Abstract
Objective:
To evaluate the impact of timing of exercise initiation on weight loss within a behavioral weight loss program.
Methods:
Adults with overweight/obesity (n=170, 18–55 years, BMI 25–42 kg/m2, 83.5% female) were enrolled in an 18-month behavioral weight loss program consisting of a reduced-calorie diet, exercise, and group-based support. The standard group (STD) received a supervised exercise program (progressing to 300 minutes/week of moderate-intensity aerobic exercise) during months 0–6. The sequential group (SEQ) was asked to refrain from changing exercise during months 0–6 and received the supervised exercise program during months 7–12. Upon completion of supervised exercise, both groups were instructed to continue 300 minutes/week of moderate-intensity exercise for the study duration.
Results:
At 6 months, STD exhibited greater reductions in body weight (-8.7±0.7 kg) versus SEQ (-6.9±0.6 kg; P=0.047). Between 6–18 months, STD regained more weight (2.5±0.8, 0.0±0.8 kg; P=0.02). At 18 months, there were no between-group differences in changes in weight (STD −6.9±1.2, SEQ −7.9±1.2 kg), fat mass, lean mass, physical activity, or attrition.
Conclusion:
Both immediate and delayed exercise initiation within a behavioral weight loss program resulted in clinically meaningful weight loss at 18 months. Thus, timing of exercise initiation can be personalized based on patient preference.
Keywords: Clinical Trials, Exercise Intervention, Weight Reduction, Physical Activity, Obesity
Introduction
Despite the short-term effectiveness of lifestyle interventions for weight loss (1, 2), many individuals regain significant weight within one year (3, 4). Regular physical activity (PA) is a strong predictor of sustained weight loss (5–7), and high levels of PA are recommended to prevent weight regain (8, 9). Most weight loss programs recommend simultaneously decreasing energy intake (EI) and increasing PA. However, many individuals with overweight/obesity are unable to achieve and sustain high levels of exercise with simultaneous delivery of diet and exercise interventions (6, 10–12).
An alternative approach, which has not been widely considered, is to deliver diet and exercise interventions sequentially. Delaying the start of an exercise intervention after diet-induced weight loss could result in enhanced exercise adherence and improved weight loss long-term because: 1) perceived enjoyment of exercise may be higher at a lower body weight (13, 14), 2) risk of exercise-related injuries may be reduced after weight loss (15), and 3) focusing on one behavioral change at a time may lead to greater long-term adherence to both diet and exercise behaviors (16, 17). However, there is limited evidence regarding the effect of simultaneous versus sequential interventions to improve diet and PA behaviors on weight loss. One previous study in individuals with class II obesity or higher (BMI >35 kg/m2) reported no differences in weight loss at 12 months with simultaneous or sequential delivery of diet and exercise interventions (18). Whether sequential delivery of diet and exercise interventions would improve weight loss in adults with less severe obesity has not been studied.
We conducted an 18-month randomized trial to evaluate the impact of timing of exercise initiation on weight loss and body composition in healthy adults with overweight/obesity (BMI 27–42 kg/m2). We hypothesized that delayed initiation of exercise would result in greater weight loss over 18 months compared to a standard approach where diet and exercise were initiated simultaneously. We also examined changes in body composition, blood pressure (BP), free-living PA, cardiorespiratory fitness, exercise perceptions, exercise-related injuries, and dietary behaviors.
Methods
Participants
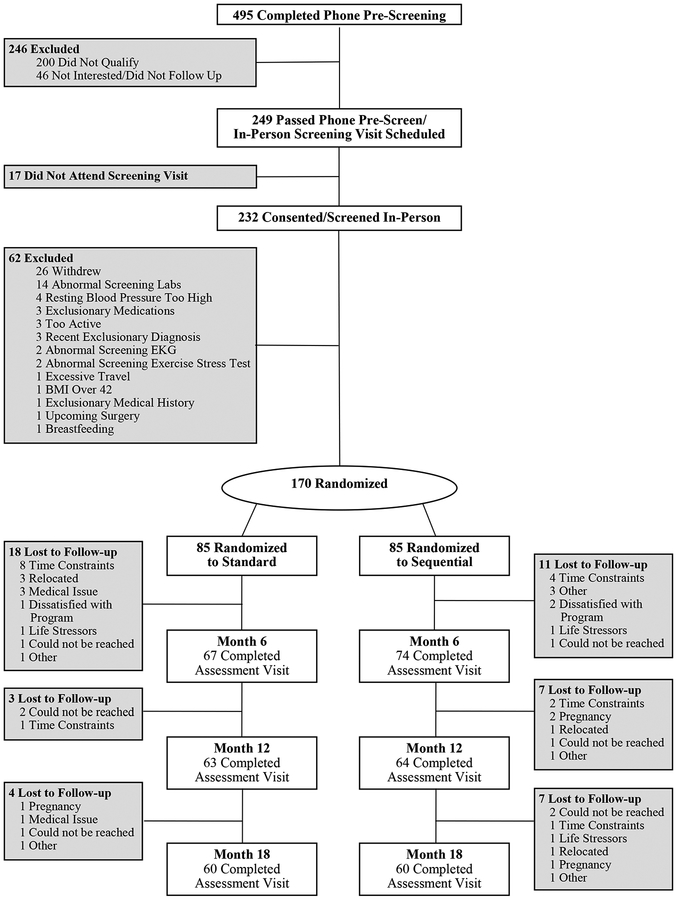
The study was approved by the Colorado Multiple Institutional Review Board and registered on ClinicalTrials.gov (). Recruitment and randomization ran from January 2014 to June 2016. Volunteers were recruited via mass mailing, emails, and flyers. Eligibility criteria included age between 18 and 55 years, BMI between 27 and 42 kg/m2, and living or working within 20 miles of the University of Colorado Anschutz Medical Campus. Exclusionary criteria included history of diabetes mellitus, cardiovascular disease, uncontrolled hypertension, uncontrolled thyroid disease, cancer within the past 5 years (except skin), any physical or medical condition that contraindicates exercise, bariatric surgery, eating disorder, current use of medications known to affect appetite, weight, or energy metabolism; current alcohol or substance abuse, regular nicotine use, weight loss of >5% over the past 6 months, or current engagement in high levels of exercise (self-report of ≥150 minutes/week at moderate intensity or greater). Pregnant or lactating women were also excluded. Volunteers meeting inclusion/exclusion criteria underwent a symptom-limited maximal exercise stress test. Those without ECG evidence of exercise-induced ischemia were invited to participate. Respondents (n=439) completed a telephone pre-screening, 232 attended an in-person screening visit, and 170 were randomized (Figure 1).
Figure 1:
Study Consort Diagram
Randomization and Intervention Groups
Participants received an 18-month weight loss intervention that provided group-based behavioral support and individualized dietary prescriptions to reduce EI throughout the intervention. Participants were randomized in a 1:1 ratio to receive a supervised exercise program delivered immediately during months 0–6 (standard, STD, n=85) or sequentially during months 7–12 (SEQ, n=85). SEQ was instructed to refrain from changing exercise habits during months 0–6. Outcome data were blinded until final data entry for 18-month assessments was completed.
The dietary intervention and behavioral support curriculum were identical in STD and SEQ and were based on a previously published behavioral weight loss program (19–21). Participants received an individualized calorie goal based on estimated resting metabolic rate (22); calorie goals ranged from 1200–1800 kcal/day and were not adjusted during the study. Targeted macronutrient content was 20–30% fat, 50–55% carbohydrate, and 20–25% protein. Group-based behavioral support was delivered by a registered dietitian and focused on dietary behavior modification using cognitive-behavioral strategies. Randomized groups met separately. Group meetings lasted approximately 60 minutes and were held weekly during weeks 0–20, every other week during weeks 21–26, and every month during weeks 27–78.
The 6-month supervised exercise program was identical in STD and SEQ. Exercise duration gradually increased to 300 minutes/week of moderate-intensity (65–75% maximum heart rate (HRmax) aerobic exercise (Table 1). Participants were provided a heart rate (HR) monitor (Polar H1 HR Sensor) and a Technogym Wellness System SmartKey to track exercise adherence. Participants were asked to attend three “in-person” exercise sessions/week, which were supervised by study staff. Supervised sessions were performed at the University of Colorado Anschutz Health and Wellness Center (AHWC) fitness facility in an area reserved for study participants. Participants could choose between treadmill, elliptical, recumbent or upright bike, rower, stepper, or arm ergometer (in the event a participant experienced an injury that precluded other forms of exercise). At weeks 16 and 21, participants were asked to add an additional one and two “on-own” sessions/week, respectively (Table 1). On-own sessions could be performed at any time, either at AHWC or outside the facility and could consist of any moderate-intensity aerobic exercise. Performance of on-own sessions was verified by study staff via review of HR monitor data and exercise logs. Exercise behavioral support was provided by an exercise specialist during six, individual, in-person sessions using a standardized curriculum. Adherence to the exercise prescription was reviewed with participants every two weeks by study staff. Upon completion of supervised exercise, both groups were instructed to continue to exercise 300 minutes/week at moderate-intensity for the remainder of the study. Participants were provided continued access to the AHWC fitness facility, however, exercise sessions were unsupervised and participants no longer had access to the reserved section of the fitness facility.
Table 1:
Description of 6-Month Supervised Exercise Program a
| Week | Days/Week | Session Duration (min/day) | Total Duration (min/week) | Exercise Sessionsa (count/week) | Exercise Behavioral Support |
|---|---|---|---|---|---|
| 1 | Establish Fitness Center Membership, Orientation | ||||
| 2 | 3 | 20 | 60 | 3 in-person | Session 1 |
| 3 | 3 | 25 | 75 | 3 in-person | |
| 4–5 | 3 | 30 | 90 | 3 in-person | Session 2 |
| 6–7 | 3 | 35 | 105 | 3 in-person | Session 3 |
| 8–9 | 3 | 40 | 120 | 3 in-person | |
| 10–11 | 3 | 45 | 135 | 3 in-person | Session 4 |
| 12–13 | 3 | 50 | 150 | 3 in-person | |
| 14–15 | 3 | 60 | 180 | 3 in-person | Session 5 |
| 16–21 | 4 | 60 | 240 | 3 in-person (+ 1 on-own) | Session 6 |
| 22–26 | 5 | 60 | 300 | 3 in-person (+ 2 on-own) | |
Participants were asked to attend three in-person exercise sessions per week during specific time windows supervised by study staff (6–8 AM M/W/F, 11:30AM-1:30 PM T/TH, 4:30–6:30 PM M-TH, and 8–10 AM SAT). Supervised sessions were performed in a 717 sq. ft. secluded area within the 30,000 sq. ft. University of Colorado Anschutz Health and Wellness Center AHWC fitness facility, accessible only to study participants. Study staff periodically checked on each participant during their exercise session; participant to staff ratio was typically <5:1. During weeks 16–20, participants were asked to add one “on-own” session (in addition to the 3 in-person sessions) for a total of 4 sessions/week. During weeks 21–26, participants were asked to add two “on-own” sessions for a total of 5 sessions/week. On-own sessions could be performed at any time, either at AHWC or outside the facility and could consist of any type of moderate-intensity aerobic exercise. Performance of on-own sessions was verified by study staff via review of HR monitor data and exercise logs. Exercise behavioral support was provided by an exercise specialist during 6 individual, in-person sessions using a standardized curriculum.
Outcome Measures
Outcomes were measured at baseline, 6, 12, and 18 months, unless stated otherwise. The primary outcome was change in body weight at 18 months, which was measured with a calibrated digital scale (to the nearest 0.2 lbs.). Waist circumference (WC, cm) was measured at the level of the superior iliac crests. Fat mass (FM) and lean mass (LM) were measured by dual-energy X-ray absorptiometry (DXA, Hologic Discovery QDR Series, Bedford, MA). BP was measured with a manual sphygmomanometer.
Free–living PA was assessed with the SenseWear Mini Armband (BodyMedia Inc., Pittsburgh, PA, version 7.0). Participants were asked to wear the device for seven consecutive days. A day was considered valid if the participant wore the SenseWear for ≥22.8 hours/day (95% wear time). To be included in the analysis, the monitoring period must have had ≥4 valid days, including ≥1 valid weekend day. Minutes of moderate-to-vigorous intensity PA (MVPA, ≥3 METs) were quantified using a proprietary algorithm (23). Outcomes included total MVPA, bout MVPA (minutes accumulated in bouts lasting ≥10 minutes where ≥80% of the entire bout was ≥3 METs (8)), non-bout MVPA (any minute ≥3 METs that did not meet the bout MVPA definition), and steps (count/day). The SenseWear has been found to have high test-retest reliability (24) and validity in a variety of settings (23, 25–27).
Cardiorespiratory fitness was assessed with a maximal aerobic capacity test (VO2max) using a modified Balke treadmill protocol (28) during which expired gases were collected and analyzed at 20-second intervals using indirect calorimetry (Parvo Medic Truemax 2400, Salt Lake City, UT). Valid tests had to meet two of three criteria: increase in VO2 <2 mL/kg/min with increase in workload; peak HR within 10 bpm of age-predicted HRmax, and respiratory exchange ratio ≥1.10.
Exercise perceptions included exercise enjoyment (modified PA Enjoyment Scale, PACES) (29), perceived benefits and barriers of exercise (Exercise Benefits and Barriers Scale, EBBS) (30), and self-efficacy for exercise (Barriers Self-Efficacy Scale, BARSE) (31). Participants were asked to report any exercise-related adverse events (AEs) or injuries that occurred at any time during the study, including date of onset, nature of the AE, and whether a specific medical diagnosis was made. The study physician rated AE severity (mild, moderate, or severe) based on standard criteria.
Dietary energy and macronutrient intake were assessed with 3-day diet records that were analyzed using Nutrition Data System for Research software (version 2016, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). Cognitive restraint (i.e. tendency to consciously restrict food intake), hunger, and disinhibition (i.e. tendency to overeat in the presence of palatable foods) were assessed with the Three Factor Eating Questionnaire (TFEQ) (32) and diet self-efficacy was assessed using the total score from the Weight Efficacy Lifestyle Questionnaire (WEL) (33).
Statistical Analysis
This study was powered to detect differences in weight loss at 18 months between SEQ and STD, which was conservatively estimated based on data involving simultaneous delivery of diet and exercise interventions over 12–24 months (10, 34). With an effect size of 0.23, correlation between baseline and month 18 weight of 0.9, α fixed at .05 for a 2-tailed test, and power set at 0.80, a sample size of 60 participants per condition was required to detect a between-group difference of 3 kg in body weight using Analysis of Covariance (ANCOVA), after adjusting for the baseline value. Assuming an attrition rate of 30%, 85 participants were randomized to each condition. Results are reported as mean±SEM unless stated otherwise.
Statistical analyses were performed with SAS version 9.4 (SAS System for Microsoft, SAS Institute Inc., Cary, NC, USA), with the type I error rate fixed at 0.05 (two-tailed). Baseline demographic and clinical characteristics were summarized using descriptive statistics. Intent-to-treat (ITT) analyses were used to examine primary and secondary outcomes. A linear mixed model with an unstructured covariance matrix was used to delineate the longitudinal profile of repeated outcomes in each randomized group. Outcomes at baseline and any subsequent time points were the dependent variable in the mixed effects modeling. Fixed effects included time (months), randomized group, and their interaction term. The between-group difference in the change from baseline to 6, 12, and 18 months was tested using contrasts. No multiple comparison adjustment test was applied. ITT analyses using baseline-observation-carried-forward were used to determine whether the association between intervention and change in weight over 18 months was moderated by baseline sex, age, BMI, VO2max, or bout MVPA. Percent of participants achieving ≥5 or ≥10% weight loss was compared using a Chi-square test. Attendance to group-based meetings and parameters related to exercise adherence during the 6-month supervised exercise program (frequency, intensity, duration, and adherence parameters within each exercise modality) were compared using a two-sample t-test. Frequency, duration, and intensity were compared across exercise modality using an analysis of variance (ANOVA). Incidence of exercise-related AEs over the 6-month supervised exercise program and over 18 months were compared using a Kaplan-Meier analysis. For those without any reported exercise-related AEs, injury-free survival time was censored by either withdrawal week for those who were lost to follow-up, or at month 18 for completers.
Results
Participant Characteristics and Attrition
At baseline, there were no significant between-group differences in age, weight, BMI, or sex (Table 2). The study consort diagram is displayed in Figure 1. There was no between-group difference in retention (70.6% in STD and SEQ). Participants lost to follow-up were similar to completers, except they were younger (mean±SD; 37±10 versus 40±9 years; P=0.02), more likely to be female (94% versus 79%; P=0.02) and had lower levels of baseline bout MVPA (mean±SD; 16±15 versus 24±25 minutes/day; P=0.02).
Table 2:
Baseline Characteristics of Study Population a
| Characteristic | STD (n=85) | SEQ (n=85) |
|---|---|---|
| Age (y) (mean ± SD) | 40 ± 10 | 39 ± 9 |
| Anthropometric Measures (mean ± SD) | ||
| BMI (kg/m2) | 34.3 ± 4.2 | 34.6 ± 4.1 |
| Total Body Fat Mass (%) | 40.5 ± 5.7 | 41. 0 ± 6.1 |
| Sex (n (%)) | ||
| Male | 14 (16.5%) | 14 (16.5%) |
| Race/Ethnicity (n (%)) | ||
| Asian | 4 (4.7%) | 2 (2.4%) |
| Other | 3 (3.5%) | 3 (3.5%) |
| Ethnicity (n (%)) | ||
| Not Hispanic or Latino | 68 (80.0%) | 60 (70.6%) |
| Behavioral Measures (mean ± SD) | ||
| Total MVPA (min/day)b | 63 ± 39 | 60 ± 38 |
| Steps (count/day) b | 6,257 ± 2520 | 6,416 ± 2226 |
| Maximal Aerobic Capacity (mean ± SD) | ||
| Absolute Maximal VO2 (L/min) | 2.35 ± 0.57 | 2.34 ± 0.54 |
Continuous variables analyzed using two sample t-tests, and categorical variables analyzed using chi-square test or Fisher’s exact test; There were no between-group differences; MVPA: moderate-to-vigorous physical activity; SEQ: Sequential; STD: Standard.
n = 74 for STD, n = 81 for SEQ.
MVPA accumulated in bouts ≥ 10 minutes.
Body Weight and Composition, Blood Pressure
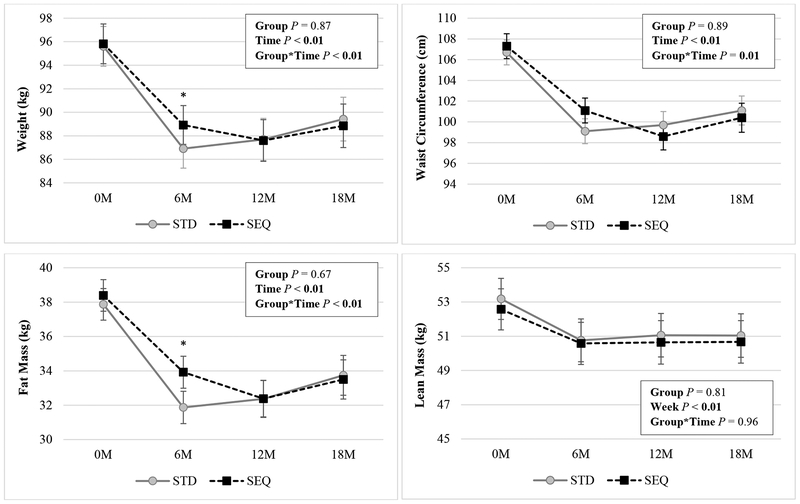
There were significant group by time interactions in weight, BMI, WC, and FM (Figure 2, Table S1) but not LM. Weight, BMI, WC, FM, and LM decreased at months 6, 12, and 18 in both STD and SEQ. At 6 months, STD had greater reductions in weight (−8.7±0.7, −6.9±0.6 kg), BMI, and FM compared to SEQ. Between months 6–18, STD regained more weight compared to SEQ (2.5±0.8, 0.0±0.8 kg). At 18 months, there were no between-group differences in changes in weight (STD −6.9±1.2, SEQ −7.9±1.2 kg), BMI, WC, FM, or LM. Change in body weight over 18 months was not moderated by sex, baseline BMI, age, VO2max, or bout MVPA. There were no between-group differences in percent of participants achieving ≥5 or ≥10% weight loss at 18 months (data not shown). There were no between-group differences in change in BP over 18 months.
Figure 2: Changes in Weight, Waist Circumference, Fat Mass, and Lean Mass by Randomized Group a.
a Results (mean ± SEM) are from linear mixed effect model with unstructured covariance using an intent-to-treat analysis; Statistically significant P values (P <0.05) are indicated in bold; SEQ: Sequential; STD: Standard.
* indicates p<0.05 for the differences of STD – SEQ in change in the parameter from baseline.
Exercise-Related Outcomes
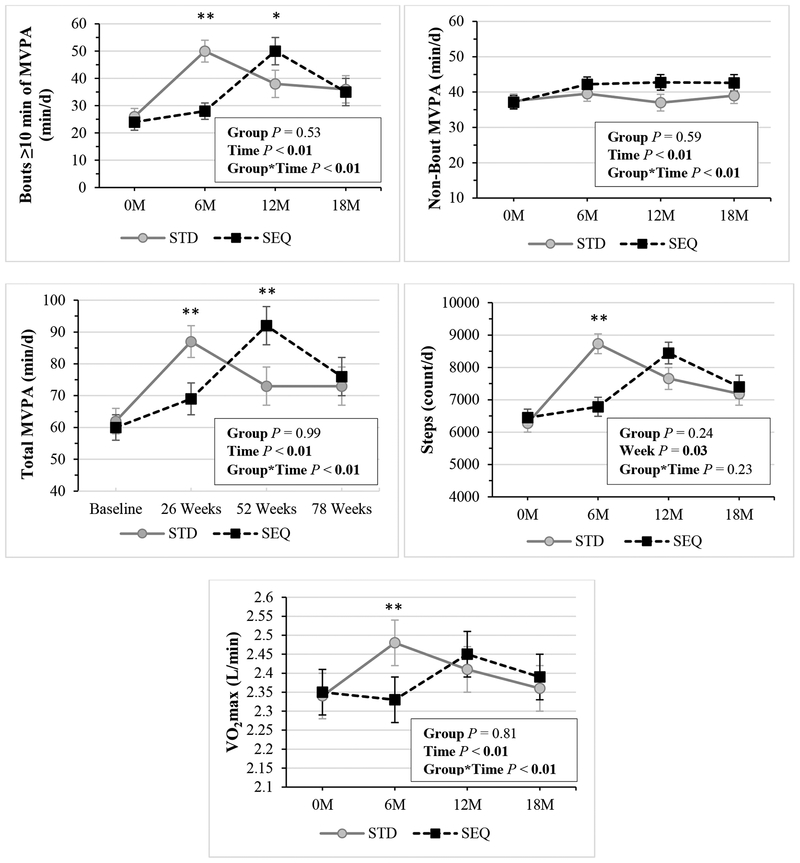
During the 6-month supervised exercise program, session compliance (mean±SD; 67±29%, 53±33%, P<0.01) and duration compliance (66±31%, 51±35%; P<0.01) were higher in STD versus SEQ, but intensity (%HRmax, STD 69±3%, SEQ 69±3%, P=0.71) was not different between groups. There were no between-group differences in frequency, duration, or intensity by exercise modality except duration on the elliptical was higher in STD versus SEQ (P=0.04, Figure S1). Free-living PA and fitness results are shown in Figure 3. Total MVPA increased at months 6, 12, and 18 in both STD and SEQ. Bout MVPA and steps increased at 6, 12, and 18 months in STD, and at 12 and 18 months in SEQ. VO2max increased at 6 and 12 months in STD; and at 12 months in SEQ. There were significant group by time interactions for total MVPA, bout MVPA, steps, and VO2max, however, there were no between-group differences in change in these measures at 18 months. Non-bout MVPA increased at 6, 12, and 18 months in SEQ, but there was no between-group difference at 18 months. There were no between-group differences in changes in MVPA, steps, or VO2max over the 6-month supervised exercise program (data not shown).
Figure 3: Changes in Physical Activity, Fitness by Randomized Group a-c.
a Results (mean ± SEM) are from linear mixed effect model with unstructured covariance using an intent-to-treat analysis; Statistically significant P values (P <0.05) are indicated in bold; Bout MVPA: bouts of ≥10 minutes in duration of moderate-to-vigorous physical activity (≥3.00 METs); Non-bout MVPA: any minute ≥3 METs that did not meet the definition for bout MVPA; Total MVPA: any minute ≥3 METs; SEQ: Sequential; STD: Standard; VO2max: maximal aerobic capacity.
b Sample sizes for bouts ≥10 minutes of MVPA, non-bout MVPA, total MVPA, and Steps are: STD: n=74 at 0M, n=63 at 6M, n=56 at 12M, n=54 at 18M; SEQ: n=81 at 0M, n=67 at 6M, n=55 at 12M, n=49 at 18M.
c Sample sizes for VO2max are: STD: n=85 at 0M, n=67 at 6M, n=57 at 12M, n=50 at 18M; SEQ: n=85 at 0M, n=73 at 6M, n=58 at 12M, n=51 at 18M.
* indicates p<0.05 for the differences of STD – SEQ in change in the parameter from baseline.
** indicates p<0.01 for the differences of STD – SEQ in change in the parameter from baseline.
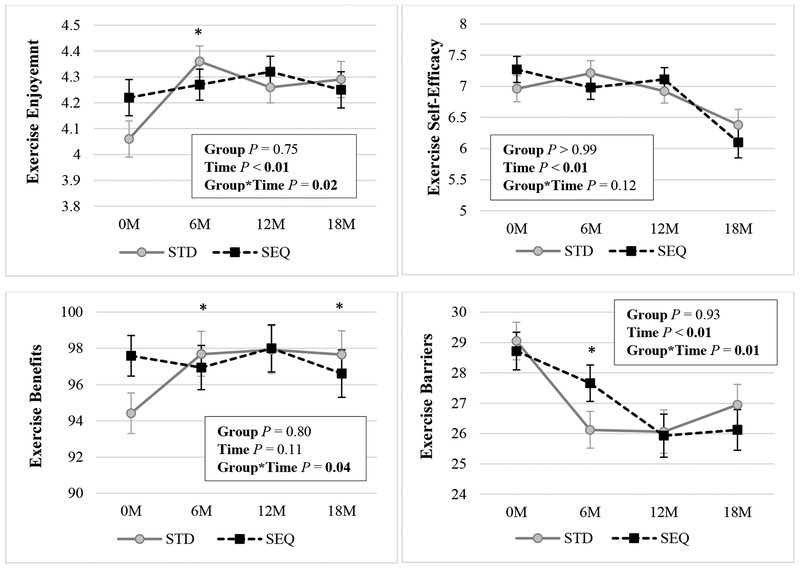
Changes in exercise perceptions are displayed in Figure 4. Exercise enjoyment increased in STD at 6, 12, and 18 months, but did not change in SEQ. At baseline, perceived benefits of exercise were lower in STD versus SEQ. At 6, 12, and 18 months, perceived benefits increased in STD, but did not change in SEQ. At 18 months, there was a near significant (P=0.05) between-group difference in change in exercise enjoyment and a significant between-group difference in change in exercise benefits. Perceived exercise barriers decreased at 6, 12, and 18 months in STD, and at 12 and 18 months in SEQ; there was no between-group difference in change in perceived exercise barriers at month 18. Exercise self-efficacy was unchanged in both groups at 6 and 12 months and decreased in both groups at 18 months, with no significant between-group differences.
Figure 4: Changes in Exercise Perceptions by Randomized Group a-e.
a Results (mean ± SEM) are from linear mixed effect model with unstructured covariance using an intent-to-treat analysis; Statistically significant P values (P <0.05) are indicated in bold; SEQ: Sequential; STD: Standard.
b Sample sizes for Exercise Enjoyment are: STD: n=84 at 0M, n=67 at 6M, n=61 at 12M, n=60 at 18M; SEQ: n=85 at 0M, n=74 at 6M, n=63 at 12M, n=59 at 18M; measured with the modified Physical Activity Enjoyment Scale (PACES); scale is 1 to 5.
c Sample sizes for Exercise Self-Efficacy are: STD: n=84 at 0M, n=67 at 6M, n=61 at 12M, n=60 at 18M; SEQ: n=85 at 0M, n=73 at 6M, n=63 at 12M, n=59 at 18M; measured with the Barriers Self-Efficacy Scale (BARSE); scale is 1 to 10.
d Sample sizes for Exercise Benefits are: STD: n=84 at 0M, n=67 at 6M, n=60 at 12M, n=59 at 18M; SEQ: n=85 at 0M, n=73 at 6M, n=62 at 12M, n=59 at 18M; measured with the Exercise Benefits and Barriers Scale (EBBS); scale is 29 to 116.
e Sample sizes for Exercise Barriers are: STD: n=82 at 0M, n=67 at 6M, n=61 at 12M, n=60 at 18M; SEQ: n=83 at 0M, n=73 at 6M, n=62 at 12M, n=59 at 18M; measured with the Exercise Benefits and Barriers Scale (EBBS); scale is 14 to 56.
* indicates p<0.05 for the differences of STD – SEQ in change in the parameter from baseline.
** indicates p<0.01 for the differences of STD – SEQ in change in the parameter from baseline.
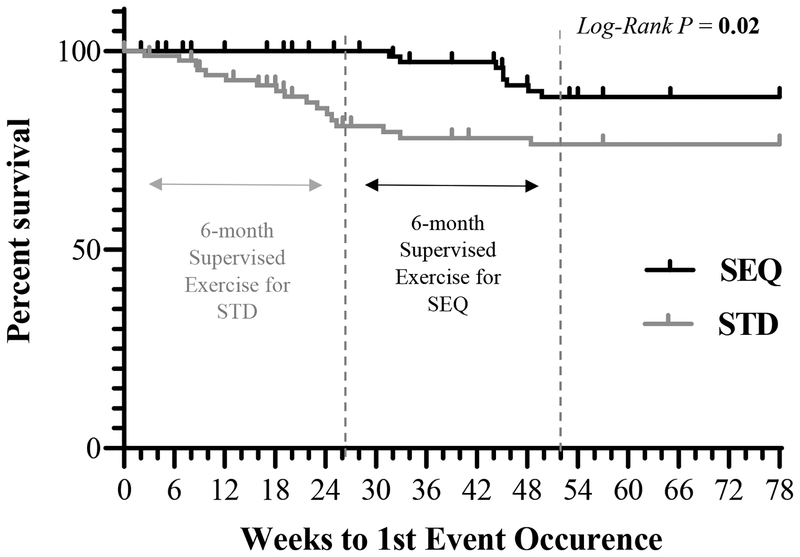
During the 18-month weight loss intervention, there were a total of 28 (19 STD, 9 SEQ) exercise-related AEs reported by 25 participants (17 STD, 8 SEQ), all of which were mild or moderate in severity. The majority (75%) of exercise-related AEs occurred during the 6-month supervised exercise phase and only 32% resulted in participants abstaining from exercise for >1 week. Over the 6-month supervised exercise program, there were no between-group differences in incidence of exercise-related AEs (AE-free survival probability±SE; STD 0.84±0.04, SEQ 0.90±0.04, P=0.30). Over 18 months, STD had a higher incidence of exercise-related AEs than SEQ (0.76±0.05, 0.88±0.04, P=0.02; Figure 5).
Figure 5: Survival Proportions for Exercise-Related Adverse Events by Randomized Group a-c.
a Results from Kaplan-Meier analysis, where survival was censored (tick marks) by either withdrawal week or at week 78 for completers; Statistically significant P values (P <0.05) are indicated in bold; The first dotted line indicates the month 6 mark; The second dotted line indicates the month 12 mark; SEQ: Sequential; STD: Standard.
b Of the total exercise-related adverse events (n=28), 89% were musculoskeletal (11 knee pain, 3 back pain, 3 foot pain, 2 hip pain, 2 plantar fasciitis, 2 foot/ankle sprain, 1 calf pain, 1 meniscus tear) and 11% were cardiopulmonary (2 instances of shortness of breath due to asthma, 1 instance of palpitations due to premature ventricular contractions).
c Of the total exercise-related adverse events (n=28), 43% were determined to be due to an acute injury, while the remainder were determined to be an overuse injury and/or exacerbation of a pre-existing condition.
Diet-Related Outcomes
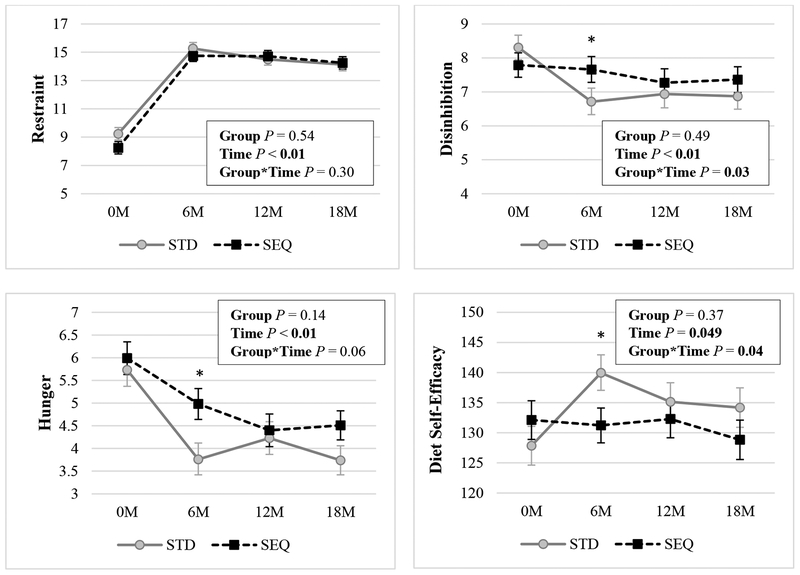
Attendance at group meetings was not different between groups over the 18-month intervention (mean±SD, STD 80±21%, SEQ 80±24%). At 18 months, EI decreased in both STD (1763±55 to 1531±53 kcal/d; P<0.01) and SEQ (1830±55 to 1465±57 kcal/d; P<0.01). There were no between-group differences in change in EI or dietary macronutrient content (% fat, carbohydrate, protein) at 18 months. Changes in dietary behaviors are displayed in Figure 6. Dietary restraint increased in both groups at 6, 12, and 18 months; there were no between-group differences. Disinhibition decreased in STD at 6, 12, and 18 months, but there were no changes in SEQ, resulting in a significant overall group by time interaction. Hunger decreased in both groups at 6, 12, and 18 months; at month 6 only, STD exhibited a greater decrease in hunger compared to SEQ. Diet self-efficacy increased in STD at 6 and 12 months; but there were no changes in SEQ; at month 6 only, STD exhibited a greater increase in diet self-efficacy compared to SEQ.
Figure 6: Changes in Dietary Behaviors by Randomized Group a-e.
a Results (mean ± SEM) are from linear mixed effect model with unstructured covariance using an intent-to-treat analysis; Statistically significant P values (P <0.05) are indicated in bold; SEQ: Sequential; STD: Standard.
b Sample sizes for Restraint are: STD: n=84 at 0M, n=65 at 6M, n=61 at 12M, n=59 at 18M; SEQ: n=84 at 0M, n=74 at 6M, n=61 at 12M, n=59 at 18M; scale is 0 to 21.
c Sample sizes for Disinhibition are: STD: n=83 at 0M, n=65 at 6M, n=60 at 12M, n=59 at 18M; SEQ: n=83 at 0M, n=74 at 6M, n=61 at 12M, n=59 at 18M; scale is 0 to 16.
d Sample sizes for Hunger are: STD: n=83 at 0M, n=65 at 6M, n=61 at 12M, n=59 at 18M; SEQ: n=85 at 0M, n=74 at 6M, n=61 at 12M, n=59 at 18M; scale is 0 to 14.
e Sample sizes for Diet Self-Efficacy are: STD: n=83 at 0M, n=66 at 6M, n=60 at 12M, n=59 at 18M; SEQ: n=84 at 0M, n=73 at 6M, n=63 at 12M, n=58 at 18M; scale is 0 to 180.
* indicates p<0.05 for the differences of STD – SEQ in change in the parameter from baseline.
** indicates p<0.01 for the differences of STD – SEQ in change in the parameter from baseline.
Discussion
While there is substantial evidence that PA is important for weight loss maintenance, how to enhance adherence to high levels of PA remains a critical question. Few studies have considered the importance of timing of the exercise intervention relative to the dietary intervention for long-term weight loss. In this study, changes in body weight, body composition, BP, PA, and fitness at 18 months did not differ between immediate versus delayed exercise initiation within a behavioral weight loss program.
It has been a long-held belief that programs targeting diet and PA simultaneously may achieve enhanced outcomes (35), as these behaviors are highly correlated (36, 37). However, some studies have suggested sequential interventions may result in improved adoption of diet and PA behaviors compared to programs that delivered these interventions simultaneously (16, 17). For example, Vandelanotte and colleagues (16) reported that sequential delivery was slightly more effective in producing sustained increases in PA and decreases in fat intake after two years. The Make Better Choices 2 Trial (17), a multicomponent mHealth intervention targeting increases in fruit and vegetable intake and MVPA, produced a composite improvement in diet and PA when interventions were delivered sequentially. However, weight loss was not an outcome in either study. In the current study, both simultaneous and sequential delivery of diet and exercise interventions resulted in clinically meaningful weight loss at 18 months, with no between-group differences. Our findings are similar to those of Goodpaster and colleagues, the only previous study that has evaluated the impact of timing of PA initiation on weight loss (18). In that study, initial initiation of PA resulted in greater weight loss at 6 months compared to delayed PA, but weight loss at 12 months was not different (18). In our study, STD exhibited greater weight loss at 6 months, but regained weight between months 6–12, whereas SEQ exhibited continued weight loss between months 6–12. Weight loss at 12 and 18 months was not different between groups. The greater initial weight loss in STD was likely due to the early addition of exercise to the dietary intervention in months 0–6, which was shown previously (34). The continued weight loss in SEQ during months 6–12 was likely due to the later occurrence of supervised exercise (and concomitant support/contact with study staff) during months 7–12. Similarly, Jakicic and colleagues (38) found that within a behavioral weight loss program targeting concurrent initial changes in diet and PA, later delivery of enhanced PA support strategies resulted in greater improvements in weight and fitness (but not PA) at 18 months as compared to a group that received these strategies earlier.
One explanation for why weight loss was not different at 18 months is the lack of between-group differences in changes in free-living PA. We hypothesized that SEQ would exhibit a greater increase in free-living PA by starting exercise at a lower body weight, as previous literature suggests (39, 40). Despite a 7.4% weight loss prior to initiating exercise, there was no between-group difference in changes in bout MVPA over the supervised exercise program (both groups increased bout MVPA by ~25 minutes/day). At 18 months, both groups maintained an increase in bout MVPA of ~10 minutes/day above baseline values. We also hypothesized that SEQ would be more adherent to the supervised exercise program due to weight-loss induced improvements in exercise perceptions, as previous literature suggested (13, 14). However, SEQ did not demonstrate significant improvements in perceived benefits and enjoyment of exercise after 6 months of diet-induced weight loss. Further, adherence to the subsequent 6-month supervised exercise program was lower in SEQ versus STD. Moreover, only STD exhibited an increase in perceived benefits and enjoyment of exercise over the 18-month intervention. Because individuals in SEQ achieved weight loss without exercise at 6 months, they may not have related their weight loss success to engaging in exercise and thus, did not exhibit the improvements in benefits and enjoyment of exercise observed in STD. Alternatively, the lack of significant increases in perceived exercise benefits and exercise enjoyment in SEQ may have been due to higher baseline levels of these parameters in this group. Exercise self-efficacy decreased in both groups, consistent with prior studies indicating that exercise self-efficacy may be artificially inflated prior to starting an exercise intervention (41).
Because injuries are a barrier to maintaining exercise (42), reducing weight prior to initiating an exercise program could facilitate exercise adherence and reduce attrition. Janney and Jakicic (15) found that participants with a higher baseline BMI exhibited an increased odds of exercise-related injuries compared to participants with a lower BMI. Despite starting the supervised exercise program at a lower weight, SEQ had a similar number of exercise-related AEs during this phase, although there were fewer exercise-related AEs in SEQ over the 18 month intervention. This latter observation may be due to the lower overall duration of exercise exposure in SEQ versus STD.
Both groups demonstrated similar reductions in dietary EI and self-reported hunger, and increases in dietary restraint over 18 months. However, STD exhibited greater increases in diet self-efficacy and decreases in hunger and disinhibition at 6 months. These results suggest that simultaneous initiation of diet and exercise interventions induced greater initial improvements in hunger, diet self-efficacy, and disinhibition compared to sequential delivery of interventions. Previous work by our colleagues suggests that in individuals with overweight/obesity, neuronal responses to food cues are attenuated after 6 months of exercise, however, there was no dietary intervention in that study (43). Despite these greater improvements in appetitive constructs observed in STD, this did not translate to greater reductions in EI or greater weight loss at 18 months; whether this would impact longer-term weight loss maintenance is unknown.
Although the supervised exercise program may limit generalizability, this program represents a strength in study design as exercise adherence in individuals with overweight/obesity is typically low when exercise is unsupervised (11, 12). Because the study sample was predominantly female (84%) and middle-aged (39±9 years), these findings may not be generalizable to males or to older adults. Despite being asked not to change exercise habits during months 0–6, SEQ exhibited a small, but significant increase in total MVPA (~9 minutes/day; P=0.03) at month 6. It is possible that some participants in SEQ were not fully compliant with the recommendation to delay exercise. Alternatively, it is also possible that movement became easier as these individuals lost weight. Lastly, while STD performed activity for a greater period of time (by design) it is unknown whether this differentially impacted other metabolic outcomes not measured in this study (e.g. lipids, insulin sensitivity).
Conclusion
Although SEQ had less overall exposure to exercise, both interventions resulted in clinically meaningful weight loss and increases in PA, with no significant between-group differences in these parameters at 18 months. Benefits of simultaneous initiation of diet and exercise interventions included greater weight loss and improvements in appetitive measures at 6 months, as well as enhanced adherence to the 6-month supervised exercise program. Conversely, benefits of sequential initiation of diet then exercise included continued weight loss during months 6–12 and fewer overall exercise-related AEs. Thus, consideration of these differential benefits along with individual-specific factors and preferences may be used to guide the timing of exercise initiation within a behavioral weight loss program.
Supplementary Material
STUDY IMPORTANCE QUESTIONS.
What is already known about this subject?
Typical behavioral weight loss programs recommend simultaneously reducing energy intake and increasing levels of exercise; however, changing two health behaviors at the same time may be challenging for some individuals.
Many individuals with overweight/obesity are unable to sustain changes in diet and exercise behaviors.
Delivering diet and exercise interventions in a sequential manner may enhance adherence to diet and exercise behaviors and improve weight loss.
What does your study add?
Both simultaneous and sequential delivery of diet and exercise interventions resulted in clinically meaningful weight loss and improvements in physical activity at 18 months.
Although weight loss at 6 months was greater when diet and exercise interventions were initiated simultaneously, there was no difference in long-term changes in body weight, body composition, device-measured physical activity, self-reported dietary energy intake, or program attrition between simultaneous and sequential delivery of diet and exercise interventions.
Based on these results, we suggest that individual-specific factors and preferences may be used to guide the timing of exercise initiation within a behavioral weight loss program.
Acknowledgements
We would like to thank Wendy Kohrt, PhD, Jere’ Hamilton, and the staff of the Colorado NORC Energy Balance Core; Janine Higgins, PhD and the staff of the CCTSI Nutrition Core; Elizabeth Kealey, RD and the Colorado NORC Clinical Intervention and Translation Core; Joseph Quatrochi, PhD and student interns from the Metropolitan State University Exercise Science Program; James O. Hill, PhD, Jan Lande, Jeanne Paradeis, Luciana Smith, and the staff of the AHWC fitness facility.
De-identified individual participant data that underlie the results reported in this article will be made available for 5 years following article publication to researchers who provide methodologically sound proposals. To gain access, data requestors will need to sign a data sharing agreement. Proposals should be directed to vicki.catenacci@ucdenver.edu.
FUNDING: This work was supported by grants from the National Institutes of Health: R01 DK097266, P30 DK048520, UL1 TR002535, T32 HL116276. Dr. Melanson is supported by resources from the Geriatric Research, Education, and the Clinical Center at the Denver VA Medical Center. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government
DISCLOSURE: HW is a partner in Shakabuku LLC, a company that provides weight management services, and Dr. Holly LLC, a company where HW provides keynote addresses and motivational speaking, outside the submitted work; HW accepts royalties from Rodale, Inc., from her book, “State of Slim”, and from Up to Date for the section HW wrote on obesity treatment, outside of the submitted work; HW receives unrelated grants from Novo Nordisk, National Cattleman’s Association, Gelesis, and DuPont. SP has an unrelated grant from WW (Weight Watchers) International. DB reports grants from University of Colorado during the conduct of the study.
Footnotes
CLINICAL TRIAL REGISTRATION:
References
- 1.National Heart Lung and Blood Institute of the National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. Obes Res. 1998;6 Suppl 2:51S–209S. [PubMed] [Google Scholar]
- 2.Eckel RH. Clinical practice. Nonsurgical management of obesity in adults. N Engl J Med. 2008;358(18):1941–1950. doi: 10.1056/NEJMcp0801652. [DOI] [PubMed] [Google Scholar]
- 3.Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119(7 Pt 2):688–693. [DOI] [PubMed] [Google Scholar]
- 4.Stunkard A, Mc L-H M. The results of treatment for obesity: A review of the literature and report of a series. AMA Arch Intern Med. 1959;103(1):79–85. [DOI] [PubMed] [Google Scholar]
- 5.Jakicic JM, Tate DF, Lang W, et al. Objective physical activity and weight loss in adults: The step-up randomized clinical trial. Obesity (Silver Spring). 2014;22(11):2284–2292. doi: 10.1002/oby.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am J Clin Nutr. 2007;85(4):954–959. [DOI] [PubMed] [Google Scholar]
- 7.Unick JL, Jakicic JM, Marcus BH. Contribution of behavior intervention components to 24-month weight loss. Med Sci Sports Exerc. 2010;42(4):745–753. doi: 10.1249/MSS.0b013e3181bd1a57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pescatello LS, American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- 9.2018 Physical Activity Guidelines Committee. 2018 physical activity guidelines advisory committee scientific report. Washington, DC: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 10.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168(14):1550–1559; 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: A randomized trial. JAMA. 1999;282(16):1554–1560. [DOI] [PubMed] [Google Scholar]
- 12.Colley RC, Hills AP, O’Moore-Sullivan TM, Hickman IJ, Prins JB, Byrne NM. Variability in adherence to an unsupervised exercise prescription in obese women. Int J Obes (Lond). 2008;32(5):837–844. doi: 10.1038/sj.ijo.0803799. [DOI] [PubMed] [Google Scholar]
- 13.Larsson UE, Mattsson E. Influence of weight loss programmes on walking speed and relative oxygen cost (%vo2max) in obese women during walking. J Rehabil Med. 2003;35(2):91–97. Epub 2003/04/15. [DOI] [PubMed] [Google Scholar]
- 14.Vuorinen AL, Strahilevitz MA, Wansink B, Safer DL. Shifts in the enjoyment of healthy and unhealthy behaviors affect short- and long-term postbariatric weight loss(dagger). Bariatr Surg Pract Patient Care. 2017;12(1):35–42. doi: 10.1089/bari.2016.0036.a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janney CA, Jakicic JM. The influence of exercise and bmi on injuries and illnesses in overweight and obese individuals: A randomized control trial. Int J Behav Nutr Phys Act. 2010;7:1. doi: 10.1186/1479-5868-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandelanotte C, De Bourdeaudhuij I, Brug J. Two-year follow-up of sequential and simultaneous interactive computer-tailored interventions for increasing physical activity and decreasing fat intake. Ann Behav Med. 2007;33(2):213–219. doi: 10.1007/bf02879903. [DOI] [PubMed] [Google Scholar]
- 17.Spring B, Pellegrini C, McFadden HG, et al. Multicomponent mhealth intervention for large, sustained change in multiple diet and activity risk behaviors: The make better choices 2 randomized controlled trial. J Med Internet Res. 2018;20(6):e10528. doi: 10.2196/10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA. 2010;304(16):1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt HR, Jortberg BT, Babbel C, et al. Weight loss in a community initiative that promotes decreased energy intake and increased physical activity and dairy consumption: Calcium weighs-in. J Phys Act Health. 2008;5(1):28–44. [DOI] [PubMed] [Google Scholar]
- 20.Peters JC, Beck J, Cardel M, et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: A randomized clinical trial. Obesity (Silver Spring). 2016;24(2):297–304. doi: 10.1002/oby.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring). 2014;22(6):1415–1421. doi: 10.1002/oby.20737. [DOI] [PubMed] [Google Scholar]
- 22.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. [DOI] [PubMed] [Google Scholar]
- 23.Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med Sci Sports Exerc. 2010;42(11):2134–2140. doi: 10.1249/MSS.0b013e3181e0b3ff. [DOI] [PubMed] [Google Scholar]
- 24.Brazeau AS, Karelis AD, Mignault D, Lacroix MJ, Prud’homme D, Rabasa-Lhoret R. Test-retest reliability of a portable monitor to assess energy expenditure. Appl Physiol Nutr Metab. 2011;36(3):339–343. 10.1139/h11-016. [DOI] [PubMed] [Google Scholar]
- 25.Fruin ML, Rankin JW. Validity of a multi-sensor armband in estimating rest and exercise energy expenditure. Med Sci Sports Exerc. 2004;36(6):1063–1069. [DOI] [PubMed] [Google Scholar]
- 26.St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr. 2007;85(3):742–749. [DOI] [PubMed] [Google Scholar]
- 27.Berntsen S, Hageberg R, Aandstad A, et al. Validity of physical activity monitors in adults participating in free-living activities. Br J Sports Med. 2010;44(9):657–664. doi: 10.1136/bjsm.2008.048868. [DOI] [PubMed] [Google Scholar]
- 28.Pollock ML, Wilmore JH, Fox SM. Exercise in health and disease. Philadelphia, PA: Saunders; 1984. [Google Scholar]
- 29.Motl RW, Dishman RK. Measuring enjoyment of physical activity in adolescent girls (vol 21, pg 110, 2001). Am J Prev Med. 2001;21(4):332–332. doi: Doi 10.1016/S0749-3797(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 30.Sechrist KR, Walker SN, Pender NJ. Development and psychometric evaluation of the exercise benefits/barriers scale. Res Nurs Health. 1987;10(6):357–365. [DOI] [PubMed] [Google Scholar]
- 31.McAuley E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. J Behav Med. 1992;15(1):65–88. [DOI] [PubMed] [Google Scholar]
- 32.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. [DOI] [PubMed] [Google Scholar]
- 33.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. J Consult Clin Psychol. 1991;59(5):739–744. [DOI] [PubMed] [Google Scholar]
- 34.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring). 2012;20(8):1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkins D, Clancy C. Multiple risk factors interventions. Are we up to the challenge? Am J Prev Med. 2004;27(2 Suppl):102–103. doi: 10.1016/j.amepre.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Gillman MW, Pinto BM, Tennstedt S, Glanz K, Marcus B, Friedman RH. Relationships of physical activity with dietary behaviors among adults. Prev Med. 2001;32(3):295–301. doi: 10.1006/pmed.2000.0812. [DOI] [PubMed] [Google Scholar]
- 37.Jakicic JM, Wing RR, Winters-Hart C. Relationship of physical activity to eating behaviors and weight loss in women. Med Sci Sports Exerc. 2002;34(10):1653–1659. doi: 10.1249/01.MSS.0000031483.65271.FD. [DOI] [PubMed] [Google Scholar]
- 38.Jakicic JM, Rickman AD, Lang W, et al. Time-based physical activity interventions for weight loss: A randomized trial. Med Sci Sports Exerc. 2015;47(5):1061–1069. doi: 10.1249/MSS.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobi D, Ciangura C, Couet C, Oppert JM. Physical activity and weight loss following bariatric surgery. Obes Rev. 2011;12(5):366–377. doi: 10.1111/j.1467-789X.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 40.King WC, Hsu JY, Belle SH, et al. Pre- to postoperative changes in physical activity: Report from the longitudinal assessment of bariatric surgery-2 (labs-2). Surg Obes Relat Dis. 2012;8(5):522–532. doi: 10.1016/j.soard.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fortier MS, Duda JL, Guerin E, Teixeira PJ. Promoting physical activity: Development and testing of self-determination theory-based interventions. Int J Behav Nutr Phys Act. 2012;9:20. doi: 10.1186/1479-5868-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tulloch H, Sweet SN, Fortier M, Capstick G, Kenny GP, Sigal RJ. Exercise facilitators and barriers from adoption to maintenance in the diabetes aerobic and resistance exercise trial. Can J Diabetes. 2013;37(6):367–374. doi: 10.1016/j.jcjd.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Cornier MA, McFadden KL, Thomas EA, et al. Differences in the neuronal response to food in obesity-resistant as compared to obesity-prone individuals. Physiol Behav. 2013;110–111: 122–128. doi: 10.1016/j.physbeh.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.