Abstract
Objective:
Radiologic predictors of post-traumatic amnesia (PTA) duration are lacking. We hypothesized that the number and distribution of traumatic microbleeds (TMBs) detected by gradient recalled echo (GRE) magnetic resonance imaging (MRI) predicts PTA duration.
Setting:
Academic, tertiary medical center.
Participants:
Adults with TBI.
Design:
We identified 65 TBI patients with acute GRE MRI. PTA duration was determined with the Galveston Orientation and Amnesia Test, Orientation Log or chart review. TMBs were identified within memory regions (hippocampus, corpus callosum, fornix, thalamus, and temporal lobe), and control regions (internal capsule and global). Regression tree analysis was performed to identify radiologic predictors of PTA duration, controlling for clinical PTA predictors.
Main Measures:
TMB distribution, PTA duration.
Results:
Sixteen patients (25%) had complicated mild, 4 (6%) moderate, and 45 (69%) severe TBI. Median PTA duration was 43 days (range 0–240 days). In univariate analysis, PTA duration correlated with TMBs in the corpus callosum (R=0.29, p=0.02) and admission GCS score (R=−0.34, p=0.01). In multivariate regression analysis, admission GCS score was the only significant contributor to PTA duration. However, in regression tree analysis, hippocampal TMBs, callosal TMBs, age and admission GCS score explained 26% of PTA duration variance and distinguished a subgroup with prolonged PTA.
Conclusions:
Hippocampal and callosal TMBs are potential radiologic predictors of PTA duration.
Introduction
Physical disabilities associated with traumatic brain injury (TBI) can be profound, but cognitive and behavioral limitations are more likely to prevent a patient from reintegrating into society.1 Post-traumatic amnesia (PTA) is a leading cause of cognitive impairment and functional disability after TBI. The impact of PTA on cognitive and functional recovery after TBI is likely related to the essential role of information storage and retrieval in nearly all aspects of cognition, including orientation, language, visuospatial and perceptual abilities.2, 3 PTA represents a specific type of memory impairment in which new information cannot be retained, leading to a state of working memory disruption. The resulting confusion and amnesia can cause or exacerbate behavioral disturbances, which are commonly observed in patients with PTA. Patients with PTA also suffer from impairments in communication, which can be associated with confabulation and perseveration, all of which prevent a return to independence in activities of daily living and hence poor long-term functional outcomes.4
PTA is transient in patients with mild TBI but may last for months in patients with severe TBI, or even indefinitely for patients with prolonged disorders of consciousness.5–7 Compared to the admission Glasgow Coma Scale (GCS) score and the duration of loss of consciousness, PTA duration is a more reliable predictor of outcomes pertaining to productivity, employment status and cognitive impairment.8–13 Many rehabilitation clinicians consider PTA duration when providing a prognosis about long-term functional outcome, since the duration of PTA has been shown to predict scores on the Functional Independence Measure (FIM) and Disability Rating Scale (DRS) at hospital discharge and 1 year post-injury, as well as Glasgow Outcome Scale (GOS) score at 6 months and 1 year.11, 13–15 Given the significant impact of PTA on patient disability and rehabilitation potential, consensus criteria have been developed for identification and objective tracking of PTA symptoms.16, 17
Despite the impact of PTA on a patient’s potential for early rehabilitation and long-term functional recovery, no acute radiological predictors of PTA duration have been identified. Specifically, it is unknown whether PTA is associated with the global burden of traumatic axonal injury (TAI) or axonal injury within specific neuroanatomic regions that are prominent in memory function. Given that memory is mediated by multiple gray matter regions and their widely distributed white matter connections, there are numerous potential mechanisms of PTA pathogenesis. However, there has been limited use of imaging methods such as MRI to investigate these mechanisms or the neuroanatomic basis for PTA.18 MRI is far more sensitive than head CT for detecting TAI, the form of traumatic pathology believed to play the most important role in disrupting the neural networks that mediate memory.19, 20 In particular, T2*-weighted gradient recalled echo (GRE) MRI provides heightened sensitivity for identifying traumatic microbleeds (TMBs), which are a hallmark of hemorrhagic TAI.
In this observational study, we investigated imaging biomarkers of PTA by measuring TMBs in the hippocampus, corpus callosum, fornix, temporal lobe and thalamus – all regions that contribute to memory acquisition, storage, and/or retrieval.3 We tested the hypothesis that the number of TMBs detected by GRE MRI in these neuroanatomic regions predicts PTA duration. We sought to provide clinicians with an acute radiologic biomarker of PTA duration, as early identification of patients who are at high risk of prolonged PTA could be used to guide rehabilitation strategies and enable early interventions for promoting recovery of memory and attention.
Methods
Subject Selection and Study Design
PTA duration data were acquired prospectively at an inpatient rehabilitation facility as part of the Traumatic Brain Injury Model Systems (TBIMS) study. TBIMS is a prospective, multicenter, longitudinal study of individuals with TBI funded by the National Institute on Disability, Independent Living, and Rehabilitation Research. During the study period 1999 to 2007, TBIMS Centers enrolled people with TBI, aged 16 years or older, who received medical care in a TBIMS-affiliated acute care hospital within 72 hours of their injury and were transferred directly from the acute care setting to the Center’s comprehensive rehabilitation program.
Upon transfer from the acute care setting to inpatient rehabilitation, each patient underwent a PTA assessment, performed by a certified speech and language pathologist. Resolution of PTA was determined by a score of at least 76 on two consecutive days using the Galveston Orientation and Amnesia Test (GOAT), or a score of at least 25 using the Orientation Log (O-LOG) on two consecutive tests with no more than 2 full calendar days between assessments. The GOAT and O-LOG scores are widely used and have similar performance characteristics for determining PTA duration.16, 17, 21 If GOAT or O-LOG data were unavailable, resolution of PTA was assessed by acute hospital chart review and defined as the time to achieve a GCS verbal score of 5 or the time to be documented as fully oriented, in either case, for two days. Of note, orientation Day 2 could be no more than 2 full calendar days from Day 1. For both methods, if any notes between Days 1 and 2 indicated that the patient did not meet criteria, Day 2 was used as the new starting point (i.e., the new Day 1) and the process was repeated. The study staff conducting PTA assessments were blinded to the MRI data and were not involved in patients’ treatment or data analysis. All study procedures were approved by the rehabilitation hospital’s Institutional Review Board, and written informed consent was provided by patients or their surrogates. A separate Institutional Review Board approval was obtained for a retrospective review of clinical records and MRI scans of patients enrolled in the rehabilitation hospital database who were treated for TBI at the acute care hospital. Written consent was waived by the Institutional Review Board for this part of the study.
The TBIMS database included 350 patients, of whom 243 were treated for TBI at the acute care hospital. All database enrollees who underwent an acute GRE MRI scan were eligible for inclusion. We operationally define “acute” as the period from admission to discharge from the acute care hospital. All MRIs were performed at the discretion of the treating clinicians. Of the 243 treated at the acute care hospital, 91 underwent MRI scans, with 82 scans containing the GRE sequence. All GRE data were evaluated for image quality, and clinical records were reviewed to identify neurologic events that could confound the relationship between TMBs and PTA duration. Five GRE MRI scans were excluded because of the presence of ischemic stroke on diffusion-weighted imaging (n=3) or motion artifact limiting interpretation of the GRE sequence (n=2). Of the remaining 77 patients, 10 were subsequently excluded because PTA data were not available, and another 2 were excluded because the patient emerged from PTA prior to the GRE MRI scan, yielding a final cohort of 65 patients with PTA duration data and acute GRE MRI.
Image Acquisition and Processing
The axial T2*-weighted 2D GRE echo-planar imaging sequence was performed on clinical 1.5 Tesla General Electric (General Electric Medical Systems, Waukesha, WI) MRI scanners. Because the MRI scans were performed for clinical purposes, multiple MRI scanners at the acute care hospital were used for data acquisition. Nevertheless, the field strength was uniform (1.5 Tesla) and the GRE sequence parameters were consistent across scanners, with the exception of TR values: TR = 450–800 msec, TE = 25 msec, flip angle = 20 degrees, slice thickness = 5 mm with a 1 mm gap, 256 × 256 acquisition matrix, in-plane resolution 0.9 × 0.9 mm.
Traumatic Microbleed Identification
Our group recently published an operational set of diagnostic criteria for TMB identification that incorporated relevant criteria for cerebral microbleeds22 while also incorporating criteria from prior TMB studies.23–25 Specifically, we defined a TMB based on the following characteristics: 1) hypointense signal on GRE without connection to the brain surface and/or the ventricular system; 2) blooming effect on GRE; and 3) distinct appearance from potential mimics (calcium and iron deposits, bone, or vessel flow voids).26 If there was uncertainty in distinguishing a TMB from a potential calcium deposit, the microbleed rater evaluated the admission head CT scan. A focal hyperdensity on CT is suggestive of a calcium deposit and thus the corresponding MRI hypointensity was not counted as a TMB. We allowed TMBs to have maximal diameters up to 25 mm, given that hemorrhagic shearing lesions in the corpus callosum may have diameters of this size.27
We counted the number of TMBs in pre-specified memory regions of interest (ROIs): the hippocampus, corpus callosum, fornix, temporal lobe (not including the hippocampus) and thalamus, because each of these memory ROIs has been shown to play a contributory role in memory acquisition, storage and/or retrieval.3 The anatomic borders of the hippocampus were defined according to a prior study of hippocampal MRI lesions.28 Although other regions such as the frontal and parietal lobes are known to contribute to memory function,3 we focused our analyses on the aforementioned ROIs to limit the number of statistical comparisons. To account for the possibility that the overall burden of TAI or the depth of TAI might underlie an association between TMBs within memory ROIs and PTA duration, we also counted global TMBs and internal capsule TMBs, respectively, as control ROIs. Global TMBs were defined as the total number of TMBs in the entire brain. The internal capsule was chosen as a control ROI because it is known to mediate motor function, not memory, and because it is at the same level as the callosum in the axial plane and therefore provides an anatomic surrogate for depth of injury (i.e. grade 2 TAI). To further explore the possibility that the grade of TAI was the primary determinant of PTA duration, we rated each patient’s GRE scan by the grade of TAI, radiologically defined in accordance with the histopathological grading system proposed by JH Adams and colleagues29: grade 1 = TAI lesions in the cerebral hemispheres; grade 2 = TAI lesions in the cerebral hemispheres and corpus callosum; and grade 3 = TAI lesions in the cerebral hemispheres, corpus callosum, and brainstem.
TMBs were identified by two neurologist raters (S.I. and S.M.) who were blinded to the clinical and PTA data. S.I. was the primary TMB rater, and S.M. the secondary TMB rater for inter-rater reliability analyses. Both raters completed a standardized cerebral microbleed detection training program of twenty GRE scans and scored > 90% on the intraclass correlation coefficient against established expert cerebral microbleed raters. Rating of microbleeds by neurologists who completed this standardized training program has been implemented in prior studies26, 30. S.I. and S.M. were tested for inter-rater reliability using the intraclass correlation coefficient for 15 randomly selected subjects in one memory ROI (corpus callosum) and one control ROI (global).
Univariate Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 7.0 (GraphPad Software; La Jolla, CA) and R version 3.5.1 (www.r-project.org). In univariate analysis, associations between the number of TMBs in memory ROIs, number of TMBs in control ROIs, clinical PTA predictors and PTA duration were assessed using Spearman’s correlation coefficient. The pre-specified clinical predictors were age and admission GCS score. Two post-hoc tests were also performed to determine if additional radiologic markers of structural injury correlated with PTA duration: 1) a Spearman correlation test was performed to determine if the number of lobar contusions correlated with PTA duration; and 2) an analysis of variance test with post-hoc Tukey’s test was performed to determine if the TAI grade (i.e. grade 1, 2 or 3) was associated with PTA duration.
Regression Statistical Analysis
It is possible that age and admission GCS score may relate to the number of TMBs and PTA duration independently, confounding interpretation of correlations. To avoid this confound, we performed a forward/backward stepwise regression analysis to determine any relation between the number of TMBs in the pre-specified memory ROIs and PTA duration while accounting for age and admission GCS score. It is also possible that while a linear relation may be weak or absent over the whole range of the dependent variable (in this case, PTA duration), this may simply reflect different levels of importance of a given independent variable across the range of the dependent one. To explore this possibility, we also performed a classification and regression tree (CART) analysis,31 which is a subset of general recursive partitioning methods wherein the dependent variable (PTA duration) is predicted from an ensemble of independent variables (pre-defined ROIs, age and admission GCS score). While a large tree risks “overfitting” the data and poorly generalizing to new samples, if it is too small, it may not reliably reflect the relationships. Therefore, once the regression tree was estimated, it was pruned via cost-complexity pruning, whereby the optimal tree was selected via 10-fold cross-validation.
Results
Subject Demographics and Clinical Characteristics
The study sample consisted of 65 patients treated for acute TBI at an academic tertiary care hospital prior to inpatient admission to the rehabilitation hospital. The mean +/− SD age of the sample was 41.3 +/− 21.8 years (range to 87 years) and 75.4% were male. The median admission GCS score was 6.5 (range 3 to 15). One quarter of the patients were classified as complicated mild TBI (i.e. admission GCS score 13–15 with an abnormal head CT), 6% as moderate TBI (i.e. admission GCS score 9–12), and 69% as severe TBI (i.e. admission GCS score 3–8). The median time to MRI scan was 3.0 days with an interquartile range (IQR) of 1–9 days. Duration of PTA ranged from 0 to 240 days, with a median of 43 days. The method of PTA duration measurement was GOAT in 3 patients, O-Log in 20 patients, and acute hospital chart review in 42 patients. Additional demographic and clinical data are reported in Table 1.
Table 1:
Patient Demographics and Clinical Characteristics (n=65)
| Age, mean +/− SD years | 41.3 +/− 21.8 years |
| Male sex, N (%) | 49 (75%) |
| Duration of acute hospitalization (range) |
0.5 – 362 days |
| Admission GCS, median (range)* | 6.5 (3 to 15) |
| Years of education (range) | 1–Doctoral Degree |
| TBI Mechanism, N (%) | |
| Motor vehicle accident | 29 (45%) |
| Fall | 19 (29%) |
| Pedestrian hit by car | 9 (14%) |
| Motorcycle accident | 4 (6%) |
| Sport related injuries | 3 (5%) |
| Assault | 1 (1%) |
| Marshall Head CT Classification | |
| Diffuse injury I | 2 (5%) |
| Diffuse injury II | 24 (61%) |
| Diffuse injury III | 5 (13%) |
| Diffuse injury IV | 0 |
| Evacuated mass lesion | 7 (18%) |
| Non-evacuated mass lesion | 1 (3%) |
| Traumatic Axonal Injury Grade | |
| Grade 1 | 35 (54%) |
| Grade 2 | 15 (23%) |
| Grade 3 | 15 (23%) |
Abbreviations: GCS, Glasgow Coma Scale.
GCS data were not available for one patient.
Traumatic Microbleed Counts and Inter-rater Reliability
The mean +/− SD number of global TMBs was 10.9 +/− 10.9 (range 0 to 53). For the memory ROIs, the mean +/− SD number of TMBs was highest in the temporal lobe (2.2 +/− 2.9) and lowest in the fornix (0.1 +/− 0.4). Mean +/− SD TMBs were 1.0 +/− 2.1 for the corpus callosum, and 0.9 +/− 1.4 for the hippocampus. Complete TMB data for all memory ROIs and the two control ROIs are provided in Table 2. In the interrater reliability analysis, the intraclass coefficient for corpus callosum TMBs was 0.97 and for global TMBs was 0.92 (both p < 0.001)
Table 2:
Univariate Correlations Between Prognostic Variables and Post-Traumatic Amnesia Duration
| Prognostic Variable |
Mean ± SD | IQR | Correlation Coefficient [95% Confidence Interval] |
P |
|---|---|---|---|---|
| Hippocampus TMBs | 0.9 ± 1.4 | 0–1 | 0.21 [−0.04, 0.44] | 0.09 |
| Corpus callosum TMBs | 1.0 ± 2.1 | 0–1 | 0.29 [0.04, 0.50] | 0.02 |
| Fornix TMBs | 0.1 ± 0.4 | 0 | 0.22 [− 0.03, 0.45] | 0.08 |
| Thalamus TMBs | 0.2 ± 0.5 | 0 | 0.20 [− 0.06, 0.43] | 0.12 |
| Temporal lobe TMBs | 2.2 ± 2.9 | 0–3 | 0.001 [−0.25, 0.25] | 0.99 |
| Global TMBs (control) | 10.9 ± 10.9 | 3–16 | 0.11 [−0.15, 0.35] | 0.40 |
| Internal capsule TMBs (control) | 0.03 ± 0.6 | 0 | 0.13 [−0.13, 0.37] | 0.31 |
| Admission GCS score* | 7.9 ± 4.3 | 4.8–11.5 | −0.34 [−0.54, −0.09] | 0.01 |
| Age | 41.3 ± 21.8 | 40 | −0.22 [−0.45, 0.03] | 0.07** |
Associations between prognostic variables and post-traumatic amnesia duration were assessed by calculating Spearman’s correlation coefficient.
GCS data were not available for one patient.
In a post-hoc analysis, older age was associated with a higher admission GCS score, indicating that higher admission GCS scores (i.e. less severe injuries) likely accounted for the inverse association between age and PTA duration. Abbreviations: GCS, Glasgow Coma Scale; IQR, interquartile range; SD, standard deviation; TMBs, traumatic microbleeds.
Univariate Correlations between Traumatic Microbleeds and Post-traumatic Amnesia Duration
The number of TMBs in the corpus callosum (R=0.29, p=0.02) correlated with PTA duration, while the number of TMBs in the hippocampus (R=0.21, p=0.09), fornix (R=0.22, p=0.08) and thalamus (R=0.20, p=0.12) trended toward correlation. TMBs within the temporal lobe (not including the hippocampus) did not correlate with PTA duration. In the control ROI analyses, neither global nor internal capsule TMBs correlated with PTA duration. Duration of PTA correlated inversely with admission GCS score (R = −0.34, p = 0.006) and showed a trend toward inverse correlation with age (R = −0.22, p = 0.07). All univariate correlation results are summarized in Table 2, and Figure 1 shows representative TMBs. In the post-hoc analyses, there was no correlation between the number of lobar contusions and PTA duration (R = 0.08, p = 0.53). TAI Grade was significantly related to PTA duration (F = 9.45, R2 = 0.23, p = 0.0003). In addition, PTA duration was significantly higher in grade 3 TAI compared to grades 1 and 2 (p = 0.003 and p = 0.0002, respectively).
Figure 1:
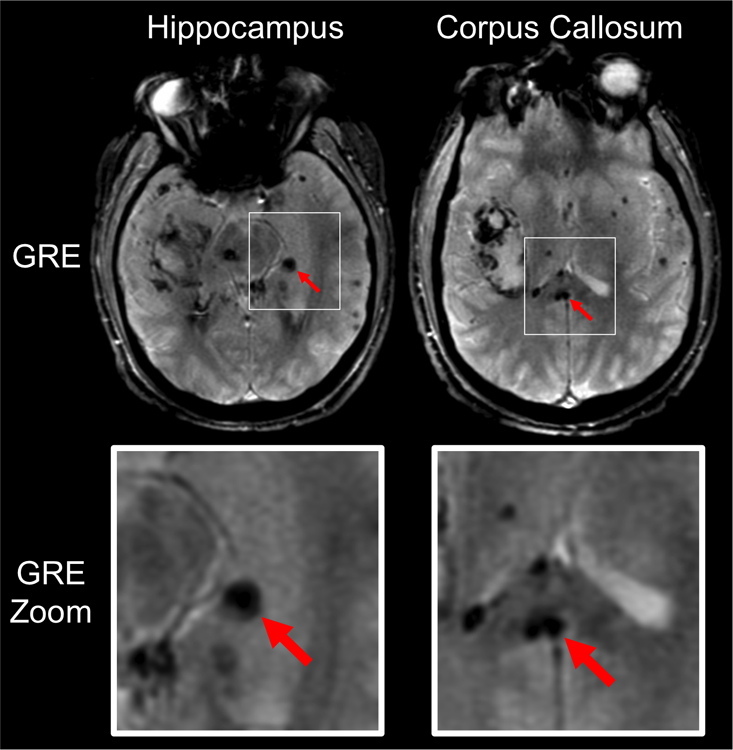
Traumatic Microbleeds (TMBs) Detected by T2* Gradient-Recalled Echo (GRE) MRI. GRE MRI data are shown for a representative patient with punctate hypointense lesions, representing TMBs, in the left hippocampus and splenium of the corpus callosum (red arrows, top panels). Zoomed views of the TMBs are shown in the bottom panels (red arrows).
Regression Tree Analysis
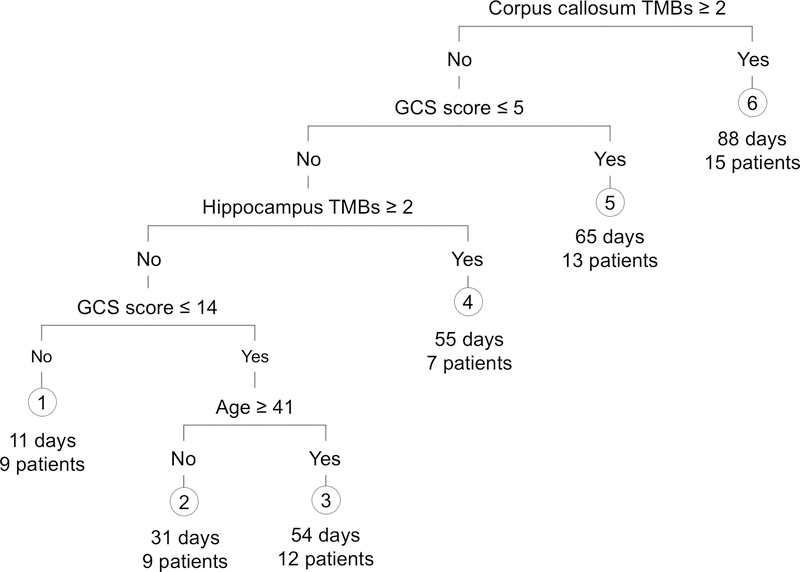
Stepwise regression analysis demonstrated that the number of TMBs in the corpus callosum and admission GCS score explained ~15% of the variance in PTA duration (F = 4.43, R2 = 0.13, p = 0.015). However, only admission GCS score was a significant contributor (p = 0.04 for GCS and p = 0.15 for corpus callosum TMBs). Subsequent regression tree analysis showed that four variables (TMBs in corpus callosum and hippocampus, admission GCS score, and age) explained 26% of the variance in PTA duration (mean prediction error 1.12 + 0.27 [S.D.], p < 0.01). Specifically, patients with at least two TMBs in the corpus callosum had, on average, 88 days of PTA, whereas patients with less than two TMBs in the corpus callosum had a duration of PTA that was related to the admission GCS score, the number of hippocampal TMBs, and age (Figure 2).
Figure 2:
Regression Tree Showing Contributions of Hippocampal and Callosal Traumatic Microbleeds, along with Admission GCS Score and Age, to Duration of Post-traumatic Amnesia. Numbers under the six group leaf-nodes indicate expected PTA duration under this model, and the number of patients in our sample that fall under that group.
Based on the regression tree results, we performed a post-hoc analysis of the subgroup of 15 patients with the longest PTA duration (mean 88 days, group 6), as well as the subgroup of 42 patients with PTA duration > 30 days (mean 78 days). The latter subgroup was selected for secondary analysis because 30 days approximates the average acute care plus rehabilitation hospital length of stay for TBI patients across age groups.32 Predicting those patients most likely to resolve PTA by the end of their inpatient rehabilitation hospitalization would identify a group for whom additional resources might be warranted due to their relatively good prognosis. Within the first subgroup (PTA > 88 days, n = 15), hippocampal, thalamic, and corpus callosum TMBs, along with age, together (but not individually) explained half of the variance in PTA duration (stepwise regression, F = 2.56, R2 = 0.50), although this relationship did not reach significance (p = 0.11) due to the limited number of patients in this group. None of the independent variables (individually or combined) explained PTA duration for the second subgroup (patients with PTA > 30 days; multiple regression F = 0.18, R2 = 0.04, p = 0.99).
Discussion
In this retrospective GRE MRI analysis of 65 patients with complicated mild-to-severe TBI enrolled in a prospective longitudinal database, we found that PTA duration was predicted by hippocampus TMBs, corpus callosum TMBs, admission GCS score, and age. The number of TMBs in the fornix, thalamus, temporal lobe and entire brain did not contribute to PTA duration. These findings suggest that TMBs within the hippocampus and corpus callosum, along with admission GCS and age, may be of greater importance than the global burden of TMBs in predicting PTA duration. If these findings are validated in larger prospective studies, early MRI detection of TMBs within these two neuroanatomic regions could serve as a radiologic biomarker to guide prognostication and clinical care paths aimed at promoting recovery of memory function. Furthermore, given the previously demonstrated association between PTA duration and long-term functional outcomes in TBI patients,11, 13–15 GRE MRI detection of TMBs within the hippocampus and corpus callosum could have implications for predicting recovery in a broad array of functional and cognitive domains.
The application of GRE MRI in this study to identify radiologic predictors of PTA duration was designed to optimize the clinical translatability of our findings in two ways. First, MRI is increasingly being used for clinical diagnosis and prognosis in patients with TBI,33–35 and GRE is currently available as a standard sequence on all clinical MRI scanners. Thus, our GRE MRI findings could inform clinical practice, in contrast to advanced imaging techniques such as resting-state functional MRI, diffusion tensor MRI, and Xenon perfusion CT, which are not commonly used in clinical practice. Second, we studied patients across the spectrum of TBI severity from complicated mild to severe injury, with the goal of increasing the generalizability of our findings to clinical practice. Although prior studies have used imaging techniques to investigate the neuroanatomic basis of PTA,36–38 most of these prior studies have focused on patients with mild TBI, limiting their generalizability.
Our GRE MRI findings in a diverse sample of TBI patients support and build upon those of prior studies that used advanced imaging techniques to investigate the neuroanatomic basis of PTA in more homogeneous samples of TBI patients. It has been long recognized that patients with hippocampal lesions have marked deficits in memory formation and memory-associated tasks.39, 40 Injury to this key node within a widely distributed memory network likely impairs the initial consolidation of memory, affecting subsequent memory retention or retrieval.3 De Simoni and colleagues used resting-state functional MRI network analysis to show that disconnections within the default mode network – particularly those linking the hippocampal and parahippocampal nodes to other nodes of the network – are associated with PTA duration.18 Our observation that hippocampal TMBs are associated with PTA duration suggests that even though a 3-dimensional resting-state fMRI analysis provides additional pathophysiologic information about network connectivity, a 2-dimensional GRE analysis may provide sufficient information to predict PTA duration. Our GRE MRI findings are also consistent with a Xenon CT perfusion study by Nariai et al, which found that in mild TBI patients with PTA, relative cerebral blood flow was elevated in the bilateral mesial temporal cortex,36 possibly suggesting disruption of the blood brain barrier or decoupling of the normal link between neuronal activity and cerebral blood flow. Although these perfusion data suggest a complex and dynamic relationship between mesial temporal lobe function and PTA, they are generally consistent with our observation that lesions within the mesial temporal lobe, particularly the hippocampus, are associated with PTA. Given that resting-state fMRI and perfusion techniques provide substantial pathophysiological data beyond that provided by standard clinical MRI, it is likely that multimodal, advanced imaging studies will be necessary to fully elucidate the complex pathogenesis of PTA.19, 20 Further studies are therefore needed to determine whether there are shared or divergent pathophysiological mechanisms underlying PTA and long-lasting memory impairment after TBI. In a clinical setting, however, it is possible that standard structural MRI with the GRE sequence may be sufficient for identifying biomarkers of PTA duration.
Notably, our observation that corpus callosum TMBs are associated with PTA duration sheds new light on the role of the callosum in PTA pathogenesis. As a primary conduit for white matter connections between the cerebral hemispheres, the corpus callosum likely contributes to PTA pathogenesis via a disconnection mechanism in which there is disruption of widely distributed bihemispheric axonal pathways that mediate memory retrieval.3, 41 This interpretation may reconcile our results with those of prior studies that found associations between PTA and abnormal perfusion within multiple grey matter regions of the cerebral cortex. For example, using SPECT in a group of 16 TBI patients, Lorberboym and colleagues found that amnesia lasting more than 30 minutes was associated with bilateral cerebral hypoperfusion in the frontal, temporal, parietal, or occipital lobes (or a combination of regions), with the left temporal lobe being most prominent.37 Similarly, a CT perfusion study found reduced blood flow in the frontal gray matter and caudate nucleus of patients with PTA compared to controls.38 It is therefore possible that white matter injury, such as that observed here in the corpus callosum, is mechanistically linked to neuronal dysfunction, and hence perfusion abnormalities, in the grey matter regions to which the white matter pathways are linked. Supporting evidence for this diaschesis phenomenon in patients with TAI was provided by a multimodal imaging study that detected cortical atrophy in grey matter regions whose axonal inputs were disrupted.42 Collectively, our results therefore suggest that there are multiple potential mechanisms by which traumatic axonal disruption, as evidenced by TMBs, contributes to PTA. Lesions in both the hippocampus, a key node in the widely-distributed memory network, and the corpus callosum, a conduit for connections linking the memory network’s nodes, predict PTA.
It is unexpected that PTA duration was not associated with the number of TMBs in the thalamus, fornix, or temporal lobe, given that these regions are known to contribute to memory function.3, 43 This observation may be attributable to a lower mean number of TMBs in the thalamus and fornix in this patient sample as compared to the mean number of hippocampal and callosal TMBs. Arguing against this explanation is the high mean number of temporal lobe TMBs, but in the case of the temporal lobe, it is possible that non-hippocampal temporal lobe injury does not contribute to PTA pathogenesis in the same manner as hippocampal injury.
There are multiple potential clinical implications of assessing TMBs to predict PTA duration. Identifying TMBs in prognostically relevant neuroanatomic regions such as the hippocampus and corpus callosum could be used to guide expectations for patients’ families about the trajectory and pace of recovery. From the standpoint of caregivers, these radiologic biomarkers could inform rehabilitation therapy plans, and help define rehabilitation needs and goals. Patients with a larger burden of hippocampal and callosal TMBs, and hence a higher probability of prolonged PTA, could also be considered for pharmacological interventions aimed at promoting recovery of memory and attention. In this regard, it is important to highlight the results of our post-hoc analysis of the relationship between TAI grade and PTA duration. We did not find that patients with grade 2 TAI have longer PTA duration than those with grade 1 TAI. This observation may be attributable to the inclusion of hippocampal TMBs in the grade 1 TAI cohort. Our results therefore suggest that TAI grade alone does not sufficiently predict PTA duration. Rather, a more neuroanatomically specific assessment of hippocampal TMBs within the grade 1 TAI subgroup may enhance the accuracy of predicting PTA duration.
Several limitations should be considered when interpreting the results of this study. Although we believe that this is the largest sample of patients with PTA imaged with GRE MRI to date, our sample size of 65 may have been insufficient to detect associations between PTA duration and injury within the thalamus and fornix. Another limitation is that although the GRE sequence is more sensitive for detecting TMBs than is head CT,44 our dataset did not include the susceptibility-weighted imaging (SWI) sequence, which is even more sensitive than GRE.45 Our MRI data were acquired during a time period when SWI was not part of standard clinical MRI protocols at our institution. The generalizability of our GRE findings to centers that use SWI awaits confirmation.
Any analysis of a complex cognitive function such as memory that limits lesion detection to a small number of ROIs and uses a 2-dimensional imaging technique is inherently subject to sampling error. Nevertheless, while advanced 3-dimensional network-mapping techniques such as resting-state fMRI are critical for future discoveries related to PTA pathogenesis, we aim here to provide initial evidence for a clinically available imaging biomarker that is readily translatable.46 Of particular interest is our exploratory analysis of those with greater than 88 days of PTA. Here the value of our imaging findings appears to be most robust. While preliminary and in need of replication, such work might lead to the use of TMB-focused MRI techniques to identify patients with the most extreme PTA prognosis early after injury.
Although the variable MRI data acquisition time in this study reflects real-world practice, it remains unclear if the timing of GRE data acquisition after TBI affects TMB visibility. While we included age as an independent predictor in our regression analysis, the possibility of cerebral amyloid angiopathy-related microbleeds or hypertensive microbleeds confounding our results is difficult to exclude. We were also unable to control for several clinical variables that are potential confounders of the relationship between TMBs and PTA, including admission hemodynamics, ICU complications, pre-morbid medical illness, and social support.47 Accordingly, it is important to consider that hippocampal TMBs, corpus callosum TMBs, admission GCS score, and age only accounted for 26% of the variance in PTA duration, highlighting the complex, multifactorial pathogenesis of PTA.
Conclusions
Our results suggest that hippocampus and corpus callosum TMBs detected by GRE MRI may predict PTA duration in patients with complicated mild, moderate, and severe TBI. The widespread availability of GRE MRI would allow rapid translation of TMB identification into clinical practice if these findings are validated in larger prospective studies. Given the individual and societal burden of PTA and its impact on recovery, larger studies are warranted.
Acknowledgments
We thank Steven L. Meisler and India A. Lissak for assistance with database quality assessment. This study was supported by the National Institute of Neurological Disorders and Stroke (R25NS065743, K23NS094538), the American Academy of Neurology/American Brain Foundation, the James S. McDonnell Foundation, and the National Institute on Disability, Independent Living, and Rehabilitation Research, Administration for Community Living, U.S. Department Health and Human Services to Spaulding Rehabilitation Hospital (H133A120085). However, the contents of this manuscript do not necessarily represent the policy of the Department of Health and Human Services and endorsement by the Federal Government should not be assumed.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Morton MV and Wehman P (1995). Psychosocial and emotional sequelae of individuals with traumatic brain injury: a literature review and recommendations. Brain Inj 9, 81–92. [DOI] [PubMed] [Google Scholar]
- 2.Squire LR, Knowlton B and Musen G (1993). The structure and organization of memory. Annu Rev Psychol 44, 453–495. [DOI] [PubMed] [Google Scholar]
- 3.Mesulam MM (1990). Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28, 597–613. [DOI] [PubMed] [Google Scholar]
- 4.Levin HS, O’Donnell VM and Grossman RG (1979). The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. J Nerv Ment Dis 167, 675–684. [DOI] [PubMed] [Google Scholar]
- 5.Russell WR and Smith A (1961). Post-traumatic amnesia in closed head injury. Arch Neurol 5, 4–17. [DOI] [PubMed] [Google Scholar]
- 6.Katz DI, Polyak M, Coughlan D, Nichols M and Roche A (2009). Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1–4 year follow-up. Prog Brain Res 177, 73–88. [DOI] [PubMed] [Google Scholar]
- 7.Giacino JT, Fins JJ, Laureys S and Schiff ND (2014). Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol 10, 99–114. [DOI] [PubMed] [Google Scholar]
- 8.Ownsworth T and McKenna K (2004). Investigation of factors related to employment outcome following traumatic brain injury: a critical review and conceptual model. Disabil Rehabil 26, 765–783. [DOI] [PubMed] [Google Scholar]
- 9.Sherer M, Sander AM, Nick TG, High WM Jr., Malec JF and Rosenthal M (2002). Early cognitive status and productivity outcome after traumatic brain injury: findings from the TBI model systems. Arch Phys Med Rehabil 83, 183–192. [DOI] [PubMed] [Google Scholar]
- 10.Sherer M, Struchen MA, Yablon SA, Wang Y and Nick TG (2008). Comparison of indices of traumatic brain injury severity: Glasgow Coma Scale, length of coma and post-traumatic amnesia. J Neurol Neurosurg Psychiatry 79, 678–685. [DOI] [PubMed] [Google Scholar]
- 11.Perrin PB, Niemeier JP, Mougeot JL, Vannoy CH, Hirsch MA, Watts JA, Rossman W, Grafton LM, Guerrier TD, Pershad R, Kingsbury CA, Bartel SW and Whitney MP (2015). Measures of injury severity and prediction of acute traumatic brain injury outcomes. J Head Trauma Rehabil 30, 136–142. [DOI] [PubMed] [Google Scholar]
- 12.Eastvold AD, Walker WC, Curtiss G, Schwab K and Vanderploeg RD (2013). The differential contributions of posttraumatic amnesia duration and time since injury in prediction of functional outcomes following moderate-to-severe traumatic brain injury. J Head Trauma Rehabil 28, 48–58. [DOI] [PubMed] [Google Scholar]
- 13.Walker WC, Ketchum JM, Marwitz JH, Chen T, Hammond F, Sherer M and Meythaler J (2010). A multicentre study on the clinical utility of post-traumatic amnesia duration in predicting global outcome after moderate-severe traumatic brain injury. J Neurol Neurosurg Psychiatry 81, 87–89. [DOI] [PubMed] [Google Scholar]
- 14.Brown AW, Malec JF, McClelland RL, Diehl NN, Englander J and Cifu DX (2005). Clinical elements that predict outcome after traumatic brain injury: a prospective multicenter recursive partitioning (decision-tree) analysis. J Neurotrauma 22, 1040–1051. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberg JH, Levin HS and Saydjari C (1996). Posttraumatic Amnesia as a predictor of outcome after severe closed head injury. Prospective assessment. Arch Neurol 53, 782–791. [DOI] [PubMed] [Google Scholar]
- 16.Frey KL, Rojas DC, Anderson CA and Arciniegas DB (2007). Comparison of the O-Log and GOAT as measures of posttraumatic amnesia. Brain Inj 21, 513–520. [DOI] [PubMed] [Google Scholar]
- 17.Novack TA, Dowler RN, Bush BA, Glen T and Schneider JJ (2000). Validity of the Orientation Log, relative to the Galveston Orientation and Amnesia Test. J Head Trauma Rehabil 15, 957–961. [DOI] [PubMed] [Google Scholar]
- 18.De Simoni S, Grover PJ, Jenkins PO, Honeyfield L, Quest RA, Ross E, Scott G, Wilson MH, Majewska P, Waldman AD, Patel MC and Sharp DJ (2016). Disconnection between the default mode network and medial temporal lobes in post-traumatic amnesia. Brain 139, 3137–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edlow BL and Wu O (2012). Advanced neuroimaging in traumatic brain injury. Semin Neurol 32, 374–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp DJ, Scott G and Leech R (2014). Network dysfunction after traumatic brain injury. Nat Rev Neurol 10, 156–166. [DOI] [PubMed] [Google Scholar]
- 21.Boake C, Millis SR, High WM Jr., Delmonico RL, Kreutzer JS, Rosenthal M, Sherer M and Ivanhoe CB (2001). Using early neuropsychologic testing to predict long-term productivity outcome from traumatic brain injury. Arch Phys Med Rehabil 82, 761–768. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R., Warach S, Launer LJ, Van Buchem MA and Breteler MM (2009). Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 8, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannion RJ, Cross J, Bradley P, Coles JP, Chatfield D, Carpenter A, Pickard JD, Menon DK and Hutchinson PJ (2007). Mechanism-based MRI classification of traumatic brainstem injury and its relationship to outcome. J Neurotrauma 24, 128–135. [DOI] [PubMed] [Google Scholar]
- 24.Firsching R, Woischneck D, Klein S, Reissberg S, Dohring W and Peters B (2001). Classification of severe head injury based on magnetic resonance imaging. Acta Neurochir (Wien) 143, 263–271. [DOI] [PubMed] [Google Scholar]
- 25.Iwamura A, Taoka T, Fukusumi A, Sakamoto M, Miyasaka T, Ochi T, Akashi T, Okuchi K and Kichikawa K (2012). Diffuse vascular injury: convergent-type hemorrhage in the supratentorial white matter on susceptibility-weighted image in cases of severe traumatic brain damage. Neuroradiology 54, 335–343. [DOI] [PubMed] [Google Scholar]
- 26.Izzy S, Mazwi NL, Martinez S, Spencer CA, Klein JP, Parikh G, Glenn MB, Greenberg SM, Greer DM, Wu O and Edlow BL (2017). Revisiting Grade 3 Diffuse Axonal Injury: Not All Brainstem Microbleeds are Prognostically Equal. Neurocrit Care 27, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gentry LR, Thompson B and Godersky JC (1988). Trauma to the corpus callosum: MR features. AJNR Am J Neuroradiol 9, 1129–1138. [PMC free article] [PubMed] [Google Scholar]
- 28.Greer DM, Scripko PD, Wu O, Edlow BL, Bartscher J, Sims JR, Camargo EE, Singhal AB and Furie KL (2013). Hippocampal magnetic resonance imaging abnormalities in cardiac arrest are associated with poor outcome. J Stroke Cerebrovasc Dis 22, 899–905. [DOI] [PubMed] [Google Scholar]
- 29.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI and McLellan DR (1989). Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology 15, 49–59. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Ramirez S, Romero JR, Shoamanesh A, McKee AC, Van Etten E, Pontes-Neto O, Macklin EA, Ayres A, Auriel E, Himali JJ, Beiser AS, DeCarli C, Stein TD, Alvarez VE, Frosch MP, Rosand J, Greenberg SM, Gurol ME, Seshadri S and Viswanathan A (2015). Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement 11, 1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hastie T, Tibshirani R, Friedman J (2001). The Elements of Statistical Learning Second ed. Springer: New York, NY. [Google Scholar]
- 32.Frankel JE, Marwitz JH, Cifu DX, Kreutzer JS, Englander J and Rosenthal M (2006). A follow-up study of older adults with traumatic brain injury: taking into account decreasing length of stay. Arch Phys Med Rehabil 87, 57–62. [DOI] [PubMed] [Google Scholar]
- 33.Moen KG, Brezova V, Skandsen T, Haberg AK, Folvik M and Vik A (2014). Traumatic axonal injury: the prognostic value of lesion load in corpus callosum, brain stem, and thalamus in different magnetic resonance imaging sequences. J Neurotrauma 31, 1486–1496. [DOI] [PubMed] [Google Scholar]
- 34.Lagares A, Ramos A, Perez-Nunez A, Ballenilla F, Alday R, Gomez PA, Kaen A and Lobato RD (2009). The role of MR imaging in assessing prognosis after severe and moderate head injury. Acta Neurochir (Wien) 151, 341–356. [DOI] [PubMed] [Google Scholar]
- 35.Yuh EL, Mukherjee P, Lingsma HF, Yue JK, Ferguson AR, Gordon WA, Valadka AB, Schnyer DM, Okonkwo DO, Maas AI, Manley GT and Investigators T-T (2013). Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 73, 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nariai T, Suzuki R, Ohta Y, Ohno K and Hirakawa K (2001). Focal cerebral hyperemia in postconcussive amnesia. J Neurotrauma 18, 1323–1332. [DOI] [PubMed] [Google Scholar]
- 37.Lorberboym M, Lampl Y, Gerzon I and Sadeh M (2002). Brain SPECT evaluation of amnestic ED patients after mild head trauma. Am J Emerg Med 20, 310–313. [DOI] [PubMed] [Google Scholar]
- 38.Metting Z, Rodiger LA, de Jong BM, Stewart RE, Kremer BP and van der Naalt J (2010). Acute cerebral perfusion CT abnormalities associated with posttraumatic amnesia in mild head injury. J Neurotrauma 27, 2183–2189. [DOI] [PubMed] [Google Scholar]
- 39.Scoville WB and Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horner AJ and Doeller CF (2017). Plasticity of hippocampal memories in humans. Curr Opin Neurobiol 43, 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catani M and Mesulam M (2008). What is a disconnection syndrome? Cortex 44, 911–913. [DOI] [PubMed] [Google Scholar]
- 42.Warner MA, Marquez de la Plata C., Spence J, Wang JY, Harper C, Moore C, Devous M and Diaz-Arrastia R (2010). Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J Neurotrauma 27, 2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller NG and Knight RT (2006). The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience 139, 51–58. [DOI] [PubMed] [Google Scholar]
- 44.Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT and Mukherjee P (2008). Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma 25, 1049–1056. [DOI] [PubMed] [Google Scholar]
- 45.Tong KA, Ashwal S, Holshouser BA, Shutter LA, Herigault G, Haacke EM and Kido DK (2003). Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology 227, 332–339. [DOI] [PubMed] [Google Scholar]
- 46.Maas AI, Harrison-Felix CL, Menon D, Adelson PD, Balkin T, Bullock R, Engel DC, Gordon W, Orman JL, Lew HL, Robertson C, Temkin N, Valadka A, Verfaellie M, Wainwright M, Wright DW and Schwab K (2010). Common data elements for traumatic brain injury: recommendations from the interagency working group on demographics and clinical assessment. Arch Phys Med Rehabil 91, 1641–1649. [DOI] [PubMed] [Google Scholar]
- 47.Marehbian J, Muehlschlegel S, Edlow BL, Hinson HE and Hwang DY (2017). Medical Management of the Severe Traumatic Brain Injury Patient. Neurocrit Care 27, 430–446. [DOI] [PMC free article] [PubMed] [Google Scholar]