Abstract
Purpose
To estimate the population-based incidence of HPV vaccination after childhood cancer.
Methods
Pediatric and young adult cancer survivors identified in the institutional Comprehensive Cancer Center registry were linked to the North Carolina Immunization Registry (NCIR). Initiation and completion of any HPV vaccine was evaluated in survivors born between 1984 and 2002 with an NCIR record by December 2014. Descriptive statistics and Kaplan-Meier estimates of cumulative incidence were stratified by sex and age at eligibility for vaccine. Cox proportional hazards were conducted and stratified by sex.
Results
Among 879 (n = 428 female; n = 451 male) study-eligible cancer survivors without prior HPV vaccination (n = 501 < 18 years, n = 378 ≥ 18 years at the time of eligibility), the cumulative incidence of HPV vaccine initiation following cancer therapy was 48.1% among females at 8.2 years and 29.2% among males at 5.0 years after vaccine eligibility among those < 18 years, and 6.2% among females at 8.1 years and 2.0% among males at 4.2 years after vaccine eligibility among those ≥ 18 years. Among those who initiated vaccination, 53% of females and 43% of males completed a 3-dose series. Younger age at cancer diagnosis (≤ 10 and 11–14 years vs. ≥ 15 years) and shorter interval from diagnosis to vaccine eligibility were more likely to initiate vaccination in models adjusted for age at eligibility, race/ethnicity, cancer type, relapse, and transplant.
Conclusions
Despite the benefit of a cancer prevention strategy, cancer survivors are sub-optimally vaccinated against HPV.
Implications for Cancer Survivors
Immunization registries can help oncologists and primary care providers identify gaps in vaccination and target HPV vaccine delivery in survivors.
Keywords: Human papilloma virus (HPV) vaccine, Childhood cancer, Adolescent and young adult (AYA) cancer survivor, Vaccine-preventable disease, Immunization registry
Introduction
The human papillomavirus (HPV) vaccine is endorsed as a cancer prevention vaccine, [1] and the quadrivalent vaccine was approved by the Food and Drug Administration (FDA) for females in 2006 and for males in 2009. The Advisory Committee on Immunization Practices (ACIP) first recommended the quadrivalent vaccine for females in 2007 and extended the recommendation to include males in 2011 [1, 2]. The ACIP recommendation recently evolved to routine HPV vaccination with the 9-valent vaccine initiated at the age of 11 or 12 years, and notes the vaccination series can be started as early as the age of 9 years. Vaccination is currently recommended for females aged 13 to 26 years, males aged 13 to 26 years who have not been vaccinated previously or who have not completed the 3-dose series, and males aged 22 to 26 who are at high risk for HPV acquisition [3, 4]. Despite the Healthy People 2020 goal of 80% vaccine completion for adolescents 13–15 years of age, HPV vaccination completion of 3 or more doses plateaued at 41.9% (confidence interval [CI] 40.1–43.7) in 2015 among US adolescent (13–17 years) females and at 28.1% (CI 26.6–29.7) for adolescent males [5].
In a recent analysis from the Childhood Cancer Survivor Study, the 30-year cumulative incidence of HPV-associated SMN was 0.2%, presenting at a median age of 27 years and representing a standardized incidence ratio (SIR) of 2.8 in cancer survivors compared with the general population [6]. Adolescent and young adult (AYA) cancer survivors represent a unique and growing part of the population who are at risk for a second malignant neoplasm (SMN) over their lifetime [7–10], and who will therefore benefit from a vaccine with cancer prevention potential. HPV vaccination, which provides protection from HPV infection, HPV-mediated cervical cancer in women, and genitourinary (GU) and head and neck cancers in males, is germane to this population. The HPV vaccine is recommended for prevention of second cancers in both males and females in the current Children’s Oncology Group Long-Term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers [11–13]. Single institution studies of self-reported uptake of HPV vaccine limited to female childhood cancer survivors indicate HPV initiation of 32–33% [14, 15]. An expanded multi-institution study among both female and male childhood cancer survivors notes that among survivors 13 to 17 years of age, HPV vaccine initiation was self-reported as significantly lower than the general population (females, 32.6% vs. 51.9%, p < 0.001; males, 14.0% vs. 33.8%, p < 0.001). Young adult (18–26 years) female survivors reported higher HPV vaccine initiation than the general population (45.7% vs. 38.2%; p = 0.04), while rates among male survivors were similarly low when compared with the general population (7.5% vs. 8.3%; p = 0.7) [16].
Traditionally, the ACIP recommended immunization schedules in children and adolescents depend on age. However, these schedules are often altered for children and adolescents on active cancer therapy [17, 18], as most routine vaccinations are stopped during treatment. A cancer survivor usually re-initiates routine vaccinations 3 to 6 months after completion of therapy, due to the immunosuppression that ensues from cancer chemotherapy [19]. Estimates of vaccine uptake in cancer survivors therefore need to account for the complexity of periods of ineligibility for vaccine delivery due to both age at cancer diagnosis (relative to ACIP eligibility age) and time since completion of therapy. In the initial period of HPV vaccine availability, eligibility windows were restricted by the upper age bound (age 26 years) of ACIP recommendations. Cancer survivors have variable periods of care under the pediatric oncologist, before transition to the primary care physician; hence, the provider responsible for advocacy and for vaccine initiation is sometimes uncertain [16, 20, 21]. There are no population-based studies to date that validate the self-reported uptake of HPV vaccine, while considering periods of eligibility for vaccination in cancer survivors.
The North Carolina Immunization Registry (NCIR) is a publicly funded web-based tool that has served as the official source for North Carolina immunization information since 2005 [22]. Given that the ACIP recommendation for routine HPV vaccination in females was made in 2007 and for males in 2011, the registry has the potential to be evaluated for HPV vaccination status which can inform interventions to increase vaccination. The registry is utilized for vaccination reporting as part of the Vaccines for Children (VFC) federal program [23] and captures immunization records for infants and young children across the state. The NCIR is utilized for provider reporting of vaccination by all state health departments and by more than 90% of pediatric and family medicine practices. It is a valid source for estimating population vaccination rates in children < 18 years of age [22, 24].
Understanding the pattern and timing of HPV vaccine up-take in cancer survivors will facilitate interventions to improve vaccination rates in this population. The objective of this study was to use linkage to the NCIR to estimate the rate of HPV vaccine initiation and completion, accounting for periods of ineligibility for vaccine delivery due to immunosuppression during cancer treatment in AYA cancer survivors.
Methods
Following approval by the Wake Forest School of Medicine institutional review board, the Wake Forest Comprehensive Cancer Center registry and a childhood cancer survivorship clinic database were searched to identify eligible cases. Eligibility criteria included a North Carolina home address at time of diagnosis and at the time of the study abstraction, and age 26 years or younger at the time of cancer diagnosis. Based on the ACIP age recommendation for HPV vaccination, we identified females and males born between January 1, 1984 and December 31, 2002 as potentially eligible. These dates of birth allowed capture of the differing gender-based age recommendations by the ACIP during the time the study was completed. Exclusion criteria for the cohort for analysis were non-malignant pathology, a diagnosis of cervical cancer, less than 6 months of follow-up after completion of therapy (as vaccines are typically not administered until 3–6 months after immunosuppressive cancer therapy), death before age of eligibility to receive the HPV vaccine, never being cancer free during the window of eligibility to receive the HPV vaccine, and inability to find treatment information in the medical record.
Valid linkage in the NCIR was confirmed by verification of match on patient name, date of birth, and address in institutional records to immunization registry record. Cases were excluded if they were not located in the NCIR, if the NCIR record was devoid of any vaccination entry, or name and date of birth in the NCIR could not be matched to those in either the cancer registry or the institutional electronic health record (EHR).
Based on FDA approval dates, NCIR data from 6/8/2006 and 10/16/2009 forward was utilized for females and males, respectively. Dates of receipt of HPV vaccine were abstracted from the NCIR between June and December 2014. Demographic characteristics, initial cancer diagnosis, and treatment information (including treatment type, and dates of initiation and cessation of treatment for the initial cancer diagnosis, and relapsed disease when applicable) were abstracted from the EHR. The start of the eligibility window for HPV vaccination for each case was the latest of the following: the date of eligibility for vaccine (either 11 years of age or date of vaccine approval by sex); 6 months after cancer treatment (surgery, chemotherapy, radiation); or 12 months following hematopoietic stem transplantation [19]. The end of the eligibility window for vaccine initiation for each subject was determined by the first of the following: the date of receipt of first dose of HPV vaccine, the date of abstraction from NCIR, the date of cancer recurrence or second malignancy, the date of death, or attained age ≥ 27 at linkage. Data was managed using Research Electronic Data Capture (REDCap), which is a secure web application [25].
Descriptive statistics of the characteristics of cancer survivors were conducted, using mean and standard deviation for continuous variables or frequency and percentage for categorical variables. The Kaplan-Meier method was applied to estimate the cumulative incidence of HPV vaccination initiation, stratified by age and sex during the eligibility window and age at eligibility. Cox proportional hazards models stratified by sex were used to estimate adjusted hazard ratios (HR) and 95% confidence intervals (CI) for HPV vaccine initiation adjusted for age at eligibility (11–17 years, 18–26 years), age at diagnosis (≤ 10 years, 11–14 years, ≥ 15 years), time between diagnosis and eligibility for HPV vaccination, relapse history, transplant history, cancer type (leukemia, Hodgkin or non-Hodgkin lymphoma vs. others), and race/ethnicity (non-Hispanic white vs. others). HPV vaccine completion rates were captured among initiators. All analyses were conducted using SAS (v 9.4, Cary, NC) and a two-sided alpha level of 0.05 was used.
Results
Among 2610 potentially eligible cases identified in the institutional cancer registry and clinic database, 1088 met inclusion and exclusion criteria prior to NCIR linkage (Supplemental Figure 1). An NCIR record was found for 94.9% (n = 1033/1088) of those who met eligibility criteria (98.0% < 18 years, and 91.7% ≥ 18 years at the time of eligibility for vaccine); and of those 91.9% (n = 949) had at least 1 vaccine noted in their record (96.5%, 530/549, < 18 years, and 86.5%, n = 419/484 ≥ 18 years) (Supplemental Figure 1). Of 234 cases who were age eligible for HPV vaccination prior to a cancer diagnosis, 19% (n = 45) had received the vaccine and were excluded from the incident analysis, including 23.5% (n = 20/85) of those < 18 years and 16.8% (n = 25/149) of those ≥ 18 years. The evaluable cohort included 879 (n = 428 female; n = 451 male) cancer survivors with an identifiable window of eligibility for vaccine receipt; of those, 501 (57%) were < 18 years and 378 (43%) were ≥ 18 years at the beginning of eligibility (Supplemental Figure 1, Table 1).
Table 1.
Characteristics of childhood and adolescent and young adult (AYA) cancer survivors eligible for receipt of human papillomavirus (HPV) vaccine after therapy
| Female n = 428 n (%) | Female < 18 years n = 259 n (%) | Female ≥ 18 years n = 169 n (%) | Male n = 451 n (%) | Male < 18 years n = 242 n (%) | Male ≥ 18 years n = 209 n (%) | |
|---|---|---|---|---|---|---|
| Age (years) in at NCIR linkage* | ||||||
| 11–12 | 14 (3.3) | 14 (5.4) | 0 (0.0) | 26 (5.8) | 26 (10.7) | 0 (0.0) |
| 13–17 | 84 (19.6) | 84 (32.4) | 0 (0.0) | 112(24.8) | 112(46.3) | 0 (0.0) |
| ≥18 | 330 (77.1) | 161 (62.2) | 169 (100.0) | 312(69.2) | 104 (43.0) | 209 (100.0) |
| Age (years) at cancer diagnosis | ||||||
| ≤10 | 232 (54.2) | 191 (73.7) | 41 (24.3) | 269 (59.7) | 191 (78.9) | 78 (37.3) |
| 11–14 | 70 (16.4) | 54 (20.8) | 16 (9.5) | 66 (14.6) | 32 (13.2) | 34(16.3) |
| ≥15 | 126 (29.4) | 14 (5.4) | 112 (66.3) | 116(25.7) | 19 (7.9) | 97 (46.4) |
| Race/ethnicity | ||||||
| Non-Hispanic/White | 342 (80.0) | 207 (79.9) | 135 (79.9) | 347 (76.9) | 175 (72.3) | 172 (82.3) |
| Black | 53 (12.4) | 27 (10.4) | 25 (14.8) | 65 (14.4) | 39 (16.1) | 26 (12.4) |
| Asian | 3 (0.7) | 2 (0.8) | 1 (0.6) | 8(1.8) | 4(1.7) | 4 (1.9) |
| Hispanic | 24 (5.6) | 18(7.0) | 6 (3.4) | 26 (5.8) | 20 (8.3) | 6 (2.9) |
| Other/unknown | 7 (1.6) | 5 (1.9) | 2(1.2) | 5(1.1) | 4(1.7) | 1 (0.5) |
| Cancer diagnosis | ||||||
| Leukemia | 105 (24.5) | 82(31.7) | 23 (13.6) | 137 (30.4) | 80 (33.1) | 57 (27.3) |
| Lymphomaa,b | 65 (15.2) | 35 (13.5) | 30 (17.8) | 83 (18.4) | 38(15.7) | 45 (21.5) |
| Solid tumorc,d | 151 (35.3) | 107 (41.3) | 44 (26.0) | 168 (37.3) | 101 (41.7) | 67 (32.1) |
| Thyroid cancer | 28 (6.5) | 5 (1.9) | 23 (13.6) | 3 (0.7) | 0 (0.0) | 3 (1.4) |
| Melanoma | 17 (4.0) | 3 (1.2) | 14 (8.3) | 7(1.6) | 4(1.7) | 3 (1.4) |
| Other cancer | 62 (14.5) | 27 (10.4) | 35 (20.7) | 53 (11.8) | 19 (7.9) | 34(16.3) |
| Relapse | ||||||
| Yes | 37 (8.6) | 22 (8.5) | 15 (8.9) | 53 (11.8) | 24 (9.9) | 29 (13.9) |
| Receipt of HSCT | ||||||
| Yes | 27 (6.3) | 18(6.9) | 9 (5.3) | 28 (6.2) | 15 (6.2) | 13 (6.2) |
Males: missing n = 1; hematopoietic stem cell transplantation (HSCT)
Females: Hodgkin lymphoma, 28(6.5); non-Hodgkin lymphoma, 37 (8.6)
Males: Hodgkin lymphoma, 27 (6.0); non-Hodgkin lymphoma, 56 (12.4)
Females: CNS tumor, 65 (15.2); renal tumor, 22 (5.1); neuroblastoma, 22 (5.1); sarcoma, 42 (9.8)
Males: CNS tumor, 55 (12.2); renal tumor, 28 (6.2); neuroblastoma, 23 (5.1); sarcoma, 62 (13.7)
The mean age at cancer diagnosis was 10.3 years (standard deviation [SD] = 7.4 years) in females and 8.8 years (SD = 6.7 years) in males. The median time from cancer diagnosis to the start of the eligibility window for vaccination was 6.3 years (range, 0.5 to 23.8 years). The majority of cancer survivors were non-Hispanic white (80% males; 77% females), consistent with cancer center demographics. The majority of patients received chemotherapy as part of their treatment. Six percent of the cohort had received a hematopoietic stem cell transplant (HSCT) as part of therapy. The mean time from initial cancer diagnosis to NCIR linkage (i.e., matching patient in the immunization registry) was 11.7 years (SD = 6.6 years) for females and 12.1 years (SD = 6.3 years) for males. The distribution of cancer diagnoses is outlined in Table 1 and is consistent with that expected in a North American cohort of pediatric and AYA cancer survivors.
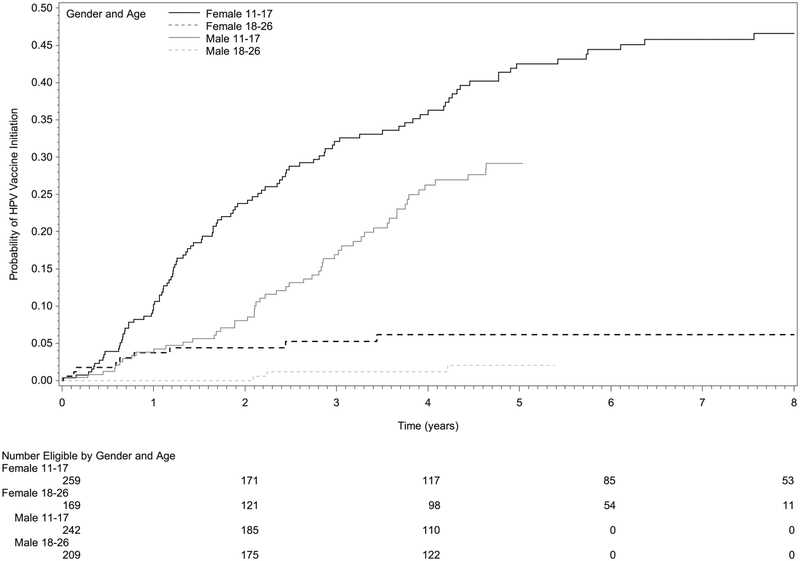
Taking into account periods of ineligibility for vaccination (e.g., during cancer therapy or projected periods of immunosuppression after therapy), the overall cumulative incidence of initiating HPV vaccination after cancer diagnosis was 34.1% among female survivors at 8 years post eligibility and 16.8% among male survivors at 5 years post eligibility. The mean age at the first HPV vaccine dose was 15.0 years (SD = 2.9 years) for females and 15.5 years (SD = 3.3 years) for males. When stratifying by age at eligibility, the cumulative incidence of vaccine initiation captured in NCIR was higher in younger cancer survivors (females 11–17 years 48.1%, 18–26 years 6.2%; males 11–17 years 29.2%; 18–26 years 2.0%; Table 2, Fig. 1).
Table 2.
Comparison of age- and sex-stratified cumulative incidence to incidence at 2.7 years follow-up of human papillomavirus (HPV) vaccine initiation among childhood and adolescent and young adult (AYA) cancer survivors after therapy
| Age at eligibility | Cumulative incidence | Incidence at 2.7 years follow-up |
|---|---|---|
| Female | ||
| 11–17 years | 48.1% | 29.3% |
| 18–26 years | 6.2% | 5.3% |
| Male | ||
| 11–17 years | 29.2% | 13.7% |
| 18–26 years | 2.0% | 1.1% |
Fig. 1.
Cumulative incidence of human papillomavirus (HPV) vaccine initiation among childhood and adolescent and young adult (AYA) cancer survivors, stratified by sex and age at vaccine eligibility. Time 0 (zero) represents the date the cancer survivor became eligible to receive the HPV vaccination based on age and completion of cancer treatment
After adjusting for age category at eligibility, race/ethnicity, treatment and cancer-related factors, and the time from diagnosis to HPV vaccine eligibility, females and males ≤ 10 years at cancer diagnosis were 3.1 times (95% CI 1.1, 8.6) and 4.0 times (95% CI 1.0, 15.6) respectively more likely to have initiated HPV vaccination compared with AYA cancer survivors aged ≥ 15 years at cancer diagnosis (Table 3). Notably, the longer the interval between cancer diagnosis and HPV vaccination eligibility, the less likely male cancer survivors were to initiate HPV vaccination (HR 0.92; 95% CI 0.86, 0.99; p = 0.03); time interval was not statistically significant for females (HR 0.95; 95% CI 0.90, 1.00; p = 0.07, Table 3). Male and female survivors who were 11–18 years at eligibility for vaccination were more likely to initiate vaccination than those ≥ 18 years (females HR 4.71; 95% CI 2.05, 10.84; males HR 9.18; 95% CI 2.56, 32.86). History of relapse status, type of cancer diagnosis, race/ethnicity, and receipt of HSCT were not significantly associated with HPV vaccine initiation (Table 3).
Table 3.
Adjusted hazard ratios (HR) for HPV vaccine initiation in childhood and adolescent and young adult (AYA) cancer survivors by sex
| Female n = 428 | Male n = 451 | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age category at eligibility (years) | ||||||
| 11–17 | 4.71 | 2.05, 10.84 | 0.0003 | 9.18 | 2.56, 32.86 | 0.0007 |
| 18–26 | 1.00 (ref) | 1.00 (ref) | ||||
| Age at cancer diagnosis (years) | ||||||
| ≤10 | 3.10 | 1.12, 8.60 | 0.0300 | 3.99 | 1.02, 15.63 | 0.0468 |
| 11–14 | 1.80 | 0.67, 4.86 | 0.24 | 2.27 | 0.56, 9.11 | 0.25 |
| ≥15 | 1.00 (ref) | 1.00 (ref) | ||||
| Time from cancer diagnosis to eligibility to receive HPV vaccination (years) | 0.95 | 0.90, 1.00 | 0.07 | 0.92 | 0.86, 0.99 | 0.03 |
| Relapse | ||||||
| Yes | 1.25 | 0.67, 2.33 | 0.49 | 0.71 | 0.25, 2.03 | 0.52 |
| Cancer diagnosis | ||||||
| Leukemia/lymphoma | 0.84 | 0.57, 1.24 | 0.38 | 0.90 | 0.53, 1.53 | 0.70 |
| Other cancera | 1.00 (ref) | 1.00 (ref) | ||||
| Race/ethnicity | ||||||
| Non-Hispanic/White | 0.82 | 0.53, 1.27 | 0.38 | 0.83 | 0.47, 1.47 | 0.53 |
| Otherb | 1.00 (ref) | 1.00 (ref) | ||||
| Receipt of hematopoietic stem cell transplantation (HSCT)c | ||||||
| Yes | 0.70 | 0.30, 1.64 | 0.41 | 0.59 | 0.14, 2.54 | 0.48 |
Solid tumor, thyroid cancer, melanoma, other cancer
Black, Asian, Hispanic, other/unknown
Prior to eligibility to receive HPV vaccination
Overall, among female cancer survivors who initiated HPV vaccination, 68.1% (77/113) completed two or more doses (Supplemental Figure 2). Among male cancer survivors who initiated HPV vaccination, 56.9% (33/58) completed two or more doses (Supplemental Figure 2). Among cancer survivors who completed 3 doses of vaccine, the mean age at dose 1 was 14.7 (SD = 2.8 years) for females and 15.8 years (SD = 3.4 years) for males.
Discussion
Childhood cancer survivors are vulnerable to HPV-associated cancer as a second malignancy; hence, efforts to understand patterns and predictors of HPV vaccination as a means of secondary cancer prevention need study. HPV vaccination prevalence rates estimated by self or parent-proxy report in patients attending survivorship clinics indicate that survivors have sub-optimal vaccine uptake, but the cross-sectional evaluation, short follow-up periods, and focus largely on female survivors [11, 14, 16, 26] support the need for further study. We present the first population-based estimates of HPV vaccine initiation after diagnosis within a childhood and AYA cancer cohort and confirm low rates of vaccination in males and females even with prolonged follow-up. We show that with increased time from cancer diagnosis, survivors are less likely to initiate vaccination. Importantly, vaccine registry linkage indicates those who are older at cancer diagnosis and not vaccinated prior to cancer diagnosis but become vaccine eligible at ≥ 18 years of age are less likely to initiate vaccination. Our study demonstrates the feasibility and utility of linking a state-based immunization registry to a comprehensive cancer center registry to assess HPV vaccination in child and AYA survivors, as 43% of survivors were >/= 18 years at the beginning of eligibility.
Among those < 18 years of age, the cumulative incidence of HPV vaccine initiation in cancer survivors was 48.1% among females at 8 years post eligibility and 29.2% among males 5 years post eligibility. These rates are both well below 2015 US population initiation rates of 62.8% (female CI 61.0–64.5) and 49.8% (male CI 48.0–51.6), among 13- to 17-year-olds in the National Immunization Survey-Teen [5].
Our approach linking a cancer registry to a state immunization registry allows a longitudinal view of the tempo from cancer diagnosis to uptake of vaccine. This methodologic approach which accounts for eligibility relative to both completion of cancer therapy and to ACIP recommended age precludes calculation of traditional “up-to-date” estimates for a vaccination. To directly compare our estimated vaccine initiation incidence rates to the adjusted rates reported by cancer survivors [16], we estimated 2.7-year incidence, and found NCIR-based rates comparable to the self-report in female (29.3% vs. 32.6%) and male (13.7% vs. 14.0%) cancer survivors < 18 years (Table 2) [14–16]. Notably, rates of vaccination continue to increase slightly over time following therapy, and while in the age eligibility window at the time, in a time period when the vaccine was recommended only up to age 26 years.
While there was no impact of cancer treatment, disease relapse status, or HSCT on vaccine initiation, age at diagnosis was a significant predictor of vaccine initiation in both sexes. While our NCIR registry rates in cancer survivors are comparable to the self-reported rates in those 11 to 17 years, the NCIR cumulative incidence of vaccine initiation in cancer survivors ≥ 18 years are dramatically lower than the general US population or than limited reports in older survivors [15, 26–35] despite the majority of the older cohort having being linked to a NCIR record with at least one other recorded vaccination. In contrast to survey-based findings, those younger at diagnosis in our cohort (≤ 10 years and 11–14 years at diagnosis) were significantly more likely than older age groups (≥ 15 years at diagnosis) to initiate HPV vaccination. The difference between our findings of younger age as a predictor of vaccine initiation compared with Klosky et al. could be explained by differences in study approach or to the source of vaccination report. Older teens and young adults motivated to be seen in a dedicated cancer survivorship program and participate in a vaccination study are likely a group who are more engaged in survivorship health and may be better insured. This group’s self-report may overestimate vaccine initiation rates [36]. Conversely, the NCIR may underestimate vaccination in those ≥ 18 years old at vaccine eligibility as this is the age threshold for national VFC coverage. However, given that 86% of those ≥ 18 in our cohort had a vaccine record, our findings highlight this as a population for intervention to enhance vaccine uptake.
Importantly, and of relevance to intervention needs, we note that increased time from cancer diagnosis to HPV vaccine eligibility was negatively associated with vaccine initiation. This underscores a window of opportunity for secondary cancer prevention initiatives while patients are still in pediatric oncology and survivorship care. This time factor, and the fact that many subspecialty clinics (and oncology clinics in particular) do not standardly resource non-influenza vaccines, highlights the need for: (1) clear communication and accountability between oncologists and primary care providers with regard to managing vaccine initiation and completion, and (2) interventions to deliver vaccines in the subspecialty setting.
The current study evaluated HPV vaccine initiation in cancer survivors during the era of early HPV vaccine availability when a 3-dose series of the quadrivalent vaccine was recommended. While it is encouraging that over half of cancer survivors who initiated vaccination completed ≥ 2 doses, completion rates for the 3-dose series was poor. This mirrors national statistics and self-report in survivors [16]. While 3 doses remains the series recommendation for immunosuppressed patients [1], studies of vaccine immunogenicity are needed in cancer survivors. Understanding immunogenicity specifically in cancer survivors with the introduction of the 9 valent 2-dose series in the general population who are < 15 years at initiation is important as we demonstrate that adherence to 3 doses is challenging.
Determining windows of eligibility for HPV vaccination in cancer survivors reflects the clinical reality of inability to vaccinate during periods of active immunosuppression, but presents methodologic challenges in retrospective analyses. Cancer relapse among survivors led to fluctuating windows of eligibility for vaccination relative to initial diagnosis, treatment duration, time of relapse, and ensuing re-initiation of cancer therapy. This intermittent eligibility is yet another source for gaps in provider delivery of vaccine. Notably, our results are incidence rates and not prevalence rates, as the small number of survivors who had initiated vaccination prior to cancer diagnosis were excluded from this analysis. Clinical guidelines for re-initiation of vaccine when dose series are interrupted by therapy or relapse are not available.
Some limitations of our study are that we could not account for vaccine contraindications, or parent- or patient-reported attitudes and beliefs regarding intent to vaccinate. While we utilized a single NCI designated cancer center, our results showing suboptimal rates of uptake of HPV vaccine were similar to studies using self-report in a multi-institution setting [14–16]. While reporting of vaccination through the NCIR is not mandated in North Carolina [37], the registry has been shown to be robust for capture of most vaccines in younger children. Validity of vaccine documentation in the NCIR has been demonstrated by a study of influenza immunization up-take, where 67–94% of children < 6 years of age had ≥ 2 recorded immunizations [38]. Due to the age eligibility window for HPV vaccine specifically, it is possible that some survivors were vaccinated by providers not in the VFC program or in a different state and therefore not mandated to report in the NCIR [22]. For example, HPV vaccination received from a pediatrician may be more likely to be reported to NCIR than a vaccine received from a gynecologist. The NCIR does not consistently indicate the location of vaccine receipt; therefore, we were not able to determine whether survivors who received the HPV vaccine did so during an oncologist visit, in survivorship clinic, at a primary care visit, or other location. Similarly, the NCIR lacks data on vaccine contraindications or exemptions. Lastly, our analysis did not adjust for: socioeconomic status or education levels (as a proxy for healthcare access), or religious preferences/beliefs (which may affect intent to initiate HPV vaccine), or dates of other adolescent vaccines.
Immunization registries, such as NCIR, are an important resource that can be used to help monitor and intervene toward improved HPV vaccine coverage, especially in younger high-risk populations [22]. The NCIR is a secure, population-based and web-based clinical tool that at the time of this study required direct data entry by medical practice personnel, such that information was unidirectional [39]. Bi-directional flow of data between immunization registries and the EHR in being piloted as a CMS meaningful use criteria is a driver of registry completeness and identifies gaps in immunization status in a timelier manner [40]. In the future, bi-directional flow of data between immunization registries and individual patient’s EHR can be utilized toward intervention strategies in childhood and AYA cancer survivors and other sub-specialty pediatric populations when they are eligible to receive the HPV vaccination [41]. Examples include standing orders, or other systems within the EHR to remind providers or members of targeted populations that vaccinations are due [40]. Such approaches can reduce missed opportunities for vaccination [42–44]. Studies of high-risk populations have utilized similar methods of immunization registry linkage, such as a study of pneumococcal and meningococcal vaccination in sickle cell disease patients [44]. An alternate approach is implementing standard orders in combination with age targeted “bundling” of the tetanus, diphtheria, and pertussis (Tdap), meningococcal conjugate (MCV4), and HPV vaccines to decrease missed opportunities for vaccination [42]. Another successful intervention strategy including subspecialty clinics at a large pediatric tertiary center indicates that patient navigators are efficacious when program is specific for: (1) provider education, (2) for HPV vaccination education to patients/parents, and (3) real-time delivery [45]. This type of intervention can play a crucial role, given that provider recommendation for HPV vaccination increases the likelihood of uptake and may be the most influential factor for vaccination [42, 43, 46–50].
There is a lack of interventions for HPV vaccination education or HPV vaccine implementation in pediatric subspecialty clinics which increasingly have a role in transition of care for AYA patients. Targeted capture of specific adolescent populations under care for chronic disease in specialty clinics may be one approach to closing the gap between current vaccination rates and the Healthy People 2020 target for HPV vaccine completion of 80% [14, 16]. An ongoing trial to evaluate vaccination and immune response to HPV vaccine in cancer survivors () may yield further information. In addition, recent consideration of expanding the age of eligibility for vaccination to 45 years will mean that family medicine clinicians and internists need to recognize cancer survivors in their clinics who have missed their earlier window to be vaccinated [51].
As cancer has become a chronic disease, coordination of cancer prevention strategies, such as vaccination, between cancer providers and the community care are essential [39, 52]. Future directions should consider interventions through immunization registries to target children and adolescents and to capture young adults at higher risk. Access to robust state immunization registries allows primary care providers and oncology and cancer survivorship clinics to assess gaps in vaccination, target vaccine delivery, and move closer to the Healthy People 2020 target of 80% HPV vaccine completion.
Supplementary Material
Acknowledgments
The authors would like to thank the Department of Emory Department of Pediatrics Grant and Manuscript Support Core.
Funding Wake Forest Vaccine Center grant (Castellino); Biostatistics and Bioinformatics Shared Resource, Wake Forest Comprehensive Cancer Center, and NCI Cancer Center Support Grant (P30 CA012197).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11764-019-00791-9) contains supplementary material, which is available to authorized users.
Data availability The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest Dr. Castellino receives research funding from Bristol Meyers Squibb for an observational study not relevant to this work. Dr. Poehling received research funding for observational studies from the National Institutes of Health and MedImmune for an observational study not relevant to this work. The remaining authors declare they have no conflict of interest.
Compliance of ethical approval All procedures performed in the study were in accordance with the ethical standards and approval of the Wake Forest School of Medicine institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The IRB determined that the retrospective nature of this study using publicly available databases did not require individual informed consent.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–8. [DOI] [PubMed] [Google Scholar]
- 2.Petrosky E, Bocchini JA, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Garland SM, Brotherton JML, Moscicki AB, Kaufmann AM, Stanley M, Bhatla N, et al. HPV vaccination of immunocompromised hosts. Papillomavirus Res. 2017. [cited 2018 May 10];4:35–8. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2405852117300253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stobbe M Vaccine panel gives nod to HPV shots for men up to age 26. Associated Press; 2019. Available from: https://www.apnews.com/e341072cbfa040369fd157bd255ed40a. [Google Scholar]
- 5.Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson TO, Nathan PC, Whitton J, Leisenring WM, Neglia JP, Fowler B, et al. Human papillomavirus (HPV)-associated malignancies as subsequent malignant neoplasms (SMN) in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study (CCSS). 15th International Conference on Long-Term Complications of Treatment of Children and Adolescents for Cancer; 2017. [Google Scholar]
- 7.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. [DOI] [PubMed] [Google Scholar]
- 8.Ojha RP, Tota JE, Offutt-Powell TN, Klosky JL, Minniear TD, Jackson BE, et al. Human papillomavirus-associated subsequent malignancies among long-term survivors of pediatric and young adult cancers. PLoS One. 2013;8:e70349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:2356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Temkin SM, Seibel NL. Are we missing an opportunity for cancer prevention? Human papillomavirus vaccination for survivors of pediatric and young adult cancers: HPV Vaccines and Childhood Cancer Survivors. Cancer. 2015. [cited 2018 Mar 29];121:3395–402. Available from: http://doi.wiley.com/10.1002/cncr.29515. [DOI] [PubMed] [Google Scholar]
- 11.Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers, Version 4.0. Monrovia, CA; 2013. Available from: http://www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf. [Google Scholar]
- 12.Smith RA, Brooks D, Cokkinides V, Saslow D, Brawley OW. Cancer screening in the United States, 2013: a review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung can. CA Cancer J Clin. 2013;63:87–105. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. Cervical Cancer. National Comprehensive Cancer Network Clinical Practice Guideline V3. 2013. [cited 2018 Jun 18]. Available from: www.nccn.org.
- 14.Hoffman L, Okcu MF, Dreyer ZE, Suzawa H, Bryant R, Middleman AB. Human papillomavirus vaccination in female pediatric cancer survivors. J Pediatr Adolesc Gynecol. 2012. [cited 2018 Mar 29];25:305–7. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1083318812000873. [DOI] [PubMed] [Google Scholar]
- 15.Klosky JL, Russell KM, Canavera KE, Gammel HL, Hodges JR, Foster RH, et al. Risk factors for non-initiation of the human papillomavirus vaccine among adolescent survivors of childhood cancer. Cancer Prev Res (Phila Pa). 2013. [cited 2018 Mar 29];6:1101–10. Available from: http://cancerpreventionresearch.aacrjournals.org/cgi/doi/10.1158/1940-6207.CAPR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klosky JL, Hudson MM, Chen Y, Connelly JA, Wasilewski-Masker K, Sun C-L, et al. Human papillomavirus vaccination rates in young cancer survivors. J Clin Oncol. 2017. [cited 2018 Mar 29];35:3582–90. Available from: http://ascopubs.org/doi/10.1200/JCO.2017.74.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger, United States, 2018. Centers for Disease Control and Prevention; 2018. Available from: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. [Google Scholar]
- 18.Recommended Immunization Schedule for Adults Aged 19 Years or Older, United States, 2018. Centers for Disease Control and Prevention; 2018. [cited 2018 Mar 29]. Available from: https://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. [Google Scholar]
- 19.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. Executive summary: 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014. [cited 2019 Jul 10];58:309–18. Available from: https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- 20.Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:4401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchhoff AC, Mann K, Warner EL, Kaddas HK, Fair D, Fluchel M, et al. HPV vaccination knowledge, intentions, and practices among caregivers of childhood cancer survivors. Hum Vaccines Immunother. 2019. [cited 2019 Jul 10];1–9. Available from: https://www.tandfonline.com/doi/full/10.1080/21645515.2019.1619407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dayton A Improving quality of health care using the North Carolina Immunization Registry. N C Med J. 2014. [cited 2019 Apr 1];75:198–203. Available from: http://www.ncmedicaljournal.com/lookup/doi/10.18043/ncm.75.3.198. [DOI] [PubMed] [Google Scholar]
- 23.VFC Eligibility Criteria. Centers for Disease Control and Prevention; 2014. [cited 2019 Apr 1]. Available from: https://www.cdc.gov/vaccines/programs/vfc/providers/eligibility.html. [Google Scholar]
- 24.Trogdon JG, Shafer P, Lindsay B, Coyne-Beasley T. Impact of introduction of the 9-valent human papillomavirus vaccine on vaccination coverage of youth in North Carolina. Vaccine. 2018;36:1304–9. [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. [cited 2018 Apr 26];42:377–81. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1532046408001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klosky JL, Gamble HL, Spunt SL, Randolph ME, Green DM, Hudson MM. Human papillomavirus vaccination in survivors of childhood cancer. Cancer. 2009. [cited 2018 Mar 29];115:5627–36. Available from: http://doi.wiley.com/10.1002/cncr.24669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13–17 years–United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:685–93. [PMC free article] [PubMed] [Google Scholar]
- 28.Elam-Evans LD, Yankey D, Jeyarajah J, Singleton JA, Curtis RC, MacNeil J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years–United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63:625–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Curtis CR, MacNeil J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:850–8. [DOI] [PubMed] [Google Scholar]
- 30.Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Singleton JA, Curtis CR, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years–United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Health Interview Survey. Centers for Disease Control and Prevention; 2018. [cited 2018 Mar 29]. Available from: https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm. [Google Scholar]
- 32.Williams WW, Lu P-J, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, et al. Surveillance of vaccination coverage among adult populations — United States, 2014. MMWR Surveill Summ. 2016. [cited 2018 Mar 29];65:1–36. Available from: http://www.cdc.gov/mmwr/volumes/65/ss/ss6501a1.htm. [DOI] [PubMed] [Google Scholar]
- 33.Williams WW, Lu P-J, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, et al. Surveillance of vaccination coverage among adult populations - United States, 2015. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002. 2017;66:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams WW, Lu P-J, O’Halloran A, Bridges CB, Pilishvili T, Hales CM, et al. Noninfluenza vaccination coverage among adults - United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63: 95–102. [PMC free article] [PubMed] [Google Scholar]
- 35.Williams WW, Lu P-J, O’Halloran A, Bridges CB, Kim DK, Pilishvili T, et al. Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:95–102. [PMC free article] [PubMed] [Google Scholar]
- 36.Martin DW, Lowery NE, Brand B, Gold R, Horlick G. Immunization information systems: a decade of progress in law and policy. J Public Health Manag Pract JPHMP. 2015;21:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.North Carolina Immunization Program (NCIP) Requirements. North Carolina Department of Health and Human Services; 2018. [cited 2018 Apr 19]. Available from: http://www.immunize.nc.gov/providers/vpdreporting.htm. [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC). Immunization information systems progress–United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:289–91. [PubMed] [Google Scholar]
- 39.The North Carolina Immunization Registry. North Carolina Department of Health and Human Services; 2017. [cited 2018 Mar29]. Available from: http://www.immunize.nc.gov/providers/ncir.htm. [Google Scholar]
- 40.Groom H, Hopkins DP, Pabst LJ, Murphy Morgan J, Patel M, Calonge N, et al. Immunization information systems to increase vaccination rates: a community guide systematic review. J Public Health Manag Pract JPHMP. 2015;21:227–48. [DOI] [PubMed] [Google Scholar]
- 41.Choi N, Curtis CR, Loharikar A, Fricchione M, Jones E, Balzer E, et al. Successful use of interventions in combination to improve human papillomavirus vaccination coverage rates among adolescents—Chicago, 2013 to 2015. Acad Pediatr. 2018. [cited 2018 Jul 11];18:S93–100. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1876285917304965. [DOI] [PubMed] [Google Scholar]
- 42.Farmar A-LM, Love-Osborne K, Chichester K, Breslin K, Bronkan K, Hambidge SJ. Achieving high adolescent HPV vaccination coverage. Pediatrics. 2016;138:e20152653. [DOI] [PubMed] [Google Scholar]
- 43.Rosenthal SL, Weiss TW, Zimet GD, Ma L, Good MB, Vichnin MD. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29: 890–5. [DOI] [PubMed] [Google Scholar]
- 44.Wagner AL, Shrivastwa N, Potter RC, Lyon-Callo SK, Boulton ML. Pneumococcal and meningococcal vaccination among Michigan children with sickle cell disease. J Pediatr. 2018. [DOI] [PubMed] [Google Scholar]
- 45.Berenson AB, Rupp R, Dinehart EE, Cofie LE, Kuo Y-F, Hirth JM. Achieving high HPV vaccine completion rates in a pediatric clinic population. Hum Vaccines Immunother. 2018;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dorell C, Yankey D, Kennedy A, Stokley S. Factors that influence parental vaccination decisions for adolescents, 13 to 17 years old: National Immunization Survey-Teen, 2010. Clin Pediatr (Phila). 2013;52:162–70. [DOI] [PubMed] [Google Scholar]
- 48.Kester LM, Zimet GD, Fortenberry JD, Kahn JA, Shew ML. A national study of HPV vaccination of adolescent girls: rates, predictors, and reasons for non-vaccination. Matern Child Health J. 2013;17:879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark SJ, Cowan AE, Filipp SL, Fisher AM, Stokley S. Parent perception of provider interactions influences HPV vaccination status of adolescent females. Clin Pediatr (Phila). 2016;55:701–6. [DOI] [PubMed] [Google Scholar]
- 50.Perkins RB, Clark JA, Apte G, Vercruysse JL, Sumner JJ, Wall-Haas CL, et al. Missed opportunities for HPV vaccination in adolescent girls: a qualitative study. Pediatrics. 2014;134:e666–74. [DOI] [PubMed] [Google Scholar]
- 51.Markowitz L HPV Vaccines. Atlanta, GA; 2018. Available from: https://www.cdc.gov/vaccines/acip/meetings/live-mtg-2018-06.html. [Google Scholar]
- 52.Choi N, Curtis CR, Loharikar A, Fricchione M, Jones E, Balzer E, et al. Successful use of interventions in combination to improve human papillomavirus vaccination coverage rates among adolescents—Chicago, 2013 to 2015. Acad Pediatr. 2018;18:S93–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.