Abstract
OBJECTIVES:
We examined the overall association as well as the dose-response relationship between leisure-time running and incident type 2 diabetes.
METHODS:
Participants were 19,347 adults aged 18–100 years who were free of cardiovascular disease, cancer, and diabetes at baseline, and who received at least two extensive preventive medical examinations between 1974–2006. Running and other types of aerobic physical activity were assessed by self-reported leisure-time activities. Type 2 diabetes was defined as fasting glucose ≥126 mg/dl (7.0 mmol/l), physician diagnosis, or insulin use.
RESULTS:
During an average follow-up of 6.5 years, 1,015 adults developed type 2 diabetes. Approximately 29.5% of adults participated in leisure-time running at baseline. Runners had a 28% (hazard ratio [HR] 0.72, 95% confidence interval [CI] 0.62–0.84) lower risk of developing type 2 diabetes compared with non-runners during follow-up. The HRs (95% CIs) of type 2 diabetes were 0.98 (0.75–1.28), 0.69 (0.51–0.92), 0.62 (0.45–0.85), 0.78 (0.59–1.04), and 0.57 (0.42–0.79) across quintiles of running time (minutes/week) compared with non-runners after adjusting for potential confounders, including levels of non-running aerobic physical activity. Similar dose-response relationships between running distance (miles/week), frequency (times/week), total amount (MET-minutes/week), and speed (mph) were also observed.
CONCLUSIONS:
Participating in leisure-time running is associated with a lower risk of developing type 2 diabetes in adults. Consistent linear dose-response relationships were observed between various running parameters and incident type 2 diabetes, supporting the prescription of running to prevent type 2 diabetes.
Keywords: Running, type 2 diabetes, aerobic exercise, cohort
INTRODUCTION
The global prevalence of type 2 diabetes, which is an established risk factor for cardiovascular diseases,1 has increased drastically over the past decade. Diabetes is projected to have an even greater growth over coming years in the United States, increasing to more than 54.9 million patients in 2030.2
Type 2 diabetes develops as a result of genetics and environmental factors, including modifiable risk factors.3 Among these lifestyle behaviors, regular physical activity has been regarded as one of the key elements to lower the risk of type 2 diabetes and delay its onset.4,5
While the benefits of moderate-intensity physical activity (defined as any physical activity requiring an energy expenditure of ≥3–6 metabolic equivalent (MET)6) on the primary prevention of type 2 diabetes is well established,7 fewer studies focus on the protective effect of vigorous-intensity physical activity (≥6 METs). Mounting evidence suggests that vigorous-intensity physical activity improves cardiorespiratory fitness, which is inversely associated with the incidence of type 2 diabetes,8 more effectively than moderate-intensity physical activity.9,10 Moreover, vigorous-intensity physical activity has been shown to attenuate weight gain more adequately than moderate-intensity physical activity,11 which is related to a higher risk of type 2 diabetes.12 Therefore, vigorous-intensity physical activity, such as running, may confer superior benefits over other types of physical activity on preventing type 2 diabetes.
Of the different types of exercise, running is the most popular among individuals who do participate in vigorous-intensity physical activity.13 Running is convenient and easily accessible, since it does not require specialized equipment or locations. Additionally, running is usually well above the MET level of 6,14 and even slow jogging is considered as vigorous-intensity physical activity.15 Current public health physical activity guidelines recommend that individuals should perform at least 500 MET-min/week of physical activity (equivalent to at least 75 minutes/week of vigorous-intensity physical activity) to obtain general health benefits, including type 2 diabetes prevention.16 Since the primary barrier preventing people from exercising is lack of time,17 performing vigorous-intensity physical activity, such as running, may motivate more people to achieve the recommended levels of physical activity.
The objective of this study was to examine the association of leisure-time running and type 2 diabetes incidence after adjusting for potential confounders, including other types of aerobic physical activity. Furthermore, the dose-response relationships of various leisure-time running parameters, such as running time, distance, frequency, total amount, and speed, with the risk of type 2 diabetes were investigated.
MATERIAL AND METHODS
Study Population
We analyzed data from the Aerobics Center Longitudinal Study (ACLS), which is a prospective cohort study designed to examine the associations of clinical and lifestyle factors with health outcomes. Participants were predominantly non-Hispanic white individuals who were well-educated and belonged to middle-to-upper socioeconomic stratum.18 The 21,350 participants aged 18–100 years received at least two extensive medical examinations at the Cooper Clinic in Dallas, Texas, between 1974–2006. Participants were excluded if they reported a history of myocardial infarction, stroke, or cancer (n = 1,005), or had diabetes (n = 998) at baseline. This resulted in a final study sample of 19,347 men and women for the analyses. The Cooper Institute Institutional Review Board approved the study protocol annually, and all participants gave their written informed consent before data collection at baseline and during follow-up examinations. Further details of the ACLS design, sampling procedures, and data collection have been previously described. 18,19
Clinical Examination
After a 12-hour overnight fast, participants underwent comprehensive medical examinations at baseline, which included body weight and height assessments, blood chemistry analyses, electrocardiography, physical examination, and a detailed medical history questionnaire. In addition, a maximal treadmill exercise test was performed to assess cardiorespiratory fitness. Smoking status, alcohol consumption, personal history of hypertension, hypercholesterolemia, myocardial infarction, stroke, cancer, and diabetes, and parental history of diabetes were assessed by standardized medical history questionnaires.
Assessment of Running
Physical activity information for the preceding 3 months was collected from the physical activity questionnaire. Participants were asked to fill in 4 questions about their running or jogging habits that addressed the duration, distance, frequency, and speed. Based on the information from the physical activity questionnaire, total weekly running time was calculated by multiplying the average duration of running and the frequency. Total amount of running was calculated by multiplying the MET value for the given speed and the weekly running time.20 Runners in this study were defined as those who reported running in all 4 questions under the running section. Participants were classified into 6 groups: non-runners and 5 quintiles of weekly running time (minutes), distance (miles), frequency (times), total amount (MET-minutes), and speed (mph) in runners, following our previous study methods.21 Total amount of other non-running aerobic physical activity (e.g. walking, cycling, swimming, aerobic dance, racquetball, skating, and other sports-related activities) was calculated and classified into 3 groups: 0, 1–499, and ≥500 MET-minutes per week based on the physical activity guidelines. Details of the physical activity assessment have been previously described.22
Ascertainment of Type 2 Diabetes
The diagnosis of type 2 diabetes was determined at a follow-up examination according to the American Diabetes Association criteria, which defines type 2 diabetes as a fasting plasma glucose concentration ≥126 mg/dl (≥7.0 mmol/l),23 physician diagnosis, or use of insulin. Follow-up time was calculated from the baseline examination to the first event of type 2 diabetes or the last follow-up observation through 2006 for participants who did not develop type 2 diabetes.
Statistical Analysis
Baseline characteristics of participants were summarized using descriptive statistics by quintiles of weekly running time. Cox proportional hazard regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) of type 2 diabetes according to exposure categories. Participants who reported no running activity were the reference group. The potential effect modification by sex on the association between running and type 2 diabetes incidence was examined using interaction terms in the Cox regression and by comparing risk estimates in the sex-stratified analyses. Because no significant interaction was found (P = .10), the pooled analyses were conducted. The proportional hazards assumption was tested and satisfied by comparing the log-log survival plots.
To determine whether the association between running status (runners versus non-runners) and incident type 2 diabetes varied by lifestyle factors or health conditions, the interactions of these dichotomized baseline characteristics were tested in the Cox regression.
SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all analyses. Two-sided tests were used, and a P value <.05 was considered statistically significant.
RESULTS
After an average follow-up of 6.5±6.1 years (minimum, 0.1 year; maximum, 31.8 years), 1,015 (5.3%) of the 19,347 participants developed type 2 diabetes. Among our sample, approximately 29.5% performed leisure-time running (n = 5,707) at baseline (Table 1). Compared with non-runners, runners were more likely to be younger, male, leaner, non-smokers, heavy alcohol drinkers, less active in other types of aerobic physical activity, had lower prevalence of chronic diseases (hypertension, hypercholesterolemia, and abnormal ECG), had less parental history of diabetes, and lower baseline fasting glucose (all P < .05). Also, runners had higher cardiorespiratory fitness levels than non-runners (P < .001).
Table 1.
Baseline characteristics of participants in the Aerobics Center Longitudinal Study, 1974–2006, by quintile of weekly running time
| Characteristics | Quintile of weekly running time (minutes/week) |
P value | ||||||
|---|---|---|---|---|---|---|---|---|
| All N = 19347 | Non-runners (0) n = 13640 | Q1 (<51) n = 1141 | Q2 (51–80) n = 1186 | Q3 (81–116) n = 1111 | Q4 (117–174) n = 1133 | Q5 (≥175) n = 1136 | ||
| Age (years) | 44.1 (9.6) | 45.0 (9.8) | 40.9 (8.7) | 41.6 (8.8) | 42.0 (8.4) | 42.8 (8.6) | 43.1 (8.9) | <.001 |
| Sex (male) | 15648 (80.9) | 10578 (77.6) | 1036 (90.8) | 1055 (89.0) | 995 (89.6) | 996 (87.9) | 988 (87.0) | <.001 |
| BMI (kg/m2)* | 25.6 (3.8) | 25.9 (4.0) | 25.3 (2.9) | 25.0 (3.1) | 24.8 (3.0) | 24.8 (3.0) | 24.1 (2.7) | <.00 1 |
| Current smokers | 2863(14.8) | 2287 (16.8) | 136 (11.9) | 145 (12.2) | 112 (10.1) | 91 (8.0) | 92 (8.1) | <.001 |
| Heavy alcohol drinking | 3451 (17.8) | 2376 (17.4) | 237 (20.8) | 202 (17.0) | 209 (18.8) | 200 (17.7) | 227 (20.0) | .022 |
| Non-running aerobic physical activity (Met-min/week)† | ||||||||
| 0 | 12241 (63.3) | 8019 (58.8) | 762 (66.8) | 856 (72.2) | 848 (76.3) | 879 (77.6) | 877 (77.2) | <.001 |
| 1–499 | 2710 (14.0) | 2282 (16.7) | 106 (9.3) | 111 (9.3) | 73 (6.6) | 81 (7.1) | 57 (5.0) | |
| ≥500 | 4396 (22.7) | 3339 (24.5) | 273 (23.9) | 219 (18.5) | 190 (17.1) | 173 (15.3) | 202 (17.8) | |
| Systolic blood pressure (mmHg) | 120 (14) | 120 (14) | 119 (13) | 119 (13) | 119 (14) | 120 (13) | 120 (14) | .014 |
| Diastolic blood pressure (mmHg) | 80 (10) | 80 (10) | 79 (9) | 79 (10) | 79 (9) | 79 (9) | 79 (9) | <.001 |
| Hypertension | 5337 (27.6) | 4007 (29.4) | 229 (20.1) | 275 (23.2) | 265 (23.9) | 284 (25.1) | 277 (24.4) | <.001 |
| Total cholesterol (mg/dl) | 206.2 (38.7) | 208.0 (39.2) | 201.4 (36.7) | 202.4 (38.6) | 203.5 (36.2) | 201.7 (37.3) | 200.5 (37.9) | <.00 1 |
| Hypercholesterol emia | 4980 (25.7) | 3797 (27.8) | 241 (21.1) | 246 (20.7) | 223 (20.1) | 249 (22.0) | 224 (19.7) | <.001 |
| Abnormal ECG‡ | 1318 (6.8) | 1056 (7.7) | 56 (4.9) | 51 (4.3) | 55 (5.0) | 49 (4.3) | 51 (4.5) | <.001 |
| Parental history of diabetes | 1308 (6.8) | 1020 (7.5) | 67 (5.9) | 54 (4.6) | 50 (4.5) | 62 (5.5) | 55 (4.8) | <.001 |
| Cardiorespiratory fitness (METs) | 11.4 (2.5) | 10.6 (2.1) | 12.6 (1.8) | 12.9 (1.9) | 13.3 (1.9) | 13.6 (2.1) | 14.7 (2.5) | <.001 |
| Impaired fasting glucose | 7618 (39.4) | 5482 (40.2) | 441 (38.7) | 452 (38.1) | 410 (36.9) | 411 (36.3) | 422 (37.2) | .012 |
| Baseline fasting glucose (mg/dl) | 97.4 (9.5) | 97.6 (9.6) | 97.4 (8.9) | 97.2 (9.0) | 97.0 (9.1) | 96.9 (9.0) | 96.7 (9.2) | .005 |
Data are presented in mean (SD) unless indicated as number (%).
BMI = body mass index.
MET = metabolic equivalent.
ECG = electrocardiogram. Smoking status (never, former, or current), heavy alcohol drinking (defined as average intake of >7 drinks per week for women, and >14 drinks per week for men); hypertension (defined by blood pressure ≥140/90 mmHg or physician diagnosed hypertension), and hypercholesterolemia (defined by total cholesterol ≥240 mg/dl or physician diagnosed hypercholesterolemia); parental history of diabetes (yes or no); and impaired fasting glucose (defined by an elevated fasting plasma glucose concentration (≥100 and <126 mg/dl)).
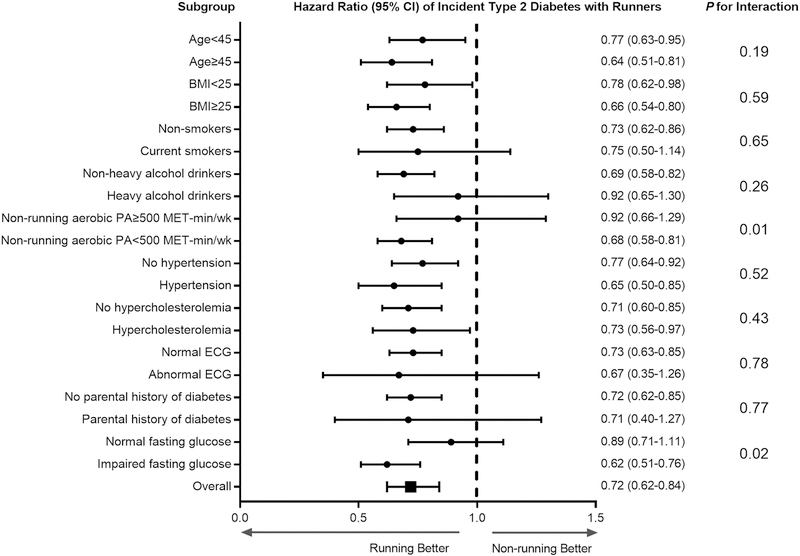
Runners had a 28% (HR 0.72 [95% CI 0.62–0.84]) reduced risk of type 2 diabetes compared with non-runners after adjustment for potential confounders (Figure 1). Stratified analyses examining the effect modifications by baseline characteristics on the association between running status (runners versus non-runners) and incident type 2 diabetes were conducted (Figure 1). We found no significant interactions in all subgroup analyses except non-running aerobic physical activity and impaired fasting glucose (both P = .02) indicating that the associations between running status and incident type 2 diabetes were consistent regardless of age, BMI, smoking status, heavy alcohol drinking, personal history of hypertension and hypercholesterolemia, ECG abnormality, and parental history of diabetes.
Figure 1. Hazard ratios of incident type 2 diabetes by running status in subgroups.
The reference group for all subgroup analyses was non-runners. All hazard ratios were adjusted for age (not in age-stratified analyses), sex, examination year, BMI (not in BMI-stratified analyses), smoking status (not in smoking-stratified analyses), heavy alcohol drinking (not in drinking-stratified analyses), non-running aerobic physical activity (not in non-running aerobic physical activity meeting the aerobic guidelines-stratified analyses), personal history of hypertension (not in hypertension-stratified analyses), personal history of hypercholesterolemia (not in hypercholesterolemia-stratified analyses), ECG abnormality (not in ECG-stratified analyses), parental history of diabetes (not in diabetes history-stratified analyses), and baseline fasting glucose (not in impaired fasting glucose-stratified analyses). The P-values for the interactions between running status and dichotomized baseline characteristics are depicted on the right.
BMI = body mass index; ECG = electrocardiogram; CI = confidence interval.
The dose-response associations between weekly running time and the incidence of type 2 diabetes are provided in Table 2. The group with the highest weekly running time (Quintile 5: ≥175 minutes/week) had a 52% (HR 0.48 [95% CI 0.35–0.66]) lower risk of developing type 2 diabetes than non-runners after adjusting for baseline age, sex and examination year (Model 1). Similar results were found after additional adjustment for baseline smoking status, heavy alcohol drinking, and levels of non-running aerobic physical activity (Model 2). Further adjustment for potential mediating factors and confounders, such as baseline BMI, medical conditions, parental history of diabetes, and baseline fasting glucose level attenuated the association, although results were still significant in most running time categories (Model 3). An inverse association between weekly running time and the risk of incident type 2 diabetes was observed in all 3 models (all P < .001). When we excluded the participants who reported any non-running aerobic physical activity (n = 7,106) to focus only on the running activity, similar associations were observed with the HRs (95% CIs) of 0.77 (0.54–1.10), 0.57 (0.40–0.82), 0.53 (0.37–0.77), 0.66 (0.47–0.93), and 0.58 (0.41–0.84) across quintiles of running time.
Table 2.
Hazard ratios of incident type 2 diabetes by quintile of weekly running time
| Quintile of weekly running time (minutes/week) |
P for linea r trend | ||||||
|---|---|---|---|---|---|---|---|
| Non-runners (0) | Q1 (<51) | Q2 (51–80) | Q3 (81–116) | Q4 (117–174) | Q5 (≥175) | ||
| No. of participantts | 13640 | 1141 | 1186 | 1111 | 1133 | 1136 | |
| No. of cases Adjusted hazardratio | 776 | 59 | 47 | 42 | 51 | 40 | |
| Model 1* | 1.00 | 0.86 | 0.60 | 0.55 | 0.68 | 0.48 | <.00 |
| [reference] | (0.66–1.12) | (0.45–0.81) | (0.40–0.75) | (0.51–0.90) | (0.35–0.66) | 1 | |
| Model 2† | 1.00 | 0.86 | 0.60 | 0.55 | 0.67 | 0.48 | <.00 |
| [reference] | (0.66–1.12) | (0.45–0.81) | (0.40–0.75) | (0.50–0.89) | (0.35–0.66) | 1 | |
| Model 3‡ | 1.00 | 0.98 | 0.69 | 0.62 | 0.78 | 0.57 | <.00 |
| [reference] | (0.75–1.28) | (0.51–0.92) | (0.45–0.85) | (0.59–1.04) | (0.42–0.79) | 1 | |
Model 1 was adjusted for baseline age (years), sex, and examination year.
Model 2 was adjusted for model 1 plus smoking status (never, former, or current), heavy alcohol drinking (average intake of >7 drinks per week for women, and >14 drinks per week for men), and levels of other non-running aerobic physical activity (0, 1–499, or ≥500 MET-minutes per week).
Model 3 was adjusted for Model 2 plus baseline BMI (kg/m2), hypertension, hypercholesterolemia, abnormal ECG, parental history of diabetes, and baseline glucose level.
MET = metabolic equivalent; BMI = body mass index; ECG = electrocardiogram.
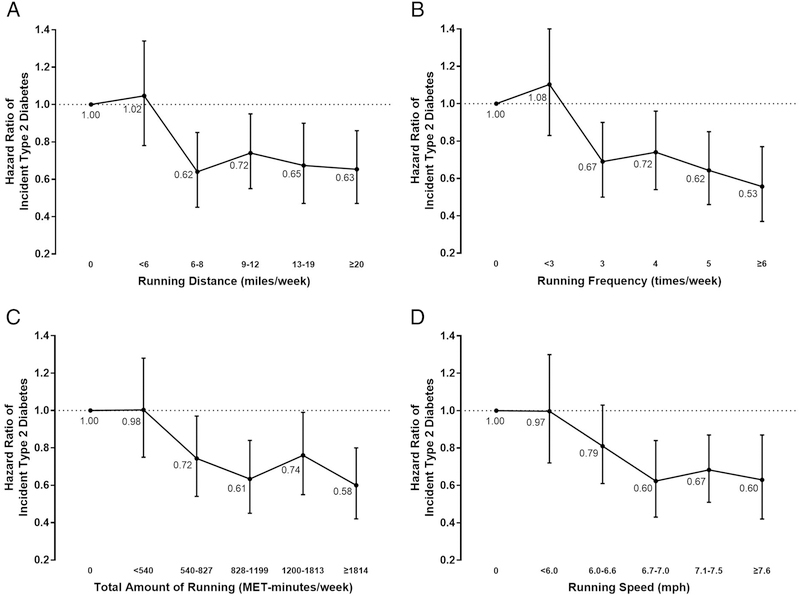
In addition to weekly running time, other running parameters were also associated with a lower risk of type 2 diabetes compared with non-runners (Figure 2). Weekly running distances ≥6 miles (Quintile 2), frequency of ≥3 times (Quintile 2), total amount ≥540 MET-min (Quintile 2), and speed ≥6.7 mph (Quintile 3), had significantly lower risks of type 2 diabetes incidence compared with non-runners.
Figure 2. Hazard ratios of incident type 2 diabetes by running distance, frequency, total amount, and speed.
Participants were categorized into 6 groups: non-runners and 5 quintiles of each running distance (A), frequency (B), total amount (C), and speed (D). All hazard ratios were adjusted for baseline age, sex, examination year, BMI, smoking status, heavy alcohol drinking, levels of non-running aerobic physical activity, personal history of hypertension, personal history of hypercholesterolemia, ECG abnormality, parental history of diabetes, and baseline fasting glucose. The bars indicate 95% confidence intervals and the dots indicate hazard ratios, which are shown next to the bars.
BMI = body mass index; ECG = electrocardiogram; MET = metabolic equivalent
The combined effects of running speed (cut point at median value of 6.7 mph) and weekly running distance (cut point at median value of 10 miles) on the risk of type 2 diabetes were examined. Compared with non-runners, the lowest risk of type 2 diabetes was found in the group with faster speed (≥6.7 mph) and longer weekly distance (≥10 miles) with the HR (95% CI) of 0.58 (0.46–0.74), followed by the faster speed (≥6.7 mph) and shorter distance (<10 miles) group: 0.73 (0.55–0.97), slower speed (<6.7 mph) and longer distance (≥10 miles) group: 0.80 (0.59–1.09), and slower speed (<6.7 mph) and shorter distance (<10 miles) group: 0.90 (0.70–1.16). Similar results were found in the joint association of running speed and total amount (cut point at median value of 989 MET-minutes/week) on the risk of developing type 2 diabetes. The combination group with the faster speed and higher total amount of running had the lowest risk (HR 0.58 [95% CI 0.45–0.74]).
DISCUSSION
In this large cohort study of adult men and women, runners had a 28% lower risk of developing incident type 2 diabetes compared with non-runners. Furthermore, consistent linear dose-response associations were observed between various running parameters and incident type 2 diabetes, indicating that more running appears to be better for the prevention of type 2 diabetes.
Because many previous investigations are limited by lack of detailed running information, there are only a few studies focusing on the health benefits of running. Several studies from the National Runners’ Health Study, which only included runners without non-runners in their analyses, have demonstrated that running is associated with a lower risk of type 2 diabetes prevalence and incidence.24,25 Our study aligns with previous reports showing the substantial health benefits of running and further indicates a possible protective effect and linear dose-response relationship between leisure-time running and incident type 2 diabetes in adults.
Previous studies with the ACLS dataset have demonstrated that relatively low weekly running doses (<51 minutes, <6 miles, 1–2 times, <506 MET-minutes, or <6 mph) are associated with reduced risks of all-cause and cardiovascular disease-mortality, after adjusting for a similar set of confounders used in our study.21,26 In the current study, the significant protective effect of running on incident type 2 diabetes arose from weekly running time of 51–80 minutes/week (Quintile 2). However, 73.4% of participants in Quintile 2 participated in running activity less than 75 minutes/week (minimum recommendation of vigorous-intensity physical activity), which may suggest that the benefits of running on the prevention of type 2 diabetes could be gained from participating in even less than the recommended amount of vigorous-intensity physical activity.
The protective effect of physical activity on incident type 2 diabetes has been well established by experimental studies and observational studies;4,5,7 however, most of these studies combined various-intensity physical activity together or focused mainly on moderate-intensity physical activity. To examine whether the benefits in type 2 diabetes prevention came from total physical activity or solely running, we conducted an additional analysis, excluding all participants who reported non-running aerobic physical activity. Similar findings were observed with this analysis, indicating that runners, apart from doing other aerobic physical activity, have a reduced risk of type 2 diabetes compared with inactive non-runners.
Several studies suggested that higher levels or greater volumes of physical activity may provide better prevention against type 2 diabetes.27,28 Moreover, one study found that the benefits of physical activity depend on both amount and intensity, and that higher intensity activities can additionally improve coronary heart disease risk factors beyond total exercise amount (kilometers run per week).29 These results suggest that while greater amounts of physical activity appear to provide more benefit, vigorous-intensity physical activity might confer unique health benefits. We also observed similar findings that runners with greater weekly running time at faster speeds, or with greater running amount with higher speeds showed the lowest risk of developing type 2 diabetes in the two joint analyses, respectively compared with non-runners.
In our earlier studies, we found that both obesity and low cardiorespiratory fitness have independently contributed to the increased risk of incident type 2 diabetes.8,30,31 In the current study, runners had a significantly lower BMI and approximately 30% higher cardiorespiratory fitness than non-runners. Similar to our running and mortality study,21 benefits of running on type 2 diabetes disappeared after further adjustment for cardiorespiratory fitness, indicating that cardiorespiratory fitness plays a significant role in the pathway between running and the development of type 2 diabetes.
The common concerns surrounding running are the occurrence of a sudden cardiac event, such as acute myocardial infarction, and musculoskeletal injuries. However, vigorous-intensity physical activity has been associated with decreased risk of incident myocardial infarction and stroke.32 Additionally, our former study indicated that runners had significantly lower risks of coronary heart disease and stroke mortality, respectively compared with non-runners.21 Regarding musculoskeletal injuries, evidence indicates that long-distance training increases the risk of musculoskeletal injuries;33 however, many forms of arthritis may actually be lower among chronic runners.14 Therefore, proper form and education as well as gradual progression in running amount and speed are necessary.
Strengths of our study include the collection of detailed running data on various running parameters, comprehensive physical assessments, and controlling of potential confounding factors, including non-running aerobic physical activity, in a large sample with a long and comprehensive follow-up. Moreover, due to the extensive and comprehensive baseline physical examination in the clinic, undetected type 2 diabetes cases are less likely to exist in the current study.
There are also several potential limitations of the current study. Since the participants in our study were predominantly non-Hispanic white individuals who were well-educated and belonged to middle-to-upper socioeconomic strata, this homogeneity of our sample may limit the generalizability of the results. However, the potential confounding by different races/ethnicities, education, and income levels may be reduced. Although reported vigorous-intensity physical activity is generally more accurate than moderate-intensity physical activity,34 self-reported running, which is considered as a desirable behavior, is still likely to be over-reported. However, over-reporting typically leads to an underestimation of the beneficial effect of running on type 2 diabetes, suggesting the effects of running on the risk of type 2 diabetes may be even larger than what we reported when running is objectively measured (e.g. accelerometer) in the future studies. Our questionnaire did not differentiate between type 2 and type 1 diabetes. However, all diabetes cases in our study were diagnosed after age 30, and the National Diabetes Statistics Report identified that more than 90% of adults with diabetes have type 2 diabetes.35 Thus, there are likely only few participants with type 1 diabetes, which occurs in early age before age 30, in our study. Additionally, we did not have sufficient dietary information to include it into our analyses, which is also considered as a significant risk factor of type 2 diabetes. However, we adjusted for BMI in our analyses as that is affected by diet, specifically total energy intake.
Our results indicate that runners have a substantially lower risk of developing type 2 diabetes compared with non-runners after adjusting for potential confounders, including other types of aerobic physical activity. We also found consistent linear dose-response relationships between various running parameters and incident type 2 diabetes. We noted significant benefits of running with lower doses and intensities supporting the prescription of running to prevent type 2 diabetes in adults, in addition to other healthy lifestyle behaviors.
Highlights:
Participating in leisure-time running is associated with a lower risk of developing type 2 diabetes in adults.
Consistent linear dose-response relationships were observed between various running parameters and incident type 2 diabetes.
We noted significant benefits of running with lower doses and intensities supporting the prescription of running to prevent type 2 diabetes in adults, in addition to other healthy lifestyle behaviors.
ACKNOWLEDGMENTS:
The authors thank the Cooper Clinic physicians and technicians for collecting the baseline data and staff at the Cooper Institute for data entry and data management.
Funding: This study was supported by the National Institutes of Health grants (AG06945, HL62508, DK088195, and HL133069). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SNB has received unrestricted research grants from The Coca-Cola Company, but these grants were not used to support this manuscript. Authors not named here have disclosed no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflict of interest.
References
- 1.Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation 1999;100(10):1132–1133. [DOI] [PubMed] [Google Scholar]
- 2.Rowley WR, Bezold C, Arikan Y, et al. Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul Health Manag 2017;20(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laaksonen DE, Lindstrom J, Lakka TA, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes 2005;54(1):158–165. [DOI] [PubMed] [Google Scholar]
- 6.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(9 Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 7.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007;30(3):744–752. [DOI] [PubMed] [Google Scholar]
- 8.Sui X, Hooker SP, Lee IM, et al. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care 2008;31(3):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swain DP, Franklin BA. VO(2) reserve and the minimal intensity for improving cardiorespiratory fitness. Med Sci Sports Exerc 2002;34(1):152–157. [DOI] [PubMed] [Google Scholar]
- 10.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol 2006;97(1):141–147. [DOI] [PubMed] [Google Scholar]
- 11.Williams PT. Greater weight loss from running than walking during a 6.2-yr prospective follow-up. Med Sci Sports Exerc 2013;45(4):706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schienkiewitz A, Schulze MB, Hoffmann K, et al. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2006;84(2):427–433. [DOI] [PubMed] [Google Scholar]
- 13.Tudor-Locke C, Johnson WD, Katzmarzyk PT. Frequently reported activities by intensity for U.S. adults: the American Time Use Survey. Am J Prev Med 2010;39(4):e13–20. [DOI] [PubMed] [Google Scholar]
- 14.Lavie CJ, Lee DC, Sui X, et al. Effects of Running on Chronic Diseases and Cardiovascular and All-Cause Mortality. Mayo Clin Proc 2015;90(11):1541–1552. [DOI] [PubMed] [Google Scholar]
- 15.Lee DC, Brellenthin AG, Thompson PD, et al. Running as a Key Lifestyle Medicine for Longevity. Prog Cardiovasc Dis 2017;60(1):45–55. [DOI] [PubMed] [Google Scholar]
- 16.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 17.Justine M, Azizan A, Hassan V, et al. Barriers to participation in physical activity and exercise among middle-aged and elderly individuals. Singapore Med J 2013;54(10):581–586. [DOI] [PubMed] [Google Scholar]
- 18.Blair SN, Kannel WB, Kohl HW, et al. Surrogate measures of physical activity and physical fitness. Evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol 1989;129(6):1145–1156. [DOI] [PubMed] [Google Scholar]
- 19.Blair SN, Kohl HW, Barlow CE, et al. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43(8):1575–1581. [DOI] [PubMed] [Google Scholar]
- 21.Lee DC, Pate RR, Lavie CJ, et al. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol 2014;64(5):472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DC, Sui X, Ortega FB, et al. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med 2011;45(6):504–510. [DOI] [PubMed] [Google Scholar]
- 23.(2) Classification and diagnosis of diabetes. Diabetes Care 2015;38 Suppl:S8–s16. [DOI] [PubMed] [Google Scholar]
- 24.Williams PT. Relationship of running intensity to hypertension, hypercholesterolemia, and diabetes. Med Sci Sports Exerc 2008;40(10):1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams PT. Changes in vigorous physical activity and incident diabetes in male runners. Diabetes Care 2007;30(11):2838–2842. [DOI] [PubMed] [Google Scholar]
- 26.Lee DC, Lavie CJ, Sui X, Blair SN. Running and Mortality: Is More Actually Worse? Mayo Clin Proc 2016;91(4):534–536. [DOI] [PubMed] [Google Scholar]
- 27.Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia 2016;59(12):2527–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA 1999;282(15):1433–1439. [DOI] [PubMed] [Google Scholar]
- 29.Williams PT. Relationships of heart disease risk factors to exercise quantity and intensity. Arch Intern Med 1998;158(3):237–245. [DOI] [PubMed] [Google Scholar]
- 30.Lee DC, Sui X, Church TS, et al. Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care 2009;32(2):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawada SS, Lee IM, Muto T, et al. Cardiorespiratory fitness and the incidence of type 2 diabetes: prospective study of Japanese men. Diabetes Care 2003;26(10):2918–2922. [DOI] [PubMed] [Google Scholar]
- 32.Chomistek AK, Cook NR, Flint AJ, Rimm EB. Vigorous-intensity leisure-time physical activity and risk of major chronic disease in men. Med Sci Sports Exerc 2012;44(10):1898–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Gent RN, Siem D, van Middelkoop M, et al. Incidence and determinants of lower extremity running injuries in long distance runners: a systematic review. Br J Sports Med 2007;41(8):469–480; discussion 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams PT, Thompson PD. Walking versus running for hypertension, cholesterol, and diabetes mellitus risk reduction. Arterioscler Thromb Vasc Biol 2013;33(5):1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Statistics Report, 2017, Estimates of Diabetes and Its Burden in the United States https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed April 30, 2018.