Abstract
Purpose:
Weight cycling, defined as intentional weight loss followed by unintentional weight regain, may attenuate the benefit of intentional weight loss on endometrial cancer risk. We summarized the literature on intentional weight loss, weight cycling after intentional weight loss, bariatric surgery, and endometrial cancer risk.
Methods:
A systematic search was conducted using MEDLINE, Embase, and Cochrane Central Register of Controlled Trials databases published between January 2000 and November 2018. We followed Preferred Reporting Items of Systematic Reviews and Meta-analysis guidelines. We qualitatively summarized studies related to intentional weight loss and weight cycling due to the inconsistent definition and quantitatively summarized studies when bariatric surgery was the mechanism of intentional weight loss.
Results:
A total of 127 full-text articles were reviewed, and 13 were included (bariatric surgery n=7, self-reported intentional weight loss n=2, self-reported weight cycling n=4). Qualitative synthesis suggested that compared to stable weight, self-reported intentional weight loss was associated with lower endometrial cancer risk (RR range=0.61–0.96), whereas self-reported weight cycling was associated with higher endometrial cancer risk (OR range=1.07–2.33). The meta-analysis yielded a 59% lower risk of endometrial cancer following bariatric surgery (OR=0.41, 95% CI=0.22, 0.74).
Conclusions:
Our findings support the notion that intentional weight loss and maintenance of a stable, healthy weight can lower endometrial cancer risk. Strategies to improve awareness and maintenance of weight loss among women with obesity are needed to reduce endometrial cancer risk.
Keywords: intentional weight loss, weight cycling, endometrial cancer risk, systematic review and meta-analysis
INTRODUCTION
Obesity is an established risk factor for endometrial cancer, conferring a two to five times higher risk compared to healthy weight women[1,2]. As the prevalence of obesity has risen over the past four decades, parallel increases in endometrial cancer incidence have occurred[3]. Empirical data suggest an inverse association between weight loss and endometrial cancer risk[4]. However, prior studies have typically not distinguished between intentional vs. unintentional weight loss. Unintentional weight loss is associated with increased morbidity and could be a consequence of malignancy[5,6], while intentional weight loss, as a result of behavioral changes (e.g., a calorie-restricted diet with physical activity) or bariatric surgery, could improve body composition, improve metabolic and hormonal regulations, and favorably affect biological pathways, resulting in lower risk of endometrial cancer development[7–10].
The usual trajectory of intentional weight loss is followed by unintentional weight re-gain[11,12]. Weight cycling occurs when weight re-gain follows intentional weight loss in repeated cycles. In addition, weight cycling is more likely to result in redistribution of body fat to upper body subcutaneous adipose tissue and visceral fat[13], which may attenuate the benefit of intentional weight loss and increase endometrial cancer risk. Bariatric surgery, an effective weight loss treatment, results in substantial weight loss that is sustainable for 10–15 years[14,15]. Moreover, bariatric surgery is associated with a 46–60% lower endometrial cancer risk[16–18]. The rapid changes in body composition and extensive weight loss from bariatric surgery differ from the gradual changes induced by behavioral approaches for weight loss. On average, behavioral weight loss interventions result in 7–15% of body weight loss within 1–2 years, while bariatric surgery results in 15–35% of weight loss within 12 months[15,19]. The substantial weight loss within a short period can provide health benefits to individuals with obesity, especially patients with morbid obesity who experience difficulties in losing weight through behavioral interventions.
Previous reviews reported the association of bariatric surgery and lower risk of endometrial cancer[18,20]. However, no systematic review or meta-analysis has summarized data on self-reported intentional weight loss and self-reported weight cycling after intentional weight loss in relation to endometrial cancer risk. We summarized this literature to assess the hypotheses that intentional weight loss is associated with lower endometrial cancer risk while weight cycling after intentional weight loss is associated with increased endometrial cancer risk. We also provide an updated synthesis of the literature on bariatric surgery and endometrial cancer risk.
MATERIALS AND METHODS
Search strategy
This study was conducted following the Preferred Reporting Items of Systematic Reviews and Meta-analyses (PRISMA) guidelines[21]. Literature searches were conducted to evaluate the impact of 1) self-reported intentional weight loss, 2) self-reported weight cycling followed by intentional weight loss, and 3) bariatric surgery on endometrial cancer risk. Searches were carried out from January 2000 to November 2018 using MEDLINE via Ovid, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases. We performed a topic-specific search by combining Medical Subject Headings (MeSH) and non-MeSH keyword terms. Our search strategy captured a comprehensive set of key terms and Medical Subject Headings pertaining to the following subjects: endometrial cancer (including uterine, neoplasm, carcinoma, malignancy, adenocarcinoma etc.), intentional weight loss strategies (including various bariatric surgeries, behavioral strategies such as diet, caloric restriction, physical activity/exercise), and weight management/control. A list of search terms used for the three databases (MEDLINE, Embase, and CENTRAL) is included in the supplementary (S1 Figure a, b, c).
Criteria for selection of studies for this review
We included all published randomized clinical trials, non-randomized trials, and observational studies evaluating any intentional weight loss (bariatric surgery or self-reported intentional weight loss) and endometrial cancer risk. Reviews, case reports, letters, commentaries, editorials, unpublished studies, or any studies not published in English were not included.
Eligible studies were those that included female participants aged 18 years or older and those that excluded participants who had preexisting endometrial or uterine cancer before intentional weight loss. Our original plan was to classify intentional weight loss as a result of surgical procedures (bariatric surgery) or behavioral interventions (caloric restricted diet with or without increased physical activity). However, the lack of long-term follow-up after behavioral weight loss interventions hindered our ability to assess the risk of developing endometrial cancer. Therefore, this systematic review and meta-analysis evaluated the relationship between self-reported intentional weight loss, self-reported weight cycling after intentional weight loss, bariatric surgery, and endometrial cancer risk. Information on self-reported intentional weight loss and weight cycling were collected through self-reported questionnaire. Bariatric procedures included Roux-en-Y gastric bypass, gastric banding, vertical banded gastroplasty, vertical sleeve gastrectomy, jejunoileal bypass, and biliopancreatic diversion. We excluded studies if: 1)the weight loss was through pharmaceutical interventions; 2)the study endpoint was not a diagnosis of endometrial cancer (e.g. endometrial cancer-related biomarkers); 3) the diagnosis of endometrial cancer was prior to or during bariatric surgery or weight loss; and 4) did not include a control group. The primary outcomes of incidence, risk ratio, odds ratio, hazard ratio, or risk difference for endometrial cancer were assessed for each study.
Data extraction
Titles and abstracts of the initial search were assessed for eligibility by two independent investigators (XZ and JR). Duplicates were removed, and articles that did not meet the inclusion criteria were excluded. Full-text articles were assessed for eligibility by XZ and JR. For studies examining the association between self-reported weight cycling, self-reported intentional weight loss and endometrial cancer risk, we extracted author’s last name, year of study, study type, population, study design, recruitment year, endometrial cancer diagnosis year, weight status measurement, weight cycling/weight loss definition, number of endometrial cancer cases, total sample size, comparison group and related effect estimate (e.g. odds ratio, relative risk, hazard ratio) with confidence intervals, and adjusted covariates (if any). For studies examining the association between bariatric surgery and endometrial cancer risk, we extracted author’s last name, year of study, study type (e.g., case-control, cohort), population, study period, comparison groups, number of cases and controls for each group, total sample size, average age of participants, and included surgical procedures.
The quality and risk of bias of each study were assessed using the Newcastle-Ottawa quality assessment scale for cohort or case-control studies[22]. Study quality assessments were conducted independently by XZ and JR. Any discrepancies regarding inclusion, exclusion and risk assessment were resolved by consensus (XZ, JR, and ASF).
Data analysis
Eligible studies were classified into two groups: self-reported intentional weight loss/weight cycling or bariatric surgery. Descriptive characteristics were reported for all studies. A qualitative synthesis was conducted for studies examining self-reported intentional weight loss, weight cycling, and endometrial cancer risk. In the meta-analysis of bariatric surgery studies, we calculated the pooled effect estimate using a random effect model due to the high likelihood of between-group heterogeneity. The heterogeneity of effect size estimates across studies was quantified using the I2 statistic, and the publication bias was assessed using the Egger’s and Begg’s tests[23]. All statistical analyses were completed using Stata MP Version 14.2 (StataCorp, College Station, TX).
RESULTS
Study selection
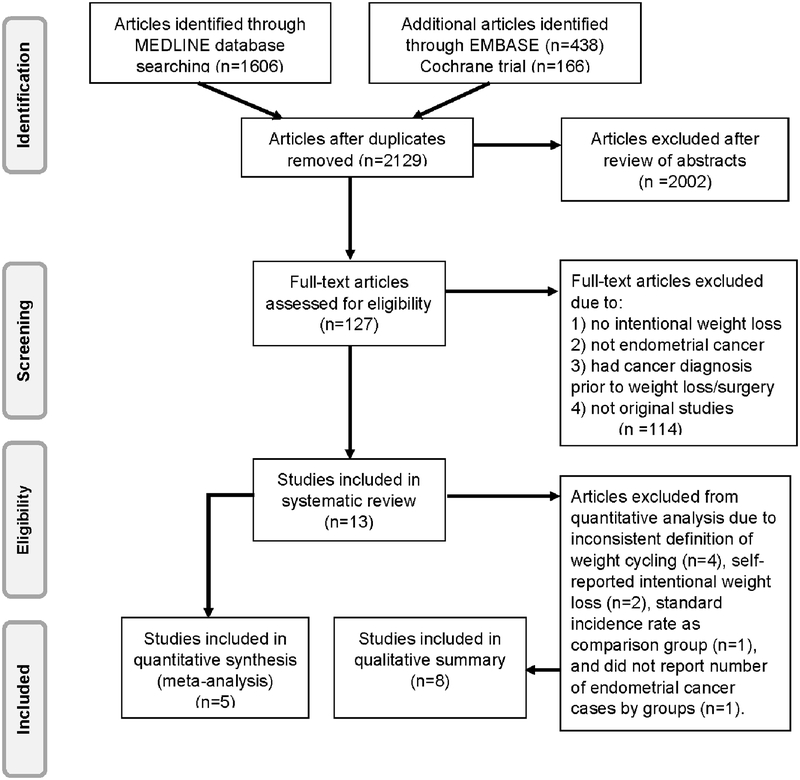
A total of 2,129 articles were identified through MEDLINE, Embase and Cochrane trial databases after duplicates were removed. After reviewing titles and abstracts, 127 full-text articles were assessed for eligibility, of which 114 were excluded for not meeting the eligibility criteria, leaving 13 eligible studies for review. Four articles examined self-reported weight cycling and endometrial cancer risk[24–27], two articles examined self-reported intentional weight loss and endometrial cancer risk[28,29], and seven articles examined bariatric surgery and endometrial cancer risk[14,17,30–34] (Figure 1). Among the 13 included articles, endometrial cancer cases were identified through state or national cancer registries, or the combination of self-report with linkage to medical records or cancer registries.
Figure 1.
PRISMA diagram of study identification and selection.
Study characteristics
Self-reported intentional weight loss and endometrial cancer risk were examined in two prospective cohort studies with follow-up time ranging between 14 and 22 years[28,29]. A total of 58,500 participants were included in these studies, among whom 708 developed endometrial cancer. Prior weight loss was classified as intentional or unintentional weight loss and assessed by a follow-up questionnaire administered six years after baseline[28] or measured using a standardized approach at clinic visits at baseline and three years after enrollment[29]. The cut point denoting self-reported intentional weight loss differed between the two studies, with Luo et al. using 10 pounds and Parker et al. using 20 pounds (Table 1a)[28,29].
Table 1a.
Study descriptions of self-reported intentional weight loss, weight cycling, and the risk of endometrial cancer
| Author, year | Study type | Population | Study design | Recruitment year | Endometrial cancer diagnosis year | Developed endometrial cancer | Total N | Weight status measures | Weight cycling/weight loss definition |
|---|---|---|---|---|---|---|---|---|---|
| Self-reported intentional weight loss | |||||||||
| Luo, 2017 | prospective cohort | WHI (U.S) | WHI cohort without history of cancer, did not have hysterectomy, did not have endometrial cancer diagnosis between baseline and year-3 visit cases: all incident endometrial cancer occurrences diagnosed after the year-3 visit | 1993–1998 | from the year-3 visit to the first of either an endometrial cancer diagnosis, a date of hysterectomy, a date of death, loss to follow-up, or end of current follow-up (9/30/2015) | 566 | 36793 | Measured at baseline and year 3 follow-up | stable weight: within ±10lbs weight gain (≥10 lbs) weight loss (≥10 lbs) intentional; unintentional |
| Parker, 2003 | prospective cohort | Iowa Women’s Health study (U.S) | Iowa Women’s Health Study randomly selected women between the age 55–69 in January 1986, had a valid driver’s license in 1985 cases: endometrial cancer was identified by computer linkage with state health registry of Iowa | 1986 | 1993–2000 | 142 | 21707 | the 1992 questionnaire | never lost ≥20lbs, intentional loss, unintentional loss, ≥ intentional plus ≥ unintentional |
| Weight Cycling | |||||||||
| Welti, 2017 | cohort | WHI observation (U.S) | WHI observational study, postmenopausal (50–79 yrs), no missing information on weight change, baseline covariates, or reported history of cancers at baseline, or reported hysterectomy at baseline | 1993–1998 | 1994–2014 | 788 | 47897 | baseline questionnaire | weight cycling: intentional weight loss and subsequent regain; stayed the same (<10lbs); steady gained; lost weight as an adult and kept it off; weight went up and down again ≥10lbs (1–3×, 4–6×, 7–10×, >10×) |
| Nagle, 2013 | population-based case-control | Australia | cases: newly diagnosed, histologically confirmed, epithelial endometrial cancer diagnosed controls: randomly selected from voter system matched by residence state and age + participants from another study | 2005–2007 | 2005–2007 | 1398 | 2936 | telephone interview/ self-administered questionnaire | weight cycling: never intentionally lost > 9kg and regained within 12 month; lost >9 kg and kept it off; >1× intentionally lost >9 kg and regain within 12 months |
| Stevens, 2012 | prospective cohort | ACS cancer prevention study II nutrition cohort (U.S) | CPS-II Nutrition Cohort (50–74 yrs) | 1992/1993 | follow-up started in 1997 and every 2 years until the date of endometrial cancer diagnosis, death, date of the last returned survey, or June 30, 2007, whichever came first | 559 | 38148 | baseline questionnaire | weight cycling was asked “how many times in your life have you purposefully …” weight cycling: # of lost ≥10lbs and regained: non-cycler, 1–4 times, 5–9 times ≥10 times |
| Trentham-Dietz, 2006 | population-based case-control | Wisconsin (U.S) | cases: Wisconsin cancer registry; controls: random selected from population lists of drivers and Medicare beneficiaries, matched by age distribution | 1992–1995 | cases: 1/1/1991–12/31/1994 | 740 | 3082 | structured telephone interview | weight cycling: intentionally lost >20lbs and regain more than half within 1 yr, frequency, age at it last happened |
Of four studies on self-reported weight cycling and endometrial cancer risk, two were prospective cohort studies with follow-up ranging from 15 to 21 years[25,27] and two were population-based case-control studies[24,26]. Data regarding weight cycling were collected from telephone or self-administered questionnaire. The time interval for weight regain varied: two studies examined regain within 12 months[24,26], one study defined the regain occurring during adulthood[27], and one study did not specify the time interval of the regain[25] (Table 1a). Among 92,063 participants involved in these studies, 3,485 cases of endometrial cancer were identified. One study did not report the number of endometrial cancer cases by weight cycling status[27]. Among weight cyclers (n=21,868), 831 (3.8%) endometrial cancer cases were identified, compared to 1,698 (8%) endometrial cancer cases identified among non-weight cyclers (n=21,225).
Of seven studies that evaluated the association between bariatric surgery and endometrial cancer risk, six were retrospective cohort studies with the recruitment period ranging between 4 and 26 years[17,30–34] and one was a prospective cohort study with follow-up spanning 26 years[14]. Four studies used frequency or propensity score matching of bariatric surgery patients to obese patients without bariatric surgery on baseline body weight or body mass index (BMI) distribution[14,17,31,34], while three did not employ matching[30,32,33]. There were a total of 7,455,757 participants involved in these studies comprising mainly patients with morbid obesity, ranging in age from 39 to 52. In these studies, a total of 44,404 cases of endometrial cancer were identified. Among women who did not have bariatric surgery (n=7,348,127), 43,541 (0.6%) cancers were identified, compared to 487 (1.9%) cases among women who underwent bariatric surgery (n=25,619). The bariatric surgical procedures were collected from surgical registry, patient registry, medical records of inpatient admissions, and health insurance database (Table 1b).
Table 1b.
Study descriptions of bariatric surgery and endometrial cancer risk
| Author, year | Study type | Population | Study period | Comparison | Developed endometrial cancer | Did not develop endometrial cancer | Total N | Age (mean) |
Surgical procedure type |
|---|---|---|---|---|---|---|---|---|---|
| Mackenzie, 2018 | retrospective cohort | Hospital Episode Statistics (England) | 1997–2012 | bariatric surgery vs. propensity-matched obese individual without surgery | 18 | 7051 | 7069 | 42 | gastric bypass, gastric banding, and sleeve gastrectomy |
| 84 | 6985 | 7069 | 42 | ||||||
| Anveden, 2017 | prospective cohort | Swedish Obese Subjects Study (Sweden) | 1987–2013 | bariatric surgery vs. | 28 | 1392 | 1420 | 47.2 | gastric banding, vertical banded gastroplasty, gastric bypass |
| conventional obesity treatment | 48 | 1399 | 1447 | 48.8 | |||||
| Schauer, 2017 | retrospective cohort | Kaiser Permanente | 2004–2014 | bariatric surgery vs. | 322 | 71568 | 17998 | 45.0 | gastric bypass, sleeve gastrectomy, laproscopic adjustable band, biliopancreatic diversion vertical gastric banding |
| obese individual without bariatric surgery | 53892 | 45.1 | |||||||
| Ward, 2014 | retrospective cohort | University Health System Consortium (U.S) | 2009–2013 | bariatric surgery vs. | 424 | 10373 | 10797 | 52.6 | history of prior bariatric surgery |
| obese women with no history of bariatric surgery | 43291 | 7284770 | 7328061 | ||||||
| Ostlund, 2010 | retrospective cohort | Swedish Patient Register (Sweden) | 1980–2006 | bariatric surgery vs. standardized incidence rate in general population | 54 | 10067 | 10121 | NA | gastric banding, vertical banding gastroplasty, or gastric bypass |
| Adams, 2009 | retrospective cohort | Utah (U.S) | 1984–2007 | bariatric surgery vs. | 14 | 5640 | 5654 | 38.9 | roux-en-y gastric bypass |
| severe obese adults | 98 | 7774 | 7872 | 39.1 | |||||
| Christou, 2008 | retrospective cohort | RAMQ (Canada, McGill University) | 1986–2002 | bariatric surgery vs. | 3 | 676 | 679 | 45.1 | roux-en-y isolated gastric bypass, vertical banded gastroplasty, roux-en-y gastric bypass, laparoscopic roux-en-y isolated gastric bypass |
| morbidly obese individuals with no history of bariatric surgery | 20 | 3658 | 3678 | 46.7 |
Synthesis of results
We used a qualitative synthesis for the two studies on self-reported intentional weight loss and endometrial cancer risk, due to the inconsistent cut points of weight loss and stable weight. Compared to those with stable weight, women reporting intentional weight loss of ten or more pounds were less likely to have a diagnosis of endometrial cancer. One study showed a 39% reduced risk (HR=0.61, 95% CI=0.40–0.92) associated with weight loss of 10 or more pounds within the last two years[29] and one showed a non-significant 4% reduced risk (RR=0.96, 95% CI=0.61–1.52)[28] associated with self-reported weight loss of 20 pounds at any point during adulthood (Table 2).
Table 2.
Qualitative synthesis: self-reported intentional weight loss, weight cycling, and endometrial cancer risk
| Author, year | Comparison | Effect estimates | Adjustment |
|---|---|---|---|
| Self-reported intentional weight loss | |||
| Luo, 2017 | weight gain vs. stable weight | HR=1.26 (1.00–1.57) | age at enrollment, race/ethnicity, education, smoking pack-years, recreational physical activity, history of hormone therapy use, parity, age of menarche, age at first birth, family history of endometrial cancer, and BMI |
| weight loss vs. stable weight | HR=0.70 (0.51–0.98) | ||
| intentional weight loss vs. stable weight | HR=0.61 (0.40–0.92) | ||
| unintentional weight loss vs. stable weight | HR=0.91 (0.56–1.46) | ||
| Parker, 2003 | weight loss ≥20lbs intentional vs. never lost ≥20lbs | RR=0.96 (0.61–1.52) | age, BMI, BMI2, waist-to-hip ratio, physical activity, education, marital status, smoking status, pack-years cigarettes, current estrogen use, alcohol use, parity, and multivitamin use |
| weight loss ≥20lbs unintentional vs. never lost ≥20lbs | RR=1.29 (0.81–2.05) | ||
| weight loss ≥20lbs intentional + ≥20lbs unintentional vs. never lost ≥20lbs | RR=1.38 (0.85–2.25) | ||
| Weight cycling | |||
| Welti, 2017 | weight gain vs. stable weight | RR=1.16 (0.95–1.42) | age, race/ethnicity, education, income, smoking status, alcohol intake, physical activity, hormone therapy use, health eating index, BMI at baseline |
| weight loss vs. stable weight | RR=1.02 (0.62–1.68) | ||
| weight cycle vs. stable weight | RR=1.23 (1.01–1.49) | ||
| Nagle, 2013 | lost and maintained >9kg vs. no intentional weight loss followed by regain | OR=1.02 (0.71–1.45) | age, age at menarche, parity, duration of oral contraceptive pill use, hormone replacement therapy use>3month, smoking status, diabetes, recent BMI |
| lost and gained >9kg 1+times vs. no intentional weight loss followed by regain | OR=2.33 (1.58–3.44) | ||
| Stevens, 2012 | 1–4 times weight cycles vs. non-cycler | RR=1.07 (0.87–1.32) | alcohol use, smoking, physical activity, family history of EC, diabetes, number of live birth/age at first birth, age at menarche, age at menopause, hormone replacement therapy use, oral contraceptive use, and total energy intake, recent BMI |
| 5–9 times weight cycles vs. non-cycler | RR=1.14 (0.85–1.53) | ||
| 10+ times weight cycles vs. non-cycler | RR=1.05 (0.77–1.42) | ||
| Trentham-Dietz, 2006 | ever weight cycling vs. never | OR=1.27 (1.00–1.61) | age, age at menarche, parity, menopausal status, age at menopause, smoking, postmenopausal hormone use, recent BMI, recent physical activity, and diabetes |
| once weight cycling vs. never | OR=1.20 (0.84–1.71) | ||
| 2–4 times weight cycling vs. never | OR=1.36 (0.98–1.88) | ||
| 5+ times weight cycling vs. never | OR=1.26 (0.80–1.97) | ||
HR: Hazard Ratio; RR: Risk Ratio; OR: Odds Ratio; BMI: Body Mass Index
Due to the inconsistent definition of the weight cycling cut point, a qualitative synthesis was used for the four studies on self-reported weight cycling and endometrial cancer risk. Overall, the findings suggest that women who reported any weight cycling were more likely to have a diagnosis of endometrial cancer. Three studies showed weight cycling was significantly associated with 1.23–2.33 times increased endometrial cancer risk[24,26,27]; while one study showed a similar magnitude of association without statistical significance[25] (Table 2).
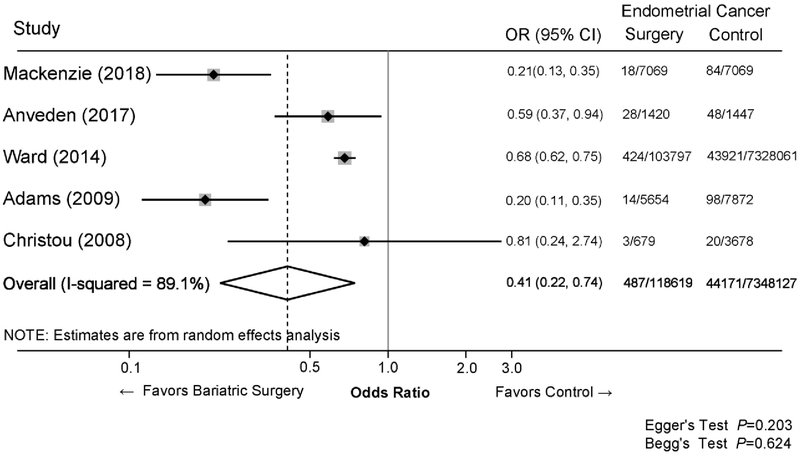
Data from five of seven articles on bariatric surgery and endometrial cancer risk were included in the quantitative synthesis using meta-analysis[14,30,31,33,34]. One article was excluded due to the use of an external comparison group (e.g., national age-standardized rate of endometrial cancer)[32]. In this study, the standardized incidence rate of endometrial cancer comparing bariatric surgery study participants to the Swedish age-standardized rate was 2.15 (95% CI=1.62–2.81). Another article was excluded due to lack of information regarding numbers of endometrial cancers according to bariatric surgery status; bariatric surgery was associated with lower endometrial cancer risk in this study (HR=0.50, P<0.05)[17]. The quantitative synthesis of the five studies demonstrated a 59% lower odds of endometrial cancer among women who had bariatric surgery compared to women who did not (pooled OR=0.41, 95% CI: 0.22, 0.74). In addition, significantly lower endometrial cancer risk, ranging from 19–80% risk reduction, was observed in the individual studies (Figure 2).
Figure 2.
Meta-analysis of the association between bariatric surgery and the risk of endometrial cancer.
Forest plot of effect estimation (Odds Ratios (OR) and 95% confident interval (95% CI)) comparing endometrial cancer risk between patients had bariatric surgery and patients did not have bariatric surgery (control). The heterogeneity of effect size estimates across studies was assessed by I2 statistic and the publication bias was assessed by Egger’s and Begg’s tests.
Quality assessment across studies
Study quality was assessed separately for the case-control and cohort studies and summarized according to participant selection, comparability across studies, and aspects related to outcomes (Table 3). The Newcastle-Ottawa Quality Assessment Scale ranges from 0–9 with a higher score indicating higher quality. In this review, the total scores of the 13 studies ranged from 6–9. Overall, studies of bariatric surgery and endometrial cancer risk were generally of higher quality compared with studies of self-reported intentional weight loss or weight cycling.
Table 3.
Quality assessment of studies included in the systematic review
| Author, year | SELECTION | COMPARABILITY | OUTCOME | TOTAL SCORE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representative-ness of the exposed | Selection of the non- exposed | Ascertainment of exposure | Outcome of interest was not present at start of intervention | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| Self-reported intentional weight loss | |||||||||
| Luo, 2017 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Parker, 2003 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 |
| Weight cycling | |||||||||
| Welti, 2017 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 7 |
| Nagle, 2013 | 1 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 6 |
| Stevens, 2012 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 7 |
| Trentham-Dietz, 2006 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 |
| Bariatric Surgery | |||||||||
| Mackenzie, 2018 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Anveden, 2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Schauer, 2017 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Ward 2014 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 6 |
| Ostlund, 2010 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 6 |
| Adams, 2009 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 |
| Christou, 2008 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
Among the two studies on self-reported intentional weight loss and endometrial cancer risk, weight loss was measured through a self-reported questionnaire in one study[28] and in the other, objectively measured weight at two time points was used[29]. One study did not account for hysterectomy status during the study period, and loss to follow-up was more than 20%[28]. Among the four studies on self-reported weight cycling and endometrial cancer risk, quality assessments were similar across studies. One study used controls recruited from two sources, including an endometrial cancer study and an ovarian cancer study, which may introduce selection bias[26]. All studies measured weight cycling once, and none of the studies specified the lag between the time of weight cycling and the time of endometrial cancer diagnosis. Although analyses in self-reported weight cycling studies all adjusted for BMI, no study matched for weight or BMI distribution between weight cyclers and controls, potentially leading to residual confounding by weight/BMI.
Of the seven studies examining bariatric surgery and endometrial cancer risk, one study used age- and calendar year-standardized rates of endometrial cancer as the comparison group[32] potentially leading to selection bias for the controls. One study did not report endometrial cancer status at baseline[33], and one did not specify whether participants had a hysterectomy at baseline[17]. Four studies specified the time lag (6 months, 1 year, 3 years, and 5 years) between bariatric surgery and endometrial cancer diagnosis, which allowed enough follow-up time to ascertain the outcome and minimize the impact of pre-existing cancer[14,17,31,34]. In addition, studies matched for weight or BMI distribution between bariatric surgery and non-surgical control groups reduced unmeasured confounding due to weight/BMI[14,17,31,34].
DISCUSSION
Findings from this review of observational studies support the notion that intentional weight loss is associated with lower endometrial cancer risk. However, weight cycling after intentional weight loss was linked with higher endometrial cancer risk. Our findings of increased endometrial cancer risk among weight cyclers demonstrate an important public health implication: avoidance of weight gain and maintenance of previous weight loss is critical for endometrial cancer prevention. Thus, strategies to sustain weight loss are needed to overcome the common trend of weight regains following intentional weight loss.
Bariatric surgery has been linked with lower overall cancer incidence and reduced risks of colorectal and breast cancers [35–37]. Consistent with two published meta-analyses, our study showed intentional weight loss through bariatric surgery was associated with lower endometrial cancer risk[18,20]. We included three recent studies on bariatric surgery and endometrial cancer risk that were not included in the previous reviews[14,17,34]. However, we excluded two studies from the Winder et al. review[20]: one study included 8 of 9 endometrial cancers diagnosed during the bariatric surgery[38] and the other did not distinguish women with previous cancer diagnosis or hysterectomy[39]. In addition, we excluded a recent study that used standardized incidence ratios to evaluate endometrial cancer risk for surgery and non-surgery patients with obesity compared to an external population[40]. In this study, the surgery group had an unexpectedly higher increased risk of endometrial cancer compared to the risk in the non-surgery group.
A recent meta-analysis of randomized controlled trials demonstrated favorable effect of weight loss interventions on risk of cardiovascular disease and cancer, without reaching statistical significance [41]. Other review articles showed intentional weight loss was associated with lower breast cancer risk, improved cardiovascular health, reduced risk of cardiovascular diseases and lower risk of liver diseases [42–44]. However, the common trajectory of intentional weight loss is followed by weight re-gain. We are the first to synthesize the association between self-reported weight cycling after intentional weight loss and endometrial cancer risk. The findings from included studies consistently suggest that weight cycling is related to increased endometrial cancer risk; however, the definition of self-reported weight cycling varied across studies as did the comparison group used for analysis. The inconsistent definition of weight cycling was also found in studies of other disease sites and study endpoints, such as cardiometabolic diseases, overall cancer risk, and mortality, which limited the possibility to quantify the pooled risk-estimates across studies [45–48]. It is critical to define a clinically meaningful threshold for the amount and timing of weight cycling and self-reported intentional weight loss. Perhaps using 5% or 10% weight loss in addition to the absolute weight change should be examined to account for individual variations in body weight. Furthermore, use of a consistent reference group would allow for meaningful comparisons of studies. Weight cycling is associated with redistribution of body fat to visceral fat[13,49], which is associated with elevated risk of endometrial cancer[50]. Future studies should investigate whether the elevated endometrial cancer risk from weight cycling is due to increased visceral fat, which can help researchers better understand underlying biological mechanisms.
Biological mechanisms linking obesity and endometrial cancer are mainly attributed to hormonal imbalances[51]. About 80% of endometrial cancers are thought to arise because of estrogen excess or progesterone deficiency[51]. Obesity alters systemic levels of insulin-like growth factors (IGFs) and hyperinsulinemia, all of which play a role in the pathogenesis of endometrial cancer[52,53]. In addition, obesity is characterized by low-grade inflammation with elevations in circulating pro-inflammation cytokines and acute phase proteins, such as CRP, TNF-alpha, soluble TNF receptors 1 and 2, IL-6, and IL-1 RA[54,55]. These serum biomarkers are consistently associated with increased endometrial cancer risk[7,9,10].
Observational studies suggest that weight loss may reverse the hormonal and metabolic imbalances associated with obesity and insulin resistance by reducing CRP, TNF-alpha, and IL-6 in the general population[8]. Behavioral weight loss interventions could normalize endometrial cancer-associated biomarkers, such as growth hormone, adiponectin, IL-6, IL-7, CA-125, and IGFBP-1, among women with severe obesity[56]. Similar findings were observed among women undergoing bariatric surgery[57,58]. In addition, resolution of endometrial hyperplasia was observed in 10 of 14 women 12 months following the bariatric surgery[58–60]. Taken together, results from biological studies of weight loss and endometrial cancer-associated biomarkers and our review of weight loss and endometrial cancer risk, indicate that endometrial cancer risk can be reduced by behavioral and surgical weight loss intervention.
We originally proposed to compare the effect of behavioral weight loss interventions (e.g., calorie restricted diet with or without physical activity prescription) to surgical weight loss intervention on endometrial cancer risk. We hypothesized that the mechanism of gradual weight loss through healthy eating and increased physical activity differs from the substantial and rapid weight loss through invasive surgical procedures. However, we only identified one article of a behavioral weight loss intervention, but this study used endometrial cancer-associated biomarkers as endpoints instead of cancer occurrence [56]. Results from the Look AHEAD study, which includes 5,145 participants with 12 years of follow-up after a behavioral intervention, are forthcoming[61]. The lack of data on behavioral weight loss interventions and cancer events as a study outcome highlights the need for future studies with long-term follow-up to build a comprehensive understanding of the mechanisms underlying cancer risk and other health benefits related to behavioral and surgical weight loss intervention.
As with most systematic reviews, the major limitation centered on the shortcomings of the reviewed literature. Due to the small number of high-quality studies and inconsistent definition of self-reported intentional weight loss and weight cycling, we were not able to perform a quantitative analytical summary for those studies. The inconsistencies prevented us from comparing results across studies. Data on self-reported weight cycling and self-reported intentional weight loss were self-reported and only collected once. Future studies using longitudinal measures and integration of electronic health record that collects standardized weight measures over time are needed. Additionally, none of the bariatric surgery studies provided information on the amount of weight loss. Dichotomizing the exposure as bariatric surgery vs. no surgery treats women within these groups as homogeneous and does not account for variation in the amount of weight reduction in the surgical group. Finally, our quantitative results related to bariatric surgery are only generalizable to that patient population. Demographic and clinical characteristics, including, age, income, obesity severity, and co-existing health conditions, likely differ between surgical and non-surgical weight loss cohorts. Therefore, the interpretation of the results needs to be cautious.
The major strengths of this systematic review include an overall rigorous approach guided by the PRISMA criteria, explicit inclusion criteria, a comprehensive search of several databases, and duplicate reviews of titles/abstracts, full-text, and data to ensure accuracy before analysis. In addition, we assessed the quality of each included study to characterize the available scientific evidence. To our knowledge, this is the first review specifically focused on self-reported weight cycling after intentional weight loss. Our finding of increased endometrial cancer risk among weight cyclers is an important extension of the literature on health outcomes of weight loss. We also updated the synthesis of literature related to bariatric surgery and endometrial cancer risk. Our topic is one of increasing importance given the obesity epidemic, and this review can help identify gaps in research and suggests the area for future research.
CONCLUSIONS
Current evidence from studies assessing intentional weight loss and weight cycling suggest that intentional weight loss is associated with lower endometrial cancer risk and weight cycling is associated with increased risk of endometrial cancer. Our study identified important research gaps which suggest future research to address current limitations. Strategies to improve awareness and maintenance of weight loss among women with obesity are needed to reduce endometrial cancer risk.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by the National Cancer Institute (K01CA21845701A1 to ASF) and Susan G. Komen Foundation (GTDR15334082 to XZ).
Footnotes
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
REFERENCE
- 1.Chang S-C, Lacey JV, Brinton LA, et al. Lifetime weight history and endometrial cancer risk by type of menopausal hormone use in the NIH-AARP diet and health study. Cancer Epidemiology and Prevention Biomarkers. 2007;16(4):723–730. [DOI] [PubMed] [Google Scholar]
- 2.Friedenreich C, Cust A, Lahmann PH, et al. Anthropometric factors and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Cancer Causes & Control. 2007;18(4):399–413. [DOI] [PubMed] [Google Scholar]
- 3.Weiderpass E, Persson I, Adami H-O, et al. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden). Cancer Causes & Control. 2000;11(2):185–192. [DOI] [PubMed] [Google Scholar]
- 4.Schouten LJ, Goldbohm RA, van den Brandt PA. Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. Journal of the National Cancer Institute. 2004;96(21):1635–1638. [DOI] [PubMed] [Google Scholar]
- 5.McMinn J, Steel C, Bowman A. Investigation and management of unintentional weight loss in older adults. Bmj. 2011;342:d1732. [DOI] [PubMed] [Google Scholar]
- 6.Wijnhoven HA, van Zon SK, Twisk J, et al. Attribution of causes of weight loss and weight gain to 3-year mortality in older adults: results from the Longitudinal Aging Study Amsterdam. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2014;69(10):1236–1243. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Rohan TE, Gunter MJ, et al. A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone non-users. Cancer Epidemiology and Prevention Biomarkers. 2011:cebp. 1222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byers T, Sedjo R. Does intentional weight loss reduce cancer risk? Diabetes, obesity and metabolism. 2011;13(12):1063–1072. [DOI] [PubMed] [Google Scholar]
- 9.Dossus L, Becker S, Rinaldi S, et al. Tumor necrosis factor (TNF)‐α, soluble TNF receptors and endometrial cancer risk: The EPIC study. International journal of cancer. 2011;129(8):2032–2037. [DOI] [PubMed] [Google Scholar]
- 10.Dossus L, Lukanova A, Rinaldi S, et al. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort—a factor analysis. American journal of epidemiology. 2013;177(8):787–799. [DOI] [PubMed] [Google Scholar]
- 11.Kraschnewski J, Boan J, Esposito J, et al. Long-term weight loss maintenance in the United States. International journal of obesity. 2010;34(11):1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens VL, Jacobs EJ, Patel AV, et al. Weight cycling and cancer incidence in a large prospective US cohort. American journal of epidemiology. 2015;182(5):394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cereda E, Malavazos AE, Caccialanza R, et al. Weight cycling is associated with body weight excess and abdominal fat accumulation: a cross-sectional study. Clinical Nutrition. 2011;30(6):718–723. [DOI] [PubMed] [Google Scholar]
- 14.Anveden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecologic Oncology. 2017;145(2):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. Jama. 2012;307(1):56–65. [DOI] [PubMed] [Google Scholar]
- 16.Anveden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145(2):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Annals of surgery. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upala S, Sanguankeo A. Bariatric surgery and risk of postoperative endometrial cancer: a systematic review and meta-analysis. Surgery for Obesity and Related Diseases. 2015;11(4):949–955. [DOI] [PubMed] [Google Scholar]
- 19.Association AD. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes care. 2007;30(6):1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obesity surgery. 2018:1–8. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 22.Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute; 2009. Available in March. 2016. [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trentham-Dietz A, Nichols HB, Hampton JM, et al. Weight change and risk of endometrial cancer. International Journal of Epidemiology. 2006;35(1):151–158. [DOI] [PubMed] [Google Scholar]
- 25.Stevens VL, Jacobs EJ, Sun J, et al. Weight cycling and risk of endometrial cancer. Cancer Epidemiology, Biomarkers & Prevention. 2012;21(5):747–752. [DOI] [PubMed] [Google Scholar]
- 26.Nagle CM, Marquart L, Bain CJ, et al. Impact of weight change and weight cycling on risk of different subtypes of endometrial cancer. European Journal of Cancer. 2013;49(12):2717–2726. [DOI] [PubMed] [Google Scholar]
- 27.Welti LM, Beavers DP, Caan BJ, et al. Weight fluctuation and cancer risk in postmenopausal women: The women’s health initiative. Cancer Epidemiology Biomarkers and Prevention. 2017;26(5):779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women’s Health Study. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2003;27(12):1447–1452. [DOI] [PubMed] [Google Scholar]
- 29.Luo J, Chlebowski RT, Hendryx M, et al. Intentional Weight Loss and Endometrial Cancer Risk. Journal of Clinical Oncology. 2017;35(11):1189–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christou NV, Lieberman M, Sampalis F, et al. Bariatric surgery reduces cancer risk in morbidly obese patients. Surgery for Obesity and Related Diseases. 2008;4(6):691–695. [DOI] [PubMed] [Google Scholar]
- 31.Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity. 2009;17(4):796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostlund MP, Lu Y, Lagergren J. Risk of obesity-related cancer after obesity surgery in a population-based cohort study. Annals of Surgery. 2010;252(6):972–976. [DOI] [PubMed] [Google Scholar]
- 33.Ward KK, Roncancio AM, Shah NR, et al. Bariatric surgery decreases the risk of uterine malignancy. Gynecologic Oncology. 2014;133(1):63–66. [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie H, Markar S, Askari A, et al. Obesity surgery and risk of cancer. British Journal of Surgery. 2018;105(12):1650–1657. [DOI] [PubMed] [Google Scholar]
- 35.Afshar S, Kelly SB, Seymour K, et al. The effects of bariatric surgery on colorectal cancer risk: systematic review and meta-analysis. Obesity surgery. 2014;24(10):1793–1799. [DOI] [PubMed] [Google Scholar]
- 36.Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of breast cancer development? A systematic review. Obesity surgery. 2017;27(11):3014–3020. [DOI] [PubMed] [Google Scholar]
- 37.Wiggins T, Antonowicz SS, Markar SR. Cancer Risk Following Bariatric Surgery-Systematic Review and Meta-analysis of National Population-Based Cohort Studies. Obes Surg. 2019;29(3):1031–1039. [DOI] [PubMed] [Google Scholar]
- 38.McCawley GM, Ferriss JS, Geffel D, et al. Cancer in obese women: potential protective impact of bariatric surgery. Journal of the American College of Surgeons. 2009;208(6):1093–1098. [DOI] [PubMed] [Google Scholar]
- 39.Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. The lancet oncology. 2009;10(7):653–662. [DOI] [PubMed] [Google Scholar]
- 40.Aravani A, Downing A, Thomas JD, et al. Obesity surgery and risk of colorectal and other obesity-related cancers: An English population-based cohort study. Cancer epidemiology. 2018;53:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. bmj. 2017;359:j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardefeldt PJ, Penninkilampi R, Edirimanne S, et al. Physical activity and weight loss reduce the risk of breast cancer: a meta-analysis of 139 prospective and retrospective studies. Clinical breast cancer. 2018;18(4):e601–e612. [DOI] [PubMed] [Google Scholar]
- 43.Hannah WN, Harrison SA. Effect of weight loss, diet, exercise, and bariatric surgery on nonalcoholic fatty liver disease. Clinics in liver disease. 2016;20(2):339–350. [DOI] [PubMed] [Google Scholar]
- 44.Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Current obesity reports. 2017;6(2):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messerli FH, Hofstetter L, Rimoldi SF, et al. Risk Factor Variability and Cardiovascular Outcome: JACC Review Topic of the Week. Journal of the American College of Cardiology. 2019;73(20):2596–2603. [DOI] [PubMed] [Google Scholar]
- 46.Mehta T, Smith DL Jr, Muhammad J, et al. Impact of weight cycling on risk of morbidity and mortality. Obesity reviews. 2014;15(11):870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhee E-J. Weight cycling and its cardiometabolic impact. Journal of obesity & metabolic syndrome. 2017;26(4):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montani JP, Schutz Y, Dulloo AG. Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obesity Reviews. 2015;16:7–18. [DOI] [PubMed] [Google Scholar]
- 49.Wallner S, Luschnigg N, Schnedl W, et al. Body fat distribution of overweight females with a history of weight cycling. International journal of obesity. 2004;28(9):1143. [DOI] [PubMed] [Google Scholar]
- 50.Ciortea R, Mihu D, Mihu CM. Association between visceral fat, IL-8 and endometrial cancer. Anticancer research. 2014;34(1):379–383. [PubMed] [Google Scholar]
- 51.Carlson MJ. Catch it before it kills: progesterone, obesity, and the prevention of endometrial cancer. Discovery medicine. 2012;14(76):215. [PMC free article] [PubMed] [Google Scholar]
- 52.Nead KT, Sharp SJ, Thompson DJ, et al. Evidence of a causal association between insulinemia and endometrial cancer: a Mendelian randomization analysis. JNCI: Journal of the National Cancer Institute. 2015;107(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vrachnis N, Iavazzo C, Iliodromiti Z, et al. Diabetes mellitus and gynecologic cancer: molecular mechanisms, epidemiological, clinical and prognostic perspectives. Archives of gynecology and obstetrics. 2016;293(2):239–246. [DOI] [PubMed] [Google Scholar]
- 54.Fantuzzi G Adipose tissue, adipokines, and inflammation. Journal of Allergy and Clinical Immunology. 2005;115(5):911–919. [DOI] [PubMed] [Google Scholar]
- 55.Das U Is obesity an inflammatory condition? Nutrition. 2001;17(11–12):953–966. [DOI] [PubMed] [Google Scholar]
- 56.Linkov F, Maxwell GL, Felix AS, et al. Longitudinal evaluation of cancer-associated biomarkers before and after weight loss in RENEW study participants: implications for cancer risk reduction. Gynecologic Oncology. 2012;125(1):114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linkov F, Goughnour SL, Ma T, et al. Changes in inflammatory endometrial cancer risk biomarkers in individuals undergoing surgical weight loss. Gynecologic Oncology. 2017;147(1):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacKintosh ML, Derbyshire AE, McVey RJ, et al. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. International journal of cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Argenta PA, Kassing M, Truskinovsky AM, et al. Bariatric surgery and endometrial pathology in asymptomatic morbidly obese women: a prospective, pilot study. BJOG: An International Journal of Obstetrics & Gynaecology. 2013;120(7):795–800. [DOI] [PubMed] [Google Scholar]
- 60.Linkov F, Elishaev E, Gloyeske N, et al. Bariatric surgery-induced weight loss changes immune markers in the endometrium of morbidly obese women. Surgery for Obesity & Related Diseases. 2014;10(5):921–926. [DOI] [PubMed] [Google Scholar]
- 61.Group LAR. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New England journal of medicine. 2013;369(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.