INTRODUCTION
Approximately one-third of inpatient operations in 2010 were performed in adults over age 65(1). As the population ages, the number of older patients undergoing surgery will continue to rise. While many surgical procedures are essential, postoperative complication rates increase with age(2). Taken together, this will require careful consideration of the risks of surgical complications, prolonged length of stay, increased discharge to post-acute facilities, and high resource utilization(3), weighed against potential benefits.
Changes in physiology with aging likely contribute to increased postoperative complications with advancing age(3, 4). Inflammation in response to surgery is associated with poor postoperative outcomes(5–8). However, there are likely other physiologic changes associated with surgery that play an important role in peri-operative outcomes but which remain poorly understood. Proteomics, which examines change in protein expression, can help identify networks of proteins that respond to surgical stress. Already, a number of studies have examined changes in protein expression in response to surgery in association with outcomes such as postoperative cognitive decline(9), or to predict estimated blood loss after coronary artery bypass surgery(10, 11). Ultimately, proteomics may lead to better understanding of complex biologic pathways, improve risk prediction and identify potential treatment strategies to improve outcomes after surgery.
Because of the abundance of potential proteins under study, proteomic assays require high sensitivity and specificity, a wide dynamic range, and accurate quantification(12). In this study, an innovative, sensitive, highly multiplexed, quantitative biomarker discovery technology “SOMAscan” (SomaLogic, Inc, Boulder, CO) was used to examine protein abundance in plasma samples from older adults undergoing major elective non-cardiac surgery. SOMAscan uses chemically modified oligonucleotides, Slow Off-rate Modified Aptamers (SOMAmers) that bind with high specificity and affinity to pre-selected proteins to quantify a protein signal, and can simultaneously measure the expression levels of 1,305 human proteins across the whole dynamic range of proteins, from very low to high concentration proteins.
The primary aim of this study was to evaluate the capacity of SOMAscan technology to identify proteins differentially expressed before and after surgery in older adults. The second aim used SOMAscan results to conduct systems biology analysis to provide insights about biological pathways, functional gene groups/networks, and regulatory mechanisms associated with surgery. Selected proteins identified by SOMAscan were then independently validated using Luminex or enzyme-linked immunosorbent assay (ELISA) methods. A final aim was to correlate the identified proteins with clinical outcome measures of surgical recovery, as another form of external validation (predictive validity).
METHODS
Study Participants
Study participants were older adults undergoing major scheduled non-cardiac surgery enrolled in the Successful Aging after Elective Surgery (SAGES) study (N=566)(13). The study design and methods have been described in detail previously(13, 14). A subcohort of 150 patients, as used in previously studies(15–17), was used for the current study; however, all data presented here are results from new analyses. In brief, eligible individuals were aged 70 and older, English speaking, scheduled to undergo elective surgery at one of two Harvard-affiliated academic medical centers with an anticipated length of stay of at least 3 days. Eligible surgical procedures were total hip or knee replacement; lumbar, cervical, or sacral laminectomy; lower extremity arterial bypass; open abdominal aortic aneurysm repair; and open or laparoscopic colectomy. Exclusion criteria included evidence of dementia, delirium at the time of study enrollment, hospitalization within 3 months, terminal condition, legal blindness, severe deafness, history of schizophrenia or psychosis, and history of alcohol abuse or withdrawal. Comorbidities were identified from medical record review. Delirious and non-delirious patients were matched for age, baseline cognitive status, surgery type, vascular comorbidity, and Apolipoprotein E (ApoE) ε4 carrier status. Thirty-six randomly selected patients from this cohort were used for the SOMAscan studies; the full subcohort (n=150) or a subset of it (n=126), due to limited sample availability, was used for validation.
The research protocol was approved by the institutional review boards of Beth Israel Deaconess Medical Center and Brigham and Women’s Hospital, the two surgical sites, and Hebrew SeniorLife, the study coordinating center, all located in Boston, Massachusetts. All participants provided written informed consent, and all experiments were performed according to relevant guidelines. Demographics and baseline clinical information such as comorbid conditions were collected preoperatively by interview and chart review(13, 14). Post-operative complications were measured daily throughout hospitalization until discharge, and length of stay and discharge to a post-acute facility was determined from the medical chart. All patients underwent phlebotomy at the pre-operative (PREOP) and post-operative day 2 (POD2) timepoints.
Sample Collection and Processing
Blood collection was incorporated into clinical blood draws taken in the pre-admitting testing center (PREOP) or drawn at home, and on the surgical ward (POD2). Blood samples were collected in heparinized tubes and stored on ice an average of 2 hours, and all processed within 4 hours of collection. Plasma was separated by low-speed centrifugation (1500g for 15 minutes at 4oC), aliquoted and stored at −80oC until analysis.
SOMAscan Assay
Heparin plasma samples from the SAGES cohort were analyzed using the SOMAscan manual assay (version 1.3k) for human plasma that measures 1305 proteins. SOMAscan kits were run in a SomaLogic trained and certified assay site. Kit provided quality control samples were run on each plate: a no protein negative control, five pooled plasma samples and the unpooled QC replicate sample. All kit control and biological samples passed each step of quality control and normalization as processed by the SomaLogic standard method using a DNA microarray readout. These include hybridization normalization, plate scaling, median normalization and calibration(18–24).
Identification and Confirmation of Protein Expression Before and After Surgery
The sequence was determined á priori. First, “surgery-related” protein expression was considered to be present when the protein expression from SOMAscan demonstrated a significant increase or decrease between PREOP and POD2 [i.e., Benjamini-Hochberg (BH) corrected p-value <0.01]. Fold-change (FC) was calculated by dividing the POD2 value by the PREOP value. If this number was <1, the FC is the (negative) reciprocal (e.g. if POD2/PREOP=0.75, FC= – (1/0.75) or −1.3) (25, 26). Thus, a positive fold-change implies upregulation, and a negative fold-change implies downregulation. If the absolute foldchange (|FC|) between PREOP and POD2 was >1.5, the protein was included in biological systems pathway analysis; if |FC| >2.0, the protein was further considered for validation with either enzyme-linked immunosorbent assay (ELISA) or Luminex (Figure 1).
Figure 1. Methodologic steps for identification of “surgery-related” protein expression profiles and ELISA confirmation and validation of proteins.
From among proteins with an absolute fold change (|FC|) >2, *three proteins were selected for confirmation and validation in a larger cohort. Proteins included C-reactive protein (CRP) and interleukin (IL-)6 based on prior work (15, 17) and chitinase 3 like 1 (CHI3L1) protein because of clinical relevance. See text for details. Proteins with change |FC|>1.5 were included in biological systems pathway analysis to allow for an analysis with a larger number of proteins.
BH=Benjamini-Hochberg; PREOP=pre-operative; POD2 = post-operative day 2
Bioinformatics and Systems Biology Analysis
Systems biology analyses of surgery-related proteins were performed using the Ingenuity Pathways Analysis (Ingenuity Systems, Redwood City, CA) Knowledge Base, a repository of biological interactions and functions created from millions of individually modeled relationships ranging from the molecular (proteins, genes) to organism (diseases) level. Ingenuity Pathway Analysis (IPA) uses enrichment analysis-based approaches(27, 28) to calculate the significance of observing a candidate protein set within the context of biological systems.
Validation of Proteomic Data by Enzyme-Linked Immunosorbent Assay (ELISA) and Luminex
From among the SOMAscan identified proteins with greater than 2-fold change, three proteins were selected for validation. C-reactive protein (CRP) and Interleukin (IL)-6 were chosen because of prior work with these inflammatory markers; Chitinase-3-like protein 1 (CHI3L1), also known as YKL-40, was selected for validation because of its association with neuro-inflammation linked to microglial activation (29) and association with postoperative outcome and clinical prognosis (30, 31). Details for the CRP and IL-6 validation (n=150) have been previously published (15–17). Measurements of the candidate protein CHI3L1 (DC3L10; 1:1000 dilution) was performed in a slightly smaller cohort (N=126) due to limited volume of the biobanked samples in 24 patients) using commercial ELISA kits from R&D Systems (Minneapolis, MN) following the manufacturer’s protocol. All standards and participant samples were run in duplicate. Each 96-well plate contained the standard curve and participant samples at 2 timepoints (PREOP and POD2). Duplicate coefficient of variations (CV’s) were typically ≤5%. Two internal calibration samples derived from two plasma samples with high and low CHI3L1 levels, frozen in small aliquots calibration purposes were present on every 96-well ELISA plate for calibration across different runs. The cross-plate variation was in the range of 5–10% based on the internal calibration value. ELISA plates were read using a BioTek MX plate reader at Optical Density (OD)=450. A 4-parameter logistic curve was used for optimizing the best-fit model.
Clinical Outcomes
As in prior studies(32), we examined post-operative complications, length of stay, and discharge to a post-acute facility. Post-operative complications were adjudicated by an expert panel, following prior classification systems as published by Gleason et al.(33) and included cardiac ischemia, stroke, sepsis, major infection, and unplanned reoperation. Length of stay was defined as the number of days from surgery to hospital discharge. Discharge to a post-acute facility was determined by medical record review.
Statistical analysis
Protein abundance data was log transformed prior to the application of the analysis methods. Participant samples and proteins were clustered using the Unweighted Pair Group Method with Arithmetic-mean (UPGMA) method using Pearson’s correlation as the similarity measure(34). Data was standardized along the individual protein expression, and average linkage clustering was used in the iterations to update the similarity matrix in the UPGMA implementation. Discriminatory power of the protein expression was also established using principal components analysis (PCA)(35). Sample classification was done using Support Vector Machines (SVM) (36) on the PCA results using a linear kernel. Differential protein abundance was assessed using both parametric and non-parametric statistical approaches to account for the degree, direction, and rank of difference between PREOP and POD2 timepoints. For all statistical tests, the BH procedure (37) was applied to correct for testing multiple hypotheses. A protein was considered to be differentially expressed if the protein was significantly dysregulated (BH p-value < 0.01) in all three statistical approaches (paired t-test, Wilcoxon signed-rank test, and binomial test). Fold-change (FC) of protein expression was calculated by applying the one-step Tukey’s biweight algorithm on the FC values (POD2/PREOP) for each paired sample (38). This provides a robust estimation of the FC for each individual protein that is unaffected by outliers.
Generalized linear models (GLM) were used to examine the associations between surgery-related changes of three ELISA/Luminex-validated proteins (IL-6, CRP, and CHI3L1) and three main clinical outcomes of interest, including the incidence of post-operative complications, length of stay (days), and post-acute facility discharge. We considered two different metrics to quantify the change in protein level from PREOP to POD2: the fold-change between PREOP and POD2 (POD2/PREOP), and the difference between POD2 and PREOP (POD2-PREOP). Generalized linear models (GLMs) with log link were applied to the outcomes of post-operative complications (yes/no) and post-acute facility discharge (yes/no) and a GLM with identity link was applied to length of stay (days). We opted to use the log link with binary or poisson error distribution to obtain relative risks from the GLM models, to avoid potential overestimation from odds ratio calculations(39–41). All models adjusted for age, gender, surgery type, vascular comorbidity, surgery duration, and anesthesia type, as in previous studies(15, 17). Relative risks/point estimates with corresponding 95% confidence intervals were reported. All analysis was performed with SAS 9.4(Cary, NC).
RESULTS
Table 1 describes detailed baseline characteristics of both the SOMAscan cohort (n=36) and the validation cohort (N=150). CRP and IL-6 were validated at timepoints PREOP and POD2 in plasma samples (n=150) and CHI3L1 was validated in plasma samples from a subcohort of these patients (n=126). The SOMAscan cohort (n=36) and the validation cohort were comparable by age, gender, surgery, anesthesia type and duration. In the SOMAscan cohort, 11% percent of the cohort developed post-operative complications, had an average length of stay of 5.0±2.2 days, and 78% were discharged to a post-acute facility. Similarly, for the validation cohort (n=150), 10% of the population experienced post-operative complications, with an average length of stay of 5.4±3.7 days, and 71% of the population was discharged to a post-acute facility. The cohort used for the CHI3L1 validation (n=126, subcohort of the 150) was not significantly different from the validation cohort (n=150)(data not shown).
Table 1.
Description of Study Cohort
| Demographics | SOMAscan group (n = 36) | Validation group (n = 150)* |
|---|---|---|
| Age, in years, mean ± SD | 76.4 ± 4.3 | 77.4 ± 4.6 |
| Female, n (%) | 20 (56) | 84 (56) |
| Non-white, n (%) | 2 (6) | 14 (9) |
| Education, mean years ± SD | 15.4 (2.3) | 15.0 (2.8) |
| Married, n (%) | 22 (61) | 88 (59) |
| Charlson score ≥ 2, n (%) | 13 (36) | 53 (35) |
| Vascular comorbidity, n (%) | ||
| Confirmed or history of myocardial infarction | 4 (11) | 17 (11) |
| Congestive heart failure | 1 (3) | 11 (7) |
| Peripheral vascular disease | 3 (8) | 18 (12) |
| Diabetes (with or without end organ damage); | 8 (22) | 32 (21) |
| Cerebrovascular disease (carotid stenosis or history of stroke or transient ischemic attack | 3 (8) | 19 (13) |
| Hemiplegia | 0 (0) | 0 (0) |
| Baseline kidney function (BUN/Creatinine ≥18), n (%) | 19 (53) | 76 (51) |
| Activities of Daily Living, n (%) | 3 (8) | 12 (8) |
| Instrumental Activities of Daily Living, n (%) | 10 (28) | 49 (33) |
|
Surgery | ||
| Surgery type, % | ||
| Orthopedic | 94 | 88 |
| Vascular | 0 | 5 |
| Gastrointestinal | 6 | 7 |
| Surgery duration, min, mean±SD | 139.7 ± 49.2 | 148.3 ± 76.0 |
| Anesthesia type, % | ||
| General or combined General/Spinal | 81 | 85 |
| Spinal | 19 | 15 |
| Anesthesia duration, minutes, mean±SD | 191.1 ± 52.5 | 199.1 ± 79.2 |
| Postoperative Apache II Score, mean±SD | 12.7 ± 2.7 | 12.9 ± 3.2 |
| Postoperative Acute Physiology Score, mean±SD | 6.9 ± 2.6 | 6.9 ± 2.9 |
| Total postoperative complications, n (%) | ||
| Unstable arrhythmia | 3 (8) | 10 (7) |
| New heart block | 0 (0) | 0 (0) |
| NSTEMI | 0 (0) | 1 (1) |
| Respiratory failure | 1 (3) | 3 (2) |
| Pulmonary embolism | 1 (3) | 3 (2) |
| Pneumonia | 0 (0) | 0 (0) |
| Sepsis | 1 (3) | 1 (1) |
| New renal failure | 1 (3) | 2 (1) |
| Stroke | 1 (3) | 1 (1) |
| Surgical complications | 0 (0) | 2 (1) |
| Any complication | 4 (11) | 16 (11) |
| Postoperative length of stay, days, mean±SD | 5.0 ± 2.2 | 5.4 ± 3.7 |
| Discharge to Post-acute Facility | 28 (78) | 107 (71) |
Charlson comorbidity score was calculated based on diagnoses abstracted from medical record review, scored from 0–35 with higher scores indicating more comorbidity. Vascular comorbidity was defined as presence or absence of at least one of the following pathological conditions: confirmed or history of myocardial infarction, congestive heart failure, peripheral vascular disease, diabetes (with or without end organ damage), cerebrovascular disease (carotid stenosis, history of stroke or transient ischemic attack), or hemiplegia
n=126 for CHI3L1
SOMAscan Enables the Detection of Surgery-related Protein Expression
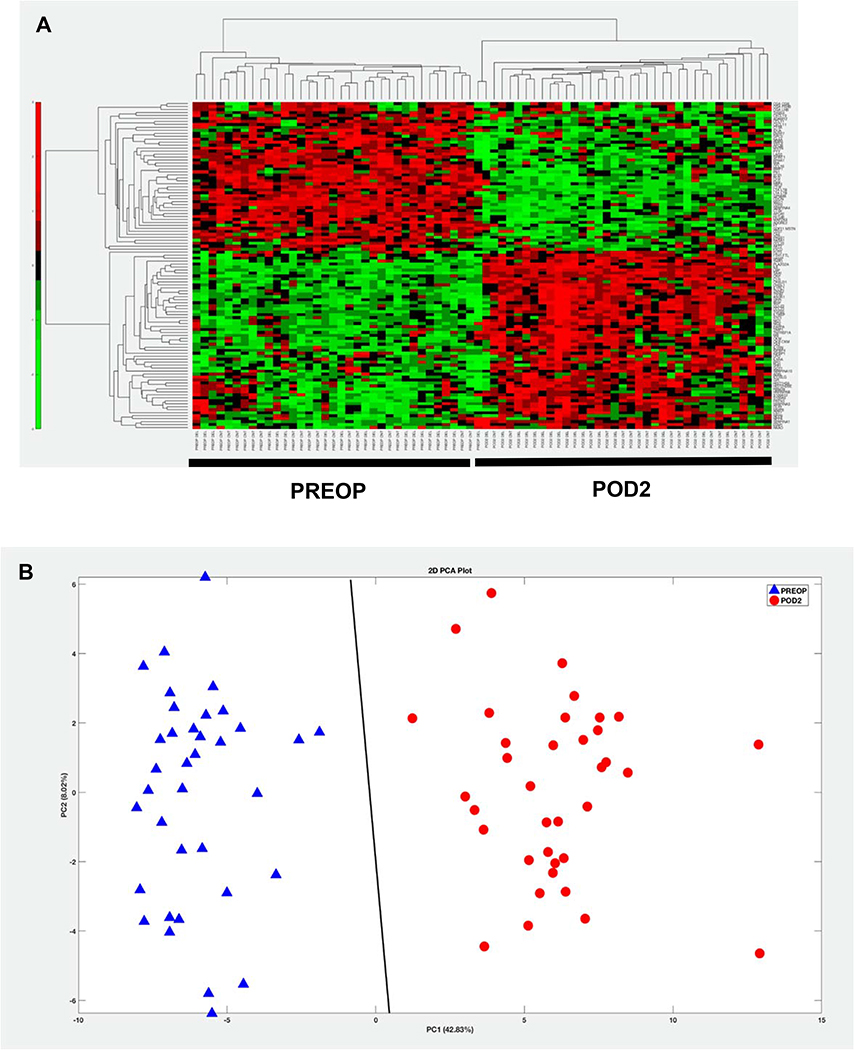
SOMAscan analysis of 36 patients at PREOP and POD2 measured expression of 1305 proteins. Of these, 564 proteins showed significantly altered levels after surgery (BH corrected p-value <0.01), with 110 proteins demonstrating |FC| >1.5 between PREOP and POD2. Hierarchical clustering of these 110 significant differentially expressed proteins accurately discriminated between PREOP and POD2 samples as shown in Figure 2A. A PCA model also showed that the detected proteomic profile effectively separated the samples into two clusters as seen by the SVM classification decision line (Figure 2B). Thirty-three of the 564 proteins demonstrated at least a two-fold change (increase or decrease (Table 2)). Twenty-five of these proteins were upregulated, with the greatest increase of 42-fold seen for Serum Amyloid-A1 protein. Fibronectin, with a 3-fold decrease in expression, was among the 8 proteins found to be downregulated after surgery. A complete list of upregulated and downregulated proteins is provided in the supplementary data (Supplement Table 1).
Figure 2: Complete discrimination between PREOP and POD2 participant samples for the 110 proteins demonstrating |FC| >1.5 by SOMAscan.
Two different unstructured learning methods, hierarchical clustering (HC) or Principal Components Analysis (PCA) was applied to the 110 significant differentially expressed proteins (Benjamini-Hochberg corrected p-value < 0.01) detected by SOMAscan in 36 participants. This demonstrates complete separation between PREOP and POD2, confirming the distinction between protein expression at these timepoints. A: In the HC colormap, red denotes up-regulation and green denotes down-regulation. B: PCA plot using the 110 proteins demonstrates complete separation in to two clusters with the Support Vector Machines (SVM) separating line based on a linear kernel. Blue triangles, PREOP; Red circles POD2
Table 2.
Fold-Change in Protein Expression Following Surgery
| Target Full Name | Gene Target | UniProtKB | Foldchange |
|---|---|---|---|
| UPREGULATED PROTEINS | |||
| Serum amyloid A1 protein | SAA | P0DJI8 | 42.64 |
| Phospholipase A2 Group IIA | NPS-PLA2 | P14555 | 8.93 |
| Interleukin 6 | IL6 | P05231 | 7.82 |
| Troponin I2, Fast Skeletal Type | TNNI2 | P48788 | 5.13 |
| Chitinase 3 Like 1 | CHI3L1 | P36222 | 5.03 |
| Hepcidin Antimicrobial Peptide | LEAP1 | P81172 | 4.56 |
| Troponin T2, Cardiac Type | TNNT2 | P45379 | 4.02 |
| Interleukin 1 Receptor Like 1 | ILIRLI | Q01638 | 3.49 |
| Creatine Kinase B; Creatine kinase M | CKB; CKM | P12277; P06732 | 3.19 |
| Complement C1r | C1R | P00736 | 3.15 |
| Creatine kinase M | CKM | P06732 | 2.82 |
| Integrin Binding Sialoprotein | IBSP | P21815 | 2.79 |
| Carbonic Anhydrase 3 | CA3 | P07451 | 2.73 |
| Erythropoietin | EPO | P01588 | 2.59 |
| Asialoglycoprotein Receptor 1 | ASGR1 | P07306 | 2.56 |
| C-Reactive Protein | CRP | P02741 | 2.52 |
| C-C Motif Chemokine Ligand 23 | CCL23 | P55773 | 2.30 |
| Insulin Like Growth Factor Binding Protein 1 | IGFBP1 | P08833 | 2.23 |
| Thrombopoietin | THPO | P40225 | 2.19 |
| Pappalysin 1 | PAPPA | Q13219 | 2.10 |
| Secreted Phosphoprotein 1 | SPP1 | P10451 | 2.08 |
| C-C Motif Chemokine Ligand 23 | CCL23 | P55773 | 2.08 |
| Interleukin 18 Binding Protein | IL18BP | O95998 | 2.07 |
| S100 Calcium Binding Protein A9 | S100A9 | P06702 | 2.04 |
| Histone Cluster 1 H3 Family Member B | HIST1H3B | P68431 | 2.04 |
| DOWNREGULATED PROTEINS | |||
| Lymphotoxin Alpha; Beta | LTA; LTB | P01374; Q06643 | −2.05 |
| N-Acylethanolamine Acid Amidase | NAAA | Q02083 | −2.07 |
| Cathepsin V | CTSV | O60911 | −2.31 |
| Lymphocyte Activating 3 | LAG3 | P18627 | −2.34 |
| Cathepsin F | CTSF | Q9UBX1 | −2.41 |
| Bone Morphogenetic Protein 1 | BMP1 | P13497 | −2.41 |
| Mediator Complex Subunit 1 | MED1 | Q15648 | −2.63 |
| Fibronectin 1 | FN1 | P02751 | −3.12 |
Significant differentially expressed proteins (Benjamini-Hochberg corrected p-value < 0.01) between PREOP and POD2 with absolute fold-change (|FC|) > 2.0 detected by SOMAscan in 36 paired samples. Fold Change is calculated by the Tukey bi-weight average of paired FC values. A positive FC denotes upregulation at POD2. Proteins shown in BOLD were later validated using Luminex or ELISA. |FC| > 1.5 (110 proteins) is shown in Supplemental Table 1
Systems Biology Analysis
In Figure 3, the upstream regulators (Figure 3A) and biological functions (Figure 3C) that are most significantly enriched by the input 110 protein list are shown. In particular, surgery affects proteins that are regulated by pro-inflammatory cytokines such as Interleukin 1 beta (IL1B), which is a likely upstream regulator of 32 out of the 110 proteins (Figure 3B). Twenty-four of these proteins are increased after surgery (red symbols) and 8 are decreased (green symbols). Among the significantly affected functional categories, enrichment for biological functions linked to inflammatory processes were most prominent, followed by functions associated with vasculature and angiogenesis (Figure 3C). Figure 3D highlights in detail the 31 proteins linked to “Inflammatory Response” among the 110 proteins. In the supplement, the significantly enriched canonical pathways, biological functions, and upstream regulators are listed (Supplement Table 2); a complete list of functional categories that are represented significantly in proteins that were upregulated or downregulated after surgery can be found in supplementary data (Supplement Table 3)
Figure 3. Systems Biology analysis of the 110 significant differentially expressed proteins was conducted using Ingenuity Pathway Analysis.
See text for details. A: Upstream transcriptional regulators that best explain the observed expression changes in the input 110 protein list as their targets. B: Upstream Regulator Analysis: Downstream targets of IL-1B from among the 110 protein list. Red indicates upregulation on POD2 and green denotes downregulation following surgery. Proteins are coded by shape; square: cytokine, vertical rhombus: enzyme, horizontal rhombus: peptidase, trapezoid: transporter, ellipse: transmembrane receptor, circle: other. Links are color-coded as orange: leads to activation, blue: leads to inhibition, gold: findings inconsistent with state of downstream protein, grey: effect not predicted. C: Biological functions that are significantly enriched by the 110 input protein list. D: Proteins among the input list that are linked to the “inflammatory response” biological function. The color, shape, and link codings are the same as part B. In parts B and D, proteins that are experimentally validated are indicated by a black arrow. The orange color of the target upstream regulator and biological function shown in the center implies “activation,” and the shade of the color implies confidence in activation with darker shades implying more confidence in the prediction.
Validation of Selected Proteins: CHI3L1, CRP, IL-6
Among the 33 surgery-related proteins with a fold change of at least two-fold, several proteins were identified via the Systems Biology analysis to be closely associated with inflammation (Figure 3D) and inflammatory upstream regulators (Figure 3B). Of particular interest among this subset of highly differentially expressed proteins were CHI3L1, CRP, and IL-6 (highlighted by arrows in Figures 3B and 3D). The surgery-related increase detected by SOMAscan in CRP and IL-6 expression was highly consistent with our previous studies using other proteomics approaches in a study of delirium following elective non-cardiac surgery(15, 17). IL-6 values were (mean±sd) 9.4±33.6pg/ml PREOP, 104.6±91.5pg/ml POD2, with a foldchange of 22.0±22.6, validated as upregulated after surgery using Luminex. CRP values were 5.3±7.4mcg/ml PREOP, 165.7±68.2mcg/ml POD2, with a foldchange of 117.2 ±177.2, validated with ELISA (Supplement Table 4).
Importantly, CHI3L1 had not been identified in our previous studies, and was uniquely discovered by SOMAscan. Similar to CRP, we validated our CHI3L1 findings with ELISA, using N=126 patients (24 of the 150 patients were excluded due to low volume of plasma remaining). CHI3L1 values were 96.2±77.5ng/ml at PREOP and 753.8±697.8ng/ml POD2 with a fold-change of 9.8±10.8, validating it as upregulated after surgery. The SOMAscan measured fold-change for CHI3L1 and IL-6 were of similar magnitude to those seen with ELISA and Luminex (respectively), but the SOMAscan fold-change for CRP was much lower (FC 2.52, Table 2) than with ELISA, likely due to saturation of the CRP aptamers on POD2.
Changes in CHI3L1, CRP, and IL-6 Predict Clinical Outcomes
PREOP levels of CHI3L1 and CRP were not associated with any clinical outcomes. For IL-6, PREOP levels were associated with post-acute facility discharge (RR 1.09, 95%CI 1.01–1.16). We then examined the association between the change in protein level from baseline to POD2 and clinical outcome. Data are reported per one standard deviation (SD) increase in FC or POD2-PREOP difference. For CHI3L1, FC was significantly associated with a 1.50-fold increased risk of post-operative complications (relative risk (RR) 1.50, 95% confidence interval (CI) 1.21–1.85), increased length of stay of 1.35 days (95%CI 0.77–1.92), and discharge to post-acute facility (RR 1.15, 95%CI 1.04–1.26). The difference between POD2 and PREOP in CHI3L1 was also significantly associated with increased length of stay by 1.34 days (95%CI 0.77–1.91) and discharge to a post-acute facility (RR 1.16, 95%CI 1.06–1.26). For CRP, the difference between POD2 and PREOP was significantly associated with an increased likelihood of post-acute discharge to a post-acute facility (RR 1.14, 95%CI 1.04–1.25). Lastly, for IL-6, FC was significantly associated with post-operative complications (RR1.63, 95%CI 1.18–2.26), incremental increase in length of stay (by 0.98 days, 95%CI 0.5–1.5), and discharge to post-acute facility (RR 1.11, 95%CI 1.04–1.18). The difference between POD2 and PREOP in IL-6 was also significantly associated with increased length of stay (1.38 days, 95%CI 0.95–1.82) and discharge to a post-acute facility (RR 1.13, 95%CI 1.03–1.25) (Figure 4, sTable 2).
Figure 4. Forest plot of associations between CHI3L1, il-6, and CRP and postoperative complications, post-acute facility discharge and length of stay.
In Panel A, the relative risk and 95% confidence interval (CI) are shown for postoperative complications (blue) and post-acute facility discharge (red). Panel B shows Length of Stay. Both RR and Days should be interpreted as the incremental risk or days with each 1 SD increase of corresponding metric. Generalized linear models (GLM) with log link were applied to the outcomes of post-operative complications (yes/no) and post-acute facility discharge (yes/no) and a GLM with identity link was applied to length of stay (days). Age, gender, surgery type, vascular comorbidity, surgery duration, and anesthesia type were adjusted in all models.
CHI3L1=Chitinase 3 Like1; CRP=C-reactive protein; IL6=interleukin-6; IQR=interquartile range; POD2= post-operative day 2; PREOP = pre-operation; RR= relative risk; SD=standard deviation.
To account for heterogeneity from the different types of surgeries in the cohort, a sensitivity analysis was conducted for the orthopedic surgery cases only (n=132). Overall, the results remain similar to the analysis with the full cohort (see Supplement, sTable 1 for details). Using this orthopedic only cohort, we performed an additional sensitivity analysis controlling for delirium, defined using daily interviews and medical record review(14) and the full Charlson co-morbidity index, obtained from medical record review (sTable 6). In this analysis, the associations of CHI3L1 and IL-6 with Post-operative Complications and Length of Stay remain statistically significant, with slightly lower effect sizes. However, the associations with Post-acute facility discharge are no longer significant; this is due to the addition of delirium to the model.
Discussion
Using SOMAscan, we identified 564 proteins (BH P-value <0.01) whose expression levels were consistently changed after surgery. Of these 564 proteins, 110 were significantly altered with at least a 1.5-fold-change in expression. Notably, most of these proteins, except CRP, were not discovered by our previous proteomics work using isobaric tags for relative and absolute quantitation-based (iTRAQ) tandem mass spectrometry(42).
SOMAscan provided a comprehensive portrayal of the physiological responses to non-cardiac surgery. Using systems biology approaches, proteins in multiple biological functions and several upstream regulators were found to be associated with surgery. The major theme emerging from this analysis is that upstream regulators involved in inflammatory processes such as cytokines, IL1B, IL6, and TNF, and transcriptional regulators, STAT3 and CEBPA, as well as various biological functions associated with the inflammatory response and immune cell regulation (i.e, leukocyte migration, cellular infiltration by leukocytes, cell movement of granulocytes, activation of leukocytes, chemotaxis of leukocytes, etc.) as well as vascular function demonstrated the greatest statistical significance (Figure 3, Supplemental Tables 2 and 3). Interestingly, one of the statistically most significant upstream regulators emerging from this analysis, IL-6, is itself induced by surgery, and many of the proteins impacted by surgery are likely downstream targets of IL-6.
Of the 110 proteins that we found were significantly dysregulated by surgery, 32 are under the regulation of the pro-inflammatory cytokine IL1B. These include IL-6, CRP, and CHI3L1. To confirm that SOMAscan accurately quantitates the expression of these proteins, and to further examine their link to surgery in a larger study cohort, surgery-related upregulation of CHI3L1, CRP, and IL-6 was validated by ELISAs or Luminex in n=126 (CHI3L1) and n=150 (CRP, IL6) patients. While increase in CRP(43, 44) and IL-6(45, 46) after surgery have been previously reported in multiple publications, the involvement of induction of CHI3L1 expression has only been reported in the context of minimally invasive colorectal cancer resection(47). Thus, this is the first demonstration that CHI3L1 is induced in response to other major surgical procedures, highlighting the importance of this protein in inflammatory processes resulting from surgery.
SOMAscan was able to detect levels for IL-6 at the pg/ml level, for CHI3L1 at the ng/ml level, and for CRP up to the mcg/ml level, thus demonstrating the sensitivity and dynamic range (>8 logs) of the SOMAscan technology. This broad dynamic range is a distinct advantage of SOMAscan for protein expression analysis compared to other technologies, as the low concentration of CHI3L1(ng/ml) and IL-6(pg/ml) might explain the lack of detection in prior studies.
The surgery-related proteins identified by SOMAscan are associated with clinically relevant surgical outcomes, providing further evidence of the clinical relevance of our findings. All three of the validated proteins were significantly upregulated by surgery such that PREOP values were much smaller than POD2. Both CHI3L1 and IL-6 (fold-change and difference) were significantly associated with all three outcome measures, while the difference in CRP was associated with discharge to post-acute facility. These findings serve to confirm and extend the prior literature, as IL-6 has been demonstrated to be elevated after surgery, and an association between elevated IL-6 levels and adverse outcomes has been demonstrated previously(48). Delirium, one such post-operative complication, is also associated with pro-inflammatory molecules IL-6 and CRP, suggesting that a preinflammatory state and heightened inflammatory response to surgery may be potential pathophysiologic mechanisms of delirium(15, 17, 49, 50).
As an indicator of microglial activation, CHI3L1 is considered to be a surrogate marker of neuroinflammation. CHI3L1 is expressed by astrocytes in Alzheimer’s disease and other neurodegenerative diseases(51), and can predict progression from prodromal mild cognitive impairment to AD dementia(52). Postoperative delirium and postoperative cognitive dysfunction are thought to result from microglial activation and neuroinflammation(53); consequently, the observation that CHI3L1 is increased after surgery and linked to clinical outcomes along with the reported association of CHI3L1 with AD supports the potential function of this protein as a neuro-inflammatory mediator as well. The association of CHI3L1 with neurocognitive outcomes after surgery was not examined in this paper, but such studies are warranted based on our findings.
Several limitations of this study are worthy of comment. The SAGES study was conducted in two academic hospitals within a single geographic region, thus, the results from this study may not be generalizable across all populations. Second, the sub-cohort used in this study was matched by delirium status (for a prior study) and therefore may not be fully representative of the full cohort, further limiting generalizability. Additionally, in our sensitivity analysis controlling delirium and the Charlson comorbidity index, (sTable 6), the associations of surgery-related proteins with Post-acute facility discharge were no longer significant. This represents mediation or effect modification by delirium, which should be explored in future studies. Third, while the SOMAscan platform measures over 1300 proteins, various proteins important for surgical effects may still be missed. Fourth, the proteins selected for ELISA or Luminex validation represent only a small subset of the proteins impacted by surgery and were chosen based on our prior work, systems biology analysis, and literature supporting a link with neuroinflammation. Fifth, correlation of CHI3L1, CRP and IL-6 with post-operative outcomes were not replicated in an independent sample. Lastly, IL-6 levels were validated using Luminex instead of ELISA, considered the “gold standard” for concentration measurement. In future work, a broader validation of surgery-related proteins should be considered, including proteins in non-inflammatory processes, changes in protein expression should all be externally validated using ELISA, and associations with post-operative outcome be replicated in an independent and diverse sample.
Conclusion
In summary, the SOMAscan platform was capable of identifying novel surgery-specific protein changes in a population of older adults undergoing major elective, non-cardiac surgery. Selected proteins validated in a larger cohort correlated with clinical outcomes, and hold promise as potential biomarkers with clinical utility. For example, CHI3L1, CRP, and IL-6 might be useful in predictive models and to monitor effectiveness of interventions aimed at improving outcomes after surgery. We observed upregulation of biomarkers over time, and further understanding of biomarker change over time will contribute towards our understanding of the physiological response to surgery and how surgical stress contributes to surgical outcomes, to ultimately inform innovative strategies to improve these outcomes, particularly for vulnerable older adults.
Supplementary Material
Sages Study Group.
[Presented in alphabetical order; individuals listed may be part of multiple groups, but are listed only once under major activity, listed in parentheses].
Overall Principal Investigator: Sharon K. Inouye, MD, MPH (HSL, BIDMC, HMS; Overall Principal Investigator, Administrative Core, Project 1).
Project and Core Leaders: David Alsop, PhD (BIDMC, HMS; Project 3 Leader); Richard Jones, ScD (Brown University; Data Core and Project 4 Leader); Thomas Travison, PhD (HSL, HMS; Data Core Leader); Edward R. Marcantonio, MD, SM (BIDMC, HMS; Overall Co- Principal Investigator, Epidemiology Core and Project 2 Leader).
Executive Committee: Steven Arnold, MD (MGH; Executive Committee); Zara Cooper, MD, MSc (HMS, BWH; Executive Committee); Bradford Dickerson, MD (MGH, HMS; Executive Committee); Tamara Fong, MD, PhD (HMS, HSL, BIDMC; Executive Committee); Eran Metzger, MD, (HMS, HSL, BIDMC; Executive Committee); Alvaro Pascual-Leone, MD (HMS, BIDMC; Executive Committee); Eva M. Schmitt, PhD (HSL; Overall Project Director); Mouhsin Shafi, MD (HMS, BIDM; Executive Committee).
Other Co-investigators: Michele Cavallari, MD, PhD (BWH; Co-investigator); Weiying Dai, PhD (BIDMC; Co-investigator); Simon T. Dillon, PhD (HMS, BIDMC; Co-investigator); Janet McElhaney, MD (UConn; Co-investigator); Charles Guttmann, MD (BWH, HMS; Co-investigator); Tammy Hshieh, MD (BWH; Co-investigator); George Kuchel, MD, FRCP, (UConn; Co-investigator); Towia Libermann, PhD (HMS, BIDMC; Co-investigator); Long Ngo, PhD (HMS, BIDMC; Co-investigator); Daniel Press, MD (HMS, BIDMC; Co-investigator); Jane Saczynski, PhD, (UMASS; Co-investigator); Sarinnapha Vasunilashorn, PhD (BIDMC; Co-investigator).
Clinical Consensus Panel: Margaret O’Connor, PhD (HMS, BIDMC; Clinical Consensus Panel); Eyal Kimchi, MD (MGH; Clinical Consensus Panel), Jason Strauss, MD (Cambridge Health Alliance; Clinical Consensus Panel); Bonnie Wong, PhD (BIDMC; Clinical Consensus Panel).
Surgical Leaders: Michael Belkin, MD (HMS, BWH; Surgical Leader); Douglas Ayres, MD (HMS, BIDMC; Surgical Leader); Mark Callery, MD (HMS, BIDMC; Surgical Leader); Frank Pomposelli, MD (HMS, BIDMC; Surgical Leader); John Wright, MD (HMS, BWH; Surgical Leader); Marc Schermerhorn, MD (HMS, BIDMC; Surgical Leader).
Epidemiology Core: Tatiana Abrantes (HSL; Epidemiology Core); Asha Albuquerque (HSL; Epidemiology Core); Sylvie Bertrand (HSL; Epidemiology Core); Amanda Brown M.Ed. (HSL; Epidemiology Core); Amy Callahan (BIDMC; Epidemiology Core), Madeline D’Aquila (HSL; Epidemiology Core); Sarah Dowal, MSW, LCSW, MPH (HSL; Epidemiology Core); Meaghan Fox (BIDMC; Epidemiology Core); Jacqueline Gallagher, MS (BIDMC; Epidemiology Core); Rebecca Anna Gersten; Ariel Hodara (BIDMC; Epidemiology Core); Ben Helfand, MPH (BIDMC; Epidemiology Core); Jennifer Inloes (HSL; Epidemiology Core); Jennifer Kettell (HSL; Epidemiology Core); Aleksandra Kuczmarska (BIDMC; Epidemiology Core); Jacqueline Nee (HSL; Epidemiology Core); Emese Nemeth (HSL; Epidemiology Core); Lisa Ochsner (BWH; Epidemiology Core); Kerry Palihnich (BIDMC; Epidemiology Core); Katelyn Parisi (HSL; Epidemiology Core); Margaret Puelle (HSL; Epidemiology Core); Sarah Rastegar, MA (HSL; Epidemiology Core); Margaret Vella (HSL; Epidemiology Core), Guoquan Xu, MD, PhD (HSL; Epidemiology Core).
Data Management and Statistical Analysis Core: Margaret Bryan (HSL; Data Management and Statistical Analysis Core); Jamey Guess (BIDMC; Data Management and Statistical Analysis Core); Dee Enghorn (HSL; Data Management and Statistical Analysis Core); Alden Gross, PhD, MHS (John Hopkins School of Medicine; Data Management and Statistical Analysis Core); Yun Gou, MA (HSL; Data Management and Statistical Analysis Core); Daniel Habtemariam (HSL; Data Management and Statistical Analysis Core); Ilean Isaza, PhD (HSL; Data Management and Statistical Analysis Core); Cyrus Kosar, MA (HSL; Data Management and Statistical Analysis Core); Christopher Rockett, PhD (HSL; Data Management and Statistical Analysis Core); Douglas Tommet, MPH (Brown University; Data Management and Statistical Analysis Core).
Fiscal Management Committee: Ted Gruen (HSL; Fiscal Management Committee); Meg Ross (HSL; Fiscal Management Committee); Katherine Tasker (HSL; Chair, Fiscal Management Committee).
Scientific Advisory Board: James Gee, PhD (University of Pennsylvania; Scientific Advisory Board); Ann Kolanowski, PhD, RN, FAAN (Pennsylvania State University; Scientific Advisory Board); Margaret Pisani, MD, MPH (Yale University; Scientific Advisory Board); Sophia de Rooij, MD, PhD (Academic Medical Center, Amsterdam; Scientific Advisory Board); Selwyn Rogers, MD, MPH (Temple University; Scientific Advisory Board), Stephanie Studenski, MD (NIA; Chair, Scientific Advisory Board); Yaakov Stern, PhD (Columbia University; Scientific Advisory Board); Anthony Whittemore, MD (BWH, HMS; Scientific Advisory Board).
Internal Advisory Board: Gary Gottlieb, MD, MBA (BWH, MGH, HMS; Internal Advisory Board); John Orav, PhD (BWH, HMS; Internal Advisory Board); Reisa Sperling, MD, MMSc (BWH, HMS; Internal Advisory Board).
Acknowledgments
The authors gratefully acknowledge the contributions of the patients, family members, nurses, physicians, staff members, and members of the Executive Committee who participated in the Successful Aging after Elective Surgery (SAGES) Study. This work is dedicated to the memory of Joshua Bryan Inouye Helfand.
Funding Sources: This research was supported by National Institute on Aging grants P01AG031720, R01AG051658(TL/ERM), K24AG035075 (ERM), K07AG041835 (SKI), R24AG054259 (SKI) and R21AG057955(TGF), and NCATS grant 3 UL1 TR001102-04S2(TGF), and a Marcus Applebaum Pilot grant from the family of Beth and Richard Marcus (TGF), National Center for Complementary and Integrative Health T32AT000051(NC), and the Alzheimer’s Association AARF-18-560786 (SV).
Abbreviations
- BIDMC
Beth Israel Deaconess Medical Center
- BWH
Brigham and Women’s Hospital
- HMS
Harvard Medical School
- HSL
Hebrew SeniorLife
- MGH
Massachusetts General Hospital
- UCONN
University of Connecticut Health Center
Literature Cited
- 1.Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory Surgery Data From Hospitals and Ambulatory Surgery Centers: United States, 2010. Natl Health Stat Report. 2017(102):1–15. [PubMed] [Google Scholar]
- 2.Gajdos C, Kile D, Hawn MT, Finlayson E, Henderson WG, Robinson TN . Advancing age and 30-day adverse outcomes after nonemergent general surgeries. J Am Geriatr Soc. 2013;61(9):1608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005;53(3):424–9. [DOI] [PubMed] [Google Scholar]
- 4.Manku K, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. II. Long-term quality of life. Anesth Analg. 2003;96(2):590–4, table of contents. [DOI] [PubMed] [Google Scholar]
- 5.Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127(2):117–26. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JL, Harken AH. Surgical genomics is here. Surgery. 2003;133(2):127–32. [DOI] [PubMed] [Google Scholar]
- 7.Gaudilliere B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, et al. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med. 2014;6(255):255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992;79(8):757–60. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Li SZ, Feng CS, Qu XD, Wang H, Zhang XN, et al. Serum proteomics of early postoperative cognitive dysfunction in elderly patients. Chin Med J (Engl). 2012;125(14):2455–61. [PubMed] [Google Scholar]
- 10.Banfi C, Parolari A, Brioschi M, Barcella S, Loardi C, Centenaro C, et al. Proteomic analysis of plasma from patients undergoing coronary artery bypass grafting reveals a protease/antiprotease imbalance in favor of the serpin alpha1-antichymotrypsin. J Proteome Res. 2010;9(5):2347–57. [DOI] [PubMed] [Google Scholar]
- 11.Clendenen N, Tollefson A, Dzieciatkowska M, Cambiaghi A, Ferrario M, Kroehl M, et al. Correlation of pre-operative plasma protein concentrations in cardiac surgery patients with bleeding outcomes using a targeted quantitative proteomics approach. Proteomics Clin Appl. 2017;11(7–8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-Based Multiplexed Proteomic Technology for Biomarker Discovery. PLoS ONE. 2010;5(12):e15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt EM, Marcantonio ER, Alsop DC, Jones RN, Rogers SO Jr., Fong TG, et al. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13(9):818 e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt EM, Saczynski JS, Kosar CM, Jones RN, Alsop DC, Fong TG, et al. The Successful Aging after Elective Surgery (SAGES) Study: Cohort Description and Data Quality Procedures. J Am Geriatr Soc. 2015;63(12):2463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillon ST, Vasunilashorn SM, Ngo L, Otu HH, Inouye SK, Jones RN, et al. Higher C-Reactive Protein Levels Predict Postoperative Delirium in Older Patients Undergoing Major Elective Surgery: A Longitudinal Nested Case-Control Study. Biol Psychiatry. 2017;81(2):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngo LH, Inouye SK, Jones RN, Travison TG, Libermann TA, Dillon ST, et al. Methodologic considerations in the design and analysis of nested case-control studies: association between cytokines and postoperative delirium. BMC Med Res Methodol. 2017;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasunilashorn SM, Ngo L, Inouye SK, Libermann TA, Jones RN, Alsop DC, et al. Cytokines and Postoperative Delirium in Older Patients Undergoing Major Elective Surgery. J Gerontol A Biol Sci Med Sci. 2015;70(10):1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Groote MA, Nahid P, Jarlsberg L, Johnson JL, Weiner M, Muzanyi G, et al. Elucidating novel serum biomarkers associated with pulmonary tuberculosis treatment. PLoS One. 2013;8(4):e61002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold L, Walker JJ, Wilcox SK, Williams S. Advances in human proteomics at high scale with the SOMAscan proteomics platform. N Biotechnol. 2012;29(5):543–9. [DOI] [PubMed] [Google Scholar]
- 20.Kiddle SJ, Sattlecker M, Proitsi P, Simmons A, Westman E, Bazenet C, et al. Candidate blood proteome markers of Alzheimer’s disease onset and progression: a systematic review and replication study. J Alzheimers Dis. 2014;38(3):515–31. [DOI] [PubMed] [Google Scholar]
- 21.Lollo B, Steele F, Gold L. Beyond antibodies: new affinity reagents to unlock the proteome. Proteomics. 2014;14(6):638–44. [DOI] [PubMed] [Google Scholar]
- 22.Mehan MR, Ayers D, Thirstrup D, Xiong W, Ostroff RM, Brody EN, et al. Protein signature of lung cancer tissues. PLoS One. 2012;7(4):e35157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menni C, Kiddle SJ, Mangino M, Vinuela A, Psatha M, Steves C, et al. Circulating Proteomic Signatures of Chronological Age. J Gerontol A Biol Sci Med Sci. 2015;70(7):809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webber J, Stone TC, Katilius E, Smith BC, Gordon B, Mason MD, et al. Proteomics analysis of cancer exosomes using a novel modified aptamer-based array (SOMAscan) platform. Mol Cell Proteomics. 2014;13(4):1050–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. [DOI] [PubMed] [Google Scholar]
- 26.Thakur PB, Vaughn-Diaz VL, Greenwald JW, Gross DC. Characterization of Five ECF Sigma Factors in the Genome of Pseudomonas syringae pv. syringae B728a. PLOS ONE. 2013;8(3):e58846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. [DOI] [PubMed] [Google Scholar]
- 28.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellwig K, Kvartsberg H, Portelius E, Andreasson U, Oberstein TJ, Lewczuk P, et al. Neurogranin and YKL-40: independent markers of synaptic degeneration and neuroinflammation in Alzheimer’s disease. Alzheimers Res Ther. 2015;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen HT, Zheng JM, Zhang YZ, Yang M, Wang YL, Man XH, et al. Overexpression of YKL-40 Predicts Poor Prognosis in Patients Undergoing Curative Resection of Pancreatic Cancer. Pancreas. 2017;46(3):323–34. [DOI] [PubMed] [Google Scholar]
- 31.Malyszko J, Koc-Zorawska E, Malyszko J. YKL-40, a marker of cardiovascular disease and endothelial dysfunction, in kidney transplant recipients. Transplant Proc. 2014;46(8):2651–3. [DOI] [PubMed] [Google Scholar]
- 32.Racine AM, Fong TG, Gou Y, Travison TG, Tommet D, Erickson K, et al. Clinical outcomes in older surgical patients with mild cognitive impairment. Alzheimers Dement. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, et al. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg. 2015;150(12):1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sneath PHA, Sokal RR. Numerical Taxonomy. The Principles and Practice of Numerical Classification. Sneath Peter H. A., Sokal Robert R. The Quarterly Review of Biology. 1975;50(4):525–6. [Google Scholar]
- 35.Pearson KL III. On lines and planes of closest fit to systems of points in space. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 1901;2(11):559–72. [Google Scholar]
- 36.Hsu C-W, Lin C-J. A comparison of methods for multiclass support vector machines. IEEE Transactions on Neural Networks. 2002;13(2):415–25. [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. [Google Scholar]
- 38.Hoaglin D Understanding Robust and Exploratory Data Analysis: Taylor & Francis; 2001. [Google Scholar]
- 39.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–3. [DOI] [PubMed] [Google Scholar]
- 40.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 41.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 42.Vasunilashorn SM, Ngo LH, Chan NY, Zhou W, Dillon ST, Otu HH, et al. Development of a Dynamic Multi-Protein Signature of Postoperative Delirium. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santonocito C, De Loecker I, Donadello K, Moussa MD, Markowicz S, Gullo A, et al. C-reactive protein kinetics after major surgery. Anesth Analg. 2014;119(3):624–9. [DOI] [PubMed] [Google Scholar]
- 44.Larsson S, Thelander U, Friberg S. C-reactive protein (CRP) levels after elective orthopedic surgery. Clin Orthop Relat Res. 1992(275):237–42. [PubMed] [Google Scholar]
- 45.Sakamoto K, Arakawa H, Mita S, Ishiko T, Ikei S, Egami H, et al. Elevation of circulating interleukin 6 after surgery: factors influencing the serum level. Cytokine. 1994;6(2):181–6. [DOI] [PubMed] [Google Scholar]
- 46.Kragsbjerg P, Holmberg H, Vikerfors T. Serum concentrations of interleukin-6, tumour necrosis factor-alpha, and C-reactive protein in patients undergoing major operations. Eur J Surg. 1995;161(1):17–22. [PubMed] [Google Scholar]
- 47.Shantha Kumara HMC, Gaita D, Miyagaki H, Yan X, Hearth SAC, Njoh L, et al. Plasma chitinase 3-like 1 is persistently elevated during first month after minimally invasive colorectal cancer resection. World Journal of Gastrointestinal Oncology. 2016;8(8):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med. 2011;26(2):73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Munster BC, Bisschop PH, Zwinderman AH, Korevaar JC, Endert E, Wiersinga WJ, et al. Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn. 2010;74(1):18–23. [DOI] [PubMed] [Google Scholar]
- 50.Vasunilashorn SM, Dillon ST, Inouye SK, Ngo LH, Fong TG, Jones RN, et al. High C-Reactive Protein Predicts Delirium Incidence, Duration, and Feature Severity After Major Noncardiac Surgery. J Am Geriatr Soc. 2017;65(8):e109–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Querol-Vilaseca M, Colom-Cadena M, Pegueroles J, San Martin-Paniello C, Clarimon J, Belbin O, et al. YKL-40 (Chitinase 3-like I) is expressed in a subset of astrocytes in Alzheimer’s disease and other tauopathies. J Neuroinflammation. 2017;14(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baldacci F, Lista S, Cavedo E, Bonuccelli U, Hampel H. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer’s disease and other neurodegenerative diseases. Expert Review of Proteomics. 2017;14(4):285–99. [DOI] [PubMed] [Google Scholar]
- 53.Cascella M, Muzio MR, Bimonte S, Cuomo A, Jakobsson JG. Postoperative delirium and postoperative cognitive dysfunction: updates in pathophysiology, potential translational approaches to clinical practice and further research perspectives. Minerva Anestesiol. 2018;84(2):246–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.