Abstract
Objective:
Clinical trial data demonstrates improved glycemic control with hybrid close loop (HCL) insulin delivery systems, yet limited real-world data exists. Data from the inaugural cohort of patients initiating a HCL system (Medtronic MiniMed™ 670G, Medtronic Canada, Brampton, ON) at a university medical center was used to examine real-world utilization and glycemic control following a standardized implementation process.
Methods:
Data from 34 adult patients with type 1 diabetes were obtained from pump downloads at 4 time points: (1) previous insulin pump, (2) HCL in manual-mode, (3) 2 weeks after HCL auto-mode transition, and (4) 6 to 12 weeks after initiation of HCL. In-person training by certified diabetes educators was performed across 3 sessions with phone and electronic messaging following auto-mode start.
Results:
Mean self-monitored blood glucose (SMBG) per day increased from 5.15 baseline to 6.49 at 6 to 12 weeks (P<.05) with 3.26 sensor calibrations per day. Time-in-auto-mode was 79.3% at 2 weeks and 72.3% at final follow-up, with 82% of patients spending >50% of time in auto-mode. There were 8.2 auto-mode exits over the final 14-day download. Time-in-target was 67.3% in manual-mode, 73.4% at 2 weeks (P = .09), and 71.7% by 6 to 12 weeks (P = .06). Hemoglobin A1c (HbA1c) decreased by 0.51% (P = .02), while total daily dose and % basal did not change. Patients with HbA1c <7.0% (53 mmol/mol) at baseline spent more time-in-target than those with HbA1c ≥7.0% (53 mmol/mol; 78.0% versus 67.5%) despite spending less time-in-auto-mode (66.5% versus 74.8%).
Conclusion:
These data illustrate real-world implementation of HCL technology using a structured education program within a major medical center. Overall benefit may vary based on baseline characteristics such as HbA1c.
INTRODUCTION
For the 1.8 million individuals in the United States living with type 1 diabetes, the importance of maintaining near normal glycemic control, measured by hemoglobin A1c (HbA1c), to prevent microvascular and macrovascular complications is well established (1). Despite this, less than 30% of individuals with type 1 diabetes currently meet HbA1c targets (2,3).
The multiple self-management behaviors necessary to achieve good glycemic control require significant time and insight. Insulin pump therapy may provide benefits in terms of glycemic control and added lifestyle flexibility (4–8). However, most individuals who use insulin pumps are still not meeting their HbA1c target (2) and reduction in hypoglycemia is dependent upon the baseline risk of the patient population (9). By comparison, continuous glucose monitoring holds more promise with respect to hypoglycemia prevention (10–13), though sensor-augmented insulin pumps have demonstrated inconsistent effects on hypoglycemia (7,11).
Automated insulin delivery has the potential to improve glycemic control while reducing the patient burden of self-management (14). The earliest systems demonstrated reduced frequency of hypoglycemia via automated suspension of insulin delivery in response to actual or predicted low sensor glucose (15,16). In 2016, the Food and Drug Administration and other regulatory agencies approved the first hybrid closed loop (HCL) insulin delivery system (Medtronic MiniMedTM 670G, Medtronic Canada, Brampton, ON) and in 2017, the device became commercially available in the United States. The device automatically modifies basal rates based on continuous glucose monitoring data. In the pivotal trial utilizing a nonrandomized before and after study design, in-home use of the system was associated with significantly reduced overall hypoglycemia and specifically nocturnal hypoglycemia (17,18). Even with the reduction in hypoglycemia, HbA1c was significantly reduced for both adolescents and adults from a range of 7.7 (61 mmol/mol) to 0.8% to a range of 7.1 (54 mmol/mol) to 0.6% (P<.001), and from a range of 7.3 (56 mmol/mol) to 0.9% to a range of 6.8 (51 mmol/mol) to 0.6% (P<.001), respectively. Both adolescent and adult participants had less glucose variability with increased time in target (70 to 180 mg/dL) on auto-basal mode compared to manual-mode (18).
However, HCL insulin delivery does not eliminate the need to perform self-care behaviors such as self-monitored blood glucose (SMBG), meal boluses, and infusion site changes (19). In addition, the user experience may be affected by additional system alerts and technical difficulties. Participants in the pivotal HCL clinical trial generally had good glycemic control at baseline (18) and were likely to be highly motivated, leaving questions about effectiveness in general clinical practice. In addition, it is not known whether insulin pump utilization and management behaviors such as glucose monitoring, calibrations, and meal boluses can predict changes in glucose control with HCL. However, a failure to perform these tasks in a timely manner can result in hyperglycemia and removal from the pump’s auto-basal system. In the in-home trial, adolescents and adults remained in auto-basal mode 75.8% and 88.0% of the time, respectively (18).
Real-world data on how patients in the general clinical population use HCL insulin delivery systems is essential for future patient selection and education. By understanding how patients use this technology and how their interactions with the device correlate with measures of glycemic control, we can better assist clinicians and patients with this technology. The objective of this study is to examine real-world utilization and glycemic control following a standardized HCL implementation process at a major academic medical center.
METHODS
This was a retrospective, observational study examining HCL insulin pump use and adherence features among an inaugural cohort of users at a single institution. The study was approved by the Ohio State University institutional review board. All patients who initiated HCL therapy in the Division of Endocrinology, Diabetes, and Metabolism before December 1, 2017 were included in the analysis.
Training Program
Prior to initiating the 670G system, patients were offered attendance at a free monthly information session that broadly described the range of available insulin pump and glucose monitoring technologies, and described expectations and procedures for training. Patients also had the option to meet with manufacturer representatives in a separate room if desired. Certified diabetes educators (CDE) conducted all training, initially with the assistance of the manufacturer. Existing insulin pump users began the 670G insulin pump and the Guardian 3 sensor (Medtronic Canada) in a single session (1 to 1.5 hours) where possible. The previous settings were transferred to the patient’s new pump at that time. Patients who did not have a sensor at the time of pump initiation returned for a separate session. Patients began in manual-mode and returned after 1 to 2 weeks for initiation of HCL auto-mode (1-hour session). Patients were instructed to upload their data weekly to CareLinkR (Medtronic Canada) for the following 4 weeks. General principles and adjustments in therapy were similar to that previously reported (20). The CDE in concert with the treating physician or nurse practitioner made further adjustments via phone or electronic messaging (approximately 30 minutes per download). Patients followed up with a combined physician and educator visit 1 to 2 months later (duration of 1 to 1.5 hours).
Data were obtained at 4 time-points: (1) previous insulin pump, (2) HCL start in manual-mode, (3) 2 weeks after HCL auto-mode transition, and (4) 6 to 12 weeks after initiation of HCL. User data were obtained directly from insulin pump downloads via the Medtronic CareLink software. Adherence, utilization, and continuous glucose monitoring data were retrieved from aggregated measures on 14-day downloads. Target blood glucose was defined as 70 to 180 mg/dL and reported as percentage of time in target range. Hypoglycemia was defined as the percentage of total time with blood glucose <70 mg/dL. HbA1c was assessed at baseline (within 3 months of HCL initiation) and 2 to 4 months post-HCL. Baseline daily carbohydrate intake was calculated using the patient’s previous insulin pump download only for patients with 90% bolus calculator use. Patients who did not consistently use the bolus calculator, and who rather relied on manual boluses did not consistently enter their carbohydrates into the pump and therefore this measure was inaccurate in these patients.
Changes in continuous variables from baseline to follow-up were analyzed using paired Student’s t tests. Differences between groups were determined using the Student’s t test (unpaired samples) or Fisher exact test, as appropriate. Univariate associations between variables were measured using Spearman’s rank-order correlation due to the nonparametric nature of variables.
RESULTS
A total of 46 patients received the HCL system between June 1 and December 31, 2017. Of these, 7 patients did not have sensors allowing them to initiate the HCL system within the study time-frame, 3 did not have a 6- to 12-week download, 1 did not have a pre-HCL download, and 1 never transitioned into HCL auto-mode for unknown reasons. Of the remaining 34 patients, all had type 1 diabetes, 50% were female, the mean age was 46 years (range 22 to 69 years) and the mean duration of diabetes was 27 years (range 22 to 48 years). Two patients self-started HCL without education or supervision of endocrinology staff.
Patient support and education was assessed by quantifying CDE education visits, physician appointments, electronic patient messages, phone calls, and the number of Carelink downloads. A summary of patient support and education is provided in Table 1. Our analysis of support is incomplete because patients received additional support from Medtronic company educators and the company helpline, which could not be quantified in our analysis.
Table 1.
Support Frequency Provided Over Initial 12 Weeks of Therapy
| Support | Frequency |
|---|---|
| CDE visits | 2.59 ± 0.50 |
| Physician appointments | 1.03 ± 0.46 |
| Electronic patient messages | 4.44 ± 2.57 |
| Phone calls | 1.38 ± 1.23 |
| Downloads | 3.85 ± 1.76 |
Abbreviation: CDE = certified diabetes educator.
Data are reported as mean ± standard deviation.
Pre-HCL and post-HCL data are detailed in Table 2. Mean SMBG per day increased from 5.15 at baseline to 6.18 at follow-up (P<.05) with 3.26 sensor calibrations per day. Time in HCL auto-mode decreased significantly falling from 79.3% at 2 weeks, to 72.3% by 6 to 12 weeks (P = .04) with 82% of patients spending >50% of the time in HCL auto-mode. Time in target was 67.3% in manual-mode, 73.4% at 2 weeks (P = .09), and 71.1% by 6 to 12 weeks (P = .08). HbA1c decreased by 0.51% (P = .001), while the total daily insulin dose in units/kg/day, and % basal insulin did not change over the study period. There was not a significant reduction in time in hypoglycemia in manual-mode (3.47%) versus at 6 to 12 weeks (3.29%) (P = .06). Carbohydrates tended to increase from 156.1 to 188.4 g/day (P = .07), while the total number of boluses tended to decrease (P = .06). Mean patient weight in the overall sample increased from 89.6 kg at baseline to 91.7 kg at 6 to 12 weeks, but this was not significant.
Table 2.
Baseline and Post-HCL Characteristics, Stratified by Baseline HbA1C
| Characteristics | Total (N = 34) | HbA1C <7.0% (53 mmol/mol)a (N = 10) | HbA1C >7.0% (53 mmol/mol)a (N = 24) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Female (N, %) | 17 (50.0) | 6 (60.0) | 11 (45.8) | ||||||
| Age (years ± SD) | 45.9 ± 11.8 | 43.9 ± 13.7 | 46.7 ± 11.2 | ||||||
| Duration of diabetes (years ± SD) | 27.8 ± 12.4 | 28.6 ± 8.4 | 16.7 ± 5.1 | ||||||
| Previous pump use (N, %) | 34 (100.0) | 10 (100) | 24 (100) | ||||||
| Prior CGM use (N, %) | 22 (65) | 7 (70.0) | 16 (69.6) | ||||||
| Current CGM use (N, %) | 18 (53) | 6 (60.0) | 12 (54.6) | ||||||
| Baseline | Follow-up | P value | Baseline | Follow-up | P value | Baseline | Follow-up | P value | |
| Weight | 89.6 ± 22.6 | 91.7 ± 22.5 | .62 | 81.1 ± 18.8 | 78.4 ± 19.0 | .68 | 93.3 ± 23.4 | 95.7 ± 22.2 | .64 |
| HbA1C (% ± SD) | 7.5 ± 1.0 | 7.0 ± 0.7 | .001b | 6.5 ± .32 | 6.6 ± 0.6 | .53 | 8.0 ± 0.9 | 7.2 ± 0.7 | .0002b |
| Pump characteristics | |||||||||
| SMBG (number ± SD) | 5.2 ± 0.4 | 6.2 ± 2.5 | .03b | 5.6 ± 0.8 | 6.1 ± 2.1 | .35 | 5.0 ± 0.5 | 6.2 ± 2.7 | .06 |
| Total insulin/day (unit ± SD) | 58 ± 32 | 57.9 ± 25.3 | .93 | 46.4 ± 16.8 | 46.8 ± 18.5 | .84 | 63.6 ± 36.9 | 63.0 ± 27.1 | .86 |
| Units/kg/day | 0.65 ± 0.05 | 0.64 ± 0.04 | .54 | 0.57 ± 0.13 | 0.54 ± 0.17 | .51 | 0.69 ± 0.27 | 0.65 ± 0.21 | .57 |
| Bolus (number ± SD) | 5.3 ± 2.1 | 4.7 ± 2.2 | .06 | 5.1 ± 1.1 | 4.7 ± 1.6 | .53 | 5.1 ± 2.1 | 4.3 ± 2.5 | .08 |
| Bolus calculator use (% ± SD) | 78.0 ± 35.1 | NA | NA | 79.9 ± 34.0 | NA | NA | 77.1 ± 36.3 | NA | NA |
| Carbohydrate (patients with >90% bolus calculator use, grams/day ± SD) | 156.1 ± 76.8 | 188.4 ± 80.4 | .07 | 176.8 ± 74.9 | 179.2 ± 41.2 | .75 | 145.1 ± 77.7 | 192.3 ± 92.5 | .003b |
| % Basal (number ± SD) | 50.8 ± 15.8 | 47.6 ± 11.8 | .33 | 50.9 ± 23.1 | 46.0 ± 12.1 | .56 | 50.8 ± 12.3 | 49.5 ± 10.6 | .67 |
| Auto-mode self-start (N, %) | NA | 2 (5.9) | NA | NA | 2 (20.0) | NA | NA | 0 (0.0) | NA |
| Time in auto-mode (% ± SD) | NA | 72.3 ± 27.3 | NA | NA | 66.5 ± 29.2 | NA | NA | 74.8 ± 26.7 | NA |
| Automode exits (number ± SD) | NA | 8.2 ± 4.4 | NA | NA | 8.4 ± 6.1 | NA | NA | 8.1 ± 3.7 | NA |
| Calibrations (number ± SD) | NA | 3.3 ± 0.8 | NA | NA | 3.5 ± 1.1 | NA | NA | 3.1 ± 0.6 | NA |
| Sensor glucose | MM | Follow-up | P value | MM | Follow-up | P value | MM | Follow-up | P value |
| Mean glucose (mg/dL ± SD) | 155.3 ± 26.1 | 150 ± 18.7 | .17 | 140.1 ± 12.6 | 138.0 ± 13.1 | .62 | 163.3 ± 27.2 | 156.5 ± 17.6 | .21 |
| Time-in-target (% ± SD) | 67.3 ± 15.2 | 71.1 ± 13.4 | .08 | 75.3 ± 7.93 | 78.0 ± 8.8 | .43 | 62.9 ± 15.7 | 68.3 ± 14.0 | .12 |
| Time <70 mg/dL (% ± SD) | 3.47 ± 4.5 | 3.3 ± 3.0 | .76 | 5.4 ± 3.6 | 4.9 ± 3.9 | .37 | 2.70 ± 3.30 | 2.6 ± 2.4 | .36 |
| Time >180 mg/dL (% ± SD) | 29.2 ± 16.3 | 25.5 ± 14.2 | .09 | 19.3 ± 8.63 | 17.1 ± 8.8 | .47 | 34.4 ± 16.6 | 29.0 ± 14.7 | .14 |
Abbreviations: CGM = continuous glucose monitoring; HbA1c = hemoglobin A1c; HCL = colesevelam hydrochloride; MM = Manual mode; SD = standard deviation; SMBG = self-monitored blood glucose.
Data are reported as mean ± SD or N (%). Differences between groups were determined using the Student’s t test or Fisher exact test, as appropriate. Baseline refers to the initial pump visit for all data points except for the sensor glucose, which was collected after initiation of manual mode.
P<.05 for HbA1c <7.0% versus >7.0%.
These comparisons were found to be statistically significant.
Patients with HbA1c <7.0% (<53 mmol/mol; n = 10) spent 66.5% of the time in HCL auto-mode compared to 74.8% for patients with HbA1c ≥7.0% (≥53mmol/mol; n = 23) (P = .14). Patients with a baseline HbA1c <7.0% (<53 mmol/mol) spent more time in target (78.0%) compared to patients whose HbA1c was ≥7.0% (≥53mmol/mol) at baseline (68.3%; P = .01). Those with HbA1c <7.0% (<53 mmol/mol) spent less time in hyperglycemia (17.1%) versus those with baseline HbA1c ≥7.0% (≥53mmol/mol) (29.0%; P = .002). Patients with HbA1c ≥7.0% (≥53mmol/mol) at baseline experienced a 0.8% decrease in HbA1c, whereas patients with a baseline HbA1c of <7.0% (<53 mmol/mol) were unchanged (0.1% increase over the study period) (P = .0001). Patients with baseline HbA1c ≥7.0% (≥53mmol/mol) had a significant increase in reported daily carbohydrates (145 ± 78 g at baseline, 192 ± 93 g at follow-up; P = .003), while those with HbA1c <7.0% (<53 mmol/mol) did not (177 ± 75 g at baseline, 179 ± 41 g at follow-up; P = .75). Patients with baseline HbA1c ≥7.0% (≥53mmol/mol) experienced an increase in weight (93.3 kg to 95.7 kg) 3 to 4 months after initiation of HCL therapy, whereas patients with baseline HbA1c <7.0% (<53 mmol/mol) experienced weight loss (81.1 kg to 78.4 kg). While the change in weight among respective subgroups was not significant, the difference in change in weight between the 2 groups was significant (P<.001). Neither baseline HbA1c subgroup experienced a significant change in time in hypoglycemia following the transition from manual-mode to auto-mode (HbA1c <7.0% [<53 mmol/mol], +0.5% change, P = .74; HbA1c >7.0 [>53 mmol/mol], –0.09%, P = .87). In addition, 100% of participants with baseline HbA1c <7.0% (<53 mmol/mol) versus 79% of participants with baseline HbA1c ≥7.0% (≥53 mmol/mol) experienced any hypoglycemia at the 6- to 12-week download. There were no significant differences in time in hypoglycemia, HCL auto-mode exits, SMBG/day, or bolus/day between the 2 groups.
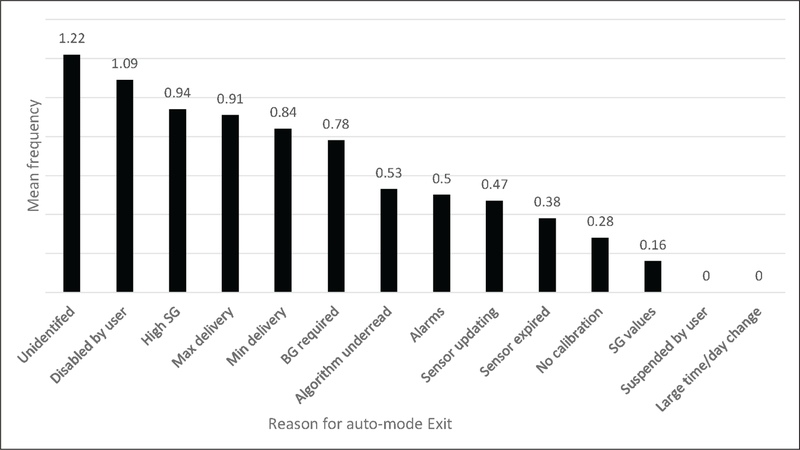
The mean number of HCL auto-mode exits was 6.8 at 2 weeks, compared to 8.2 HCL auto-mode exits at 6 to 12 weeks (range 1 to 19; P = .036). The number of HCL auto-mode exits at the 2-week download was associated with a decrease in time in HCL auto-mode at 6 to 12 weeks (P = .015). The most common reasons for exit on the final 14-day HCL download was “BG required” (9.89%), “unidentified” (9.51%), “disabled by user” (9.13%), “max delivery” (8.75%), and “high SG” (7.98%) (Fig. 1).
Fig. 1.
Mean frequency of reasons for HCL auto-mode exit per 14 day download. BG = blood glucose; SG = sensor glucose.
Educational and support factors (i.e., frequency of physician visits, CDE visits, MyChart messages, phone calls, or CareLink downloads) were not significantly correlated with a change in HbA1c, time-in-target, or time-in-HCL-auto-mode. The 2 patients who self-started HCL had a HbA1c level below the participant mean (6.9% [52 mmol/mol] and 6.2% [44 mmol/mol]). The participants spent 79% and 14% of the time in auto-mode but a similar amount of time in target (66% and 68%, respectively).
Univariate associations with change in HbA1c, time in auto-mode, time-in-target, and time in hypoglycemia are presented in Table 3. CGM use immediately prior to HCL initiation was associated with a greater percentage of time spent in auto-mode (P = .011). The amount of time spent in auto-mode was not significantly associated with previous insulin pump use and adherence variables (i.e., SMBG/day, bolus/day, bolus calculator use, % basal), change in HbA1c, time-in-target, or time in hypoglycemia. A higher baseline HbA1c was associated with a greater decline in HbA1c (P<.0001), but less time in target (blood glucose of 70 to 180 mg/dL; P = .002). Older age, greater percentage of bolus calculator use at baseline, and higher baseline HbA1c were all associated with less percentage of time spent in hypoglycemia (P = .043, .032, and .018, respectively).
Table 3.
Correlations Between Baseline Characteristics and Clinical Outcomes
| Change in HbA1c | Time in auto-mode | Time in target | Time in hypoglycemia | |||||
|---|---|---|---|---|---|---|---|---|
| Spearman | P value | Spearman | P value | Spearman | P value | Spearman | P value | |
| Age | 0.246 | .175 | 0.157 | .376 | 0.038 | .833 | –0.349 | .043a |
| DM duration | 0.199 | .301 | 0.207 | .264 | 0.057 | .762 | –0.158 | .397 |
| Current CGM use | 0.220 | .251 | 0.450 | .011a | –0.160 | .391 | 0.278 | .130 |
| SMBG/day | 0.024 | .897 | 0.147 | .413 | 0.211 | .239 | 0.029 | .874 |
| Bolus/day | 0.080 | .665 | 0.250 | .155 | 0.236 | .179 | 0.298 | .087 |
| Bolus calculator use | –0.243 | .195 | –0.296 | .101 | –0.039 | .833 | –0.379 | .032a |
| Carb/day | 0.320 | .119 | –0.133 | .519 | 0.203 | .319 | –0.023 | .912 |
| Total daily insulin | –0.190 | .297 | 0.074 | .676 | 0.106 | .550 | 0.015 | .933 |
| Basal-bolus | 0.073 | .691 | 0.036 | .842 | 0.013 | .941 | 0.107 | .548 |
| HbA1c | –0.698 | <.0001a | 0.169 | .349 | –0.524 | .002a | –0.409 | .018a |
| Time in auto-mode | 0.074 | .687 | 0.302 | .083 | –0.176 | .320 | ||
| # auto-mode exits | 0.133 | .484 | 0.103 | .575 | –0.286 | .113 | 0.073 | .691 |
| Time in target | 0.315 | .079 | 0.302 | .083 | 0.196 | .266 | ||
| Time in hypoglycemia | 0.092 | .618 | –0.176 | .320 | 0.196 | .266 | ||
Abbreviations: CGM = continuous glucose monitoring; DM = diabetes mellitus; Ha = alternative hypothesis; HbA1c = hemoglobin A1c; Pr = probability; SMBG = self-monitoring of blood glucose.
These comparisons were found to be statistically significant.
Ha: mean ! = 188.4412; Ha: mean>188.4412; Pr(T < t) = 0.0208; Pr(|T| > |t|) = 0.0415
DISCUSSION
In this study, HCL therapy was associated with a reduction in HbA1c and a trend of an increase in time in target range, but no change in total daily insulin dose or percentage of basal insulin. Patients initiating HCL who had an elevated HbA1c at baseline had a larger reduction in HbA1c and spent more time in auto-mode than those with baseline HbA1c <7.0% (<53 mmol/mol), but a similar length of time in hypoglycemia. Unlike previous clinical trials, our real-world analysis did not show a reduction in time spent in hypoglycemia, though there was a trend in the overall analysis (15,16,18). This is of particular significance for participants who were achieving HbA1c targets at baseline as this appears to be the factor incentivizing therapy selection. These data suggest a benefit in terms of glycemic control in a real-world patient population, provided that appropriate education and support is available.
Not surprisingly, patients with a HbA1c <7.0% (<53 mmol/mol) at baseline spent more time in target throughout the study compared to participants with baseline HbA1c ≥7.0% (≥53 mmol/mol), but spent significantly less time in auto-mode. These participants did not experience a reduction in HbA1c or time spent in hyperglycemia or hypoglycemia with HCL therapy. However, it should be noted that the study is limited by sample size especially in the subset of patients with a baseline HbA1c <7.0% (<53 mmol/mol) and it is unknown if these findings would be replicated in a larger pool of participants. The significant increase in carbohydrates that participants recorded on HCL versus baseline is of interest given clinical reports of patients entering “fake carbs” into the HCL system to facilitate a correction bolus (20). However, the mean carbohydrate consumption among participants in this study was 179.2 g/day and therefore the increase could also be attributed to improved adherence with carbohydrate counting or fewer missed carbohydrate boluses. This is supported by the observation that only patients with a baseline HbA1c ≥7.0% (≥53 mmol/mol) had a significant increase in reported carbohydrates.
By comparison, in the pivotal clinical study, subjects spent a larger percentage of time in auto-mode (87.2% versus 72% in the present study) (17). However, a similar reduction in HbA1c was observed (0.6% versus 0.5% in the present analysis). The change in % time in target was nearly identical. These data suggest similar benefits in terms of glycemic control in a real-world patient population compared to that observed in the pivotal clinical trial.
Higher baseline HbA1c was the only predictor of initial HbA1c reduction and time in target range among patients beginning HCL. This supports a role for broad adoption of the HCL technology. On the other hand, older age, higher baseline HbA1c, and more frequent bolus calculator use at baseline were predictors of less time spent in hypoglycemia. The latter suggests that experience and behaviors may at least in part play a role for hypoglycemia avoidance. Further complicating matters is the observation that time spent in auto-mode was not associated with time in target. In fact, sensor use at baseline was the only baseline predictor of time spent in auto-mode, suggesting that previous familiarity or success with the technology may predict greater use of (or tolerance to) HCL. In focus groups of a subset of patients enrolled in the pivotal trial, patients with low a priori knowledge of the device capability reported unexpected increase in treatment burden that did not meet their expectations for an artificial pancreas (19). The potential criticism of the pivotal trial is that an increase in SMBG mandated for successful operation of HCL could have been the mediator of improvement in glycemic control. However, despite an increase in SMBG following HCL initiation in this analysis, SMBG frequency was not associated with multiple measures of glycemic control. Thus, while the study cannot confirm that HCL itself results in improved time in target range or whether other factors may play a role, the data support a role for HCL for improving glucose control among patients with varying behaviors at baseline.
Use of HCL auto-mode decreased significantly over time. It is possible this decline in use was a function of increased treatment burden as described in previous studies (19). Patients in our study had a significant increase in the number of SMBG checks per day and experienced a high number of HCL auto-mode exits forcing them out of the HCL system. A full summary report of all alarms is not readily available with routine CareLink downloads. Since most exits are preceded by multiple alarms, quantifying alarms would be an important treatment burden measurement. The conjecture that treatment burden played a role in time in HCL auto-mode is supported by our findings showing a significant correlation between number of HCL auto-mode exits on the 2-week download and time spent in HCL auto-mode at 6 to 12 weeks. Additional studies aimed at examining treatment burden and its effects on quality of life and therapy utilization are needed. This information, along with the time required for education and support, should be considered when initiating HCL therapy.
Additional randomized studies are needed. A recent meta-analysis of single hormone and dual hormone closed loop systems support the potential benefits from closedloop therapy but noted that clinical trials thus far were limited by inconsistent study designs and glucose metrics as well as small sample size and treatment duration (14,21).
There are several limitations of the study worth noting. First, the sample size is small and study participants may not be representative of the general population in several respects including their relatively good glycemic control at baseline and the fact that all participants were using insulin pump therapy prior to starting HCL. In addition, the follow-up period was short, and additional time would be needed to establish long-term adherence, particularly since we observed a reduction in HCL use over this short time span. As a retrospective analysis, we were unable to assess quality of life. However, examples of treatment burden were analyzed, including the frequency of auto-mode exits and SMBG checks. The findings warrant further inquiry with larger randomized studies among a more diverse population with greater variability in baseline glycemic control and among device naïve participants. Nevertheless, we present a detailed description of the implementation of HCL and analysis of baseline and on treatment predictors of outcomes in a real-world setting. Interestingly, despite experiencing a significant reduction in HbA1c, our findings did not show a significant improvement in time-in-target. It is important to note that most patients were not regular CGM users. Therefore, baseline time-in-target was captured while patients were in manual-mode, after the first education visit. While this was the approach used in the pivotal clinical trial (17), it limits our ability to attribute the reduction in HbA1c exclusively to the HCL system versus the initiation of CGM therapy. Previous studies have shown a significant reduction in HbA1c with initiation of CGM alone (22).
CONCLUSION
In conclusion, the findings in this study support the use of HCL in patients with a range of self-management behaviors. Patients not yet meeting their glycemic targets may experience the greatest clinical benefit from Medtronic’s 670G HCL system. The HCL system shows promise in improved glycemic control for patients while the data in this study suggest benefit may vary based on patient baseline characteristics such as HbA1c level.
Acknowledgments
K.D. reports consulting and advisory activities with Eli Lilly, GSK, and Mannkind corporations, and research support from GSK, Novo Nordisk, and Sanofi Aventis.
Abbreviations:
- CDE
certified diabetes educator
- HbA1c
hemoglobin A1c
- HCL
hybrid closed loop
- SMBG
self-monitored blood glucose
Footnotes
DISCLOSURE
E.F is the founder of A1Control, LLC. J.Z. has no multiplicity of interest to disclose.
REFERENCES
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 2.McKnight JA, Wild SH, Lamb MJ, et al. Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32:1036–1050. [DOI] [PubMed] [Google Scholar]
- 3.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38:971–978. [DOI] [PubMed] [Google Scholar]
- 4.Ahern JA, Boland EA, Doane R, et al. Insulin pump therapy in pediatrics: a therapeutic alternative to safely lower HbA1c levels across all age groups. Pediatr Diabetes. 2002;3:10–15. [DOI] [PubMed] [Google Scholar]
- 5.Phillip M, Battelino T, Rodriguez H, et al. Use of insulin pump therapy in the pediatric age-group: consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2007;30:1653–1662. [DOI] [PubMed] [Google Scholar]
- 6.Karges B, Schwandt A, Heidtmann B, et al. Association of insulin pump therapy versus insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318:1358–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care. 2014;37:2114–2122. [DOI] [PubMed] [Google Scholar]
- 8.Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157:336–347. [DOI] [PubMed] [Google Scholar]
- 9.Pickup JC. Is insulin pump therapy effective in type 1 diabetes? Diabet Med. 2019;36:269–278. [DOI] [PubMed] [Google Scholar]
- 10.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371–378. [DOI] [PubMed] [Google Scholar]
- 12.Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317:379–387. [DOI] [PubMed] [Google Scholar]
- 13.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:501–512. [DOI] [PubMed] [Google Scholar]
- 15.Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224–232. [DOI] [PubMed] [Google Scholar]
- 16.Battelino T, Nimri R, Dovc K, Phillip M, Bratina N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: a randomized controlled trial. Diabetes Care. 2017;40:764–770. [DOI] [PubMed] [Google Scholar]
- 17.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407–1408. [DOI] [PubMed] [Google Scholar]
- 18.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iturralde E, Tanenbaum ML, Hanes SJ, et al. Expectations and attitudes of individuals with type 1 diabetes after using a hybrid closed loop system. Diabetes Educ. 2017;43:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver KW, Hirsch IB. The hybrid closed-loop system: evolution and practical applications. Diabetes Technol Ther. 2018;20:S216–S223. [DOI] [PubMed] [Google Scholar]
- 21.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benkhadra K, Alahdab F, Tamhane S, et al. Real-time continuous glucose monitoring in type 1 diabetes: a systematic review and individual patient data meta-analysis. Clin Endocrinol. 2017;86:354–360. [DOI] [PubMed] [Google Scholar]