Summary
Background
Targeting combination HIV interventions to locations and populations with high HIV burden is a global priority, but the impact of these strategies on HIV incidence is unclear. We assessed the impact of combination HIV interventions on HIV incidence in four HIV hyperendemic communities in Uganda.
Methods
From November 4th, 2011 to August 16th, 2017, data were collected from five open population-based cohort surveys of persons aged 15–49 years residing in four fishing communities on Lake Victoria. We evaluated trends in HIV testing coverage among all participants, circumcision coverage among male participants, antiretroviral therapy (ART) coverage and HIV viral load among HIV-positive participants, and sexual behaviors and HIV incidence among HIV-negative participants.
Findings
Overall, 8,941 participants were surveyed and contributed 20,721 person visits; 52% (n=4,619) were male. HIV prevalence was 41% (1598/3870) in the 2011–2012 baseline survey. A total of 3,222 initially HIV-negative participants with at least one repeat visit contributed 9,475 person-years (pys) of follow-up and 230 incident HIV infections. Over ~5 years, HIV testing coverage increased from 68% (2613/3870) to 95% (4520/4733) (p<0.0001); male circumcision coverage increased from 35% (698/2011) to 65% (1630/2524) (p<0.0001); ART coverage increased from 16% (254/1598) to 82% (1420/1739) (p<0.0001); and, population HIV viral load suppression in all HIV-positive persons increased from 34% (546/1596) to 80% (1385/1736) (p<0.0001). There were no decreases in risky sexual behaviors. Overall, HIV incidence decreased from 3.43/100 pys (95% CI: 2.45–4.67) in 2011–2012 to 1.59/100 pys (95% CI: 1.19–2.07) in 2016–2017 (adjusted incidence rate ratio (adjIRR) 0.52; 95% CI 0.34–0.79). Declines in HIV incidence were similar among men (adjIRR 0.53; 95% CI 0.30–0.93) and women (adjIRR=0.51; 95% CI 0.27–0.96). The risk of incident HIV infection in circumcised men was lower than in uncircumcised men (adjIRR 0.46; 95% CI 0.32–0.67).
Interpretation
Rapid expansion of combination HIV interventions in HIV hyperendemic fishing communities is feasible and can have substantial impact on HIV incidence. However, incidence remains higher than HIV epidemic control targets, and additional efforts will be needed to achieve this global health priority.
Introduction
The Joint United Nations Programme on HIV/AIDS (UNAIDS), the World Health Organization (WHO), and the United States President’s Emergency Plan for AIDS Relief (PEPFAR) have all emphasized the need to target HIV treatment and prevention resources to populations and locations with the highest HIV burden.1–3 In Africa, HIV hyperendemic geographical areas, sometimes referred to as hotspots,3 have included fishing, urban slum, and peri-urban communities, which are often overrepresented by priority and key populations such as sex and migrant workers.4–10 Many of these communities have also been historically underserved by HIV treatment and prevention services.4,10
Combination HIV Interventions (CHI), including antiretroviral therapy (ART), voluntary medical male circumcision (MC), HIV testing services (HTS) and behavior change interventions, have been shown to reduce HIV incidence in some lower risk populations in Africa.11–14 However, knowledge of CHI impact in HIV hyperendemic areas is limited, and these communities have unique social-behavioral, demographic, and structural characteristics such as high levels of mobility, transactional sex, multiple sexual partnerships, and alcohol use and greater proportions of men which may hinder CHI scale-up and moderate its effects.4,7,15,16
Here, we report on a prospective cohort study from 2011 to 2017 in four HIV hyperendemic Lake Victoria fishing communities in Uganda (HIV prevalence ~41%) to evaluate trends in CHI coverage and CHI impact on HIV incidence.4 This study was embedded within the Rakai Community Cohort Study (RCCS), a population-based, observational study of HIV incidence, sexual behaviors and health service utilization in south-central Uganda. We have previously shown that scale-up of CHI has led to HIV incidence reductions in nearby, lower HIV burden agrarian and trading RCCS communities (HIV prevalence ~13%);11 however, CHI scale-up and HIV incidence trends in these high burden fishing communities have not been previously reported.
Methods
Study design and participants
The RCCS, conducted by the Rakai Health Sciences Program (RHSP), is an open (participants can age in and out of the cohort, participants can also enter or exit the cohort based upon residency), population-based cohort of individuals aged 15–49 years in 40 communities located in Rakai and neighboring districts of south-central Uganda.4,11 RCCS methods have been previously reported.4,11 In brief, the RCCS conducts a household census and interviews consenting individuals to collect self-reported demographic, behavioral, and service uptake data. Free HTS is provided at the time of interview with referral to CHI services as appropriate; HIV status is determined using a validated three rapid HIV test algorithm.4,11 Blood samples are also taken for HIV confirmation and further tests, e.g. HIV viral load. Established in 1994, the RCCS initially focused on rural agrarian and trading communities. In 2011, the four most populous Lake Victoria fishing communities in the region were added to the cohort. This study uses data from these four communities which were surveyed five times between November 4th, 2011 to August 16th, 2017. Viral load testing on all HIV-positive participants was performed at baseline and the final two surveys. Residency in the fishing villages was defined as 1 month or longer residence with intention to stay.
This study was approved by the Research and Ethics Committee of the Uganda Virus Research Institute, the Ugandan Council of Science and Technology, and the Western Institutional Review Board.
Prior to November 2011, HIV services in the four fishing communities were limited.4 With support from PEPFAR and the U.S. Centers for Disease Control and Prevention Uganda, CHI services were subsequently rapidly expanded with RHSP as lead implementer. For example, a new community-based HIV clinic was established in the largest of the four fishing communities and MC was provided through mobile camps and referrals to outreach MC facilities. From 2011 to 2013, ART was initiated in these communities at a CD4 count of <350 cells per μL. In 2013, fisherfolk were classified by the Ugandan Ministry of Health as a priority population in which ART should be started regardless of CD4 count, i.e. Universal Test and Treat.17 The largest of the four fishing communities is the site of DREAMS programming featuring HTS, community mobilization, economic and vocational strengthening, and condom promotion, as well as a community health worker program promoting CHI services.18–21 All HIV services are provided free of charge to the recipient. Pre-exposure Prophylaxis (PrEP) was not available in these communities at the time of these surveys.
Statistical analysis
Self-reported CHI coverage was assessed at each survey. ART coverage was defined as the proportion of all HIV-positive participants who self-reported ART use and was assessed overall and by sex and age groups. Self-reported ART use has been validated in this setting by plasma detection of antiretroviral drugs showing a specificity of 99% (95% Confidence Intervals (CI): 97–100%) and sensitivity of 77% (95% CI: 70–83%).22 MC coverage was defined as the proportion of men who self-reported being circumcised. Self-reported MC status has been previously validated from clinical records with 100% specificity.23 Viral suppression was defined as <1000 copies per mL per WHO recommendations.24
The unit of exposure for HIV incidence was person-years (pys) of follow-up between surveys for individuals who were HIV-negative at their baseline and had at least one subsequent follow-up visit. HIV incident cases were persons who first tested HIV-seropositive at a follow-up visit allowing for up to one missed visit. HIV incidence was calculated per 100 pys, with incident infections assumed to occur at the mid-point of the follow-up interval. After validation of underlying assumptions, multivariable Poisson regression with generalized estimating equations and an exchangeable correlation structure to account for repeated measurements was used to estimate incidence rate ratios (IRR) and 95% CIs comparing mean incidence rates after scale-up of CHI (2016–17) to the baseline interval before CHI scale-up (2011–12).
The final multivariable model included variables previously shown to be associated with HIV incidence including individual-level information on demographics (sex, age, marital status, education) and sexual behaviors (number of sexual partners in the previous 12 months, sex with partners outside the community of residence, sex with non-marital partners, condom use with non-marital partners, and self-reported genital ulceration). Secondary analyses were stratified by sex, age group, and by MC status. Sensitivity of results to both selective participation and loss to follow-up were evaluated using inverse probability weighting (appendix p 14).11 Analysis was performed using STATA 15 Statistical Software (StataCorp LLC, College Station, Texas).
Role of the funding source
Some funders (CDC-Uganda and Karolinska Institutet) contributed to study design, data interpretation and analysis. All other funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. JK, LC, and SJR had overall scientific oversight, full access to all the data in the study, and final responsibility for the decision to submit for publication.
Results
At baseline, the four study communities (appendix p 1) varied by size (census populations of persons age 15–49, n=600, 605, 1249, and 2876) but had similar HIV prevalence (range: 38–43%).4 Over the five surveys, 8,941 total study participants contributed 20,721 person-visits. 52% (n=4,619) of participants were male and the median age was 30 years (interquartile range=25–37). Overall, HIV prevalence was 41% (1598/3870) at the first cohort survey. A total of 3,222 participants who were HIV-negative at the baseline visit and had at least one follow-up visit (i.e. the HIV incidence cohort) were followed for a total of 9,475 pys with 230 seroconversions observed. Table 1 shows aggregated eligibility, participation, and follow-up data.
Table 1.
Eligibility, participation, and follow-up by survey round, 2011–2017.
| Survey | Interview Date | Census Eligibleα |
Eligible and present for surveyβ |
Eligible and present who participated in surveyγ |
HIV-negative participants eligible for incidence cohort* |
Eligible HIV- negative participants who out-migrated prior to the subsequent survey |
Incidence cohort |
||
|---|---|---|---|---|---|---|---|---|---|
| Median (range) | no. | no. | no. (%) | no. | no. (%) | no. | |||
| 1 | Jan 20, 2012 (Dec 7, 2011-Jun 4, 2012) |
5,330 | 3,913 (73) | 3870(99%) | - | - | - | ||
| 2 | Sep 6, 2012 (Aug 16, 2012-Mar 18, 2013) |
5,910 | 4,044 (68) | 3965(98%) | 2,272 | 314 (14) | 1674 (74) | ||
| 3 | Oct 8, 2013 (Sep 23, 2013-Jul 4, 2014) |
6,214 | 3,947 (64) | 3926(99%) | 2,539 | 535 (21) | 1735 (68) | ||
| 4 | May 20, 2015 (Apr 23, 2015-Nov 13, 2015) |
7,191 | 4,243 (59) | 4222(99%) | 2,581 | 516 (20) | 1752 (68) | ||
| 5 | Jan 20, 2017 (Dec 21, 2016-Aug 14, 2017) |
7,462 | 4,806 (64) | 4738(98%) | 2,843 | 719 (25) | 1849 (65) | ||
Residents aged 15–49 in the census.
Eligible census population present at time of survey,
Eligible census population present and participated in survey.
Includes all age-eligible HIV-negative participants from prior survey and any HIV-negative participants from two surveys prior if participant was absent at the most recent survey.
The number of eligible individuals living in the four communities grew by 40% over the course of the study. The proportion of eligible residents who participated in the specific surveys ranged from 59%−73% across survey rounds, but maintained similar composition by sex and age (appendix p 1). The major reason for non-participation was absence for work or school at time of survey rather than refusal (appendix p 2). Among eligible individuals present in the community at time of survey, ~99% participated in the survey. Among individuals who were HIV-negative, follow-up rates ranged from 65–74% with out-migration and absence for work or school contributing to almost all of the losses-to-follow up (appendix p 3).
From 2011–2012 to 2016–2017, among HIV-negative participants, there were no significant changes in the proportion of individuals who were ever sexually active or in the number of non-marital sexual partners, overall or by sex. There was a modest decline in consistent condom use with non-marital sex partners from 41% (340/832) initially to 35% (387/1119) by the last survey (p<0.0048) (appendix p 4). The proportion of respondents reporting ever having received an HIV test increased from 68% (2613/3870) in 2011–2012 to 89% (3536/3965) by the second survey in 2012, and to 96% (4520/4733) by the final survey in 2016–2017 (p<0.0001) (appendix p 5).
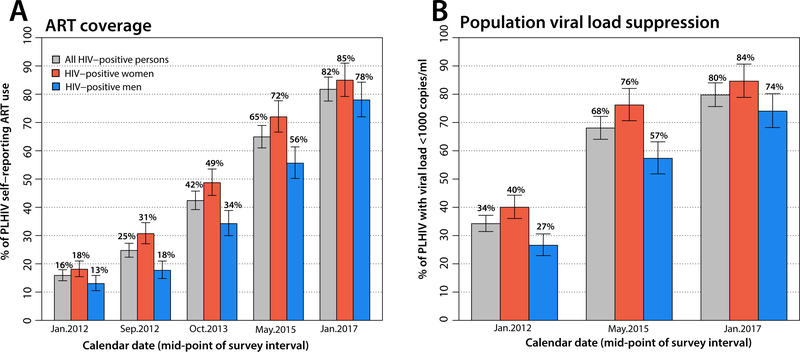
Figure 1A shows ART coverage scale-up over the study period (appendix p 5). ART use increased from the 2011–2012 baseline of 16% (254/1598) to 82% (1420/1739) in 2016–2017 (p<0.0001). ART coverage was higher in women than in men at all surveys (p<0.0001) and by 2016–2017, 85% (797/940) of HIV-infected women reported ART use compared to 78% (623/799) of men. Figure 1B also show trends in viral suppression among all HIV-positive individuals (appendix p 5), i.e. population viral load, which increased from 34% (546/1596) in 2011–2012 to 80% (1385/1736) by 2016–2017 (p<0.0001). Viral suppression was greater in women compared to men at each time point (p<0.0001).
Figure 1. ART scale-up and viral suppression.
Panel A shows ART coverage overall and by sex from 2011–2017. Panel B shows population viral load suppression (viral suppression among all HIV-positive participants) overall and by sex from 2011–2017. Bars indicate 95% confidence intervals.
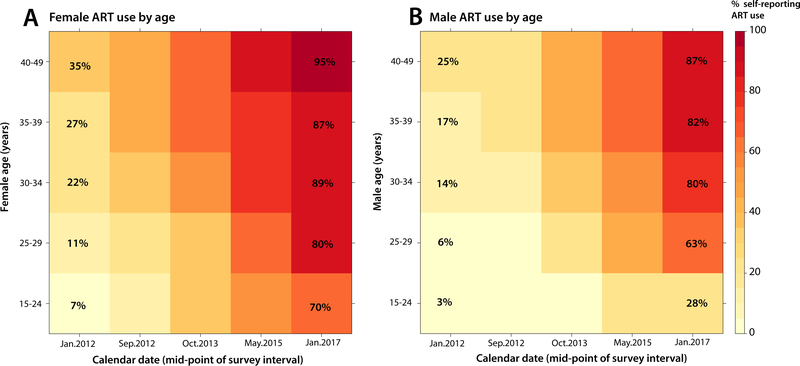
Figure 2 shows a heat map of ART coverage by sex and age group. In both men and women, coverage was lower in younger age groups, but this differential was more pronounced and persistent in men. For example, by 2016–2017, ART coverage was 59% lower in men aged 15–24 years compared to men aged 40–49 years; in contrast, the differential in women comparing the same age groups was 25%.
Figure 2. ART coverage by gender and age.
Panel A shows female ART coverage heat maps by age group from 2011 to 2017. Panel B shows male ART coverage heat maps by age group from 2011 to 2017.
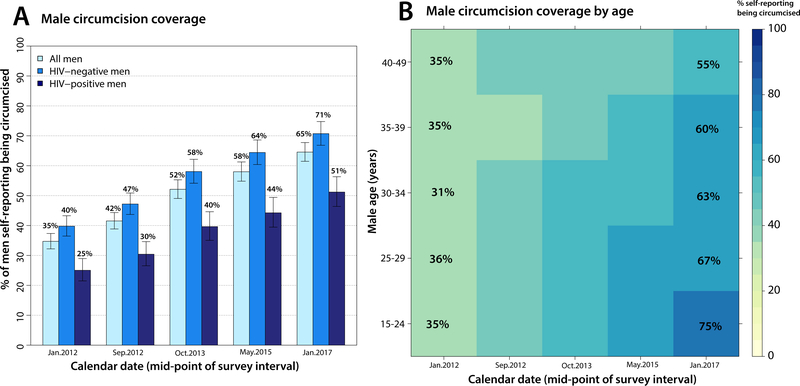
Figure 3A shows self-reported MC stratified by HIV status (appendix p 5). Overall, MC coverage increased from 35% (698/2011) to 65% (1630/2524) over the study period (p<0.0001). Coverage was consistently higher in HIV-negative compared to HIV-positive men (p<0.0001), but coverage in both groups progressively increased over time. Figure 3B shows MC coverage stratified by age. In 2011–2012, MC coverage was uniform and modest across age groups. However, by 2016–2017, coverage was significantly greater in younger age groups compared to older men (p<0.0001).
Figure 3. Male circumcision scale-up.
Panel A shows male circumcision coverage overall and by HIV status from 2011–2017. Panel B shows male circumcision coverage heat maps by age group from 2011–2017.
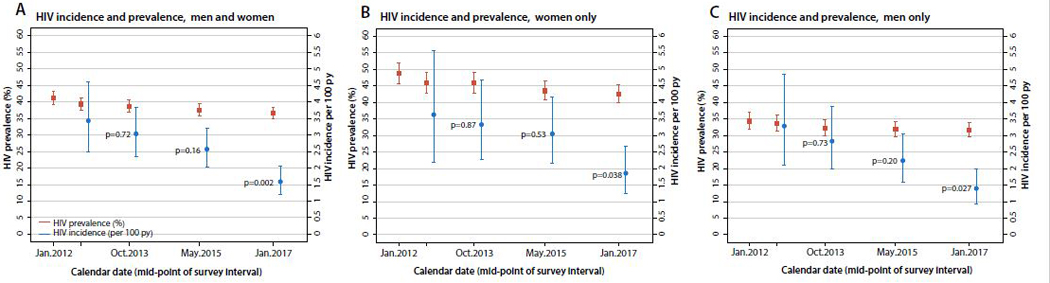
Figure 4 (appendix p 6) shows trends in HIV incidence and prevalence. Consistent declines in HIV incidence at each study interval were observed in both sexes and were significantly lower at the last follow-up interval compared to baseline (p<0.00021). HIV prevalence declined from 41% (1598/3870) at baseline to 37% (1738/4737) (p<0.0001) by the final survey (appendix p 7–9).
Figure 4. HIV incidence and prevalence trends.
Panel A shows trends in HIV incidence and prevalence among men and women from 2011–2017. Panel B shows trends in HIV incidence and prevalence among women only from 2011–2017. Panel C shows trends in HIV incidence and prevalence among men only from 2011–2017. Bars indicate 95% confidence intervals.
Table 2 shows unadjusted and adjusted incidence rate ratios (adjIRR) of factors associated with HIV incidence (appendix p 10–13 for data stratified by sex and MC status). Baseline HIV incidence in 2012 was 3.43/100 pys (95% CI: 2.45–4.67). By 2016–2017, HIV incidence was 1.59/100 pys (95% CI: 1.19–2.07), a decline of 48% (adjIRR 0.52; 95% CI: 0.34–0.79). Previous marriage, self-reported genital disease, and having more than one sex partner were associated with increased HIV incidence. MC and secondary/tertiary education were associated with lower HIV incidence.
Table 2.
Factors associated with HIV incidence in four Ugandan fishing communities, 2011–2017.α
| Variable | Incident cases |
Person-years (py) | Incidence per 100 py (95%CI) |
IRR (95%CI) | p-value | adjIRR (95%CI) | p-value |
|---|---|---|---|---|---|---|---|
| Survey(s)β | |||||||
| Sep.2012 (2) | 40 | 1,165.5 | 3.43(2.45–4.67) | 1 | - | 1 | - |
| Oct.2013 (3) | 64 | 2,108.1 | 3.04(2.34–3.88) | 0.88(0.60–1.31) | 0.54 | 0.93(0.63–1.38) | 0.72 |
| May.2015 (4) | 72 | 2,799.8 | 2.57(2.01–3.24) | 0.75(0.51–1.10) | 0.14 | 0.76(0.51–1.12) | 0.16 |
| Jan.2017 (5) | 54 | 3,403.7 | 1.59(1.19–2.07) | 0.46(0.31–0.69) | 0.00021 | 0.52(0.34–0.79) | 0.0021 |
| Age (years) | |||||||
| 15–19 | 12 | 673.2 | 1.78(0.92–3.11) | 1 | - | 1 | - |
| 20–24 | 64 | 1,831.2 | 3.49(2.69–4.46) | 1.96(1.06–3.63) | 0.032 | 1.40(0.72–2.69) | 0.32 |
| 25–29 | 68 | 2,178.2 | 3.12(2.42–3.96) | 1.75(0.95–3.24) | 0.073 | 1.23(0.63–2.42) | 0.55 |
| 30–34 | 32 | 1,842.4 | 1.74(1.19–2.45) | 0.97(0.50–1.89) | 0.94 | 0.66(0.32–1.35) | 0.26 |
| 35–39 | 31 | 1,403.4 | 2.21(1.50–3.14) | 1.24(0.64–2.42) | 0.53 | 0.83(0.40–1.73) | 0.62 |
| >39 years | 23 | 1,548.5 | 1.49(0.94–2.23) | 0.83(0.41–1.67) | 0.609 | 0.61(0.29–1.30) | 0.20 |
| Sex | |||||||
| Women | 108 | 3,915.5 | 2.76(2.26–3.33) | 1 | - | 1 | - |
| Uncircumcised men | 69 | 1,926.2 | 3.58(2.79–4.53) | 1.30(0.96–1.76) | 0.094 | 0.95(0.66–1.37) | 0.782 |
| Circumcised men | 53 | 3,635.3 | 1.46(1.09–1.91) | 0.53(0.38–0.74) | 0.00017 | 0.42(0.28–0.64) | 0.0098 |
| Marital status | |||||||
| Never married | 24 | 1,329.7 | 1.80(1.16–2.69) | 1 | - | 1 | - |
| Married | 126 | 6,085.6 | 2.07(1.73–2.47) | 1.15(0.74–1.78) | 0.54 | 1.10(0.56–2.16) | 0.78 |
| Previously married | 80 | 2,061.7 | 3.88(3.08–4.83) | 2.15(1.36–3.40) | 0.0011 | 1.99(1.18–3.37) | 0.0098 |
| Education | |||||||
| None | 19 | 624.0 | 3.04(1.83–4.75) | 1 | - | 1 | - |
| Primary | 182 | 6,842.4 | 2.66(2.29–3.08) | 0.87(0.54–1.41) | 0.58 | 0.82(0.52–1.31) | 0.41 |
| Secondary/Tertiary | 29 | 2,010.5 | 1.44(0.97–2.07) | 0.47(0.26–0.85) | 0.012 | 0.50(0.28–0.89) | 0.019 |
| Sex with partners outside of the community in last year | |||||||
| None | 162 | 6,589.5 | 2.46(2.09–2.87) | 1 | - | 1 | - |
| One or more | 68 | 2,887.5 | 2.36(1.83–2.99) | 0.96(0.72–1.28) | 0.77 | 0.85(0.61–1.18) | 0.32 |
| Self-reported genital ulcer disease in the last year | |||||||
| No | 179 | 8,531.8 | 2.10(1.80–2.43) | 1 | - | 1 | - |
| Yes | 51 | 945.2 | 5.40(4.02–7.09) | 2.57(1.88–3.51) | <0.0001 | 1.88(1.36–2.60) | 0.0012 |
| # sex partners in the last year | |||||||
| None | 6 | 685.0 | 0.88(0.32–1.91) | 1 | - | 1 | - |
| One | 103 | 5,142.7 | 2.00(1.64–2.43) | 2.29(1.00–5.22) | 0.049 | 2.10(0.81–5.48) | 0.13 |
| Two | 62 | 2,007.4 | 3.09(2.37–3.96) | 3.53(1.52–8.18) | 0.0033 | 4.02(1.44–11.26) | 0.0081 |
| Three or more | 59 | 1,642.0 | 3.59(2.74–4.64) | 4.10(1.77–9.52) | 0.0010 | 4.64(1.57–13.76) | 0.0056 |
| Non-marital relationships and consistent condom use | |||||||
| Stable partners only/no sex partners | 124 | 6,277.7 | 1.98(1.64–2.36) | 1 | - | 1 | - |
| Non-stable partner, inconsistent use | 71 | 2,028.1 | 3.50(2.73–4.42) | 1.77(1.32–2.38) | 0.00013 | 1.06(0.62–1.82) | 0.82 |
| Non-stable partner, consistent use | 35 | 1,171.2 | 2.99(2.08–4.16) | 1.51(1.04–2.20) | 0.031 | 0.98(0.54–1.78) | 0.95 |
CI=Confidence Interval; IRR=Incidence Rate Ratio; adjIRR=adjusted Incidence Rate Ratio
Dates listed are the survey interval midpoint; 1st survey (not shown) midpoint interval was Jan2012.
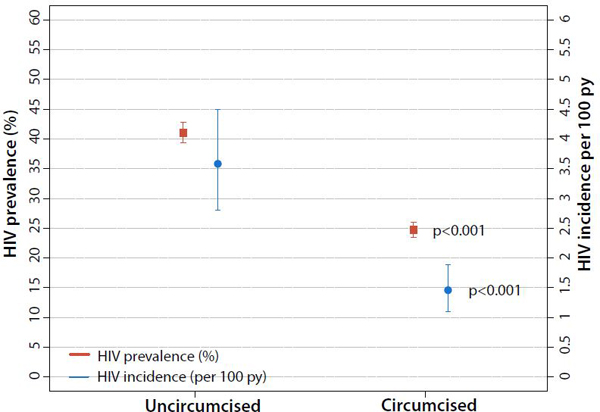
Declines in HIV incidence (appendix p 6) were similar in men (adjIRR 0.53; 95% CI: 0.30–0.93) and women (adjIRR 0.51; 95% CI 0.27–0.96). Incident HIV infection across all surveys was lower among circumcised compared to uncircumcised men (adjIRR 0.46, 95% CI: 0.32–0.67) (Figure 5, appendix p 11). Results did not change substantially with inclusion of inverse probability weights to account for selective participation and follow-up (appendix p 15).
Figure 5. Male circumcision status and HIV prevalence and incidence.
Male HIV prevalence and incidence by circumcision status over the full study period, 2011–2017.
Discussion
This study demonstrated the feasibility of rapidly expanding CHI services in HIV hyperendemic fishing communities in Uganda and observed a concurrent ~48% (95% CI: 21%−66%) reduction in HIV incidence. Declines in HIV incidence with CHI scale-up have been reported in some lower-risk populations,11,12,14 but this is the first report, to our knowledge, of prospectively observed declines in overall HIV incidence with rapid CHI scale-up in HIV hyperendemic communities.
We previously reported a 42% (95% CI: 24%−55%) reduction in HIV incidence to 0.66/100 pys by 2016 relative to a period prior to CHI scale-up (1999–2004) amongst an RCCS cohort of lower-risk agrarian and trading communities (HIV prevalence ~13%). In this prior study, ART coverage was 69%, HIV population viral load suppression was 75%, MC coverage was 59% by 2015–2016 compared to 80%, 82%, and 65%, respectively, in the fishing communities by 2016–2017. This prior study observed greater declines in HIV incidence in men compared to women; however, this disparity was not observed in the fishing communities. Future modeling studies based on empiric population-level data are needed to better understand individual CHI contributions to HIV incidence declines and reasons for sex and community-level differences.
Other studies which help to contextualize our findings include the SEARCH trial which assessed a universal test and treat, multi-disease care model in both lower-risk rural communities and higher-risk fishing communities. In SEARCH, HTS and ART were rapidly scaled, with population viral load suppression reaching ~79% by Year 3 of the study. Annual measured HIV incidence declined by ~32% over three years, although there were no incidence differences between the two study arms.12 POPART, a cluster-randomized trial in South Africa and Zambia found mixed results on the impact of UTT on HIV incidence despite reaching the first two 90–90 targets in both intervention arms.25 In western Kenya, a population-based cohort analysis found that HIV incidence in a lower risk population (HIV prevalence 12%−15%) declined by roughly half between 2011 and 2016 as CHI was scaled up.26 In the population-based, nationally representative HIV impact assessments (PHIAs), HIV incidence measured using cross-sectional incidence assays also appears to be declining in several sub-Saharan African countries.14,27 However, a separate cohort study in South Africa from 2004–2015 observed HIV incidence declines only in males, and, surprisingly, found increasing female HIV incidence.28 Continued reporting of HIV incidence-based impact evaluations are needed to monitor and better understand the impact and sustainability of CHI.
In this study, CHI was rapidly scaled in high-risk communities. However, important disparities were apparent. Women were more likely than men and older persons more likely than younger persons to report being on ART, whereas younger men were more likely than older men to be circumcised. These findings are consistent with reports elsewhere in Africa.29–31 Continued characterization of “hard-to-reach” populations as well as targeted interventions are needed.32
Despite surpassing 90–90-90 goals and almost obtaining 95–95-95 goals of ~86% population viral suppression,31 HIV incidence in these fishing communities remained fifteen times higher than the suggested incidence required for HIV epidemic control based upon prior modeling exercises, i.e. 0.1/100 pys.29,30 Of note, MC increased in these communities to 65% coverage but remained short of the 80% UNAIDS/PEPFAR coverage goals.1 MC in this study was strongly protective among men, decreasing their risk of HIV acquisition by ~54%. Thus, hyperendemic communities may need exceptionally high coverage levels of ART and MC to approach epidemic control, and/or newer interventions such as oral Pre-Exposure Prophylaxis (PrEP) or novel interventions such as long-acting injectable PrEP or a vaccine.33 Continued monitoring of these communities will also be needed to demonstrate sustainability of CHI expansion, possibilities for further CHI scale-up, and the role of newer CHI interventions.
This study has limitations. Follow-up rates were moderate, mostly due to out-migration. However, study findings did not change with use of inverse probability weights suggesting there was limited bias due to selective follow-up. This study was also not able to ascertain the impact of mortality, secular trends, or other potential unmeasured confounders which may have affected incidence declines. However, the strong temporal association between CHI scale-up and HIV incidence declines supports causality. ART and MC coverage were self-reported and may have resulted in misclassification though both measures have been validated in the Rakai setting.22,23
In summary, significant HIV incidence declines in HIV hyperendemic fishing villages were likely due to CHI scale-up, suggesting that existing HIV treatment and prevention interventions can be rapidly expanded and have substantial population-level impact on HIV incidence in high burden settings. However, HIV incidence remained higher than that needed for HIV epidemic control and additional efforts will be needed to achieve this global health priority.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for studies on longitudinal HIV cohort studies which included HIV hypderendemic communities in sub-Saharan Africa published up to December 1, 2018. Key search terms included “HIV or AIDS”, “cohort”, “observational”, “fishing”, “hotspot”, “hyperendemic” and “Africa”. No language limitation was placed. Several studies were found reporting high levels of HIV seroprevalence and associated risk factors in varied hyperendemic communities; however studies reporting longitudinal HIV incidence and the population-level impact of HIV interventions in hyperendemic communities were not found.
Added value of this study
This study is, to our knowledge, the first prospective study to provide evidence that combined HIV interventions such as antiretroviral therapy and male circumcision can be rapidly scaled in HIV hyperendemic communities and have a substantial impact on HIV incidence. We also confirmed the population-level benefits of male circumcision in preventing HIV acquisition. Finally, despite observing significant HIV incidence declines, HIV incidence remained much higher than what is needed for HIV epidemic control.
Implications of all the available evidence
The available evidence indicates the need for strong HIV surveillance programs which regularly survey a range of community types, additional population-level observational and modeling studies of heterogeneous communities to better understand the varied impacts of HIV interventions in different risk settings, the need for further efforts, such as increased antiretroviral therapy and male circumcision scale-up and pre-exposure prophylaxis roll-out, and additional resources from national governments and international and global organizations in order to reach HIV epidemic control.
Acknowledgements.
This work was supported by the National Institute of Allergy and Infectious Diseases (grant numbers U01AI100031, U01AI075115, R01AI110324, R01AI102939, R01AI128779, R01AI123002, and K01AI125086), the National Institute of Mental Health (grant numbers R01MH107275, R01MH105313), the National Center for Child Health and Human Development (grant numbers R01HD091003), the Division of Intramural Research of the National Institute for Allergy and Infectious Diseases, the National Cancer Institute (contract number HHSN261200800001E), the Johns Hopkins University Center for AIDS Research (grant number P30AI094189), the Karolinska Institutet, and the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) (cooperative agreement number NU2GGH000817). We thank the cohort participants, staff, and local community leaders who have made this study possible. We also thank the personnel at the Office of Cyberinfrastructure and Computational Biology at the National Institute of Allergy and Infectious Diseases for data management support.
Funding
The National Institute of Mental Health, the National Institute of Allergy and Infectious Diseases, the National Institute of Child Health and Development, the National Cancer Institute, and the Division of Intramural Research and the Division of AIDS, National Institute for Allergy and Infectious Diseases, National Institutes of Health, Centers for Disease Control and Prevention-Uganda, Karolinska Institutet, and the Johns Hopkins University Center for AIDS Research.
Footnotes
Declarations of interest
We declare that we have nothing to disclose.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.PEPFAR 2018 Annual Report to Congress. The Office of the U.S. Global AIDS Coordinator and Health Diplomacy. April 2018. http://www.pepfar.gov/documents/organization/234744.pdf. Last access date: April 30, 2019.
- 2.UNAIDS. Global AIDS Update 2017. Geneva, Switzerland: United National Programme on HIV/AIDS; 2017. [Google Scholar]
- 3.Lessler J, Azman AS, McKay HS, Moore SM. What is a Hotspot Anyway? The American Journal of Tropical Medicine and Hygiene 2017; 96(6): 1270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang LW, Grabowski MK, Ssekubugu R, et al. Heterogeneity of the HIV epidemic in agrarian, trading, and fishing communities in Rakai, Uganda: an observational epidemiological study. The Lancet HIV 2016; 3(8): e388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clift S, Anemona A, Watson-Jones D, et al. Variations of HIV and STI prevalences within communities neighbouring new goldmines in Tanzania: importance for intervention design. Sexually transmitted infections 2003; 79(4): 307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimani J, McKinnon LR, Wachihi C, et al. Enumeration of sex workers in the central business district of Nairobi, Kenya. PloS one 2013; 8(1): e54354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiwanuka N, Ssetaala A, Mpendo J, et al. High HIV-1 prevalence, risk behaviours, and willingness to participate in HIV vaccine trials in fishing communities on Lake Victoria, Uganda. Journal of the International AIDS Society 2013; 16(1): 18621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messina JP, Emch M, Muwonga J, et al. Spatial and socio-behavioral patterns of HIV prevalence in the Democratic Republic of Congo. Social science & medicine 2010; 71(8): 1428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wand H, Ramjee G. Targeting the hotspots: investigating spatial and demographic variations in HIV infection in small communities in South Africa. Journal of the International AIDS Society 2010; 13: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanser F, de Oliveira T, Maheu-Giroux M, Barnighausen T. Concentrated HIV subepidemics in generalized epidemic settings. Curr Opin HIV AIDS 2014; 9(2): 115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grabowski MK, Serwadda DM, Gray RH, et al. HIV Prevention Efforts and Incidence of HIV in Uganda. N Engl J Med 2017; 377(22): 2154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havlir D, Charlebois E, Balzer L, Clark TD, Kamya M. SEARCH community cluster randomized study of HIV “test and treat” using multi-disease approach and streamlined care in rural Uganda and Kenya. Abstract no. WEAX0106LB. International AIDS Conference; July 23–27, 2018, Amsterdam, Netherlands. [Google Scholar]
- 13.Karim SS. Assessing progress with HIV incidence in national cohorts. The Lancet HIV 2016; 4(2): e56–e8. [DOI] [PubMed] [Google Scholar]
- 14.Justman JE, Mugurungi O, El-Sadr WM. HIV Population Surveys — Bringing Precision to the Global Response. New England Journal of Medicine 2018; 378(20): 1859–61. [DOI] [PubMed] [Google Scholar]
- 15.Lubega M, Nakyaanjo N, Nansubuga S, et al. Risk Denial and Socio-Economic Factors Related to High HIV Transmission in a Fishing Community in Rakai, Uganda: A Qualitative Study. PloS one 2015; 10(8): e0132740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seeley JA, Allison EH. HIV/AIDS in fishing communities: challenges to delivering antiretroviral therapy to vulnerable groups. AIDS Care 2005; 17(6): 688–97. [DOI] [PubMed] [Google Scholar]
- 17.Uganda AIDS Commission. HIV and AIDS Uganda Country Progress Report 2014. Kampala; 2015. [Google Scholar]
- 18.Chang LW, Nakigozi G, Billioux VG, et al. Effectiveness of peer support on care engagement and preventive care intervention utilization among pre-antiretroviral therapy, HIV-infected adults in Rakai, Uganda: a randomized trial. AIDS and behavior 2015; 19(10): 1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long A, Mbabali I, Hutton HE, et al. Design and Implementation of a Community Health Worker HIV Treatment and Prevention Intervention in an HIV Hot Spot Fishing Community in Rakai, Uganda. J Int Assoc Provid AIDS Care 2017; 16(5): 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang LW, Mbabali I, Kong X, et al. Impact of a community health worker HIV treatment and prevention intervention in an HIV hotspot fishing community in Rakai, Uganda (mLAKE): study protocol for a randomized controlled trial. Trials 2017; 18(1): 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PEPFAR. Dreaming of an AIDS-Free Future. 2018. https://www.pepfar.gov/documents/organization/287807.pdf. Last access date: April 30, 2019.
- 22.Grabowski MK, Reynolds SJ, Kagaayi J, et al. The validity of self-reported antiretroviral use in persons living with HIV: a population-based study. AIDS 2018; 32(3): 363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong X, Ndyanabo A, Nalugoda F, et al. The accuracy of women’s reports of their partner’s male circumcision status in Rakai, Uganda. AIDS 2013; 27(4): 662–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2016. [PubMed] [Google Scholar]
- 25.Hayes RJ, Donnell D, Floyd S, et al. Impact of Universal Testing and Treatment in Zambia and South Africa: HPTN071 (POPART). Abstract No. 92. Conference on Retroviruses and Opportunistic Infections; March 4–7, 2019; Seattle, Washington. [Google Scholar]
- 26.Borgdorff MW, Kwaro D, Obor D, et al. HIV incidence in western Kenya during scale-up of antiretroviral therapy and voluntary medical male circumcision: a population-based cohort analysis. The Lancet HIV 2018; 5(5):e241–e249. [DOI] [PubMed] [Google Scholar]
- 27.Justman J, Reed JB, Bicego G, et al. Swaziland HIV Incidence Measurement Survey (SHIMS): a prospective national cohort study. The Lancet HIV 2016; 4(2): e83–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandormael A, Adam NA, Dobra A, de Oliveira T, Tanser F. Sharp Decline in Male HV Incidence in a Rural South African Population (2004–2015). Abstract No. 46. Conference on Retroviruses and Opportunistic Infections; March 4–7, 2018, Boston, Massachusetts. [Google Scholar]
- 29.Ghys PD, Williams BG, Over M, Hallett TB, Godfrey-Faussett P. Epidemiological metrics and benchmarks for a transition in the HIV epidemic. PLoS Med 2018; 15(10): e1002678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galvani AP, Pandey A, Fitzpatrick MC, Medlock J, Gray GE. Defining control of HIV epidemics. The Lancet HIV 2018; 5(11): e667–e670 [DOI] [PubMed] [Google Scholar]
- 31.UNAIDS. Ending AIDS Progress Towards the 90–90-90 Targets, 2017.
- 32.Bassett IV, Regan S, Mbonambi H, et al. Finding HIV in hard to reach populations: mobile HIV testing and geospatial mapping in Umlazi township, Durban, South Africa. AIDS and behavior 2015; 19(10): 1888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisinger RW, Fauci AS. Ending the HIV/AIDS Pandemic. Emerging Infectious Diseases 2018; 24(3): 413–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.