proanthocyanidins, and phenolic acids. Chokeberry fruit and products have great antioxidant and health-promoting potential as they reduce the occurrence of free radicals. This publication reviewed the scientific research regarding the phenolic compounds and the antioxidant potential of chokeberry fruits, products and isolated compounds. These findings may be crucial in future research concerning chokeberry based functional food products. Chokeberry fruits can be considered as promising component of designed food with enhanced antioxidant potential. However, like other plants and medicinal products of natural origin, black chokeberry requires extensive studies to determine its antioxidant potential, safety and mechanisms of action.
Keywords: chokeberry, Aronia melanocarpa, polyphenols, antioxidants, free radicals
1. Introduction
Black chokeberry (Aronia melanocarpa L.) is a perennial shrub of the Rosaceae family. The chokeberry plant is native to eastern parts of North America, but it was introduced in Europe at the beginning of the twentieth century. The usable parts of chokeberry are mainly fruits [1]. There are many cultivated chokeberry varieties: ‘Viking’, ‘Nero’, ‘Galicjanka’, ‘Hugin’, ‘N’, ‘Rubin’, ‘Aron’ and others [2,3,4,5]. Fresh, unprocessed chokeberry fruits are rarely consumed due to their astringent taste, but they are used in the food industry for the production of juices, nectars, syrups, jams, preserves, wines, tinctures, fruit desserts, jellies, fruit teas and dietary supplements [6]. Chokeberry anthocyanins can be added to foods as natural dyes [7,8]. The following paper provides a synthetic and systematic review of the current literature on the subject of selected antioxidative and bioactive compounds found in black chokeberry. The study involved a comparative analysis of the concentration of selected compounds in black chokeberry Aronia melanocarpa L. fruits, products and isolated compounds. The study involved a comparison of phenolics composition, e.g., anthocyanins, proanthocyanidins, flavonols, flavanols, proanthocyanidins, and phenolic acids. Additionally, the antioxidant activity of chokeberry fruits and products by mean of various assays had been reviewed. The latest findings in the subject have not been studied so far. The review’s findings may be crucial in future research concerning chokeberry based functional food products, a promising component of designed food with enhanced antioxidant potential.
2. Botanical and Chemical Characteristics
Chokeberry is a shrub which may grow as high as three meters. Young bushes are compact, whereas mature ones resemble a sprawling tree. Leaves covering branches are oval. They are green in the spring and summer, but in the autumn they become reddish brown. Fruits in clusters reach maturity in late August and September. Mature chokeberry fruits are black and blue from the outside and on the cross section. Fruits are spherical. Depending on the variety, their diameter ranges from 6.1 to 17.8 mm, whereas the weight of 100 fruits ranges from 32 to 111.7 g, sometimes even to about 280 g [1].
The composition and health value of chokeberry fruit depends on many factors, e.g., variety, maturity, environmental and climatic conditions [9,10]. The dry weight of chokeberry fruit, juice and pomace analysed by Mayer-Miebach, Adamiuk, and Behsnilian [11] was respectively 17.9–26%, 11.1–17.4% and 44.6–50%. Ochmian et al. [1,12] found that chokeberry fruits contained 15.3–19.5% of dry matter, including 14.2–18.7% of soluble substances. Skupień and Oszmiański [13] found high content of dry matter in fruit, i.e., 26.67–30.76%. The amount of protein in the fruit was low and amounted to 3.7 g/100 g DM (dry matter) of fruit [14]. Arginine, tyrosine, histidine, lysine, cysteine, α-alanine, asparagine, serine, glutamic acid and threonine are some of the amino acids in chokeberry. Most amino acids, including essential ones, were found in pomace. Their total weight amounted to 28.9 g/kg DM [15,16]. The total lipid content in fresh chokeberry fruit was 0.09–0.17% [17,18]. The largest amounts were found in stones and skin fractions, i.e., 13.9% and 2.9–9.8%, respectively [19]. The content of lipids in chokeberry pomace amounted to 5.5 g/100 g, including 65.0% of polyunsaturated fatty acids (PUFA). The pomace chiefly contained linoleic and oleic acids. Chokeberry seed oil contains sterols and phospholipids [20,21]. Sugars and dietary fibre are chokeberry carbohydrates. Studies have shown that the total content of carbohydrates in fresh fruits ranges from 6.21 to 20.92 g/100, total reducing sugars from 5.71 to 19.36 g/100 g, including fructose 1.38–4.71 g/100 g and glucose 1.09–5.70 g/100 g [1,11,13,22,23,24]. The content of sucrose ranges from 0 to 1.53 g/100 g of fruit. Chokeberry products and fruit contain relatively large amounts of sorbitol, i.e., 8.56 g/100 g of fruit. Chokeberry pomace, which consists of peels, stones and internal fruit cell structures, is a rich source of dietary fibre, which amounts to about 70% of dry matter.
More than 60% of chokeberry dietary fibre is composed of insoluble fraction (lignin, cellulose, hemicellulose) [25,26,27]. Chokeberries also contain vitamin B, carotenoids, tocopherols, vitamin C and vitamin K [17,21,28]. Ash amounts to 6.8 g/100 g DM in chokeberry fruit. Chokeberries also contain macroelements (K, Ca, P, Mg and Na), essential trace elements (Zn, Fe, Se, Cu, Mo, Cr), trace elements that are probably essential (Mn, Si, Ni, B, V) and toxic elements (Pb, Cd, Hg, As). Chokeberry fruit and products are potentially rich sources of K, Ca, P, Mg, Na, Fe and Zn [14,25,29]. Table 1 shows detailed data on the composition of chokeberry fruit and products.
Table 1.
The qualitative composition of fruits, juice and other black chokeberry products.
| Components | Fruits | Juice | Other Black Chokeberry Products |
|---|---|---|---|
| Dry matter | 15.30–19.50% [1], 17.9–26% [11], 26.67–30.76% [13], 15.7% [18], 22.14–23.45% [24], 21.0–26.0% [30], 18.3–23.5 g/100 g FW [31], 18.92–20.14% [32] | 19.22–26.94% [5], 11.1–17.4% [11], 15.46–16.87% [33], 13.42–21.54% [34], 18.1% [35] |
44.6–50% [11], 90.21% dried pomace [16], 93.6–94.9% dried pomace fractions [19], 96.86% fruit powder [33], 97.63–98.30% pomace powder [33], 90.44–94.797% powder [34], 93.60–93.96% capsules [34], 88.32–96.01% fruit tea [34], 82.00–84.61% dried berries [34], 5.7% extract [36], 90.8% pomace [37], 108 g/kg nectar [38] |
| Solube solids (ºBrix) | 14.20–18.70 [1], 16.0 [12], 21.7–24.1 [13], 8.9–11.2 [17], 15.2–22.9 [23], 15.5–18.2 g/100 g FW [39], 14.4–15.6 [32], | 18.15–25.61 [5], 13.30–20.99 [34], 12.50–20.10 [40], 7.8–14.3 [41] | 26.75–37.53 powder, 31.91–83.71 capsules, 12.17–38.22 fruit tea, 20.93–25.49 dried berries [34] |
| Protein | 3.71 g/100 g DM [14], 0.60–0.81 g/100g [17], 0.7 g/100g FW [18] | 0.2 g/100 g [42,43] | 10.77% dried pomace [16], 4.9–24.1% DM dried pomace fractions [19] |
| Formol number (total amino acid) |
10.0–19.9 (ml 0.1 M NaOH/100 g) [23] | - | - |
| Fat | 0.09–0.17 g/100g [17], 0.14% [18] | < 0.1 g/100g [42,43] | 5.15% dried pomace [16], 2.9–13.9% DM dried pomace fractions [19], 19.3 g/kg seeds [21] |
| SFA | - | - | 9.51% TFA dried pomace [16] |
| UFA | - | - | 90.49% TFA dried pomace [16] |
| PUFA n-6 | - | - | 43.60% TFA dried pomace [16] |
| PUFA n-3 | - | - | 29.97% TFA dried pomace [16] |
| Sterols | - | - | 1.2 g/kg seed oil [21] |
| Phospholipids | - | - | 2.8 g/kg seed oil [21] |
| Carbohydrates | 13.73–15.06 g/100g [17] | 17.9 g/100g [42,43] | - |
| Total dietary fibre | - | 0.3 g/100g [42,43] | 21.79% dried pomace [16], 63.5–77.9% DM dried pomace fractions [19], 72.0% DM fibre [27], 95.8 g/100 g DM pomace [37] |
| Soluble fibre | 0.81–1.03 g/100 g [17] | - | 5.4% DM fibre [27] |
| Insoluble fibre | 4.01–5.25 g/100 g [17] | - | 66.6% DM fibre [27] |
| NDF | - | - | 34.65% dried pomace [16], 41.9–48.5% fibre [25], 87.48 g/100 g DM pomace [26] |
| ADF | - | - | 35.59% dried pomace [16], 34.6–45.6% fibre [25], 57.24 g/100 g DM pomace [26] |
| ADL | - | - | 17.58% dried pomace [16] |
| Lignin | - | - | 15.0–26.8% fibre [25], 22.68 g/100 g DM pomace [26], 23.03 g/100 g DM pomace [37] |
| Cellulose | - | - | 18.5–25.1% fibre [25], 34.56 g/100 g DM pomace [26], 33.14 g/100 g DM pomace [37] |
| Hemicellulose | - | - | 0.81–7.72% fibre [25], 30.24 g/100 g DM pomace [26], 32.08 g/100 g DM pomace [37] |
| Total pectins | - | - | 10.7–14.0% fibre [25], 7.52 g/100 g DM pomace [37] |
| Water soluble pectins | - | - | 1.00–2.86% fibre [25] |
| Total sugars | 9.16–13.79 g/100 g [1], 68–158 g/kg [11], 19.32–20.92 g/100 g [13], 83.0–111.6 g/kg [24], 44.596–125.851 g/L [44], 6.21–6.91 g/100 g [32] | 110–143 g/L [11], 85.24–87.31 g/100 g DM [33], 12.0–19.6 g/100 mL [45], 89.49–162.37 g/L [40], 14.84% [35] | 46.15 g/100 g DM fruit powder [33], 25.05–31.83 g/100 g DM pomace powder [33], 84 g/kg pomace 11, 38.2 g/kg nectar [38] |
| Solube sugars | - | - | 0.35% dried pomace [16] |
| Reducing sugars | 8.83–12.48 g/100 g [1], 18.21–19.36 g/100 g [13], 23.00 g/100 g DM [14], 71.5–102.4 g/kg [24], 6.8–9.0 mg/100 g [30], 5.71–6.58 g/100 g [32] |
- | - |
| Sucrose | ND-1.53 g/100 g [13], ND-0.7 g/kg [23], 0.22–0.48 g/100 g [32] | 5.4–6.8 g/100 mL [45], ND-3.03 g/L [40], ND [43] | 0.03–0.43% DM dried pomace fractions [19], 0.16 g/L extract [36] |
| Glucose | 10.9–40.1 g/kg [11], 36.3–57.0 g/kg [23], 9.794–18.429 g/L [44] | 31.7–40.4 g/L [11], 26.15–26.91 g/100 g DM [33], 1.5–3.3 g/100 mL [45], 26.29–42.86 g/L [40], 22.0–34.0 g/kg [41], 4.25 g/100 g [42,43], 3.69% [35] |
22.8 g/kg pomace [11], 0.39–0.80% DM dried pomace fractions [19], 14.64 g/100 g DM fruit powder [33], 5.08–12.55 g/100 g DM pomace powder [33], 6.29 g/L extract [36] |
| Fructose | 13.8–41.6 g/kg [11], 26.0–47.1 g/kg [23], 13.828–40.080 g/L [44] | 30.2–39.1 g/L [11], 19.04–19.18 g/100 g DM [33], 1.5–3.3 g/100 mL [45], 27.44–41.18 g/L [40], 22.0–32.0 g/kg [41], 3.87 g/100 g [42,43], 2.15% [35] | 23.6 g/kg pomace [11], 0.48–0.58% DM dried pomace fractions [19], 10.43 g/100 g DM fruit powder [33], 6.64–6.73 g/100 g DM pomace powder [33], 9.58 g/L extract [36] |
| Sorbitol | 43.6–76.1 g/kg [11], 46.3–85.6 g/kg [23], 20.974–67.342 g/L [44] | 47.8–63.8 g/L [11], 39.29–41.99 g/100 g DM [33], 3.6–6.3 g/100 mL [45], 35.77–77.30 g/L [40], 27.4–48.1 g/kg [41], 7.39 g/100 g [42,43], 9.00% [35] |
37.6 g/kg pomace [11], 1.06–2.32% DM dried pomace fractions [19],21.08 g/100 g DM fruit powder [33], 12.55–13.33 g/100g DM pomace powder [33], 20.1 g/L extract [36] |
| pH | 3.36–3.79 [17], 3.3–3.7 [18], 3.23–3.57 [31] | 3.77–3.96 [5], 3.54–3.92 [34], 3.5 [45], 3.42–3.72 [40], 3.15–3.45 [41], 3.5 [35] | 4.02–4.13 powder [34], 3.31–4.10 capsules [34], 4.01–4.13 fruit tea [34], 4.01–4.28 dried berries [34], 3.3 nectar [38] |
| Titratable acidity (citric acid eq) |
0.75–1.05 g/100 g [1], 1.42 g/100 g [12], 0.493–0.548 g/100 g [13], 6.7–11.9 g/kg (eq malic acid) [23], 1.9–2.6 g/kg [24], 1.03–1.44 g/100 g FW (eq malic acid) [31], 1.24–1.31 g/100 g [32] | 0.89–1.06% [5], 0.29–1.32% [34], 0.85–1.22% [41], 0.89% [35] | 0.52–0.58% DM dried pomace fractions [19], 1.67–2.30% powder [46], 2.10–4.66% capsules [34], 1.08–1.60% fruit tea [34], 1.13–1.37% dried berries [34], 13.2 g/L extract [36], 3.9 g/kg nectar [38] |
| Organic acids total | 8.21–16.812 g/L [44] | 12.27–21.87 g/L [40] | - |
| Citric acid | 0.18–0.25 g/100 g [17], 0.7–1.3 g/kg [23], 0.584–2.399 g/L [44] | 0.72–2.55 g/L [40] | 48.9–94.2 mg/100 g DM dried pomace fractions [19], 10.5 g/L extract [36] |
| Isocitric acid | 17.2–37.3 g/kg [23] | - | - |
| Malic acid | 0.56–1.63 g/100 g [17], 4.5–12.8 g/kg [23], 5.112–11.695 g/L [44] | 9.32–13.33 g/L [40] | 124.5–301.0 mg/100 g DM dried pomace fractions [19] |
| Oxalic acid | - | 0.17–0.79 g/L [40] | - |
| Tartaric acid | 0.321–2.068 g/L [44] | 4.66–5.20 g/L [40] | - |
| Quinic acid | 4.1–6.8 g/kg [23] | - | - |
| Succinic acid | 0.478–0.977 g/L [44] | - | - |
| Fumaric acid | 0.051–0.107 g/L [44] | - | - |
| Galacturonic acid | - | - | 535.4–1561.2 mg/100 g DM dried pomace fractions [19] |
| Ash | 6.83 g/100 g DM [14], 0.37–0.49 g/100g [17], 4.2–11.8 g/kg [23] | 0.5 g/100g [42,43] | 1.95% dried pomace [16], 1.4–3.9% DM dried pomace fractions [19] |
| Na | 2.0–3.7 mg/100 g [17], 2.6% [18], 12.5–16.8 mg/kg [29] | 19.6–56.3 mg/kg [29], 19 ppm [45] | 0.037 g/kg dried pomace [16], 52.5–89.0 mg/kg DM dried pomace fractions [19], 9.4–40.8 mg/kg fruit tea [29] |
| K | 164–265 mg/100 g [17], 218 mg% [18], 1356.3–3659.7 mg/kg [23], 2707–4977 mg/kg [29] | 848–3204 mg/kg [29], 1242 ppm [45] | 2.78 g/kg dried pomace [16], 1814.3–3075.9 mg/kg DM dried pomace fractions [19], 385–2792 mg/kg fruit tea [29] |
| Ca | 22.8–43.9 mg/100 g [17], 32.2 mg% [18], 119.0–552.3 mg/kg [23], 601–1167 mg/kg [29] | 138–1225 mg/kg [29], 151 ppm [45] | 2.75 g/kg dried pomace [16], 2186.8–4080.4 mg/kg DM dried pomace fractions [19], 469–1395 mg/kg fruit tea [29] |
| Mg | 15.5–17.4 mg/100 g [17], 16.2 mg% [18], 83.3–314.2 mg/kg [23], 164–578 mg/kg [29] | 209–589 mg/kg [29], 85 ppm [45] | 0.88 g/kg dried pomace [16], 370.8–2501.0 mg/kg DM dried pomace fractions [19], 99–338 mg/kg fruit tea [29] |
| P | 15.9–21.7 mg/100 g [17], 257.0–417.5 mg/kg [23], 239–956 mg/kg [29] | 167–1037 mg/kg [29] | 2.39 g/kg dried pomace [16], 282–526 mg/kg fruit tea [29] |
| Zn | 0.090–0.220 mg/100 g [17], 4.09–8.40 mg/kg [29] | 0.89–3.45 mg/kg [29] | 15.7 mg/kg dried pomace [16], 5.6–36.9 mg/kg DM dried pomace fractions [19], 2.41–8.27 mg/kg fruit tea [29] |
| Fe | 0.33–1.68 mg/100 g [17], 0.93 mg% [18], 9.4–14.2 mg/kg [29] | 7.2–25.2 mg/kg [29] | 197 mg/kg dried pomace [16], 68.9–86.2 mg/kg DM dried pomace fractions [19], 22.8–58.1 mg/kg fruit tea [29] |
| Se | 0.21–0.28 mg/kg [29] | ND-1.73 mg/kg [29] | 0.26–0.56 mg/kg fruit tea [29] |
| Cu | 0.035–0.056 mg/100 g [17], 0.82–2.11 mg/kg [29] | 0.68–4.51 mg/kg [29] | 1.95 mg/kg dried pomace [16], 5.0–12.4 mg/kg DM dried pomace fractions [19], 1.76–4.00 mg/kg fruit tea [29] |
| Mo | 0.016–0.021 mg/kg [29] | ND-0.064 mg/kg [29] | 0.050–0.290 mg/kg fruit tea [29] |
| Mn | 0.132–0.263 mg/100 g [17], 5.49–17.89 mg/kg [29] | 2.98–11.77 mg/kg [29] | 31.5 mg/kg dried pomace [16], 2.63–52.2 mg/kg fruit tea [29] |
| Ni | 0.143–0.740 mg/kg [29] | 0.130–0.860 mg/kg [29] | 0.204–0.568 mg/kg fruit tea [29] |
| V | 0.40–1.58 mg/kg [29] | 0.47–1.43 mg/kg [29] | 0.31–0.96 mg/kg fruit tea [29] |
| Si | 2.37–6.37 mg/kg [29] | ND-7.4 mg/kg [29] | 2.18–6.30 mg/kg fruit tea [29] |
| Cr | 0.49–0.53 mg/kg [29] | 0.55–0.74 mg/kg [29] | 0.44–0.85 mg/kg fruit tea [29] |
| Li | ND-6.75 mg/kg [29] | ND-0.072 mg/kg [29] | ND-0.075 mg/kg fruit tea [29] |
| Sr | 1.57–7.05 mg/kg [29] | 0.34–3.67 mg/kg [29] | 1.01–9.10 mg/kg fruit tea [29] |
| Al | 2.88–4.40 mg/kg [29] | 1.64–9.70 mg/kg [29] | 3.60–25.47 mg/kg fruit tea [29] |
| Sn | 0.62–0.72 mg/kg [29] | 0.86–1.09 mg/kg [29] | 0.58–0.89 mg/kg fruit tea [29] |
| As | 0.20–0.36 mg/kg [29] | 0.37–0.79 mg/kg [29] | 0.30–0.98 mg/kg fruit tea [29] |
| Cd | 0.016–0.041 mg/kg [29] | 0.050–0.064 mg/kg [29] | 0.035–0.059 mg/kg fruit tea [29] |
| Ba | 1.48–6.66 mg/kg [29] | 0.77–2.06 mg/kg [29] | 1.48–9.62 mg/kg fruit tea [29] |
| Pb | 0.048–0.091 mg/kg [29] | ND-0.143 mg/kg [29] | 0.053–0.205 mg/kg fruit tea [29] |
| Sb | ND-0.29 mg/kg [29] | ND-0.54 mg/kg [29] | ND-0.66 mg/kg fruit tea [29] |
| Co | 0.019–0.043 mg/kg [29] | ND-0.092 mg/kg [29] | ND-0.144 mg/kg fruit tea [29] |
| B | 2.88–14.22 mg/kg [29] | ND-9.32 mg/kg [29] | 2.60–4.96 mg/kg fruit tea [29] |
| Vitamin C | 31 mg/100 g [12], 1.9–8.4 mg/100 g [13], 4.0–19.3 mg/100 g [17], 13.7 mg/100 g [18], 2.3–13.7 mg/100 g [30], 0.911–1.552 g/L [44], 3.5–7.2 mg/100 g FW [31] | 29 g/L [47] | 1100 mg/kg nectar [38] |
| Vitamin B1 | 0.017–0.019 mg/100 g [17] | - | - |
| Vitamin B2 | 0.016–0.027 mg/100 g [17] | - | - |
| Niacin | 0.27–0.34 mg/100 g [17] | - | - |
| Panthotenic acid | 0.225–0.382 mg/100 g [17] | - | - |
| Vitamin B6 | 0.024–0.029 mg/100 g [17] | - | - |
| Folate | 0.002–0.004 mg/100 g [17], 20.4–20.6 µg/100 g FW [48] | - | - |
| Vitamin A | 0.77 mg/100 g [18] | - | - |
| Carotenoids total | 48.6 mg/kg [49] | 97.8 µg/L [47] | - |
| Lycopene | ND 31, 0.6 mg/kg [49] | - | ND nectar [38] |
| α-Carotene | 6.4–10.6 µg/100 g [17] | - | |
| β-Carotene | 495–887 µg/100 g [17], 46.4 µg/g DW [28], 16.7 mg/kg [49] | - | ND nectar [38] |
| ζ-Carotene | 0.3 mg/kg [49] | - | - |
| β-Cryptoxanthin | 234–649 µg/100 g [17], ND [28], 12.2 mg/kg [49] | - | - |
| Lutein | 9.1 µg/g DW [28], 3.4 mg/kg [49] | - | - |
| 5,6-Epoxylutein | 0.4 mg/kg [49] | - | - |
| trans-Violaxanthin | 4.5 mg/kg [49] | - | - |
| cis-Violaxanthin | 8.5 mg/kg [49] | - | - |
| Neoxanthin | 2.0 mg/kg [49] | - | - |
| Xanthophyll | 2.7 µg/g DW [28] | - | - |
| Vitamin E | - | 55.5 mg/kg seed oil [21] | |
| α-Tocopherol | 1.35–1.47 mg/100 g [17] | - | 70.6 mg/kg seed oil [21] |
| β-Tocopherol | 0.10–0.16 mg/100 g [17] | - | 28.2 mg/kg seed oil [21] |
| γ-Tocopherol | 0.08–0.10 mg/100 g [17] | - | 0.2 mg/kg seed oil [21] |
| γ-Tocotrienol | - | - | 0.8 mg/kg seed oil [21] |
| δ-Tocopherol | 0.05–0.07 mg/100 g [17] | - | 0.2 mg/kg seed oil [21] |
| Vitamin K | 17.8–28.8 µg/100 g [17] | - | - |
| Chloride | - | 13 ppm [45] | - |
| Nitrate | 45.20–98.50 mg/kg [1], 62.7–64.7 mg/kg [32] | 9 ppm [45] | - |
| Nitrite | 0.62–1.87 mg/kg [1], 0.90–1.24 mg/kg [32] | - | - |
| Phosphate | - | 184 ppm [45] | - |
| Sulfate | - | 1368 ppm [45] | - |
ADF: acid dietary fibre, ADL: acid detergent lignin, DM: dry matter, FW: fresh weight, NDF: neutral dietary fibre, PUFA: polyunsaturated fatty acids, SFA: saturated fatty acids, TFA: total fatty acids, UFA: unsaturated fatty acids.
3. Polyphenol Components
Polyphenols are biofactors that determine the high bioactivity of black chokeberries. Chokeberry fruits are some of the richest sources of polyphenols, which include anthocyanins, flavonols, flavanols, proanthocyanidins, and phenolic acids [33,50,51,52]. Various authors found the following content of polyphenols in chokeberry fruit: 7849 mg/100 g DM [53]; 6351,38 mg/100 g DM [54]; 37,600 mg/kg DM [55]; 1079–2996 mg Gallic Acid Equivalents/100 g fresh mass [56]; 819–1330 mg GAE/100 g FW (fresh weight) [1]; 778–1285 g GAE/kg FW [3]. Chokeberry products and waste are also rich in polyphenols [5,7,11,34,57,58]. The dark blue colour of chokeberry fruit is caused by the high concentration of anthocyanins, which include cyanidine 3-glucoside, 3-galactoside, 3-xyloside and 3-arabinoside. A small proportion of anthocyanins is attributed to pelargonidine-3-galactoside and pelargonidine-3-arabinoside [46,59,60]. Chokeberry flavonols are a diverse group of compounds, which mainly consist of quercetin derivatives. The main quercetin derivatives in chokeberries are: quercetin-3-glucoside, 3-galactoside, 3-rutinoside, 3-robinobioside and 3-vicianoside. Chokeberry fruit also contain flavonols in the form of isorhamnetin 3-galactoside, 3-glucoside, 3-neohesperidoside and 3-rutinoside; myricetin and kaempherol 3-galactoside and 3-glucoside [61,62,63]. The degree of proanthocyanidin polymerisation is a characteristic feature of chokeberries. Proanthocyanidins are composed of (−)-epicatechin and trace amounts of (+)-catechin, which may occur only at the end of a molecular chain of proanthocyanidins. Individual subunits are linked by C4 → C6 and C4 → C8 bonds [64,65,66].
The degree of proanthocyanidin polymerisation is DP > 10, which translates to the mean procyanidin polymerisation degree (mDP). The mDP of proanthocyanidin in fruits was 19–59; in juice it was 12–52 and in pomace it was 18–34 [11,13,40,45,53,65]. Hellström et al. [66] observed an exceptionally high degree of proanthocyanidin polymerisation in chokeberry juice and extract (mDP > 100). In chemical terms, proanthocyanidins are oligomers and polymers of flavan-3-ol, linked by B-type and A-type bonds. Therefore, the results of many studies have confirmed that the biological and chemical properties of proanthocyanidins depend on their structure, and in particular the molecular weight also expressed as the degree of polymerisation (DP). Part of (−)-epicatechin in chokeberry fruit has the form of monomers [53,59,67], while powders and chokeberry juice epicatechin can be found in combination with cyanidin glycosides [33].
Chokeberries mostly contain chlorogenic and neochlorogenic acids. Other phenolic acids are cryptochlorogenic acid, p-coumaric acid and its derivatives, caffeic acid and its derivatives, protocatechuic, vanillic, ferulic, salicylic, syringic, 4-hydroxybenzoic and ellagic acids. Dried juice also contains methyl esters of chlorogenic and neochlorogenic acids, 2,4,6-trihydroxybenzaldehyde, 3-hydroxybenzoic acid and phenylacetic acid derivatives [68]. Gentizinic and synapic acids were identified in chokeberry honey [69]. There were also reports on the content of eriodictyol 7-glucuronide; 3,7-diglycuronide, 7-xylose and naringenin-7-O-glucopyranoside and 7-O-β-d-glucopyranoside-5,7,3′,5′-tetrahydroxyflavanone, as flavanones found in chokeberry fruit and products [13,39,68,70,71,72]. Table 2 shows the polyphenol composition of chokeberry fruit and products.
Table 2.
The qualitative composition of selected polyphenols present in black chokeberry fruits, juices and other products.
| Compound | Fruit | Juice | Other Products (e.g., Dried Fruits, Extracts, Pomaces, Juice Concentrates, Teas) |
|---|---|---|---|
| Polyphenols total (spectrophotometric method) |
1845–2340 mg GAE/100 g [1], 7.78–12.85 g GAE/kg FW [3], 13.3 g GAE/kg [7], 15.0–17.9 g CE/kg FW [11], 603 mg GAE/100 g FW [51], 1079–1921 mg GAE/100 g FW [56], 8008 mg GAE/100 g DM [58], 20.1 mg GAE/g FW [59], 127–197 mg GAE/g DM [67], 1540.01 mg GAE/100 g FW [73], 10637.20 mg GAE/kg [74], 8563.8–12055.7 mg GAE/kg FW [75] | 8834–11093 mg GAE/L [5], 6.3–6.6 g GAE/kg [7], 4.7–9.0 g CE/kg FW [11], 3002–6639 mg GAE/L DM [34], 675–755 mg GAE/100 mL [45], 2.73–10.35 g GAE/L [40], 4772.2 mg GAE/L [35], 4.00 g GAE/L [76,77], 8.7 mg GAE/100 g [78], 6484 mg GAE/L [79], 386 mg GAE/100 g [80], 373.5 µg GAE/mL [81], 386 mg GAE/100 g [82], 709.3 mg GAE/100 mL [83], 6652 mg GAE/L [84], 5461 mg GAE/L [85], 3172–7340 mg GAE/L [86] |
39.9–50.1 g GAE/kg dried fruits [7], 29.6 g GAE/kg concentrate [7], 63.1 g GAE/kg pomace [7], 6.9–12 g GAE/kg jam [7], 6.7 g GAE/kg compote [7], 2.6 g GAE/kg syrup [7], 31–63 g CE/kg FW pomace [11], 19.64–27.82 mg GAE/g DM extract [57], 4954–7265 mg GAE/100 g DM dried fruits [58], 4233–4951 mg GAE/100 g DM powder [34], 1494–3436 mg GAE/100 g DM fruit tea [34], 1954–2466 mg GAE/100 g DM dried berries [34], 4511–5292 mg GAE/100 g DM capsules [34], 46.8 g GAE/L juice concentrate [87], 27.63–34.28 mg GAE/100 mg DM powder [88], 44.87 mg GAE/g extract [89], 3.38–3.77 g GAE/L juice concentrate [76], 3.68–3.87 g GAE/L juice concentrate [77], 30.9 mg GAE/100 g dried fruit [78], 7.7 mg GAE/100 g compote [78], 792.3–919.7 mg GAE/g DM dried fruits [90], 712 mg GAE/g extract [91], 612.4 mg GA-E/g extract [92], 483 mg CE/g DM extract [93], 757–910 mg GAE/g extract [94], 85.55 mg GAE/100 mL fruit tea (decoction) [95], 88.77 mg GAE/100 mL fruit tea (infusion) [95], 745 mg GAE/g extract [96], 477.72 mg GAE/g extract [97], 714 mg SAE/g extract [98], 2995.20 mg GAE/100 g DM dried fruits [99] |
| Polyphenols total/sum (chromatographic method) |
819.2–1329.5 mg/100 g [1], 672.4 mg/100 g [12], 2574.47–2773.41 mg/100 g FW [13], 7849.21 mg/100 g DM [53], 6351.38 mg/100 g DM [54], 2477.0–6930.5 mg/kg FW [75] | 4521.18–6686.69 g/100 g DM [33], 3729.07 mg/100 g DM [53], 996.33–1450 mg/L [40], 1277.09 mg/L [76,77], 6.95 g/L [100], 1296.8–3545.6 µg/mL [101] | 8044–15058 mg/100 g DM dried pomace fractions [19], 24723.67 g/100 g DM fruit powder [33], 15607.48–24447.77 g/100 g DM pomace powder [33], 10583.27 mg/100 g DM pomace [53], 63.58 g/kg extract [70], 27.0 g/L juice concentrate [87], 1043.89–1162.77 mg/L juice concentrate [76], 1003.37–1188.62 juice concentrate [77], 0.88 g/L wine [100], 5.29–6.51 g/L juice microcapsules [100], 2.05–2.41 g/L wine microcapsules [100], 42.9–233.9 mg/100 mL liqueur [102], 309.6 mg/g extract [103] |
| Nonflavonoids total (spectrophotometric method) |
- | 1383–1840 mg GAE/L [5], 808–1527 mg GAE/L DM [34] | 1602–1906 mg GAE/100 g DM powder [34], 2051–2300 mg GAE/100 g DM capsules [34], 479–1557 mg GAE/100 g DM fruit tea [34], 1072–1086 mg GAE/100 g DM dried berries [34] |
| Phenolic acids total/sum | 121.9 mg/100 g [12], 63.9 mg/100 g FW [51], 669.03 mg/100 g DM [54], 183 ng/g DM Y [104], 32.43 mg/100 g DM [105], 77–96 mg/100 g FW Y [106] | - | 110.92 mg ChAE/g extract [92], 34.5–49.0 mg ChAE/100 mL liqueur [102], 158 ng/g DM compote Y [104], 74–109 ng/g DM jam Y [104], 149.2 mg ChAE/g extract [103] |
| Hydroxycinnamic acids total/sum | 1.4–1.5 g ChAE/kg FW [11], 116.4 mg ChAE/100 g FW frozen [63], 127.0 mg ChAE/100 g FW blanched [63], 6.38–9.85 mg/g DM [67], 739.3–1670.3 mg ChAE/kg FW [75] | 0.45–0.59 g ChAE/kg FW [11], 48.9–77.9 mg ChAE/100 g FW X [63] | 0.72–0.82 g ChAE/kg FW pomace [11], 89.7–231.6 mg ChAE/100 g DM dried pomace fractions [19], 43.7 mg ChAE/100 g FW presscake X [63], 0.078 g ChAE/l juice concentrate [87], 271 mg ChAE/100 g juice concentrate [107], 56.7 mg ChAE/g DM extract [93], 135.14 mg ChAE/g extract [97] |
| Caffeic acid (3,4-Dihydroxycinnamic acid) |
0.13 mg/100 g FW Z [51], ND [62], 3.96 mg/100 g DM Z [105], 60–75 mg/100 g FW Z Y [106] | 1.2–1.8 mg/L Z [77], Tr [101] | 118.9 µg/100 g herbhoney Z [69], 0.067–1.26 mg/g tea infusion Z [108], 0.35 g/L wine [100], 0.6 mg/g extract [91], 0.41–0.48 g/L wine microcapsules [92], 53.85 mg/L extract Z [109], 0.736 mg/g extract [96], ND dried fruits Y [99] |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) |
0.77 mg/100 g FW Z [51], ND [110], 11 ng/g DM Y [104], 8.3–13.0 mg/100 g FW Z Y [106] | 24.93–57.35 mg/L Z [40], 14.3–103.6 µg/mL Z [101] | ND-0.176 mg/g tea infusion Z [108],0.08 g/L wine [100], 0.56–0.70 g/L wine microcapsules [100], 2.4 mg/g extract [91], 14 ng/g DM Y [104], 2–9 ng/g DM Y [104], 1.94 mg/g extract [96] |
| Chlorogenic acid (3-Caffeoylquinic acid) 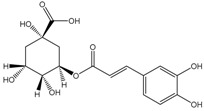
|
72.0–96.6 mg/100 g [1], 1131.15–1960.72 mg/kg FW [3], 0.69–0.74 g/kg FW Z [11], 65.42 mg/100 g [12], 83.97–110.62 mg/100 g FW Z [13], 20.4 mg NChaE/100 g FW [51], 301.85 mg/100 g DM Z [53], 164.42 mg/100 g FW Z [62], 70.2 mg/100 g FW frozen Z [63], 80.2 mg/100 g FW blanched Z [63], 3.32–6.42 mg/g DM Z [67], 218 mg/100 g FW Z [110], 61 mg/100 g FW Z [72], 416.9–1000.6 mg/kg FW Z [75] |
0.2–0.3 g/kg FW Z [11], 470.51–642.74 mg/100 g DM Z [33], 415.86 mg/100 g DM Z [53] 30.4–47.7 mg/100 g FW Z X [63], 58.9–67.8 mg/100 mL [45], 463.31–642.28 mg NChAE/l [40], 390.5 mg/L Z [35], 10.3–36.3 mg/L Z [111] 370.06 mg/L Z [76,77], 0.51 mg/g Z [78], 1389 mg/L Z [79], 0.97 g/L [100], 32 mg/100 g [82], 691 mg/L [84], 858 mg/L [85], 453.3–628.1 µg/mL Z [101], 45.50 mg/100 mL Z [112] |
0.42–0.50 g/kg FW pomace Z [11],33.2–84.5 mg/100 g DM dried pomace fractions Z [19], 769.25 mg/100 g DM fruit powder Z [33], 848.17–1192.69 mg/100 g DM dried pomace powder Z [33], 204.35 mg/100 g DM pomace Z [53], 3.60–4.60 mg/g DM dried fruits [54], 28.8 mg/100 g FW presscake Z X [63], 13.7 µg/100 g herbhoney Z [69], 6.24 g/kg extract [70], 43.95 mg NChAE/g DM extract [113], 0.0377 g/L juice concentrate Z [87], 603.1 mg/kg extract Z [114], 310.59–354.07 mg/L juice concentrate Z [76], 318.19–352.34 mg/L juice concentrate Z [77], 2.33 mg/g dried fruit Z [78], 0.37 mg/g compote Z [78], 20.6 μg/mg extract Z [115], 0.94–0.99 g/L juice microcapsules [100], 63.8 mg/g extract [91], 68.32 mg/g extract Z [92], 2181.05 mg/L extract [109], 79.0 mg/g extract [96], 3.90 mg/g extract Z [97], 63.5 mg/g extract [98], 58.23 mg/100 g DM dried fruits Z Y [99] |
| Neochlorogenic acid (5-Caffeoylquinic acid) 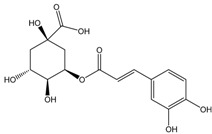
|
59.3–79.1 mg/100 g [1], 883.31–1156.59 mg/kg FW [3], 0.71–0.72 g ChAE/kg FW [11], 56.51 mg/100 g [12], 74.60–99.76 mg ChAE/100 g FW [13], 37.5 mg/100 g FW Z [51], 290.81 mg ChAE/100 g DM [53], 79.88 mg/100 g FW Z [62], 46.2 mg ChAE/100 g FW frozen [63], 46.8 mg ChAE/100 g FW blanched [63], 2.16–6.54 mg ChAE/g DM [67], 123 mg ChAE/100 g FW [72], 189 mg ChAE/100 g FW [110], 322.4–669.7 mg ChAE/kg FW [75] | 0.21–0.29 g ChAE/kg FW [11], 891.56–1048.49 mg/100 g DM [33], 393.10 mg ChAE/100 g DM [53], 18.4–30.1 mg ChAE/100 g FW X [63], 65.5–80.6 mg/100 mL [45], 323.67–442.33 mg/L Z [40], 415.7 mg/L Z [35], 21.8–57.3 mg/L [111], 426.57 mg ChAE/l [76,77], 0.47 mg ChAE/g [78], 1057 mg/L Z [79], 1.18 g/L [100], 28 mg/100 g [82], 840 mg/L [84], 830 mg/L [85], 361.3–449.0 µg ChAE/mL [101], 49.21 mg ChAE/100 mL [112] | 25.5–68.1 mg ChAE/100 g DM dried pomace fractions [19], 728.81 mg/100 g DM fruit powder [33], 1161.53–1174.35 mg/100 g DM pomace powder [33], 169.20 mg ChAE/100 g DM pomace [53], 14.9 mg ChAE/100 g FW presscake X [63], 6.03 g/kg extract [70], 1.13 mg/g DM extract Z [113], 0.0401 g ChAE/l juice concentrate [87], 257.2 mg/kg extract Z [114], 355.51–402.08 mg ChAE/l juice concentrate [76], 334.52–422.32 mg ChAE/l juice concentrate [77], 1.82 mg ChAE/g dried fruit [78], 0.26 mg ChAE/g compote [78], 1.25–1.28 g/L juice microcapsules [100], 7 mg/g extract [91], 21.6 mg/g extract [96], 0.31–0.32 g ChAE/kg FW pomace [96], 24.05 mg ChAE/g extract [97], 41.7 mg/g extract [98] |
| Cryptochlorogenic acid (4-Caffeoylquinic acid) |
2.22 mg NChAE/100 g FW [51], ND [62] | 28.21–32.66 mg/100 g DM [33], 34.56–86.51 mg NChAE/l [40], 0.13 mg ChAE/g [78] | 19.91 mg/100 g DM fruit powder [33], 41.57–53.60 mg/100 g DM pomace powder [33], ], ND extract [113], 0.17 mg ChAE/g dried fruit [79], 0.03 mg ChAE/g compote [79] |
| Gallic acid (3,4,5-Trihydroxybenzoic acid) |
ND [51], ND Y [106] | 0.04 g/L [100], 6.9 mg/L [84], ND-197.8 µg/mL Z [101] | 111.3 µg/100 g herbhoney Z [69], ND-0.596 mg/g tea infusion Z [108], 3.15 mg/100 g DM dried fruits Z Y [99] |
| Vanillic acid (3-Methoxy-4-hydroxybenzoic acid) |
0.37 mg/100 g DM Z [105], ND-1.31 mg/100 g FW Z Y [106] | - | 0.07–0.09 mg/g DM dried fruits [90], ND dried fruits Y [99] |
|
p-Coumaric acid (4-hydroxycinnamic acid) |
ND [12], 0.02 mg/100 g FW Z [51], ND [62], 27 ng/g DM Z Y [104], 3.05 mg/100 g DM Z [105], 5.5–7.61 mg/100 g FW Z Y [106] | - | 446.4 µg/100 g herbhoney Z [69], ND extract [113], 4.3 μg/mg extract Z [115], 24 ng/g DM compote Z Y [104], 12–25 ng/g DM jam Z Y [104], 7.33 mg/100 g DM dried fruits Z Y [99] |
| Cinnamic acid | ND-0.90 mg/100 g FW Z Y [106] | - | 7.5 μg/mg extract Z [115] |
| p-Coumaric acid derivatives | - | 0.4 mg ChAE/100 mL [112] | - |
| Coumaric acid glucoside | 1.29 mg CoAE/100 g FW [51] | - | - |
| Caffeoylquinic acid derivative | - | 19.3–128.3 µg ChAE/mL [101] | - |
| Caffeic acid glucoside | 0.04 mg CaE/100 g FW [51] | - | - |
|
p-Coumaroylquinic acid 3-O-p-Coumaroylquinic acid |
- | 8.31–9.32 mg/100 g DM [33], 2.72–3.66 mg CoAE/l [40] | 6.81 mg/100 g DM fruit powder [33], 10.96–12.70 mg/100 g DM pomace powder [33], ND extract [113] |
| dicaffeoylquinic acid | 3.74 mg/100 g FW [62] | - | 0.26 mg NChAE/g DM extract [113] |
| di-Caffeic quinic acid | - | 1.00–1.33 mg/100 g DM [33] | 4.88 mg/100 g DM fruit powder [33], 3.09–5.35 mg/100 g DM pomace powder [33] |
| Ferulic acid (3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid) |
0.01 mg/100 g FW Z [51], 1.9–2.8 mg/100 g FW Z Y [106] | 19.9 mg/L [84] | 41.4 µg/100 g herbhoney Z [69], 15.09 mg/L extract [109], ND dried fruits Y [99] |
| Depside | - | 2.15–6.30 PCAE mg/L [40] | - |
| Gentisic acid (2,5-Dihydroxybenzoic acid) |
- | - | 229.2 µg/100 g herbhoney Z [69] |
| Sinapic acid (3,5-Dimethoxy-4-hydroxycinnamic acid) |
ND Y [106] | - | 44.6 µg/100 g herbhoney Z [69] |
| Salicylic acid (o-Hydroxybenzoic acid) |
15.60 mg/100 g DM Z [67] | - | - |
| Syringic acid (4-Hydroxy-3,5-dimethoxybenzoic acid) |
65 ng/g DM Y [104], 4.16 mg/100 g DM Z [105], ND Y [106] |
- | 7.8 µg/100 g herbhoney Z [69], 53 ng/g DM compote Y [104], 34–45 ng/g DM jam Y [104] |
| 4-Hydroxybenzoic acid | ND [51], 38 ng/g DM Y [104], 5.29 mg/100 g DM Z [105], ND Y [106] | - | 35 ng/g DM compote Y [104], 9–26 ng/g DM jam Y [104], 13 ng/g DM dried homogenate prepared from fresh fruit Y [104] |
| Ellagic acid | 1.57 mg/100 g FW Z [51], 42 ng/g DM Y [104] | - | ND extract [71], ND extract [113], 32 ng/g DM compote Y [104], 11–15 ng/g DM jam Y [104], ND dried fruits Y [99] |
| Flavonoids total (spectrophotometric method) | 5.3 g CE/kg [7], 18.31 mg QE/100 g FW [73] | 6994–9710 mg GAE/L [5], 2.7–2.9 g CE/kg [7], 2180–5271 mg GAE/L DM [34], 2.50 g CE/l [76,77], 189.4 mg CE/100 mL [83] | 12.5–19.9 g CE/kg dried fruits [7], 6.1 g CE/kg concentrate [7], 9.3 g CE/kg pomace [7], 2.9–6.4 g CE/kg jam [7], 3.3 g CE/kg compote [7], 1 g CE/kg syrup [7], 2327–3317 mg GAE/100 g DM powder [34], 2459–2992 mg GAE/100 g DM capsules [34], 878–2322 mg GAE/100 g DM fruit tea [34], 867–1394 mg GAE/100 g DM dried berries [34], 3.45–5.22 mg QE/100 mg DM powder [88], 5.65 mg CE/g extract [89], 2.01–2.27 g CE/l juice concentrate [76], 1.91–2.27 g CE/l juice concentrate [77], 52.0–66.1 mg CE/g DM dried fruits [90], 21.94 mg/g extract [92], 62.0–90.1 mg CE/g extract [94] |
| Flavonoids total (chromatographic method) | 556.0 mg/100 g [12], 71 mg QRE/100 g FW [72] | - | 49.7 mg QRE/g extract [103] |
| Anthocyanins total (spectrophotometric method) | 4.5 g CGlE/kg [7], 488.8 mg CGlE/100 g FW frozen [8], 3917 mg CGlE/100 g DM [58], 498.98 mg/100 g FW [73], 4341.06 mg CGlE/kg [74] | 1829–2768 mg CGlE/l [5], 130.5–210.3 mg CGlE/100 g FW X [8], 150–1228 mg CGlE/l DM [34], 0.10–0.67 g CGlE/l [40], 0.4–0.7 g CGlE/kg [41], 456.2 CGlE/l [35], 369.47 mg CGlE/l [76,77], 0.6 CGlE mg/100 g [78], 106.8 mg CGlE/100 mL [83], 508–1087 mg CGaE/l [86] | 1.4–3.1 g CGlE/kg dried fruits [7], 3.6 g CGlE/kg concentrate [7], 10 g CGlE/kg pomace [7], 0.2–0.4 g CGlE/kg jam [7], 0.2 g CGlE/kg compote [7], 0.1 g CGlE/kg syrup [7], 515.6–652.9 mg CGlE/100 g FW of mash [8], 1581.7–2495.2 mg CGlE/100 g FW of pomace [8], 238.5–383.6 mg CGlE/100 g FW of pomace X [8], 781–2227 mg CGlE/100 g DM dried fruits [58], 1165–1641 mg CGlE/100 g DM powder [34], 1997–2468 mg CGlE/100 g DM capsules [34], 282–675 mg CGlE/100 g DM fruit tea [34], 141–147 mg CGlE/100 g DM dried berries [34], 2.93–4.80 mg CGlE/100 mg DM powder [88], 0.203–0.273 CGlE% DM extract [111], 271.35–330.32 g CGlE/l juice concentrate [76], 270.10–352.30 g CGlE/l juice concentrate [77], 7.1 CGlE mg/100 g dried fruit [79], 0.8 CGlE mg/100 g compote [78], 1146–3715 mg CGlE/g DM dried fruits [90], 202.28 mg/g extract [92], 0.07–0.14 mg CGlE/100 g extract [94], 8.12 mg CGlE/100 mL fruit tea (decoction) [95], 8.63 mg CGlE/100 mL fruit tea (infusion) [95] |
| Anthocyanins total/sum (chromatographic method) |
256.4 mg/100 g FW frozen [8], 6.2–6.7 g CGlE/kg FW [11], 529.3 mg/100 g [12], 357 mg CGlE/100 g FW [51], 1265.48 mg CRE/100 g DM [54], 249–447 mg CGaE/100 g FW [56], 1480.0 mg CGlE/kg [59], 619.2 mg CGlE/100 g FW frozen [63], 281.2 mg CGlE/100 g FW blanched [63], 3.37–14.87 mg CGaE/g DM [67], 4056.22 mg CGlE/kg [74], 1500.9–5486.2 mg CGlE/kg FW [75] | 63.9–98.7 mg/100 g FW X [8], 58–473 g CGlE/kg FW [11], 7.2–104.8 mg CGlE/100 g FW X [63], 2.8–45.2 mg CGaE/100 mL [45], 373.06 mg/L [76,77], 4.76 g/L [100], 59.3–1118 mg CGlE/l [116], 221.4 mg/L [85], 2.0–855.5 µg CGlE/mL [101], 19.10 mg/100 mL [117] | 274.5–310.6 mg/100 g FW mash [8], 738.7–1221.1 mg/100 g FW pomace [8], 114.4–186.0 mg/100 g FW pomace X [8], 11.9–19.5 g CGlE/kg FW pomace [11], 616.2–1239.0 mg CGlE/100 g DM dried pomace fractions [19], 138.3 mg CGlE/100 g FW presscake X [63], 8.0 g CRE/l juice concentrate [87], 8384 mg CGlE/kg extract [114], 957.2 mg/100 g juice concentrate [107], 238.35–317.02 mg/L juice concentrate [76], 258.84–316.50 mg/L juice concentrate [77], 0.45 g/L wine [100], 3.07–4.27 g/L juice microcapsules [100], 1.02–1.30 g/L wine microcapsules [100], 147 mg CGlE/g DM extract [93], 5.9 mg CGlE/g extract capsule [116], 188 mg CGlE/l syrup [116], 1.2–170.7 mg CGlE/100 mL liqueur [102], 93.75 mg CGlE/g extract [97], 110.7 mg CGaE/g extract [103], 23.6–192.1 mg/L dried fruits infusions [118], 272.2–342.1 mg/L dried pomace infusions [118] |
Cyanidin-3-O-arabinoside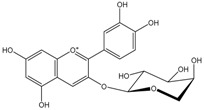
|
128.0–299.4 mg/100 g [1], 941.82–1553.29 mg/kg FW [3], 1359.4 mg CGlE/kg [7], 74.3 mg/100 g FW frozen Z [8], 1.9–2.1 g CGlE/kg FW [11], 116.39 mg/100 g [12], 220.27–249.46 mg CGaE/100 g FW [13], 112 mg CGlE/100 g FW [51], 581.50 mg CGaE/100 g DM [53], 52–149 mg CGaE/100 g FW [56], 399.3 mg CGlE/100 g FW [59], 1243.2 mg CyE/kg FW [60], 159.21 mg/100 g FW Z [62], 154.7 mg CGlE/100 g FW frozen [63], 57.5 mg CGlE/100 g FW blanched [63], 0.18–4.06 mg CGaE/g DM [67], 146 mg CGaE/100 g FW [72], 544 mg CGlE/100 g FW [110], 993.77 mg CGlE/kg [74], 367.2–1532.4 mg CGlE/kg FW [75] | 117.8–172.6 mg CGlE/kg [7], 16.9–27.7 mg/100 g FW Z X [8], 14–108 g CGlE/kg FW [11], 248.72–554.90 mg/100 g DM [33], 324.37 mg CGaE/100 g DM [53], 1.0–20.4 mg CGlE/100 g FW Z [63], 0.7–10.7 mg CGaE/100 mL [45], 11.47–32.59 CGlE mg/L [40], 28.8–48.5 mg/L Z [111], 78.47 mg/L [76,77], 101 mg/L Z [79], 1.44 g/L [100], 36.2 mg CGaE/100 g [80], 1.93 mg/100 mL Z [81], 11.3–249.9 mg CGlE/l [116], 36.2 mg/100 g [82], 8.2 mg/L [84], 61.7 mg/L [85], Tr-190.2 µg CGlE/mL [101], 5.18 mg/100 mL Z [117], 110.1–178.7 mg CGaE/l [86], 5.12 mg CGaE/100 mL [112] |
186–477.7 mg CGlE/kg dried fruits [7], 1447.6 mg CGlE/kg concentrate [7], 1651.1 mg CGlE/kg pomace [7], 22–85.2 mg CGlE/kg jam [7], 41 mg CGlE/kg compote [7], 27.2 mg CGlE/kg syrup [7], 84.2–93.4 mg/100 g FW mash Z [8], 217.5–366.7 mg/100 g FW pomace Z [8], 36.0–55.8 mg/100 g FW pomace Z X [8], 3.7–5.7 g CGlE/kg FW pomace [11], 23.38 mg CGlE/g extract [11], 191.7–389.9 mg CGlE/100 g DM dried pomace fractions [19], 3328.79 mg/100 g DM fruit powder [33], 1835.62–3116.02 mg/100 g DM pomace powder [33], 532.64 mg CGaE/100 g DM pomace [53], 0.14–0.32 mg/g DM extract Z [57], 29.3 mg CGlE/100 g FW presscake Z [63], 6.17 g/kg extract [70], 77.08 mg CGlE/g DM extract [113], 0.0274 g CRE/l juice concentrate [87],1.9–17.7 mg/g tea infusion Z [108], 2143.0 mg CGlE/kg extract [114], 187.9 mg/100 g juice concentrate Z [107], 45.19–64.55 mg/L juice concentrate [76], 49.76–67.77 mg/L juice concentrate [77], 51.6–370.9 mg/g DM dried fruits [90], 159.6 μg CGlE/mg extract [115], 0.25 g/L wine [100], 1.03–1.26 g/L juice microcapsules [100], 0.46–0.59 g/L wine microcapsules [100], 23.58 mg/g extract [91], 33.21 mg/g extract Z [92], 1.42 mg CGlE/g extract capsule [116], 36.3 mg CGlE/l syrup [116], 2.43 mg/100 mL fruit tea (decoction) Z [95], 2.32 mg/100 mL fruit tea (infusion) Z [95], 36.7 mg CGaE/g extract [96], 81.8 mg/g extract [98], 5.9–54.6 mg/L dried fruits infusions [118], 77.3–98.7 mg/L dried pomace infusions [118] |
| Cyanidin-3-O-galactoside (Idaein) 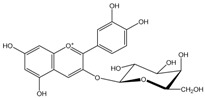
|
417.3–636.0 mg/100 g [1], 1010.80–1203.56 mg/kg FW [3], 2917.2 mg CGlE/kg [7], 157.1 mg/100 g FW frozen Z [8], 4.1–4.4 g CGlE/kg FW [11], 379.36 mg/100 g [12], 473.54–515.22 mg/100 g FW Z [13], 229 mg CGlE/100 g FW [51], 1282.41 mg/100 g DM Z [53],168–282 mg/100 g FW Z [56], 989.7 mg CGlE/100 g FW [59], 2482.4 mg/kg FW Z [60], 222.11 mg/100 g FW Z [62], 424.7 mg CGlE/100 g FW frozen [63], 205.5 mg CGlE/100 g FW blanched [63], 2.21–14.50 mg/g DM Z [67], 315 mg CGaE/100 g FW Z [72], 1243 mg CGlE/100 g FW [110], 2794.74 mg CGlE/kg [74], 1055.3–3621.0 mg CGlE/kg FW [75] | 286.6–441.4 mg CGlE/kg [7], 40.1–60.3 mg/100 g FW Z X [8], 43–341 g CGlE/kg FW [11], 702.15–1451.55 mg/100 g DM Z [33], 787.00 mg/100 g DM Z [53], 5.5–77.1 mg CGlE/100 g FW X [63], 1.7–29.5 mg/100 mL Z [45], 46.58–96.88 CGlE mg/L [40], 82.1–133.0 mg/L Z [111], 278.43 mg/L Z [76,77], 301 mg CArE/l [79], 3.16 g/L [100], 107.6 mg/100 g Z [80], 5.48 mg/100 mL Z [81], 44.0–822.1 mg CGlE/l [116], 107.6 mg/100 g [82], 20.0 mg/L [84], 143.7 mg/L [85], 1.9–616.0 µg CGlE/mL [101], 12.60 mg/100 mL Z [117], 319.4–506.1 mg/L Z [86], 12.49 mg/100 mL Z [112] | 475.7–928 mg CGlE/kg dried fruits [7], 3349.7 mg CGlE/kg concentrate [7], 4600.5 mg CGlE/kg pomace [7], 81.2–237.4 mg CGlE/kg jam [7], 120.4 mg CGlE/kg compote [7], 81.6 mg CGlE/kg syrup [7], 162.0–187.4 mg/100 g FW mash Z [8], 437.2–754.6 mg/100 g FW pomace Z [8], 68.4–114.9 mg/100 g FW pomace Z X [8], 7.6–12.5 g CGlE/kg FW pomace [11], 376.5–749.4 mg CGlE/100 g DM dried pomace fractions [19], 8286.4 mg/100 g DM fruit powder Z [33], 4521.34–7961.70 mg/100 g DM pomace powder Z [33], 1119.70 mg/100 g DM pomace Z [53], 0.40–0.85 mg/g DM extract Z [57], 99.8 mg CGlE/100 g FW presscake X [63], 15.53 g/kg extract [70], 181.01 mg CGlE/g DM extract [113], 0.0432 g CRE/l juice concentrate [87], 3.8–37.6 mg/g tea infusion Z [108], 5456.0 mg CGlE/kg extract [114], 733.3 mg/100 g juice concentrate Z [107], 182.32–238.69 mg/L juice concentrate Z [76], 196.63–240.36 mg/L juice concentrate Z [77], 119.6–798.1 mg/g DM dried fruits [90], 314.0 μg CGlE/mg extract [115], 0.20 g/L wine [100], 1.99–2.81 g/L juice microcapsules [100], 0.56–0.71 g/L wine microcapsules [100], 80.07 mg/g extract Z [92], 4.03 mg CGlE/g extract capsule [116], 144.1 mg CGlE/l syrup [116], 6.66 mg/100 mL fruit tea (decoction) Z [95], 5.04 mg/100 mL fruit tea (infusion) Z [95], 84.5 mg/g extract Z [96], 64.04 mg CGlE/g extract [97], 270.2 mg/g extract [98], 16.0–126.2 mg/L dried fruits infusions [118], 178.2–222.7 mg/L dried pomace infusions [118] |
| Cyanidin 3-O-glucoside (Chrysanthemin) 
|
7.8–27.2 mg/100 g [1], 127 mg/kg Z [7], 11.1 mg/100 g FW frozen Z [8], 0.08–0.09 g/kg FW Z [11], 7.11 mg/100 g [12], 18.15–21.51 mg/100 g FW Z [13], 7.66 mg/100 g FW Z [51], 42.14 mg CGaE/100 g DM [53], Tr-4.7 mg CGaE/100 g FW [56], 37.6 mg/100 g FW Z [59], 200.0 mg/kg FW Z [60], 10.87 mg/100 g FW Z [62], 19.8 mg/100 g FW frozen Z [63], 10.9 mg/100 g FW blanched Z [63], 0.049–0.469 mg CGaE/g DM [67], 10 mg CGaE/100 g FW [72], 46.2 mg/100 g FW Z [110], 121.69 mg/kg Z [74], 34.1–113.5 mg/kg FW Z [75] | 15.2–19.9 mg/kg Z [7], 2.9–4.8 mg/100 g FW Z X [8], 0.5–9.9 g/kg FW Z [11], 19.71–39.99 mg/100 g DM [33], 28.15 mg CGaE/100 g DM [53], 0.4–4.6 mg/100 g FW Z X [63], 0.3–2.0 mg CGaE/100 mL [45], 2.01–4.37 mg/L Z [40], 3.7–5.7 mg/L Z [111], 9.28 mg/L Z [76,77], 21 mg/L Z [79], 0.16 g/L [100], 0.72 mg/100 mL Z [81], 2.4–41.9 mg/L Z [116], 4.9 mg/100 g [82], 4.4 mg/L [84], 4.4 mg/L [85], Tr-25.1 µg/mL Z [101], 0.73 mg/100 mL Z [117], 24.0–43.7 mg CGaE/l [86], 0.71 CGaE/100 mL [112] |
19.3–60.6 mg/kg dried fruits Z [7], 214.7 mg/kg concentrate Z [7], 237.7 mg/kg pomace Z [7], 3.3–10 mg/kg jam Z [7], 4 mg/kg compote Z [7], 3.6 mg/kg syrup Z [7],12.2–13.3 mg/100 g FW mash Z [8], 33.9–52.0 mg/100 g FW pomace Z [8], 5.1–7.9 mg/100 g FW pomace Z X [8], 0.24–0.44 g/kg FW pomace Z [11], 21.0–43.7 mg/100 g DM dried pomace fractions Z [19], 5.4 mg/100 g FW presscake Z X [63], 225.80 mg/100 g DM fruit powder [33], 125.91–220.06 mg/100 g DM pomace powder [33], 79.44 mg CGaE/100 g DM pomace [53], 0.07–0.14 mg/g DM extract Z [57], 0.79 g/kg extract [70], 7.09 mg/g DM extract Z [113], 0.0055 g CRE/l juice concentrate [87], 0.52–5.6 mg/g tea infusion Z [108], 13.84–21.10 mg/100 mg DM powder Z [88], 415.0 mg/kg extract Z [114], 34.1 mg/100 g juice concentrate Z [107], 6.52–7.52 mg/L juice concentrate Z [76], 6.87–8.15 mg/L juice concentrate Z [77], 6.1–49.1 mg/g DM dried fruits [90], 14.5 μg/mg extract Z [115], 0.05–0.20 g/L juice microcapsules [100], 3.68 mg/g extract Z [92], 0.196 mg/g extract capsule Z [116], 7.5 mg/L syrup Z [116], 1.29 mg/100 mL fruit tea (decoction) Z [95], 0.85 mg/100 mL fruit tea (infusion) Z [95], 4.79 mg CGaE/g extract [96], 3.14 mg/g extract Z [97], 12.5 mg/g extract [98], 0.7–4.3 mg/L dried fruits infusions [118], 6.9–8.0 mg/L dried pomace infusions [118] |
Cyanidin-3-O-xyloside
|
29.0–38.2 mg/100 g [1], 165.8 mg CGlE/kg [7], 13.7 mg CGlE/100 g FW frozen [8], 0.13–0.14 g CGlE/kg FW [11], 26.40 mg/100 g [12], 30.27–33.39 mg CGaE/100 g FW [13], 8.12 mg CGlE/100 g FW [51], Tr-12 mg CGaE/100 g FW [56], 52.71 mg CGaE/100 g DM [53], 51.5 mg CGlE/100 g FW [59], 38.7 mg CyE/kg FW [60], 9.92 mg/100 g FW [62], 20.1 mg CGlE/100 g FW frozen [63], 7.3 mg CGlE/100 g FW blanched [63], ND-0.391 mg CGaE/g DM [67], 10 mg CGaE/100 g FW [72], 73 mg CGlE/100 g FW [110], 146.02 mg CGlE/kg [74], 44.3–233.1 mg CGlE/kg FW [75] | 14.7–19.3 mg CGlE/kg [7], 3.7–5.8 mg CGlE/100 g FW X [8], 1–13 g CGlE/kg FW [11], 17.32–48.35 mg/100 g DM [33], 33.63 mg CGaE/100 g DM [53], 0.21–2.7 mg CGlE/100 g FW X [63], 1.24–4.74 CGlE mg/L [40], 0.2–2.1 mg CGaE/100 mL [45], 3.2–5.2 mg/L [111], 6.88 mg/L [76,77], 13 mg CArE/l [79], 5.2 mg CGaE/100 g [80], 0.8–3.8 mg CGlE/l [116], 5.2 mg/100 g [82], 0.6 mg/L [84], 11.6 mg/L [85], Tr-24.3 µg CGlE/mL [101], 0.59 mg/100 mL Z [117], 19.8–29.4 mg CGaE/l [86], 0.59 CGaE/100 mL [112] | 21.8–62.5 mg CGlE/kg dried fruits [7], 201.1 mg CGlE/kg concentrate [7], 223.4 mg CGlE/kg pomace [7], 3–8.7 mg CGlE/kg jam [7], ND mg CGlE/kg compote [7], 2.8 mg CGlE/kg syrup [7], 15.8–17.2 mg CGlE/100 g FW mash [8], 36.7–63.3 mg CGlE/100 g FW pomace [8], 4.7–7.3 mg CGlE/100 g FW pomace X [8], 0.3–0.6 g CGlE/kg FW pomace [11], 3.19 mg CGlE/g extract [11], 27.0–57.1 mg CGlE/100 g DM dried pomace fractions [19], 294.14 mg/100 g DM fruit powder [33], 166.86–275.41 mg/100 g DM pomace powder [33], 105.06 mg CGaE/100 g DM pomace [53], 3.7 mg CGlE/100 g FW presscake X [63], 1.03 g/kg extract [70], 8.17 mg CGlE/g DM extract [113], 0.0031 g CRE/l juice concentrate [87], 0.77–4.4 mg CGlE/g tea infusion [108], 334.0 mg CGlE/kg extract [114], 4.32–6.43 mg/L juice concentrate [76], 4.70–6.55 mg/L juice concentrate [77], 5.2–71.2 mg/g DM dried fruits [90], 16.6 μg CGlE/mg extract [115], 1.52 mg/g extract [91], 0.255 mg CGlE/g extract capsule [116], 0.45 mg CGlE/l syrup [116], 0.96 mg CGlE/100 mL fruit tea (decoction) [95], 0.99 mg CGlE/100 mL fruit tea (infusion) [95], 5.05 mg CGaE/g extract [96], 40.0 mg/g extract [98], 0.7–7.0 mg/L dried fruits infusions [118], 10.1–12.7 mg/L dried pomace infusions [118] |
| Cyanidin-3-O-galactoside + Cyanidin-3-O-glucoside | - | - | 65.04 mg/g extract [91] |
| Cyanidin-3,5-hexoside-(epi)catechin | - | 9.16–9.87 mg/100 g DM [33] | 12.04 mg/100 g DM fruit powder [33], 14.33–20.43 mg/100 g DM pomace powder [33] |
| Cyanidin-3-pentoside-(epi)catechin | - | 3.95–4.24 mg/100 g DM [33] | 5.76 mg/100 g DM fruit powder [33], 7.26–10.30 mg/100 g DM pomace powder [33] |
| Cyanidin-3-hexoside-(epi)cat-(epi)cat | - | 6.77–7.74 mg/100 g DM [33] | 10.98 mg/100 g DM fruit powder [33], 13.61–20.23 mg/100 g DM pomace powder [33] |
| Cyanidin | - | 0.22 mg CGaE/100 mL [112] | 387.43 mg CyE/100 g DM dried fruits Y [99] |
| Pelargonidin-3-arabinoside | 2.3 mg CGlE/100 g FW [59], 50.4 mg PE/kg FW [60] | - | 7.6 mg/100 g juice concentrate Z [107] |
| Pelargonidin-3-arabinoside + Pelargonidin-3-galactoside | - | - | 0.473 mg CGaE/g extract [96] |
| Pelargonidin-3-galactoside | Tr [59], ND [60] | - | - |
| Flavanones | |||
| Eriodictyol-7-glucuronide Eriodictyol-glucuronide |
22.11–26.43 mg/100 g FW [13] | 19.24–28.97 mg/100 g DM [33], 24.31–64.88 NE mg/L [40] | 81.36 mg/100 g DM fruit powder [33], 57.61–84.40 mg/100 g DM pomace powder [33] |
| Eriodictyol-3,7-O-diglucuronide | - | 7.86 mg/100 mL [112] | 1.86 g/kg extract [70] |
| Eriodictyol | 51.4 mg/100 g FW [119] | - | - |
| Flavonols total (chromatographic method) |
21.2 mg/100 g [12], 273.96 mg/100 g DM [54], 34.7 mg/100 g FW frozen [63], 35.0 mg/100 g FW blanched [63], 71 mg QRE/100 g FW [72], 76.43 mg/kg [74], 192.4–408.4 mg/kg FW [75] | 16.5–21.3 mg/100 g FW X [63] | 57.0–126.8 mg/100 g DM dried pomace fractions [19], 16.7 mg/100 g FW presscake X [63], 0.021 g QRE/l juice concentrate [87], 308.9 mg QRE/100 g juice concentrate [107], 4.3–16.4 mg QE/100 mL liqueur [102], 23.93 mg QE/g extract [97] |
| Kaempferol-3-O-galactoside | 0.54 mg KE/kg [61] | - | - |
| Isorhamnetin-3-O-galactoside | ND 1, 1.5 mg IE/kg [61] | - | - |
| Isorhamnetin-3-O-glucoside | ND 1, 1.2 mg IE/kg [61] | - | ND extract [113] |
| Isorhamnetin-3-O-rhamnosylhexoside | - | 0.81–4.80 IGaE mg/L [40] | - |
| Isorhamnetin pentosylhexoside | - | 0.30–0.81 mg/100 g DM [33] | 4.33 mg/100 g DM fruit powder [33], 0.81–12.20 mg/100 g DM pomace powder [33] |
| Isorhamnetin pentoside hexoside | 1.12 mg/100 g FW [62] | - | - |
| Isorhamnetin rhamnosylhexosideisomer sum | - | 0.74–1.85 mg/100 g DM [33] | 18.41 mg/100 g DM fruit powder [33], 7.95–14.27 mg/100 g DM pomace powder [33] |
| Isorhamnetin 3-O-neohesperidoside | 1.16 mg/100 g FW [62] | - | - |
| Isorhamnetin 3-O-rutinoside | ND [61], 0.83 mg/100 g FW Z [62] | - | - |
| Myricetin-3-O-galactoside | 0.55 mg ME/kg [61], ND [62] | - | ND extract [113] |
| Myricetin-3-O-glucoside | 0.20 mg ME/kg [61], ND [62] | - | ND extract [113] |
| Myricetin-glucoside/galactoside | 0.03 mg ME/100 g FW [51] | - | - |
| Quercetin-3-O-arabinopyranoside Quercetin 3-arabinoside |
ND [51], 5.0 mg QGaE/kg [61], 3.1 mg/100 g FW [62] | - | ND extract [70] |
| Morin (3,5,7,2′,4′-Pentahydroxyflavonol) | - | - | ND-0.501 mg/g tea infusion Z [108] |
| Kaempferol | ND [62], 5.30 mg/kg Z [74] | - | 14.5 µg/100 g herb honey Z [69], 1.12 mg/100 g DM dried fruits Z Y [99] |
| Kaempferol-3-O-glucoside (Astragalin) | ND [12], ND [61], 0.38 mg/100 g FW Z [62] | - | ND extract [70], ND extract [113] |
| Kaempferol-glucoside/galactoside | 0.40 mg KE/100 g FW [51] | - | - |
| Quercetin-3-O-galactoside (Hyperoside) 
|
6.6–9.9 mg/100 g [1], 8.31 mg/100 g [12], 9.91–14.57 mg QRE/100 g FW [13], 8.90 mg QE/100 g FW [51], 36.98 mg QRE/100 g DM [53], 65.6 mg/kg Z [61], 19.09 mg/100 g FW Z [62], 10.6 mg QRE/100 g FW frozen [63], 10.2 mg QRE/100 g FW blanched [63], 0.320–0.558 mg/g DM Z [67], 28.3 mg QGlE/100 g FW [110] | 6.77–16.46 mg/100 g DM [33], 49.76 mg QRE/100 g DM [53], 4.1–5.7 mg QRE/100 g FW X [63], 7.0–10.3 mg/L Z [111], 0.05 mg QGlE/g [78], 97 mg/L Z [79], 9.8 mg/100 g [82], 76.0–94.8 µg/mL Z [101], 2.83 mg QRE/100 mL [112] | 28.1–62.5 mg QRE/100 g DM dried pomace fractions [19], 104.11 mg/100 g DM fruit powder [33], 48.97–102.43 mg/100 g DM pomace powder [33], 47.44 mg QRE/100 g DM pomace [53], 0.17–0.27 mg/g DM extract Z [57], 5.2 mg QRE/100 g FW presscake Z [63], 2.83 g/kg extract [70], 7.68 mg QRE/g DM extract [113], 0.00032 g QRE/l juice concentrate [87], 0.06 mg QGlE/g compote [78], 0.31 mg QGlE/g dried fruit [78], 7.2 mg/g extract [91], 0.43 mg/100 mL fruit tea (decoction) Z [95], 0.27 mg/100 mL fruit tea (infusion) Z [95], 4.64 mg/g extract [96], 8.9 mg/g extract [98] |
| Quercetin-3-O-glucoside (Isoquercitrin) 
|
4.4–11.3 mg/100 g [1], 4.03 mg/100 g [12], 7.07–8.87 mg QRE/100 g FW [13], 15.27 mg QE/100 g FW [51], 21.64 mg QRE/100 g DM [53], 43.8 mg QGaE/kg [61], 12.73 mg/100 g FW Z [62], 7.6 mg QRE/100 g FW frozen [63], 7.2 mg QRE/100 g FW blanched [63], 0.239–0.424 mg/g DM Z [67], 20.8 mg/100 g FW Z [110] | 7.08–13.54 mg/100 g DM [33], 31.24 mg QRE/100 g DM [53], 3.2–4.2 mg QRE/100 g FW X [63], 21.4 mg/L Z [35], 4.8–5.8 mg/L Z [111], 0.03 mg/g Z [78], 53 mg/L Z [79], 4.8 mg/100 g [82], 2.25 mg QRE/100 mL [112] | 63.27 mg/100 g DM fruit powder [33], 32.75–67.14 mg/100 g DM pomace powder [33], 26.50 mg QRE/100 g DM pomace [53], 0.10–0.15 mg/g DM extract Z [57], 3.6 mg QRE/100 g FW presscake X [63], 2.25 g/kg extract [70], 0.00050 g QRE/l juice concentrate [87], 0.22 mg/g dried fruit Z [78], 0.03 mg/g compote Z [78], 5.8 mg/g extract [91], 0.28 mg/100 mL fruit tea (decoction) Z [95], 0.22 mg/100 mL fruit tea (infusion) Z [95], 4.02 mg/g extract [96], 21.5 mg/g extract [98] |
| Quercetin-3-O-glucoside + Quercetin-3-O-rutinoside |
- | - | 16.7–37.4 mg QRE/100 g DM dried pomace fractions [19] |
| Quercetin-dihexoside | 33.3 mg QGaE/kg [61], 5.67 mg/100 g FW [62], 4.4 mg QRE/100 g FW frozen [63], 5.2 mg QRE/100 g FW blanched [63] | 2.89–6.36 mg/100 g DM [33], 2.3–3.3 mg QRE/100 g FW X [63], 5.00–29.39 mg QGlE/l [40] | 43.58 mg/100 g DM fruit powder [33], 21.99–43.15 mg/100 g DM pomace powder [33], 2.3 mg QRE/100 g FW presscake X [63] |
| Quercetin-3-O-rutinoside (Rutin) 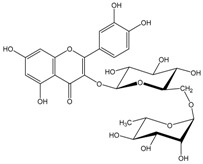
|
3.9–6.1 mg/100 g [1], 5.51 mg/100 g [12], 5.50–6.27 mg/100 g FW Z [13], 14.1 mg QE/100 g FW Z [51], 15.10 mg/100 g DM Z [53], 42.4 mg QGaE/kg [61], 5.13 mg/100 g FW Z [62], 3.9 mg QRE/100 g FW frozen Z [63], 4.1 mg QRE/100 g FW blanched Z [63], 0.158–0.189 mg/g DM Z [67], 12.6 mg QGlE/100 g FW [110], 192.1–403.0 mg/kg FW Z [75] | 4.29–8.98 mg/100 g DM [33], 27.53 mg/100 g DM Z [53], 2.3–2.8 mg/100 g FW Z X [63], 13.33–53.42 QGlE mg/L [40], 70.9 mg/L Z [35], 5.9–6.9 mg/L Z [111], 107.13 mg/L Z [76,77], 194 mg/L Z [79], 3.4 mg/100 g [82], 93.6–141.7 µg/mL Z [101], 1.68 mg/100 mL Z [112] | 44.31 mg/100 g DM fruit powder [33], 22.74–43.68 mg/100 g DM pomace powder [33], 13.55 mg/100 g DM pomace Z [53], 0.31–0.42 mg/g DM extract Z [57], 1.7 mg/100 g FW presscake Z X [63], 1.68 g/kg extract [70], 16.77 mg/g DM extract [113], 0.00091 g/L juice concentrate Z [87], 0.032–0.738 mg/g tea infusion Z [108], 153.8 mg/kg extract Z [114], 79.95–100.21 mg/L juice concentrate Z [76], 86.37–97.69 mg/L juice concentrate Z [77], 0.08–0.10 mg/g DM dried fruits [90], 5.2 mg/g extract [91], 0.94 mg/100 mL fruit tea (decoction) Z [95], 0.58 mg/100 mL fruit tea (infusion) Z [95], 498.80 mg/L extract Z [109], 18.3 mg/g extract [98] |
| Quercetin-3-O-glucuronide (Miquelianin) |
5.6 mg QGaE/kg [61], ND [62] | - | ND extract [113] |
| Quercetin-3-O-vicianoside (Peltatoside) 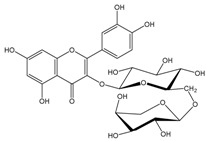
|
2.6–4.3 mg/100 g [1], 2.36 mg/100 g [12], 3.84–5.41 mg QRE/100 g FW [13], 36.4 mg QGaE/kg [61], 4.0 mg QRE/100 g FW frozen [63], 4.5 mg QRE/100 g FW blanched [63], 8.5 mg QGlE/100 g FW [110] | 1.95–5.50 mg/100 g DM [33], 2.5–3.1 mg QRE/100 g FW X [63], 8.80–43.48 QGlE mg/L [40], 1.8–2.0 mg/L [111], 1.15 mg QRE/100 mL [112] | 45.32 mg/100 g DM fruit powder [33], 20.41–43.20 mg/100 g DM pomace powder [33], 1.9 mg QRE/100 g FW presscake X [63], 1.15 g/kg extract [70], 3.78 mg QRE/g DM extract [113] |
| Quercetin-3-O-xyloside | 0.40 mg QE/100 g FW [51], 1.5 mg QGaE/kg [61], ND [62] | - | ND extract [70] |
Quercetin-3-O-robinobioside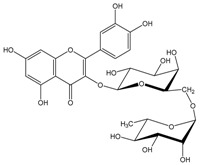
|
1.1–11.3 mg/100 g [1], 1.03 mg/100 g [12], 5.42–5.76 mg QRE/100 g FW [13], 29.6 mg QGaE/kg [61], 3.5 mg QRE/100 g FW frozen [63], 3.1 mg QRE/100 g FW blanched [63], 11.1 mg QGlE/100 g FW [110] | 4.94–10.75 mg/100 g DM [33], 1.6–2.1 mg QRE/100 g FW X [63], 23.59–118.89 QGlE mg/L [40], 1.17 mg QRE/100 mL [112] | 47.45 mg/100 g DM fruit powder [33], 25.60–50.52 mg/100 g DM pomace powder [33], 1.4 mg QRE/100 g FW presscake X [63], 1.17 g/kg extract [70] |
| Quercetin-O-dihexoside | - | 0.19–0.58 mg/100 g DM [33] | 1.96 mg/100 g DM fruit powder [33], 1.31–3.04 mg/100 g DM pomace powder [33] |
| Quercetin-O-deoxyhexoside-hexoside | 3.64 mg/100 g FW [62] | - | - |
| Quercetin hexoside pentoside | - | - | 0.24 g QRE/l juice concentrate [87] |
| Quercetin-arabinoglucoside Quercetin 3-O-arabinoglucoside |
6.63 mg/100 g FW [62] | 0.22 mg QGlE/g [78] | 0.13 mg QGlE/g dried fruit [78], 0.02 mg QGlE/g compote [78] |
| Quercetin 3-O-(6”-malonyl)-glucoside | 1.52 mg/100 g FW Z [62] | - | ND extract [113] |
| Quercetin | 0.21 mg/100 g FW Z [51], ND [62], 0.74 mg/100 g FW frozen Z [63], 0.66 mg/100 g FW blanched Z [63], 71.13 mg/kg Z [74], 0.3–17.4 mg QRE/kg FW [75] | 0.19–0.40 mg/100 g FW Z X [63], 1.75–22.73 mg/L [40], 64.4 mg/L Z [35], 0.27 mg QRE/l [76,77], 11.8 mg/100 mL [83], ND-26.9 µg/mL Z [101] | ND [8,9], 6.7–16.4 mg/100 g DM dried pomace fractions Z [19], 0.66 mg/100 g FW presscake Z X [63], 11.6 µg/100 g herbhoney Z [69], 0.00006 g QRE/l juice concentrate [87], ND-0.243 mg/g tea infusion Z [108], 1.9 mg/kg extract Z [114], 1.6 mg/g extract [91], 117.60 mg/L extract Z [109], 1.56 mg/g extract [96], 1.8 mg/g extract [98], 42.28 mg/100 g DM dried fruits Z Y [99] |
| Quercetin-diglucoside | 9.24 mg QE/100 g FW [51] | - | - |
| Chrysin (5,7-Dihydroxyflavone) |
- | - | 3.5 µg/100 g herbhoney Z [69] |
| Hesperetin | - | - | 10.5 µg/100 g herbhoney Z [69] |
| Naringenin | - | - | 31.3 µg/100 g herbhoney Z [69] |
| Flavan-3-ols total/sum | - | - | 7281–13504 mg/100 g DM dried pomace fractions [19], 6.6 g CE/l juice concentrate [87], 3940.1 µg CE/g extract [89], 93.90 mg/g extract [97] |
(-)-Epicatechin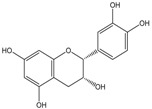
|
467.35–862.50 mg/kg FW [3], 15.76–32.18 mg/100 g FW [13], 0.12 mg/100 g FW Z [51], 15.04 mg/100 g DM [53], ND [62] | 12.71 mg/100 g DM [53], 213.58–235.28 mg/100 g DM Z [33], 40.2 mg CE/l [35], 1.48 mg/100 mL [112] | 6.6–12.0 mg/100 g DM dried pomace fractions [19], 174.53 mg/100 g DM fruit powder Z [33], 236.19–260.13 mg/100 g DM pomace powder Z [33], 11.41 mg/100 g DM pomace [53], 1.95 g/kg extract [70], ND extract [113], 12.77 mg/g extract Z [97], 7.6 mg/g extract [98] |
| (+)-Catechin | ND [51,62] | 87.66–107.18 mg/100 g DM Z [33] | 122.70 mg/100 g DM fruit powder Z [33], 142.81–180.27 mg/100 g DM pomace powder Z [33], ND extract [70], ND extract [113], 19.93 mg/g extract Z [97] |
| Proanthocyanidins total (spectrophotometric method) |
8–178 g CE/kg FW [11], 2.46–3.74 g PCB2E/100 g FW [56], 845.2 mg CE/100 g FW frozen [63], 868.6 mg CE/100 g FW blanched [63], 9.25–13.5 mg CE/g DM [67] | 4.6–15 g CE/kg FW [11], 392.6–464.8 mg CE/100 g FW X [63], 60–72 CyE mg/100 mL [45], 3529.1 mg CE/l [47], 0.64–4.17 g CyE/l [40], 240 mg CE/l [79], 34.2 mg CE/100 mL [81], 442 mg CE/100 g [82], 3122.5 mg/L [85] | 524.2 mg CE/100 g FW presscake X [63], 30.87–59.22 mg CE/100 mg DM powder [88], 5.6 mg CE/g extract [89], 83.8 mg CE/g extract [91], 129.87 mg/g extract [92], 305 mg CE/g DM extract [93], 39.2 mg CE/g extract [93], 24–129 g CE/kg FW pomace [93] |
| Proanthocyanidins total/sum (chromatographic method) |
1426.66–1645.64 mg/100 g FW [13], 80.50 mg ECE/100 g FW [51], 5181.60 mg/100 g DM [53], 663.7 mg/100 g FW [59], 1564–3259μg CE/g DM [67] | 1472.27–2371.07 mg/100 g DM [33], 1578.79 mg/100 g DM [53], 3926.2 mg/L [84], 293.38 mg/100 mL [112] | 7274–13492 mg/100 g DM dried pomace fractions [19], 9977.84 mg/100 g DM fruit powder [33], 6201.73–9714.57 mg/100 g DM pomace powder [33], 8191.58 mg/100 g DM pomace [53], 390.0 mg CGlE/kg extract [114], 532.8 mg/100 g juice concentrate [107], 5.14 mg CE/g extract [91], 146.4 mg/g extract [98] |
| Mean degree of polymerisation (mDP) polymeric procyanidins | 19 [11], 42–59 [13], 23 [53] | 24 [11], 23 [53], 41 [45], 12–52 [40] | 18 pomace [11], 15.5–37.5 dried pomace fractions [19], 34 pomace [53] |
| Mono-, di-, oligomeric flavan-3-ols | 326.55 mg/100 g DM [54] | - | - |
| Polymeric proanthocyanidins | 3816.36 mg/100 g DM [54], 1562–3258 µg CE/g DM [67] | - | 14.9 g/kg extract [70] |
| Procyanidin B1 | - | - | 10.27 mg/g extract Z [97] |
| Procyanidin B2 | - | 21.90–28.19 mg/100 g DM Z [33] | 24.86 mg/100 g DM fruit powder Z [33], 36.40–42.13 mg/100 g DM pomace powder Z [33], ND extract [70], ND extract [97] |
| Monomers | 5.89 mg ECE/100 g FW [51], 5.17 mg/100 g FW [59], 0.01–0.02 µg CE/g DM [67] | - | 60.0 mg/100 g juice concentrate [107], 0.0088 mg CE/g extract [91] |
| Dimers | 0.57 mg ECE/100 g FW [51], 12.48 mg/100 g FW [59], 0.10–0.39 µg CE/g DM [67] | ND-423 µg/mL [101] | 101.5 mg/100 g juice concentrate [107], 0.36 mg CE/g extract [91] |
| Trimers | 0.79 mg ECE/100 g FW [51], 10.29 mg/100 g FW [59], 0.22–0.86 µg CE/g DM [67] | - | 69.6 mg/100 g juice concentrate [107], 1.66 mg CE/g extract [91] |
| 4˗6-mers | 40.32 mg/100 g FW [59], 0.48–1.75 µg CE/g DM [67] | - | - |
| Tetramers | 0.70 mg ECE/100 g FW [51] | - | 83.4 mg/100 g juice concentrate [107], 1.24 mg CE/g extract [91] |
| Pentamers | 0.75 mg ECE/100 g FW [51] | - | 68.8 mg/100 g juice concentrate [107], 0.76 mg CE/g extract [91] |
| Hexamers | 1.04 mg ECE/100 g FW [51] | - | 129.9 mg/100 g juice concentrate [107], 0.34 mg CE/g extract [91] |
| 7˗10-mers | 52.87 mg/100 g FW [59], 0.10–0.36 µg CE/g DM [67] | - | - |
| Heptamers | 0.56 mg ECE/100 g FW [51] | - | 60.7 mg/100 g juice concentrate [107], 0.16 mg CE/g extract [91] |
| Oktamers | 0.51 mg ECE/100 g FW [51] | - | 0.064 mg CE/g extract [91] |
| ≥Nonamers | - | - | 0.54 mg CE/g extract [91] |
| Decamers | 0.16 mg ECE/100 g FW [51] | - | - |
| >10-mers | 69.0 mg ECE/100 g FW [51], 542.6 mg/100 g FW [59], 1562–3258 µg CE/g DM [67] | - | - |
DM: dry matter, FW: fresh weight, ND: not detected, Tr: trace, Z: value calculated from the curve for the standard corresponding to the determined compound, X: concentration on original berry weight basis, Y: after hydrolysis. Equivalents: CaE: caffeic acid, CArE: cyanidin 3-O-arabinoside, CE: catechin, CGaE: cyanidin 3-O-galactoside, CGlE: cyanidin 3-O-glucoside, ChAE: chlorogenic acid, CoAE: p-coumaric acid, CRE: cyanidin 3-O-rutinoside, CyE: cyanidine, ECE: epicatechin, GAE: gallic acid, IE: isorhamnetin, IGaE: isorhamnetin 3-O-galactoside, KE: kaempferol, ME: myricetin, NChAE: neochlorogenic acid, NE: naringenin, PCAE: protocatechuic acid, PCB2E: procyanidin B2, PE: pelargonidin, QE: quercetin, QGaE: quercetin 3-O-galactoside, QGlE: quercetin 3-O-glucoside, QRE: quercetin 3-O-rutinoside, SAE: sinapic acid.
4. Antioxidative Activity
Chokeberry fruits have high antioxidative potential, usually higher than other plant materials tested. The antioxidative activity of chokeberries was confirmed in various radical scavenging assays, the effects of transition metals on changes in the state of oxidation, and the ability to inhibit lipid peroxidation in a variety of model systems (Table 3). Tarko et al. [110] studied chokeberries, apples, plums, pears, bananas and melons. They found that chokeberry fruit components were the most active scavengers of the ABTS radical cation. The analysis of blackberry, blackcurrant, chokeberry, raspberry and redcurrant antioxidants (DPPH•) indicated a relatively high potential of chokeberries and selected blackcurrant varieties [120]. Nakajima, Tanaka, Seo, Yamazaki, and Saito [121] confirmed the DPPH radical scavenging ability using ethanol extracts of five berries, which were rich in anthocyanins: blackberries, black chokeberries, blackcurrants, bilberries and elderberries. The antioxidative activity of the chokeberry extract at the highest concentration (2 mg/mL) was lower than that of the other extracts, except the elderberry extract. Other studies tested the potential of 26 fruits to scavenge superoxide radicals (ROO•) in the ORAC assay. Chokeberry fruits were less active than elderberries and wild roses—their values amounted to 160.8, 205.4 and 201.1 µmol TE/g FW, respectively. The antiradical activity of the other fruits ranged from 2.3 to 98.9 µmol TE/g FW [36]. Wu et al. [59] evaluated the antioxidative potential of different fruits using ORAC for hydrophilic (H-ORAC) and lipophilic compounds (L-ORAC). Among 15 fruit samples, chokeberries and blackberries exhibited the highest antiradical activity. The H-ORAC values noted for chokeberries were many times greater than the L-ORAC values, i.e., 158.2 and 2.42 µmol TE/g FW, respectively.
Table 3.
Antioxidant activity of chokeberry fruits and products.
| Product | Experimental model | Method | Result | Reference |
|---|---|---|---|---|
| Chokeberry juices from different growing seasons | Chokeberries (200 g) were mixed in a house blender. Juice was separated from the mash by subsequent pressing, bottled and stored at 4 °C. |
DPPH [mmol TE/l] |
juice2012 14.6, juice2013 13.4, juice2014 12.9 | Tolić et al. [5] |
| FRAP [mmol Fe2+/l] |
juice2013 179.5, juice2012 166.7, juice2014 128.2 | |||
| Chokeberry products | Powdered fruits and pomace (2 g) were extracted with 50 mL of MeOH acidified with 2.0% formic acid. The extraction was performed twice by incubation for 20 min under sonication. Next, the slurry was centrifuged and the supernatant was filtered. | ABTS [mmol TE/100 g DM] |
powder from dried fruit 81.66, powder from pomace of uncrushed fruit 81.63, powder from pomace of crushed fruit 59.94, juice from crushed fruit 32.73, juice from uncrushed fruit 20.11 | Oszmiański and Lachowicz [33] |
| DPPH [mmol TE/100 g DM] |
powder from dried fruit 53.78, powder from pomace of uncrushed fruit 52.22, powder from pomace of crushed fruit 32.61, juice from crushed fruit 20.20, juice from uncrushed fruit 9.81 | |||
| Dried chokeberry fruits by freeze drying (FD), vacuum-microwave drying (VMD), vacuum drying (VD), convection drying (CD), convection-vacuum-microwave drying (CVM) | Chokeberry powder (5 g) was weighed into a test tube, 25 mL of 80% MeOH with 1% HCl was added, and the suspension was stirred slightly. Tubes were sonicated for 15 min twice and left at 4 °C for 24 h. Afterward, the extract was centrifuged and supernatants were collected. | ABTS [mmol TE/100 g DM] |
fresh 234.9, FD 114.7, CVM2h+360/240 83.0, CVM6h+360/120 82.7, CVM6h+360/240 80.5, VMD120W 78.4, CVM2h+360/120 77.9, VMD240W 77.2, VMD480/120W 76.3, VMD480/240W 76.2, VMD360/240W 75.9, VD 75.3, CD70 °C 75.0, VMD240/120W 73.2, VMD360/120W 72.3, CD60 °C 52.4, CD50 °C 41.9 | Samoticha et al. [58] |
| FRAP [mmol TE/100 g DM] |
Fresh 39.0, FD 26.3, CVM2h+360/240 23.8, CVM6h+360/240 22.1, CVM6h+360/120 21.5, VMD360/240W 20.7, VMD480/240W 19.7, VD 19.5, CVM2h+360/120 19.3, VMD480/120W 19.3, VMD240W 19.1, VMD120W 18.5, VMD240/120W 17.8g, CD70 °C 17.7, VMD360/120W 17.5, CD60 °C 16.1, CD50 °C 15.4 | |||
| Commercial chokeberry juices | Not specified. | ABTS [mmol TE/1] |
20.39–91.21 | Sosnowska et al. [40] |
| DPPH [mmol TE/1] |
19.02–106.13 | |||
| FRAP [mmol TE/1] |
12.19–61.09 | |||
| Fruit extracts | Freeze-dried fruits were disintegrated with mill and were triplicate extracted with 70% EtOH. Samples were dried, then dissolved in distilled water and percolated through an Amberlite XAD4 column to adsorb polyphenols. Polyphenols were obtained after washing the column with 70% ethanol. The collected fraction was evaporated in a vacuum evaporator until dry mass. | phosphatidylcholine (PC) liposome oxidation inhibition [IC50 μg/mL] | chokeberry 24.6, blackcurrant 30.9, rosehip 33.5, hawthorn 45.9 | Strugała et al. [113] |
| Dried pomaces | Dried, milled and sieved fruits pomace (6–10 g) were extracted in two steps with 100 mL of eluent (50 + 50) deionized H2O (temp. 100 °C) and left for 5 min. Mixture was shaken for 15 min and then filtered through a 45 μm filter. The extracts were collected and evaporated on a rotary evaporator at 40 °C. | DPPH [%] |
blackcurrant 68.2, chokeberry 67.0, apple 47.3, strawberry 39.3, carrot 37.7 | Pieszka et al. [16] |
| TRAP [μg TE/g] |
chokeberry 179, apple 96, black currant 82, strawberry 61, carrot 50 | |||
| Selected edible fruits and their leaves | Fruits (0.5 g) and leaves (0.2 g) were freeze dried and extracted by 10 mL of mixture containing MeOH (30 mL/100 mL), ascorbic acid (2.0 g/100 mL) and acetic acid (1.0 mL/100 mL) of reagent. The extracts were sonicated for 15 min, left for 24 h at 4 °C in darkness, sonicated again for 15 min and centrifuged. | ABTS [mM TE/100 g DM] |
quinceleaves 116.49, cranberryleaves 96.02, bilberryleaves 79.30, Japanese quinceleaves 60.30, chokeberryfruits 52.31, chokeberryleaves 50.01, appleleaves 35.94, bilberryfruits 35.34, blackcurrantleaves 32.91, Japanese quincefruits 32.88, blackcurrantfruits 22.47, cranberryfruits 14.61, applefruits 8.72, quincefruits7.85 | Teleszko and Wojdyło [54] |
| FRAP [mM TE/100 g DM] |
quinceleaves 65.25, bilberryleaves 59.58, cranberryleaves 43.17, chokeberryleaves 40.55, Japanese quinceleaves 40.09, chokeberryfruits 36.64, bilberryfruits 26.81, Japanese quincefruits 19.51, blackcurrantleaves 19.16, appleleaves 15.30, blackcurrantfruits 11.82, cranberryfruits 8.40, quincefruits 5.43, applefruits 3.44 | |||
| Chokeberry products: (juices (J), powders (P), capsules (C), fruit teas (FT), dried berries (DB)) | Samples (6 g) were mixed with 20 mL of MeOH/2% HCl (95:5 v/v). After 60 min the solution was filtered under vacuum in a 50 mL. Extraction of the residue was repeated using the same conditions. The filtrates were combined and adjusted to 50 mL with MeOH/2% HCl (95:5 v/v). | DPPH [mmol TE/l juice or mmol TE/100 g DM powder, capsules, fruit teas, dried berries] |
DB2 191.31, DB1 183.52, FT3 163.33, FT4 153.96, FT1 149.44, P3 131.06, FT2 111.43, P2 105.68, P1 95.00, C2 80.93, C1 58.49, J10 40.19, J8 34.22, J1 33.37, J11 28.12, J9 26.25, J2 23.03, J6 20.66, J4 19.47, J5 18.29, J7 16.51, J2 12.09 | Tolić et al. [34] |
| FRAP [mmol of Fe2+/l juice or mmol of Fe2+/100 g DM powder, capsules, fruit teas, dried berries] |
J6 79.86, J1 76.14, J4 72.43, J8 71.50, P2 68.60, C1 65.82, J10 62.92, P1 60.66, C2 60.35, J11 60.13, J2 51.50, J2 48.76, J5 48.64, P3 47.38, FT2 43.12, J7 38.98, J9 38.71, FT1 32.74, DB1 21.51, DB2 17.40, FT4 15.94, FT3 13.50 | |||
| Different fruits extracts | Powdered, freeze dried fruits (5 g) were extracted with 100 mL 80% acetone in 0.2% formic acid at room temperature for 1 h. After that, the samples were centrifuged and concentrated via rotary evaporation to a volume of 15 mL in order to fully remove the acetone. Then the volume was adjusted to 50 mL with ultra clean H2O and the extracts were centrifuged. | ORAC [μmol TE/l] |
rosehip 93677.6; hawthorn 73804.6; blueberry 72487.2; chokeberry 55505.7; blackcurrant 46421.7; rowanberry 23689.6 | Denev et al. [122] |
| TRAP [μmol TE/l] |
rosehip 87109.4; hawthorn 51125.1; blueberry 43433.6; chokeberry 43217.1; rowanberry 34612.0; blackcurrant 33510.6 | |||
| H-ORAC [μmol GAE/L] |
rosehip 76069.4; hawthorn 31328.5; blueberry 26339.0; chokeberry 22506.0; blackcurrant 20019.3; rowanberry 15373.4 | |||
| Lipid peroxidation [% of control] |
blackcurrant> rowanberry> blueberry > chokeberry > rosehip > hawthorn autoxidation of linoleic acid was effectively inhibited by blueberry, rowanberry and blackcurrant extracts at the end of the first day of storage. The inhibition of hydroperoxide formation by these fruits and, in addition by chokeberry, was observed also after the third day of linoleic acid autoxidation (less than 50% of control). Finally, hydroperoxide formation was inhibited to more than 50% of the control value by all extracts analysed after 6 days of linoleic acid autoxidation. Extract from blueberry was the most effective as it diminished the hydroperoxides to 8% of control value. |
|||
| Dried (D) and candied (C) fruits | Dried and homogenized whole edible parts of fruits (15 g) were dispersed in 20 mL of 62.5% aqueous methanol containing 2 g/L of TBHQ. To this extract 5 mL of 6M HCl was added, the hydrolysis was carried out in a shaking water bath at 85 °C for 2 h. Then the sample was filtered, made up to 50 mL with methanol, and sonicated (5 min.). | ABTS [mmol/100 g DM] |
chokeberriesD 21.378, bilberriesD 17.996, cherriesC 3.038, plumsD 2.913, grapes (amber light) D 2.188, apricotsD 1.377, cranberriesC 0.835, grapes (amber dark)D 0.648, datesC 0.621f, figsD 0.388 | Miletić et al. [99] |
| DPPH [μmol/100 g DM] |
bilberriesD 2130.23, chokeberriesD 1815.08, plumsD 503.65, datesC 388.98, apricotsD 317.56, grapes (amber light) D 264.56, cherriesC 254.64, grapes (amber dark) D 152.53, cranberriesC 139.80, figsD 129.55 | |||
| Wild and cultivated small fruits | Frozen fruits (6 g) were homogenized and mixed with 10 mL of ethyl acetate. The procedure was repeated four times. Extract (20 mL) was evaporated to dryness and the residue was dissolved in 4 mL of MeOH. | DPPH [μmol TE/mg DM] |
blackberry 0.2125, chokeberry 0.1065, cherry 0.1030, blackthorn 0.0785, raspberry 0.0725 | Mitic et al. [123] |
| ABTS [μmol TE/mg DM] |
blackberry 0.3616, cherry 0.2552, chokeberry 0.1808, blackthorn 0.1704, raspberry 0.1576 | |||
| total reducing power [μmol AAE/mg DM] |
blackberry 0.1920, cherry 0.1720, aronia 0.1540, blackthorn 0.1440, raspberry 0.1180 | |||
| FRAP [μmol Fe/mg DM] |
blackberry 1.0900, aronia 0.6120, cherry 0.5660, blackthorn 0.4100, raspberry 0.3780 | |||
| Different fruits | Fruits (20 g) were extracted with 70% acetone (200 mL) at room temperature for 60 min with stirring. After centrifugation, and filtration, the supernatants were concentrated by vacuum rotary evaporator. The aqueous phase was diluted to 25 mL with H2O. | ABTS [μmol TE/g] |
chokeberry 124.66, bilberry 54.17, blue honeysuckle 51.54, blackcurrant 35.75, lingonberry 34.82, blackberry 28.91, blueberry 27.09, red gooseberry 24.39, red currant 23.45, cranberry 20.43, raspberry 20.36, green gooseberry 18.56, strawberry 16.56, pomegranate 14.02, sour cherry 10.70, grape pink 10.38, apple 8.77, sweet cherry 6.00, orange 4.80, pineapple 4.02, red grapefruit 3.69, mandarine 3.54, pomelo 3.25, plum ‘Węgierka Zwykła’ 2.03, kiwi 1.97, banana 1.83, pear ‘Nashi’ 1.65, peach 1.59, pear ‘Lukasówka’ 1.46, plum ‘Renkloda’ 0.84 | Podsędek et al. [124] |
| FRAP [μmol TE/g] |
chokeberry 94.24, blue honeysuckle 49.52, bilberry 41.70, blackcurrant 29.93, blackberry 23.36, lingonberry 22.28, red gooseberry 16.99, blueberry 16.86, raspberry 16.81, red currant 16.68, cranberry 12.74, green gooseberry 12.28, strawberry 9.95, sour cherry 9.12, pomegranate 7.62, grape pink 6.10, apple 4.98, sweet cherry 4.34, pineapple 4.01, orange 3.50, red grapefruit 3.08, pomelo 2.22, mandarine 1.66, plum ‘Węgierka Zwykła’ 1.49, kiwi 1.46, banana 1.15, pear ‘Lukasówka’ 0.81, peach 0.79, pear ‘Nashi’ 0.76, plum ‘Renkloda’ 0.66 | |||
| Commercially available Aronia melanocarpa tea infusions (TI) | Tea sample (2 g) were infused with 200 mL deionized H2O heated to 95 °C for 10 min. The solutions were filtered and washed with deionized H2O, cooled to room temperature and diluted to 250 mL with deionized H2O. | DPPH [mmol TE/g] |
TI2 0.074, TI3 0.068, TI1 0.067, TI5 0.058, TI4 0.055 | Veljković et al. [108] |
| ABTS [mmol TE/g] |
TI2 2.744, TI3 2.731, TI1 2.715, TI5 0.089, TI4 0.076 | |||
| FRAP [mmol Fe/g] |
TI4 0.153, TI1 0.147, TI3 0.147, TI5 0.144, TI2 0.136 | |||
| reducing power [mmol AAE/g] |
TI4 3.48, TI5 2.14, TI1 1.36, TI3 0.88, TI2 0.53 | |||
| Different fruits | Homogenized fruits (2 g) were mixed with 20 mL of 0.2% formic acid in 80% acetone solution. Extraction was conducted at room temperature for 1 h. After that, the samples were centrifuged and supernatants were removed. The solid residues were subjected to the second extraction under the same conditions. Both supernatants were combined. | ORAC [μmol TE/g FW] |
elderberry 205.4, brier 201.1, chokeberry 160.8, hawthorn 153.6, blueberry 98.8, blackcurrant 96.0, rowanberry 80.9, blackthorn 79.1, blackberry 74.2, cranberry 70.0, sour cherry 58.6, cornel cherry 49.0, strawberry 47.2, raspberry 38.9, red grapes 26.8, cherry 25.8, pomegranate 19.7, apple 13.8, fig 13.6, plum 10.8, apricot 7.2, white grapes 6.3, peach 6.2, pumpkin 4.9, watermelon 3.8, honeydew melon 2.3 | Denev et al. [50] |
| Black chokeberry (Aronia melanocarpa) powders from commercial pure clack chokeberry juice (Rabenhorst, Germany), obtained by different drying processes: freeze drying (FD), spray drying (SD), oven vacuum drying (OV) | Juice (5 l) was centrifuged (15 min, 5950× g). The supernatant was loaded into XAD-16 column, sugars were removed by H2O, phenolic compounds were removed by EtOH. The remaining solvent was evaporated at 40 °C up to the final volume of aqueous extract 2 l. Extracts was subjected to different drying processes. Dry powders was resolubilized with 5 mL of 20% MeOH (v/v) by 60 sec sonication followed 60 sec of vortexing. The procedure was repeated (3 ×). In case of PCL assay extraction of the hydrophilic antioxidants required about 10 mg of the powders followed by the addition of 10 mL of deionized H2O treated in an ultrasonic bath for 2 min, vortexed for 1 min (3 ×) and centrifuged. The same procedure was applied to extraction of lipophilic compounds with MeOH. All extracts obtained were filtered (0.45μm PTFE filter). | ABTS [μmol TE/100 mg DM] |
SD 251.34, FD 180.45, OV40 °C 175.85, OV60 °C 165.47, OV80 °C 158.08 | Horszwald et al. [88] |
| DPPH [μmol TE/100 mg DM] |
SD 26.49, FD 24.68, OV40 °C 22.81, OV60 °C 20.20, OV80 °C 15.80 | |||
| FRAP [μmol TE/100 mg DM] |
SD 248.56, FD 193.69, OV60 °C 179.91, OV80 °C 171.38, OV40 °C 165.27 | |||
| PCL ACW [μmol TE/100 mg DM] |
OV40 °C 291.94, OV60 °C 289.79, FD 282.34, SD 279.33, OV80 °C 238.72 | |||
| PCL ACL [μmol TE/100 mg DM] |
FD 476.15, OV40 °C 447.06, OV60 °C 436.79, OV80 °C 427.36, SD 411.73 | |||
| Black chokeberry fruits and products | Sample (2 g) was extracted in a cooled ultrasonic bath for 15 min using 5 mL of 75% MeOH containing 0.1% (v/v) formic acid. Samples were then centrifuged for 10 min at 83 Hz and the supernatant was collected. This procedure was repeated four times until the total volume reached 20 mL. | ABTS [g TE/kg FW] |
dried fruit1 74.0, dried fruit2 54.4, pomace 49.6 concentrate 22.0, fruit 11.0, juice2 10.8, juice3 10.8, juice1 9.8, jam2 9.8, compote 9.4, jam1 9.0, syrup 3.7, sour cherry-chokeberry syrup 2.0, raspberry-chokeberry syrup 1.2 | Kapci et al. [7] |
| DPPH [g TE/kg FW] |
dried fruit1 36.3, dried fruit2 30.5, pomace 25.2, fruit 11.3, concentrate 10.8, jam2 8.7, juice2 6.2, juice3 5.8, juice1 5.7, jam1 5.0, compote 4.8, syrup 2.2, sour cherry-chokeberry syrup 2.0, raspberry-chokeberry syrup 0.7 | |||
| CUPRAC [g TE/kg FW] |
dried fruit1 257.2, dried fruit2 233.2, pomace 192.4, concentrate 74.5, fruit 67.7, jam2 57.4, juice2 35.1, juice1 33.8, jam1 33.6, compote 33.2, juice3 30.7, syrup 13.4, sour cherry-chokeberry syrup 5.2, raspberry-chokeberry syrup 3.0 | |||
| Berry fruit ethanol extracts | Not specified. | ABTS [µM TE/g FW] |
blackcurrant ‘Titania’ 56.8, chokeberry ‘Nero’ 53.2, blackberry ‘Polar’ 51.7, raspberry ‘Polana’ 28.5, highbush blueberry ‘Bluecrop’ 27.3, red chokeberry ‘Brilliant’ 23.8, red currant ‘Heros’ 22.1, white currants ‘Blanca’ 7.4 | Najda and Łabuda [73] |
| DPPH [µM TE/g FW] |
chokeberry ‘Nero’ 199.4, blackcurrant ‘Titania’138.90, blackberry ‘Polar’ 129.3, red chokeberry ‘Brilliant’ 63.2, raspberry ‘Polana’ 59.4, red currant ‘Heros’ 48.3, highbush blueberry ‘Bluecrop’ 40.4, white currants ‘Blanca’ 19.4 | |||
| FRAP [µM Fe/g FW] |
chokeberry ‘Nero’ 112.5, blackcurrant ‘Titania’ 108.4, blackberry ‘Polar’ 97.1, highbush blueberry ‘Bluecrop’ 34.5, red chokeberry ‘Brilliant’ 34.1, red currant ‘Heros’ 31.7, raspberry ‘Polana’ 27.5, white currants ‘Blanca’ 12.3 | |||
| Berry extracts: aqueous (W), 100% acetone (Ac) and 100% hexane (He) | Lyophilized berries were extracted with water, 100% of acetone and 100% of hexane (concentration 25 mg/mL) at room temperature twice during 3 h. | ABTS [μM TE/g] |
murtilla non-ripe 620.7, murtilla ripe 446.6, blueberries Poland 254.8, chokeberry 219.3, murteola ripe 200.6, blueberries Chile 197.7, murteola non-ripe 144.4, raspberries 82.5, murtilla non-ripe 56.42, murtilla ripe 29.89, blueberries Poland 26.59, murteola non-ripe 19.79, chokeberry 15.14, murteola ripe 10.08, blueberries Chile 9.79, raspberries 7.61, raspberries 3.25, chokeberry 2.80, murtilla non-ripe 2.30, murteola non-ripe 1.54, blueberries Chile 1.11, murtilla ripe 1.10, blueberries Poland 0.98, murteola ripe 0.60 | Arancibia-Avila et al. [125] |
| DPPH [μM TE/g] |
murtilla non-ripe 334.7, murtilla ripe 208.9, murteola ripe 102.4, blueberries Chile 94.5, chokeberry 87.2, blueberries Poland 75.1, murteola non-ripe 64.6, raspberries 27.7, murtilla non-ripe 23.76, murtilla ripe 16.43, blueberries Poland 15.01, murteola non-ripe 9.32, murteola ripe 4.00, chokeberry 3.55, raspberries 3.29, blueberries Chile 3.01, raspberries 2.14, chokeberry 1.19, murtilla non-ripe 1.15, murteola non-ripe 0.98, blueberries Poland 0.44, blueberries Chile 0.37, murtilla ripe 0.35, murteola ripe 0.18 | |||
| FRAP [μM TE/g] |
murtilla non-ripe 327.3, murtilla ripe 208.9, blueberries Poland 177.3, murteola ripe 76.0, blueberries Chile 73.3, chokeberry 57.4, murteola non-ripe 43.0, murtilla non-ripe 41.46, raspberries 27.7, murtilla ripe 20.87, blueberries Poland 11.59, murteola non-ripe 8.38, blueberries Chile 5.59, murteola ripe 2.94, chokeberry 2.84, raspberries 2.80, raspberries 1.99, chokeberry 0.89, murtilla non-ripe 0.89, blueberries Chile 0.58, murteola non-ripe 0.52, blueberries Poland 0.31, murtilla ripe 0.30, murteola ripe 0.16 | |||
| CUPRAC [μM TE/g] |
murtilla non-ripe 600.5, murtilla ripe 428.5, blueberries Poland 250.9, chokeberry 212.9, blueberries Chile 154.0, murteola ripe 116.8, murteola non-ripe 82.9, murtilla non-ripe 38.79, blueberries Poland 33.27, raspberries 30.4, murtilla ripe 28.65, murteola non-ripe 14.11, blueberries Chile 13.45, murteola ripe 7.72, chokeberry 7.16, raspberries 6.09, raspberries 4.56, murtilla non-ripe 3.18, chokeberry 3.12, murteola non-ripe 2.86, blueberries Poland 2.78, blueberries Chile 2.68, murtilla ripe 0.77, murteola ripe 0.12 | |||
| Different fruits | Fruits were extracted with H2O containing 200 ppm of SO2 (ratio of solvent to fruits 3:10). Then the extract was adsorbed on Purolite AP 400 resin for further purification. The polyphenols were then eluted out with 80% EtOH, concentrated and freeze dried. | ABTS [µmol TE/mg DM] |
apple 5.65, strawberry 4.80, chokeberry 4.15 | Bonarska-Kujawa et al. [70] |
| Linoleic acid oxidation [antioxidant activity index min/(µg DM/mL)] |
apple 47.1, chokeberry 44.4, strawberry 43.1 | |||
| Erythrocyte membranes UV oxidation [I50 mg/mL DM] |
Trolox 0.0146, apple 0.0286, chokeberry 0.0520, strawberry 0.0529 | |||
| Erythrocyte membranes AAPH oxidation [I50 mg/mL DM] | Trolox 0.00390, apple 0.00794, chokeberry 0.00955, strawberry 0.02423 | |||
| Wild chokeberry and cultivars | Homogenized berries (15 g) were extracted (3 × 15mL) in the EtOH solution containing 1% hydrochloric acid. Combined extracts were shaken for 30 min and left to stand 24 h. Than the extracts were evaporated using a rotary evaporator. | DPPH [EC50 g fruit/g DPPH] |
wild 0.46, ‘Nero’ 0.91, ‘Viking’ 0.94, ‘Galicianka’ 2.46 | Jakobek et al. [2] |
| Fruit juices | Fresh juice (1 mL) was diluted up to the volume of 25 mL. Part of solution was centrifuged at 15,000 rpm for 20 min at 4 °C. Supernatant solution was used for analysis. Additionally, non-centrifuged juices were analysed. | ORAC [μmol TE/100 mL] |
non-centrifuged samples: black chokeberry 1086.60, blackcurrant 500.80, red currant 422.60, apple 389.40, cranberry 379.70, pomegranate 340.50, blueberry 297.80, lime 285.10, lemon 223.10, grapefruit 105.04, aloe vera 81.35, red orange 71.72, black grapes 58.60, kumquat 38.16, white grapes 30.82 Centrifuged samples: blackcurrant 1271.80, black chokeberry 666.60, red currant 540.50, cranberry 336.60, blueberry 206.80, grapefruit 200.30, apple 196.40, pomegranate 151.40, kumquat 133.50, lemon 125.90, lime 111.90, black grapes 94.30, red orange 35.68, white grapes 31.10, aloe vera 27.07 |
Keskin-Šašić et al. [126] |
| Different berry extracts | Lyophilized berries were extracted with MeOH (concentration 25 mg/mL) at room temperature twice during 3 h. | ABTS [μM TE/g] |
murtilla non-ripe 878.18, murtilla ripe 405.76, blueberries Poland 265.92, murtilla-like berries non-ripe 244.22, chokeberry 152.63, blueberries Chile 150.45, raspberries 80.04, murtilla-like berries ripe 65.35 | Arancibia-Avila et al. [89] |
| FRAP [μM TE/g] |
murtilla non-ripe 486.92, murtilla ripe 204.21, blueberries Poland 149.04, chokeberry 100.81, murtilla-like berries non-ripe 81.32, blueberries Chile 67.12, murtilla-like berries ripe 34.12, raspberries 33.98 | |||
| CUPRAC [μM TE/g] |
murtilla non-ripe 1012.42, murtilla ripe 507.89, blueberries Poland 265.76, chokeberry 215.85, murtilla-like berries non-ripe 203.83, blueberries Chile 141.36, murtilla-like berries ripe 92.36, raspberries 69.91 | |||
| Fruit and vegetable snacks extracts (chips and puffings) | Chips and puffings (100 g) were freeze dried, minced and macerated with 80% EtOH (500 mL) for 24 h at room temperature (repeated 3 times). Collected extracts were filtered, centrifuged, then the EtOH was evaporated. Powdered extracts were kept frozen until further use (−18 °C). | ABTS [mg TE/g DM extract] |
chokeberrypuffing 37.44, blackcurrantpuffing 18.61, strawberrypuffing 16.16, apple-bananachips 13.16, applechips 11.76, apple-blackcurrantchips 10.74, apple-orangechips 10.59, carrotpuffing 2.23 | Gramza-Michałowska and Człapka-Matyasik [127] |
| ABTS [EC50 mg/mL] |
chokeberrypuffing 20.11, blackcurrantpuffing 38.92, strawberrypuffing 46.28, apple-bananachips 55.62, applechips 64.79, apple-orangechips 69.95, apple-blackcurrantchips 71.92, carrotpuffing 437.12 | |||
| DPPH [mg TE/g DM extract] |
chokeberrypuffing 6.82, strawberrypuffing 6.40, apple-orangechips 5.38, apple-blackcurrantchips 5.31, apple-bananachips 5.29, blackcurrantpuffing 5.28, applechips 5.02, carrotpuffing 3.47 | |||
| DPPH [EC50 mg/ml] |
chokeberrypuffing 10.04, strawberrypuffing 11.23, apple-orangechips 12.67, blackcurrantpuffing 12.71, apple-bananachips 13.11, apple-blackcurrantchips 14.28, applechips 14.72, carrotpuffing 23.19 | |||
| Berry fruits | Homogenized fruits (50 g) were mixed with 150 mL 1% citric acid in H2O and extracted on an orbital shaker at 60ºC for 1 h. | ORAC [μmol TE/g DM extract] |
elderberry 5783, blueberry 5646, chokeberry 5165, blackberry 4042, blackcurrant 3949 | Denev et al. [36] |
| TRAP [μmol TE/g DM extract] |
chokeberry 4051, elderberry 3230, blueberry 2860, blackberry 2771, blackcurrant 2132 | |||
| H-ORAC [μmol GAE/g DM extract] |
blueberry 1293, chokeberry 1265, elderberry 1264, blackcurrant 874, blackberry 834 | |||
| TBARS [inhibition of induced lipid peroxidation] [nmol/mL] |
Chokeberry > elderberry > blackcurrant > blueberry > blackberry > control | |||
| NO scavenging activity time [sec] | blueberry 183, chokeberry 215, blackcurrant 280, elderberry 290, blackberry 363, control 947 | |||
| Fruit products: purees, concentrates, juices | Puree (1.5 g) was mixed with 5 mL H2O, and vortexed for 1 min. Afterwards, the solution was centrifuged at 3800 g for 5 min. The supernatants were collected in 20 mL volumetric flasks (procedure was repeated 3 times). After the last extraction, the flasks were filled up to the mark and aliquots of 1.5 mL were centrifuged. | FRAP [mmol Fe2+/100 g] |
acerola 17.23, chokeberry 9.79, elderberry 9.33, boysenberry 5.90, blackcurrant 5.24, blackberry 4.53, açai-lime 4.09, lingonberry 3.90, strawberry 2.93, grape 2.91, cranberry 2.28, pomegranate 1.66, apple 1.33, orange 0.48 | Müller et al. [128] |
| ABTS [mmol TE/100 g] |
acerola 10.57, chokeberry 9.73, elderberry 9.66, grape 5.92, blackcurrant 5.50, boysenberry 4.38, açai-lime 4.00, lingonberry 3.94, blackberry 3.08, cranberry 2.21, strawberry 2.08, pomegranate 1.75, apple 1.10, orange 0.40 | |||
| ORAC [mmol TE/100 g] |
chokeberry 11.45, elderberry 10.27, acerola 9.42, açai-lime 7.68, blackcurrant 6.99, boysenberry 5.31, strawberry 3.83, lingonberry 3.74, grape 3.49, blackberry 3.44, cranberry 2.57, pomegranate 2.50, apple 1.66, orange 1.07 | |||
| Chokeberry fruits- different cultivars | Fresh samples (10 g) were homogenized for 10 s in 100 mL of MeOH. The resulting paste was placed into Erlenmeyer flasks (120 mL) for 24 h at 25 °C, and the residue was then extracted with two additional portions of MeOH. The combined MeOH extracts were evaporated at 40 °C and redissolved in MeOH at a concentration of 100 mg/mL. | DPPH [g AAE/kg] |
‘Viking’ 15.96, ‘Nero’ 15.32, ‘Hugin’ 11.15, ‘Aron’ 9.02, ‘Fertödi’ 8.89 | Rop et al. [3] |
| hydroxyl radical (OH•) scavenging activity [% inhibition] |
‘Viking’ 34.15, ‘Nero’ 33.51, ‘Hugin’ 31.12, ‘Aron’ 25.01, ‘Fertödi’ 22.08 | |||
| nitric oxide (NO•) scavenging activity [% inhibition] |
‘Viking’ 41.46, ‘Nero’ 37.30, ‘Hugin’ 33.10, ‘Aron’ 28.42, ‘Fertödi’ 27.59 | |||
| superoxide anion (O2•−) scavenging activity [% inhibition] |
‘Viking’ 36.92, ‘Nero’ 35.96, ‘Hugin’ 30.48, ‘Aron’ 22.22, ‘Fertödi’ 21.24 | |||
| lipid peroxidation (TBARS) [inhibition activity%] |
‘Viking’ 19.81, ‘Nero’ 19.22, ‘Hugin’ 16.19, ‘Aron’ 12.57, ‘Fertödi’ 12.05 | |||
| Berry fruits | Ground berries (5 g) with 25 mL of MeOH at ambient temperature for 2 h with constant shaking. The solution was filtered, and the residue was repeatedly extracted with 20 mL of MeOH for 2 h. Finally, extracts were combined. | DPPH [inhibition%] |
chokeberry var. ‘cleata’≥ chokeberry ‘Viking’≥ chokeberry ‘Aron’ > raspberry ‘Bristol’> raspberry ‘Meeker’> elderberry ‘Lacimiata’> raspberry ‘Poranna Rosa’> elderberry ‘Aurea’ | Viskelis et al. [129] |
| Different berries | Berries (20 g) were grinded in MeOH (20 mL) acidified with HCl (0.1%). After 60 min the solution was filtered. The residue was extracted again, and the extracts were combined and diluted to volume of 50 mL with MeOH acidified with HCl (0.1%). | DPPH [inhibition %] |
chokeberry > blackberry> red raspberry> strawberry | Jakobek et al. [74] |
| Fruit juices | Fruits (500 g) were thawed at room temperature, then processed in juice extractor natural fruit juice was centrifuged. | DPPH [μmol TE/mL] |
chokeberry 72.44, elderberry 62.14, blackcurrant 30.15, sour cherry 12.52, blackberry 8.75, red raspberry 8.20, strawberry 4.39, sweet cherry 4.07 | Jakobek et al. [74] |
| Fruit pomaces | The pomace was thawed and dried in the air dryer (50 °C, 2 h) before the analysis. Subsequently dried pomaces were ground and subjected to extraction in 80% EtOH. | ABTS [µM TE/g DM] |
honeysuckle 62.24, blackcurrant 56.88, chokeberry 53.2, strawberry 23.32, Japanese quince 13.97 | Nawirska et al. [119] |
| DPPH [µM TE/g DM] |
chokeberry 199.4, blackcurrant 138.81, honeysuckle 65.27, strawberry 58.67, Japanese quince 18.21 | |||
| FRAP [µM Fe3+/g DM] |
chokeberry 12.53, honeysuckle 11.13, Japanese quince 6.12, blackcurrant 5.24, strawberry 2.75 | |||
| Black chokeberry fruits, juice and pomace | Freeze-dried sample (1 g) was homogenized in 20 mL of MeOH. The slurry was filtered and filtrate was diluted in MeOH. | ABTS [μM TE/100 g DM] |
pomace 779.58, fruits 439.49, juice 314.05 | Oszmiański and Wojdyło [53] |
| DPPH [μM TE/100 g DM] |
pomace 301.89, fruits 279.38, juice 127.45 | |||
| Berry fruits | 20 mL of MeOH/HCl 2% (95:5 v/v) were added to 20 g frozen berries. After 60 min, the berries were homogenized and centrifuged for 15 min at 3000 rpm. The supernatant solution was filtered under vacuum, and the residue was extracted again the same way. The solution was diluted to volume with MeOH/HCl 2%. | DPPH [EC50 mg fruit] |
black currant ‘Tsema’ 1.0, black chokeberry ‘Nero’ 1.8, blackcurrant ‘Ben Lomond’ 1.8, blackcurrant ‘Silvergieters’ 2.5, blackcurrant ‘Burga’ 2.7, blackcurrant ‘Baldwin’ 2.9, blackcurrant ‘Tenah’ 3.4, blackcurrant ‘Noir De Bourgogne’ 3.6, blackcurrant ‘Black Down’ 4.2, redcurrant ‘Rotet’ 4.3, blackberry ‘Smoothstem’ 4.6, blackberry ‘Thornless Boy Sembes’ 5.2, raspberry ‘Sumner’ 5.5, blackberry ‘Black Diamond’ 5.7, blackberry ‘Darrow’ 5.7, red currant ‘Rosetta’ 5.7, red currant ’Red Lake’ 5.9, blackberry ‘Hull Thornless’ 6.2, blackberry ‘Chester’ 7.6, blackberry ‘Black Satin’ 9.5, raspberry ‘September’ 10.9 | Benvenuti et al. [120] |
| Juice concentrates | Not specified. | ABTS [mg TE/mL] |
blackcurrant 104.3, chokeberry 103.2, elderberry 98.7, redcurrant 36.0, strawberry 30.0, raspberry 24.7, red grape 23.1, cherry 18.7, plum 8.8 | Bermúdez-Soto and Tomás-Barberán [87] |
| DPPH [mg TE/mL] |
chokeberry 60.0, blackcurrant 55.3, elderberry 43.3, redcurrant 23.1, strawberry 16.6, raspberry 13.4, red grape 10.4, cherry 10.0, plum 4.6 | |||
| Berry fruits | Sample (1 g) was mixed with 5 g of sea sand, transferred to a 22 mL extraction cell, and extracted with hexane/dichloromethane (1:1v/v) followed by acetone/water/acetic acid (70:29.5:0.5 v/v) extraction (ASE200). The extracts from hexane/dichloromethane were used to measure lipophilic ORACFL, acetone/water/acetic acid extracts were used to measure the hydrophilic ORACFL. | L-ORAC [μmol TE/g FW] |
chokeberry 2.42, elderberry 1.97, red currant 1.27, blackcurrant ‘Titania’ 1.15, blackcurrant ‘Ben Alder’ 0.84, blackcurrant ‘Ben Nevis’ 0.75, blackcurrant ‘Ban Tirran’ 0.75, blackcurrant ‘Ben Lomond’ 0.68, blackcurrant ‘Ukarine’ 0.68, gooseberry ‘Marigold’ 0.45, gooseberry ‘Leveller’ 0.43, gooseberry ‘Careless’ 0.35, gooseberry ‘Dan’s Mistake’ 0.28, gooseberry ‘Whinham’ 0.15, gooseberry ‘Lancashine’ 0.15 | Wu et al. [59] |
| H-ORAC [μmol TE/g FW] |
chokeberry 158.2, elderberry 145.0, blackcurrant ‘Ben Alder’ 100.6, blackcurrant ‘Ben Lomond’ 92.3, blackcurrant ‘Ben Nevis’ 90.6, blackcurrant ‘Ban Tirran’ 86.6, blackcurrant ‘Ukarine’ 53.7, blackcurrant ‘Titania’ 49.0, gooseberry ‘Lancashine’ 41.3, gooseberry ‘Whinham’ 39.2, gooseberry ‘Dan’s Mistake’ 37.1, gooseberry ‘Marigold’ 33.7, red currant 32.6, gooseberry ‘Leveller’ 26.4, gooseberry ‘Careless’ 20.4 | |||
| Berry fruits | Berries (3–5 g) were extracted twice with 10 mL of 80% acetone containing 0.2% formic acid using a Polytron for 2 min and then centrifuged. The supernatants were combined. | ORAC [μmol TE/g FW] |
chokeberry 160.2, lingonberry ‘Amberland’ 38.1, blueberry ‘Serra’ 28.9, cranberry ‘Ben Lear’ 18.5 | Zheng and Wang [130] |
AAE: ascorbic acid equivalents, ACL: antioxidant capacity of lipid soluble compounds, ACW: antioxidant capacity of water soluble compounds, CUPRAC: cupric reducing antioxidant capacity, DM: dry matter, EC50: half maximum effective concentration, EtOH: ethanol, FRAP: ferric reducing antioxidant power, FW: fresh weight, GAE: gallic acid equivalents, H-ORAC: hydroxyl radical averting capacity, I50: extract concentrations which cause 50% inhibition, MeOH: methanol, ORAC: oxygen radical absorbance capacity, PCL: photochemiluminescence, TE: Trolox equivalents, TRAP: total peroxyl radical trapping parameter.
The high antioxidative potential of chokeberry fruit was confirmed using ORAC, TRAP, hydroxyl radical (HO•) and nitric oxide (NO) assay. The ability of chokeberry extract to scavenge radicals (ROO•) in the ORAC assay amounted to 5165 µmol TE/g of the extract and it was only 10.7% lower than that of the elderberry extract. The TRAP assay showed that the ROO• radical was more effectively scavenged by the chokeberry extract (4051 μmol TE/g extract) than the elderberry fruit extract (3230 μmol TE/g extract). The antiradical activity of the chokeberry, black elderberry and bilberry extracts against the hydroxyl radical HO• did not differ significantly (1264–1293 μmol GAE/g extract). The chokeberry extract exhibited high nitrogen oxide (NO) scavenging capacity and inhibited the oxidation of α-linolenic acid [36]. The chokeberry fruits also inhibited liposomal oxidation effectively, exhibiting superior antioxidative properties to blackcurrants, rosehips and hawthorns [113].
Najda and Łabuda [73] investigated the antioxidative activity of different fruits and found that chokeberry fruits exhibited higher antioxidative activity than the other eight fruits. The activity was measured by determining the ability to reduce Fe in the FRAP assay and to scavenge the DPPH radical. The ABTS cation was effectively scavenged only by the ingredients of ‘Titania’ blackcurrants. The chokeberry fruit extracts exhibited antioxidative activity in DPPH, hydroxyl (HO•), superoxide anion (O2•−), and nitric oxide (NO) assay, and inhibited lipid oxidation. The ‘Viking’ and ‘Nero’ varieties had the greatest antioxidative potential, whereas the ‘Fertödi’ and ‘Aron’ cultivars exhibited the lowest potential [3]. Viskelis et al. [129] compared the antioxidative activity of different fruit varieties of chokeberries, raspberries and elderberries. The chokeberry extracts showed the highest antioxidative potential, where the ‘Viking’, ‘Aron’ and ‘Cleata’ varieties scavenged the DPPH radical comparably.
Apart from chokeberry fruit, fruit products and post-production waste also exhibit the antioxidative potential. The analysis of the antiradical activity of chokeberry fruit, juice and pomace against ABTS•+ and DPPH• indicated the highest activity of the pomace, followed by the fruit and juice. The antiradical activity was correlated with the polyphenol content in the analysed material [53]. The antioxidative potential of fruit juices was tested. The chokeberry juice had the greatest ability to scavenge the DPPH radical (72.44 µmol TE/mL). The elderberry fruit juice exhibited high activity (62.14 µmol TE/mL), while the activity of other juices was at least 50% lower (4–30 µmol TE/mL) [74]. The ORAC assay confirmed the high antiradical activity of chokeberry juice, which was greater than the activity of 14 other juices [126]. The chokeberry juice concentrate also exhibited the highest antiradical activity, as it scavenged the radicals more effectively than the other fruit juice concentrates [87].
The chokeberry pomace scavenged DPPH radicals and reduced Fe in the FRAP assay much more than the honeysuckle, Japanese quince, blackcurrant and strawberry pomaces. In comparison with the other pomaces the chokeberry pomace exhibited moderate activity in the ABTS•+ assay [119]. According to Pieszka et al. [16] dried chokeberry pomace exhibited better antioxidative properties (TRAP) than apple, blackcurrant, strawberry and carrot pomaces. Chokeberry groups differ in their antioxidative activity considerably [45]. Sosnowska et al. [40] studied the antioxidative activity of juices and observed that the results differed about 5 times in ABTS, FRAP and DPPH assays, while chokeberry teas differed 1.1, 1.3, 6.6 and 36.1 times in FRAP, DPPH, reducing power and ABTS assays [108]. There were also differences in the antioxidative activity of dried chokeberry fruits, powders, capsules, and jams [7,34].
The analysis of the results of studies on chokeberry juice shows that the antioxidative potential of chokeberry products depends on the period and the year of raw material harvesting [5,41]. However, the main factor affecting the antioxidative properties of the products that needs to be considered is the technological production processes. The antioxidative activity is influenced by crushed raw material used for the production of juices and chokeberry powders [33], the method and drying parameters during the production of dried fruit and powder extracts [58,88], as well as the extraction solvent and temperature [89,125]. The antioxidative potential of chokeberry fruit and products is mainly attributed to polyphenols. The activity of individual chokeberry polyphenols: anthocyanins, proanthocyanidins, phenolic acids and flavonols was also determined. The major contributor to DPPH radical scavenging values was the anthocyanin fraction (66.7%), followed by the proanthocyanidin fractions (25.1%), flavonols and phenolic acids (8.2% of the total activity) [74,114]. Zheng and Wang [130] assessed the antioxidative activity of chokeberry in the ORAC assay and found that it resulted from anthocyanins (53.1%), phenolic acids (38.2%) and flavonols (8.7%). Proanthocyanidin was not included in the study.
5. Conclusions
Black chokeberry (Aronia melnocarpa) is a source of many bioactive compounds with a wide spectrum of antioxidant and health-promoting properties. Like other berries, chokeberry is a source of polyphenols, which exhibit the antioxidant potential, demonstrated in numerous in vitro and in vivo experiments. Black chokeberry has the potential to inhibit the activity of various types of radicals, through different mechanisms of action. Fresh, unprocessed black chokeberry fruits are used in the food production, e.g., juices, syrups, wines, preserves, various dietary supplements, and natural dyes. However, they are rarely consumed due to their bitter taste, resulting from the presence of polyphenols. Black chokeberry fruits and flowers are used as traditional remedies based on the health-promoting actions against influenza and immunity enhancer. Numerous studies confirmed the beneficial effects of Aronia melanocarpa L. varieties consumption on hypertension, glucose metabolism disorders, dyslipidaemia, proinflammatory conditions, and reducing the risk factors of the metabolic syndrome. Results also showed the probable potential of black chokeberry to inhibit the development of some types of cancers.
Further research is necessary to understand the interactions with other compounds which may affect the activity of chokeberry components. The antioxidant potential of chokeberry fruit and its products indicates that all fractions could be utilized as a source of antioxidants and valuable nutrients with potential applications in food industry. So far the literature has not provided clear answers to questions concerning the mechanism of interaction between chokeberry components and their stability in complex systems, e.g., in food. Studies conducted to date have indicated numerous benefits resulting from chokeberry and its polyphenolic compounds inclusion in a daily diet. Like other plants and medicinal products of natural origin, chokeberry requires extensive studies on humans to determine its efficacy, safety and mechanisms of action.
Author Contributions
A.S., conceptualization, writing and editing and A.G.-M., conceptualization, writing, editing, supervision and funding acquisition.
Funding
This research was supported by statutory funds of the Department of Gastronomy Sciences and Functional Foods of Poznań University of Life Sciences, grant number 506.751.03.00.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Ochmian I., Grajkowski J., Smolik M. Comparsion of some morphological features, quality and chemical content of four cultivars of chokeberry fruits (Aronia melanocarpa) Not. Bot. Horti Agrobot. Cluj-Napoca. 2012;40:253–260. doi: 10.15835/nbha4017181. [DOI] [Google Scholar]
- 2.Jakobek L., Drenjančević M., Jukić V., Šeruga M. Phenolic acids, flavonols, anthocyanins and antiradical activity of “Nero”, “Viking”, “Galicianka” and wild chokeberries. Sci. Hortic. 2012;147:56–63. doi: 10.1016/j.scienta.2012.09.006. [DOI] [Google Scholar]
- 3.Rop O., Mlcek J., Jurikova T., Valsikova M., Sochor J., Reznicek V., Kramarova D. Phenolic content, antioxidant capacity, radical oxygen species scavenging and lipid peroxidation inhibiting activities of extracts of five Black chokeberry (Aronia melanocarpa (Michx.) Elliot) cultivars. J. Med. Plants Res. 2010;4:2431–2437. [Google Scholar]
- 4.Smolik M., Ochmian I., Smolik B. RAPD and ISSR methods used for fingerprinting selected, closely related cultivars of Aronia melanocarpa. Not. Bot. Horti Agrobot. Cluj-Napoca. 2011;39:276–284. doi: 10.15835/nbha3926268. [DOI] [Google Scholar]
- 5.Tolić M.T., Krbavčić I.P., Vujević P., Milinović B., Jurčević I.L., Vahčić N. Effects of weather conditions on phenolic content and antioxidant capacity in juice of chokeberries (Aronia melanocarpa L.) Pol. J. Food Nutr. Sci. 2017;67:67–74. [Google Scholar]
- 6.Kitryte V., Kraujaliene V., Sulniute V., Pukalskas A., Rimantas Venskutonis P. Chokeberry pomace valorization into foodingredients by enzyme-assisted extraction: Processoptimization and product characterization. Food Bioprod. Process. 2017;105:36–50. doi: 10.1016/j.fbp.2017.06.001. [DOI] [Google Scholar]
- 7.Kapci B., Neradová E., Čížková H., Voldřich M., Rajchl A., Capanoglu E. Investigating the antioxidant potential of chokeberry (Aronia melanocarpa) products. J. Food Nutr. Res. 2013;52:219–229. [Google Scholar]
- 8.Vagiri M., Jensen M. Influence of juice processing factors on quality of black chokeberry pomace as a future resource for colour extraction. Food Chem. 2017;217:409–417. doi: 10.1016/j.foodchem.2016.08.121. [DOI] [PubMed] [Google Scholar]
- 9.Sidor A., Drożdżyńska A., Gramza-Michałowska A. Black chokeberry (Aronia melanocarpa) and its products as potential healthpromoting factors - an overview. Trends Food Sci. Technol. 2019;89:45–60. doi: 10.1016/j.tifs.2019.05.006. [DOI] [Google Scholar]
- 10.Kader A.A., Barrett D.M. Classification, composition of fruits and postharvest maintenance of quality. In: Barrett D.M., Somogyi L.P., Ramaswamy H.S., editors. Processing Fruits: Science and Technology. 2nd ed. CRC Press; Boca Raton, FL, USA: 2005. pp. 3–22. [Google Scholar]
- 11.Mayer-Miebach E., Adamiuk M., Behsnilian D. Stability of chokeberry bioactive polyphenols during juice processing and stabilization of a polyphenol-rich material from the by-product. Agriculture. 2012;2:244–258. doi: 10.3390/agriculture2030244. [DOI] [Google Scholar]
- 12.Ochmian I., Oszmiański J., Skupień K. Chemical composition, phenolics and firmness of small black fruis. J. Appl. Bot. Food Qual. 2009;83:64–69. [Google Scholar]
- 13.Skupień K., Oszmiański J. The effect of mineral fertilization on nutritive value and biological activity of chokeberry fruit. Agric. Food Sci. 2007;16:46–55. doi: 10.2137/145960607781635822. [DOI] [Google Scholar]
- 14.Červenka L. Moisture adsorption characteristics of black currant (Ribes nigrum L.), black elderberry (Sambucus nigra L.) and chokeberry (Aronia melanocarpa, [minchx.] Ell.) samples at different temperatures. J. Food Process. Eng. 2011;34:1419–1434. [Google Scholar]
- 15.Boncheva M., Georgiev G., Shishkov V. Effects of Aronia melanocarpa fruit juice in improving medical test results and creating feeling of health in patients with non-alcoholic fatty liver disease – NAFLD. J. Gene Med. 2013;2:21–30. [Google Scholar]
- 16.Pieszka M., Gogol P., Pietras M., Pieszka M. Valuable components of dried pomaces of chokeberry, black currant, strawberry, apple and carrot as a source of natural antioxidants and nutraceuticals in the animal diet. Ann. Anim. Sci. 2015;15:475–491. doi: 10.2478/aoas-2014-0072. [DOI] [Google Scholar]
- 17.Tanaka T., Tanaka A. Chemical components and characteristics of black chokeberry. Nippon Shokuhin Kagaku Kogaku Kaishi. 2001;8:606–610. doi: 10.3136/nskkk.48.606. [DOI] [Google Scholar]
- 18.Lancrajan I. Aronia melanocarpa a potential therapeutic agent. Studia Universitatis “Vasile Goldiş”, Seria Ştiinţele Vieţii (Life Sciences Series) 2012;22:389–394. [Google Scholar]
- 19.Sójka M., Kołodziejczyk K., Milala J. Polyphenolic and basic chemical composition of black chokeberry industrial by-products. Ind. Crop. Prod. 2013;51:77–86. doi: 10.1016/j.indcrop.2013.08.051. [DOI] [Google Scholar]
- 20.Dulf F.V., Andrei S., Bunea A., Socaciu C. Fatty acid and phytosterol contents of some Romanian wild and cultivated berry pomaces. Chem. Pap. 2012;66:925–934. doi: 10.2478/s11696-012-0156-0. [DOI] [Google Scholar]
- 21.Zlatanov M.D. Lipid composition of Bulgarian chokeberry, black currant and rose hip seed oil. J. Sci. Food Agric. 1999;79:1620–1624. doi: 10.1002/(SICI)1097-0010(199909)79:12<1620::AID-JSFA410>3.0.CO;2-G. [DOI] [Google Scholar]
- 22.Skupień K., Kostrzewa-Nowak D., Oszmiański J., Tarasiuk J. In vitro antileukaemic activity of extracts from chokeberry (Aronia melanocarpa [Michx] Elliott) and mulberry (Morus alba L.) leaves against sensitive and multidrug resistant HL60 cells. Phytother. Res. 2008;22:689–694. doi: 10.1002/ptr.2411. [DOI] [PubMed] [Google Scholar]
- 23.Šnebergrová J., Čížková H., Neradová E., Kapci B., Rajchl A., Voldřich M. Variability of characteristic components of aronia. Czech. J. Food Sci. 2014;32:25–30. doi: 10.17221/540/2012-CJFS. [DOI] [Google Scholar]
- 24.Wichrowska D., Wojdyła T., Rolbiecki S., Rolbiecki R. Influence of drip irrigation and microsprinkler irrigation on the yield and quality of chokeberry fruits. Zesz. Nauk. Inst. Sadow. I Kwiaciarstwa. 2007;15:63–71. [Google Scholar]
- 25.Borycka B., Stachowiak J. Relations between cadmium and magnesium and aronia fractional dietary fibre. Food Chem. 2008;107:44–48. doi: 10.1016/j.foodchem.2007.07.014. [DOI] [Google Scholar]
- 26.Nawirska A., Uklańska C. Waste products from fruit and vegetable processing as potential sources for food enrichment in dietary fibre. Acta Sci. Pol. Technol. Aliment. 2008;7:35–42. [Google Scholar]
- 27.Wawer I., Wolniak M., Paradowska K. Solid state NMR study of dietary fiber powders from aronia, bilberry, black currant and apple. Solid State Nucl. Magn. Reson. 2006;30:106–113. doi: 10.1016/j.ssnmr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Olsson M.E., Gustavsson K.E., Andersson S., Nilsson Å., Duan R.D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2004;52:7264–7271. doi: 10.1021/jf030479p. [DOI] [PubMed] [Google Scholar]
- 29.Pavlović A.N., Brcanović J.M., Veljković J.N., Mitić S.S., Tošić S.B., Kaličanin B.M., Kostić D.A., Dordević M.S., Velimirović D.S. Characterization of commercially available products of aronia according to their metal content. Fruits. 2015;70:385–393. doi: 10.1051/fruits/2015038. [DOI] [Google Scholar]
- 30.Andrzejewska J., Sadowska K., Klóska Ł., Rogowski L. The effect of plant age and harvest time on the content of chosen components and antioxidative potential of black chokeberry fruit. Acta Sci. Pol. Technol. Aliment. 2015;14:105–114. [Google Scholar]
- 31.Skrede G., Martinsen B.K., Wold A.B., Birkeland S.E., Aaby K. Variation in quality parameters between and within 14 Nordic tree fruit and berry species. . Acta Agric. Scand. Sect. B—Soil Plant. Sci. 2012;62:193–208. [Google Scholar]
- 32.Skupień K., Ochmian I., Grajkowski J. Influence of mineral fertilization on selected physical features and chemical composition of aronia fruit. Acta Agrophys. 2008;11:213–226. [Google Scholar]
- 33.Oszmiański J., Lachowicz S. Effect of the production of dried fruits and juice from chokeberry (Aronia melanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules. 2016;21:1098. doi: 10.3390/molecules21081098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolić M.T., Jurčević I.L., Krbavčić I.P., Marković K., Vahčić N. Phenolic content, antioxidant capacity and quality of chokeberry (Aronia melanocarpa) products. Food Technol. Biotechnol. 2015;53:171–179. doi: 10.17113/ftb.53.02.15.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daskalova E., Delchev S., Peeva Y., Vladimirova-Kitova L., Kratchanova M., Kratchanov C., Denev P. Antiatherogenic and cardioprotective effects of black chokeberry (Aronia melanocarpa) juice in aging rats. Evid. Based Complement. Altern. Med. 2015;2015:717439. doi: 10.1155/2015/717439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denev P., Ciz M., Ambrozova G., Lojek A., Yanakieva I., Kratchanova M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010;123:1055–1061. doi: 10.1016/j.foodchem.2010.05.061. [DOI] [Google Scholar]
- 37.Nawirska A., Kwaśniewska M. Frakcje błonnika w wytłokach z owoców. Acta Sci. Pol. Technol. Aliment. 2004;3:13–20. [Google Scholar]
- 38.Atanasova-Goranova V.K., Dimova P.I., Pevicharova G.T. Effect of food products on endogenous generation of N-nitrosamines in rats. Br. J. Nutr. 1997;78:335–345. doi: 10.1079/BJN19970151. [DOI] [PubMed] [Google Scholar]
- 39.Sueiro L., Yousef G.G., Seigler D., DeMejia E.G., Grace M.H., Lila M.A. Chemopreventive potential of flavonoid extracts from plantation-bred and wild Aronia melanocarpa (black chokeberry) fruits. J. Food Sci. 2006;71:480–488. doi: 10.1111/j.1750-3841.2006.00152.x. [DOI] [Google Scholar]
- 40.Sosnowska D., Podsędek A., Kucharska A.Z., Redzynia M., Opchowska M., Koziołkiewicz M. Comparison of in vitro anti-lipase and antioxidant activities, and composition of commercial chokeberry juices. Eur. Food Res. Technol. 2016;242:505–515. doi: 10.1007/s00217-015-2561-4. [DOI] [Google Scholar]
- 41.Bolling B.W., Taheri R., Pei R., Kranz S., Yu M., Durocher S.N., Brand M.H. Harvest date affects aronia juice polyphenols, sugars, and antioxidant activity, but not anthocyanin stability. Food Chem. 2015;187:189–196. doi: 10.1016/j.foodchem.2015.04.106. [DOI] [PubMed] [Google Scholar]
- 42.Yamane T., Kozuka M., Wada-Yoneta M., Sakamoto T., Nakagaki T., Nakano Y., Ohkubo I. Aronia juice suppresses the elevation of postprandial blood glucose levels in adult healthy Japanese. Clin. Nutr. Exp. 2017;12:20–26. doi: 10.1016/j.yclnex.2017.01.002. [DOI] [Google Scholar]
- 43.Yamane T., Kozuka M., Yamamoto Y., Nakano Y., Nakagaki T., Ohkubo I., Ariga H. Effectiveness of aronia berries for reduction of mild fibrosis and gene expression analysis in livers from mice fed a high-fat diet with aronia berries. Funct. Foods Health Dis. 2016;6:144–157. [Google Scholar]
- 44.Djuric M., Brkovic D., Miloševic D., Pavlovic M., Curčic S. Chemical characterisation of the fruit of black chokeberry grown on different types of soil. Rev. De Chim. 2015;66:178–181. [Google Scholar]
- 45.Handeland M., Grude N., Torp T., Slimestad R. Black chokeberry juice (Aronia melanocarpa) reduces incidences of urinary tract infection among nursing home residents in the long term- a pilot study. Nutr. Res. 2014;34:518–525. doi: 10.1016/j.nutres.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Kim B., Ku C.S., Pham T.X., Park Y., Martin D.A., Xie L., Taheri R., Lee J., Bolling B.W. Aronia melanocarpa (chokeberry) polyphenol–rich extract improves antioxidant function and reduces total plasma cholesterol in apolipoprotein E knockout mice. Nutr. Res. 2013;33:406–413. doi: 10.1016/j.nutres.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Oprea E., Manolescu B.N., Fărcăşanu I.C., Mladin P., Mihele D. Studies concerning antioxidant and hypoglycaemic activity of Aronia melanocarpa fruits. Farmacia. 2014;62:254–263. [Google Scholar]
- 48.Strålsjö L., Åhlin H., Witthöft C.M., Jastrebova J. Folate determination in Swedish berries by radioprotein-binding assay (RPBA) and high performance liquid chromatography (HPLC) Eur. Food Res. Technol. 2003;216:264–269. [Google Scholar]
- 49.Razungles A., Oszmianski J., Sapis J.C. Determination of carotenoids in fruits of Rosa sp. (Rosa canina and Rosa rugosa) and of chokeberry (Aronia melanocarpa) J. Food Sci. 1989;54:774–775. doi: 10.1111/j.1365-2621.1989.tb04709.x. [DOI] [Google Scholar]
- 50.Denev P., Lojek A., Ciz M., Kratchanova M. Antioxidant activity and polyphenol content of Bulgarian fruits. Bulg. J. Agric. Sci. 2013;19:22–27. [Google Scholar]
- 51.Dudonné S., Dubé P., Anhê F.F., Pilon G., Marette A., Lemire M., Harris C., Dewailly E., Desjardins Y. Comprehensive analysis of phenolic compounds and abscisic acid profiles of twelve native canadian berries. J. Food Compos. Anal. 2015;44:214–224. doi: 10.1016/j.jfca.2015.09.003. [DOI] [Google Scholar]
- 52.Gramza-Michałowska A., Sidor A., Kulczyński B. Berries as a potential anti-influenza factor – A review. J. Funct. Foods. 2017;37:116–137. doi: 10.1016/j.jff.2017.07.050. [DOI] [Google Scholar]
- 53.Oszmiański J., Wojdylo A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. J. Food Res. Technol. 2005;221:809–813. doi: 10.1007/s00217-005-0002-5. [DOI] [Google Scholar]
- 54.Teleszko M., Wojdyło A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods. 2015;14:736–746. doi: 10.1016/j.jff.2015.02.041. [DOI] [Google Scholar]
- 55.Hudec J., Bakoš D., Mravec D., Kobida L., Burdová M., Turianica I., Hlušek J. Content of phenolic compounds and free polyamines in black chokeberry (Aronia melanocarpa) after application of polyamine biosynthesis regulators. J. Agric. Food Chem. 2006;54:3625–3628. doi: 10.1021/jf060299q. [DOI] [PubMed] [Google Scholar]
- 56.Wangensteen H., Bräunlich M., Nikolic V., Malterud K.E., Slimestad R., Barsett H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods. 2014;7:746–752. doi: 10.1016/j.jff.2014.02.006. [DOI] [Google Scholar]
- 57.Ćujić N., Šavikin K., Janković T., Pljevljakušić D., Zdunić G., Ibrić S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016;194:135–142. doi: 10.1016/j.foodchem.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Samoticha J., Wojdyło A., Lech K. The influence of different the drying methods on chemical composition and antioxidant activity in chokeberries. Lwt – Food Sci. Technol. 2016;66:484–489. doi: 10.1016/j.lwt.2015.10.073. [DOI] [Google Scholar]
- 59.Wu X., Gu L., Prior R.L., McKay S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004;52:7846–7856. doi: 10.1021/jf0486850. [DOI] [PubMed] [Google Scholar]
- 60.Veberic R., Slatnar A., Bizjak J., Stampar F., Mikulic-Petkovsek M. Anthocyanin composition of different wild and cultivated berry species. Lwt- Food Sci. Technol. 2015;60:509–517. doi: 10.1016/j.lwt.2014.08.033. [DOI] [Google Scholar]
- 61.Mikulic-Petkovsek M., Slatnar A., Stampar F., Veberic R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012;135:2138–2146. doi: 10.1016/j.foodchem.2012.06.115. [DOI] [PubMed] [Google Scholar]
- 62.Tian Y., Liimatainen J., Alanne A.L., Lindstedt A., Liu P., Sinkkonen J., Kallio H., Yang B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017;220:266–281. doi: 10.1016/j.foodchem.2016.09.145. [DOI] [PubMed] [Google Scholar]
- 63.Wilkes K., Howard L.R., Brownmiller C., Prior R.L. Changes in chokeberry (Aronia melanocarpa L.) polyphenols during juice processing and storage. J. Agric. Food Chem. 2014;62:4018–4025. doi: 10.1021/jf404281n. [DOI] [PubMed] [Google Scholar]
- 64.Bräunlich M., Slimestad R., Wangensteen H., Brede C., Malterud K.E., Barsett H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients. 2013;5:663–678. doi: 10.3390/nu5030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esatbeyoglu T., Winterhalter P. Preparation of dimeric procyanidins B1, B2, B5, and B7 from a polymeric procyanidin fraction of black chokeberry (Aronia melanocarpa) J. Agric. Food Chem. 2010;58:5147–5153. doi: 10.1021/jf904354n. [DOI] [PubMed] [Google Scholar]
- 66.Hellström J.K., Shikov A.N., Makarova M.N., Pihlanto A.M., Pozharitskaya O.N., Ryhänen E.L., Kivijärvi P., Makarov V.G., Mattila P.H. Blood pressure-lowering properties of chokeberry (Aronia mitchurinii, var. Viking) J. Funct. Foods. 2010;2:163–169. [Google Scholar]
- 67.Taheri R., Connolly B.A., Brand M.H., Bolling B.W. Underutilized chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) accessions are rich sources of anthocyanins, flavonoids, hydroxycinnamic acids, and proanthocyanidins. J. Agric. Food Chem. 2013;61:8581–8588. doi: 10.1021/jf402449q. [DOI] [PubMed] [Google Scholar]
- 68.Li J., Deng Y., Yuan C., Pan L., Chai H., Keller W.J., Kinghorn A.D. Antioxidant and quinone reductase-inducing constituents of black chokeberry (Aronia melanocarpa) fruits. J. Agric. Food Chem. 2012;60:11551–11559. doi: 10.1021/jf303712e. [DOI] [PubMed] [Google Scholar]
- 69.Socha R., Juszczak L., Pietrzyk S., Fortuna T. Antioxidant activity and phenolic composition of herbhoneys. Food Chem. 2009;113:568–574. doi: 10.1016/j.foodchem.2008.08.029. [DOI] [Google Scholar]
- 70.Bonarska-Kujawa D., Pruchnik H., Oszmiański J., Sarapuk J., Kleszczyńska H. Changes caused by fruit extracts in the lipid phase of biological and model membranes. Food Biophys. 2011;6:58–67. doi: 10.1007/s11483-010-9175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parzonko A., Oświt A., Bazylko A., Naruszewicz M. Anthocyans-rich Aronia melanocarpa extract possesses ability to protect endothelial progenitor cells against angiotensin II induced dysfunction. Phytomedicine. 2015;22:1238–1246. doi: 10.1016/j.phymed.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 72.Slimestad R., Torskangerpoll K., Nateland H.S., Johannessen T., Giske N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Compos. Anal. 2005;18:61–68. doi: 10.1016/j.jfca.2003.12.003. [DOI] [Google Scholar]
- 73.Najda A., Łabuda H. Content of phenolic compounds and antioxidant properties of fruits of selected orchard shrub species. Mod. Phytomorphology. 2013;3:105–109. [Google Scholar]
- 74.Jakobek L., Šeruga M., Medvidović-Kosanović M., Novak I. Anthocyanin content and antioxidant activity of various red fruit juices. Deutshe Lebensm. -Rundsch. 2007;103:58–64. [Google Scholar]
- 75.Jakobek L., Drenjančević M., Jukić V., Šeruga M., Turalija A., Milić M. Polyphenols, anthocyanins and antiradical activity of chokeberries. Electron. J. Environ. Agric. Food Chem. (Ejeafche) 2012;11:76–84. [Google Scholar]
- 76.Popović K., Pozderović A., Jakobek L., Rukavina J., Pichler A. Concentration of chokeberry (Aronia melanocarpa) juice by nanofiltration. J. Food Nutr. Res. 2016;55:159–170. [Google Scholar]
- 77.Pozderović A., Popović K., Pichler A., Jakobek L. Influence of processing parameters on permeate flow and retention of aroma and phenolic compounds in chokeberry juice concentrated by reverse osmosis. Cyta – J. Food. 2016;14:382–390. [Google Scholar]
- 78.Romani A., Vignolini P., Ieri F., Heimler D. Polyphenols and volatile compounds in commercial chokeberry (Aronia melanocarpa) products. Nat. Prod. Commun. 2016;11:99–102. doi: 10.1177/1934578X1601100129. [DOI] [PubMed] [Google Scholar]
- 79.Tomić M., Ignjatović D., Tovilović-Kovačević G., Krstić-Milošević D., Ranković S., Popović T., Glibetić M. Reduction of anxiety-like and depression-like behaviors in rats after one month of drinking Aronia melanocarpa berry juice. Food Funct. 2016;7:3111–3120. doi: 10.1039/C6FO00321D. [DOI] [PubMed] [Google Scholar]
- 80.Kardum N., Milovanović B., Šavikin K., Zdunić G., Mutavdžin S., Gligorijević T., Spasić S. Beneficial effects of polyphenol- rich chokeberry juice consumption on blood pressure level and lipid status in hypertensive subjects. J. Med. Food. 2015;18:1231–1238. doi: 10.1089/jmf.2014.0171. [DOI] [PubMed] [Google Scholar]
- 81.Stanisavljević N., Samardžić J., Janković T., Šavikin K., Mojsin M., Topalović V., Stevanović M. Antioxidant and antiproliferative activity of chokeberry juice phenolics during in vitro simulated digestion in the presence of food matrix. Food Chem. 2015;175:516–522. doi: 10.1016/j.foodchem.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 82.Kardum N., Takić M., Šavikin K., Zec M., Zdunić G., Spasić S., Konić-Ristić A. Effects of polyphenol-rich chokeberry juice on cellular antioxidant enzymes and membrane lipid status in healthy women. J. Funct. Foods. 2014;9:89–97. doi: 10.1016/j.jff.2014.04.019. [DOI] [Google Scholar]
- 83.Valcheva-Kuzmanova S.V., Beronova A.B., Momekov G.T. Protective effect of Aronia melanocarpa fruit juice in a model of cisplatin-induced cytotoxicity in vitro. Folia Med. 2013;55:76–79. doi: 10.2478/folmed-2013-0031. [DOI] [PubMed] [Google Scholar]
- 84.Valcheva-Kuzmanova S., Eftimov M., Denev P., Krachanova M., Belcheva A. Effect of Aronia melanocarpa fruit juice on alcohol-induced depressive-like behavior in rats. Scr. Sci. Med. 2013;45:7–13. [Google Scholar]
- 85.Sainova I., Pavlova V., Alexieva B., Vavrek I., Nikolova E., Valcheva-Kuzmanova S., Markova T., Krachanova M., Denev P. Chemoprotective, antioxidant and immunomodulatory in vitro effects of Aronia melanocarpa total extract on laboratory-cultivated normal and malignant cells. J. Biosci. Biotechnol. 2012:35–43. [Google Scholar]
- 86.Borowska E.J., Szajdek A., Czaplicki S. Effect of heat and enzyme treatment on yield, phenolic content and antioxidant capacity of juices from chokeberry mash. Ital. J. Food Sci. 2009;21:197–209. [Google Scholar]
- 87.Bermúdez-Soto M.J., Tomás-Barberán F.A. Evaluation of commercial red fruit juice concentrates as ingredients for antioxidant functional juices. Eur. J. Food Res. Technol. 2004;219:133–141. doi: 10.1007/s00217-004-0940-3. [DOI] [Google Scholar]
- 88.Horszwald A., Julien H., Andlauer W. Characterisation of Aronia powders obtained by different drying processes. Food Chem. 2013;141:2858–2863. doi: 10.1016/j.foodchem.2013.05.103. [DOI] [PubMed] [Google Scholar]
- 89.Arancibia-Avila P., Toledo F., Werner E., Suhaj M., Leontowicz H., Leontowicz M., Martinez-Ayala A.L., Paśko P., Gorinstein S. Partial characterization of a new kind of Chilean Murtilla-like berries. Food Res. Int. 2011;44:2054–2062. doi: 10.1016/j.foodres.2011.01.016. [DOI] [Google Scholar]
- 90.Thi N.D., Hwang E.S. Effects of drying methods on contents of bioactive compounds and antioxidant activities of black chokeberries (Aronia melanocarpa) Food Sci. Biotechnol. 2016;1:55–61. doi: 10.1007/s10068-016-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie L., Lee S.G., Vance T.M., Wang Y., Kim B., Lee J.Y., Chun O.K., Bolling B.W. Bioavailability of anthocyanins and colonic polyphenol metabolites following consumption of aronia berry extract. Food Chem. 2016;211:860–868. doi: 10.1016/j.foodchem.2016.05.122. [DOI] [PubMed] [Google Scholar]
- 92.Brzóska M.M., Rogalska J., Galazyn-Sidorczuk M., Jurczuk M., Roszczenko A., Tomczyk M. Protective effect of Aronia melanocarpa polyphenols against cadmium-induced disorders in bone metabolism: A study in a rat model of lifetime human exposure to this heavy metal. Chem. -Biol. Interact. 2015;229:132–146. doi: 10.1016/j.cbi.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 93.Hirth M., Preiß R., Mayer-Miebach E., Schuchmann H.P. Influence of HTST extrusion cooking process parameters on the stability of anthocyanins, procyanidins and hydroxycinnamic acids as the main bioactive chokeberry polyphenols. Lwt - Food Sci. Technol. 2015;62:511–516. doi: 10.1016/j.lwt.2014.08.032. [DOI] [Google Scholar]
- 94.Hwang E.S., Thi N.D. Antioxidant contents and antioxidant activities of hot-water extracts of aronia (Aronia melancocarpa) with different drying methods. Korean J. Food Sci. Technol. 2014;46:303–308. doi: 10.9721/KJFST.2014.46.3.303. [DOI] [Google Scholar]
- 95.Šavikin K., Zdunić G., Janković T., Gođevac D., Stanojković T., Pljevljakušić D. Berry fruit teas: Phenolic composition and cytotoxic activity. Food Res. Int. 2014;62:677–683. doi: 10.1016/j.foodres.2014.04.017. [DOI] [Google Scholar]
- 96.Kim B., Park Y., Wegner C.J., Bolling B.W., Lee J. Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in Caco-2 cells. J. Nutr. Biochem. 2013;24:1564–1570. doi: 10.1016/j.jnutbio.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 97.Bijak M., Bobrowski M., Borowiecka M., Podsędek A., Golański J., Nowak P. Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds. Fitoterapia. 2011;82:811–817. doi: 10.1016/j.fitote.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 98.Frejnagel S. Comparison of polyphenolic composition of extracts from honeysuckle, chokeberries and green tea - a short report. Pol. J. Food Nutr. Sci. 2007;57:83–86. [Google Scholar]
- 99.Miletić N., Popović B., Mitrović O., Kandić M., Leposavić A. Phenolic compounds and antioxidant capacity of dried and candied fruits commonly consumed in Serbia. Czech. J. Food Sci. 2014;32:360–368. doi: 10.17221/166/2013-CJFS. [DOI] [Google Scholar]
- 100.Wilkowska A., Ambroziak W., Adamiec J., Czyżowska A. Preservation of antioxidant activity and polyphenols in chokeberry juice and wine with the use of microencapsulation. J. Food Process. Preserv. 2017;41:e12924. doi: 10.1111/jfpp.12924. [DOI] [Google Scholar]
- 101.Piasek A., Kusznierewicz B., Grzybowska I., Malinowska-Pańczyk E., Piekarska A., Azqueta A., Collins A.R., Namieśnik J., Bartoszek A. The influence of sterilization with EnbioJet® Microwave Flow Pasteurizer on composition and bioactivity of aronia and blue-berried honeysuckle juices. J. Food Compos. Anal. 2011;24:880–888. doi: 10.1016/j.jfca.2011.04.005. [DOI] [Google Scholar]
- 102.Sokół-Łętowska A., Kucharska A.Z., Wińska K., Szumny A., Nawirska-Olszańska A., Mizgier P., Wyspiańska D. Composition and antioxidant activity of red fruit liqueurs. Food Chem. 2014;157:533–539. doi: 10.1016/j.foodchem.2014.02.083. [DOI] [PubMed] [Google Scholar]
- 103.Olas B., Wachowicz B., Nowak P., Kedzierska M., Tomczak A., Stochmal A., Oleszek W., Jeziorski A., PiekarskI J. Studies on antioxidant properties of polyphenol-rich extract from berries of Aronia melanocarpa in blood platelets. J. Pysiol. Pharmacol. 2008;59:823–835. [PubMed] [Google Scholar]
- 104.Szwajgier D., Halinowski T., Helman E., Tylus K., Tymcio A. Influence of different heat treatments on the content of phenolic acids and their derivatives in selected fruits. Fruits. 2014;69:167–178. doi: 10.1051/fruits/2014004. [DOI] [Google Scholar]
- 105.Szopa A., Ekiert H., Muszyńska B. Accumulation of hydroxybenzoic acids and other biologically active phenolic acids in shoot and callus cultures of Aronia melanocarpa (Michx.) Elliott (black chokeberry) Plant. CellTissue Organ. Cult. 2013;113:323–329. doi: 10.1007/s11240-012-0272-0. [DOI] [Google Scholar]
- 106.Mattila P., Hellström J., Törrönen R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006;54:7193–7199. doi: 10.1021/jf0615247. [DOI] [PubMed] [Google Scholar]
- 107.Baum J.I., Howard L.R., Prior R.L., Lee S.O. Effect of Aronia melanocarpa (black chokeberry) supplementation on the development of obesity in mice fed a high-fat diet. J. Berry Res. 2016;6:203–212. doi: 10.3233/JBR-160134. [DOI] [Google Scholar]
- 108.Veljković J., Brcanović J., Pavlović A., Mitić S., Kaličanin B., Mitić M. Bagged Aronia melanocarpa tea: Phenolic profile and antioxidant activity. Acta Fac. Med. Naiss. 2014;31:245–252. [Google Scholar]
- 109.Skarpańska-Stejnborn A., Basta P., Sadowska J., Pilaczyńska-Szcześniak Ł. Effect of supplementation with chokeberry juice on the inflammatory status and markers of iron metabolism in rowers. J. Int. Soc. Sports Nutr. 2014;11:48. doi: 10.1186/s12970-014-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tarko T., Duda-Chodak A., Sroka P., Satora P., Michalik J. Transformations of phenolic compounds in an in vitro model simulating the human alimentary tract. Food Technol. Biotechnol. 2009;47:456–463. [Google Scholar]
- 111.Bursać Kovačević D., Gajdoš Kljusurić J., Putnik P., Vukušić T., Herceg Z., Dragović-Uzelac V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016;212:323–331. doi: 10.1016/j.foodchem.2016.05.192. [DOI] [PubMed] [Google Scholar]
- 112.Krajka-Kuźniak V., Szaefer H., Ignatowicz E., Adamska T., Oszmiański J., Baer-Dubowska W. Effect of chokeberry (Aronia melanocarpa) juice on the metabolic activation and detoxication of carcinogenic N-nitrosodiethylamine in rat liver. J. Agric. Food Chem. 2009;57:5071–5077. doi: 10.1021/jf803973y. [DOI] [PubMed] [Google Scholar]
- 113.Strugała P., Gładkowski W., Kucharska A.Z., Sokół-Łętowska A., Gabrielska J. Antioxidant activity and anti-inflammatory effect of fruit extracts from blackcurrant, chokeberry, hawthorn, and rosehip, and their mixture with linseed oil on a model lipid membrane. Eur. J. Lipid Sci. Technol. 2016;118:461–474. doi: 10.1002/ejlt.201500001. [DOI] [Google Scholar]
- 114.Jakobek L., Šeruga M., Krivak P. The influence of interactions among phenolic compounds on the antiradical activity of chokeberries (Aronia melanocarpa) Int. J. Food Sci. Nutr. 2011;62:345–352. doi: 10.3109/09637486.2010.534438. [DOI] [PubMed] [Google Scholar]
- 115.Wang Y., Zhao L., Wang D., Huo Y., Ji B. Anthocyanin-rich extracts from blackberry, wild blueberry, strawberry, and chokeberry: Antioxidant activity and inhibitory effect on oleic acid-induced hepatic steatosis in vitro. J. Sci. Food Agric. 2016;96:2494–2503. doi: 10.1002/jsfa.7370. [DOI] [PubMed] [Google Scholar]
- 116.Vlachojannis C., Zimmermann B.F., Chrubasik-Hausmann S. Quantification of anthocyanins in elderberry and chokeberry dietary supplements. Phytother. Res. 2015;29:561–565. doi: 10.1002/ptr.5284. [DOI] [PubMed] [Google Scholar]
- 117.Wiczkowski W., Romaszko E., Piskula M.K. Bioavailability of cyanidin glycosides from natural chokeberry (Aronia melanocarpa) juice with dietary-relevant dose of anthocyanins in humans. J. Agric. Food Chem. 2010;58:12130–12136. doi: 10.1021/jf102979z. [DOI] [PubMed] [Google Scholar]
- 118.Bober I., Oszmiański J. Zastosowanie wytłoków aronii do naparów herbat owocowych. Acta Sci. Pol. Technol. Aliment. 2004;3:63–72. [Google Scholar]
- 119.Nawirska A., Sokół-Łętowska A., Kucharska A. Właściwości przeciwutleniające wytłoków z wybranych owoców kolorowych. Żywnosć. Nauka. Technologia Jak. 2007;4:120–125. [Google Scholar]
- 120.Benvenuti S., Pellati F., Melegari M., Bertelli D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Robus, Ribes and Aronia. J. Food Sci. 2004;69:164–169. [Google Scholar]
- 121.Nakajima J., Tanaka I., Seo S., Yamazaki M., Saito K. LC/PDA/ESI-MS profilng and radical scavnging activity of anthocyanins in various berries. J. Biomed. Biotechnol. 2004;5:241–247. doi: 10.1155/S1110724304404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Denev P., Kratchanova M., Ciz M., Lojek A., Vasicek O., Nedelcheva P., Blazheva D., Toshkova R., Gardeva E., Yossifova L., et al. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 2014;157:37–44. doi: 10.1016/j.foodchem.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 123.Mitic V., Stankov Jovanovic V., Dimitrijevic M., Cvetkovic J., Simonovic S., Nikolic Mandic S. Chemometric analysis of antioxidant activity and anthocyanin content of selected wild and cultivated small fruit from Serbia. Fruits. 2014;69:413–422. doi: 10.1051/fruits/2014026. [DOI] [Google Scholar]
- 124.Podsędek A., Majewska I., Redzynia M., Sosnowska D., Koziołkiewicz M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014;62:4610–4617. doi: 10.1021/jf5008264. [DOI] [PubMed] [Google Scholar]
- 125.Arancibia-Avila P., Namiesnik J., Toledo F., Werner E., Martinez-Ayala A.L., Rocha-Guzmán N.E., Gallegos-Infante J.A., Gorinstein S. The influence of different time durations of thermal processing on berries quality. Food Control. 2012;26:587–593. doi: 10.1016/j.foodcont.2012.01.036. [DOI] [Google Scholar]
- 126.Keskin-Šašić I., Tahirović I., Topčagić A., Klepo L., Salihović M., Ibragić S., Toromanović J., Ajanović A., Velispahić E. Total phenolic content and antioxidant capacity of fruit juices. Bull. Chem. Technol. Bosnia Herzeg. 2012;39:25–28. [Google Scholar]
- 127.Gramza-Michałowska A., Człapka-Matyasik M. Evaluation of the antiradical potential of fruit and vegetable snacks. Acta Sci. Pol. Technoloia Aliment. 2011;10:63–72. [PubMed] [Google Scholar]
- 128.Müller L., Gnoyke S., Popken A.M., Böhma V. Antioxidant capacity and related parameters of different fruit formulations. Lwt-Food Sci. Technol. 2010;43:992–999. [Google Scholar]
- 129.Viskelis P., Rubinskienė M., Bobinaitė R., Dambrauskienė E. Bioactive compounds and antioxidant activity of small fruits in Lithuania. J. Food Agric. Environ. 2010;8:259–263. [Google Scholar]
- 130.Zheng W., Wang S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003;51:502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]


