Abstract
It has been shown that dystrobrevin-binding protein 1 gene, that encodes the protein dysbindin-1, is associated with risk for cognitive deficits and studies have shown decreases in glutamate and correlated decreases in dysbindin-1 protein in the prefrontal cortex (PFC) and hippocampus of post-mortem tissue from schizophrenia patients. The PFC and the hippocampus have been shown to play a fundamental role in cognition, and studies in dysbindin-1 null mice have shown alterations in NMDAR located in pyramidal neurons as well as perturbation in LTP and cognitive deficits. The balance between excitatory and inhibitory transmission is crucial for normal cognitive functions, however, there is a dearth of information regarding the effects of loss of dysbindin-1 in GABAergic transmission.
Using in vitro whole cell clamp recordings, Western blots and immunohistochemistry, we report here that dysbindin-1 deficient mice exhibit a significant decrease in the frequency of sIPSCs and in the amplitude of mIPSCs and significant decreases in PV staining and protein level. These results suggest that loss of dysbindin-1 affects GABAergic transmission at pre- and post-synaptic level and decreases parvalbumin markers
Keywords: dysbindin-1, prefrontal cortex, GABA, interneurons
Introduction
The dystrobrevin-binding protein 1 gene encodes the protein dysbindin-1, a protein that has been found to play important roles in brain development (Cox et al, 2009; Jia et al, 2014; Mullin et al, 2015). Furthermore, it has been suggested that dysbindin-1 plays a role in cognitive deficits (Baek et al, 2012; Burdick et al, 2007; Varela-Gomez et al, 2015; Wolf et al, 2011; Zinkstok et al, 2007; Zhang et al, 2010), and recent studies have shown that genetic variations in dysbindin-1 expression correlate with the effects of antipsychotics (Scheggia et al., 2018).
Interestingly, studies have shown decreases in glutamate and correlated decreases in dysbindin-1 protein in the prefrontal cortex (PFC) and hippocampus of post-mortem tissue from schizophrenia patients (Talbot et al., 2004; Tang et al., 2009; Weickert et al., 2004, 2008), and decreases in glutamate release have been demonstrated in dysbindin-1 deficient mice (Chen et al., 2008; Jentsch et al., 2009). Furthermore, we have reported that dysbindin-1 deletions elicit alterations in NMDAR’s located in pyramidal neurons (Karlsgodt et al., 2011) and affects LTP (Glen et al., 2014). Dysbindin-1 has a widespread distribution in the brain, being expressed by many neuronal populations, including pyramidal neurons in the hippocampus and the dorsolateral PFC (Chiba et al., 2006). Within these areas, dysbindin-1 is expressed both pre-as well as postsynaptically. Studies have shown that the dystrophin glycoprotein complex (DGC; of which dysbindin-1 is part) is concentrated at the postsynaptic density (PSD;Talbot et al., 2004) and therefore it has been suggested that dysbindin-1 may be involved in regulating some PSD functions, including trafficking and tethering of receptors (including NMDA, nicotinic, and GABAA receptors) and signal transduction proteins (Camargo et al., 2007; Kumamoto et al., 2006).
The PFC and the hippocampus have been shown to play a fundamental role in cognition (Guo et al., 2019; Bast et al, 2017;Hiser and Koenigs, 2018), however normal cognitive function depends not only on glutamatergic transmission but in a balance between excitatory and inhibitory transmission. A considerable body of work indicates that dysfunction within cortical inhibitory circuitry contributes to cognitive deficits (for review see Lewis, DA, 2014). Indeed, several laboratories have reported decreases in both the levels of the GABA synthesizing enzyme GAD-67 as well as decreases in the expression of the GABA transporter (GAT) in the PFC of schizophrenia patients (Akbarian and Huang, 2006; Gonzalez-Burgos et al., 2011; Lewis et al., 2012; Mirnics et al., 2000). Furthermore, several studies have also shown changes in GABAA receptor binding in the prefrontal cortex of schizophrenia patients (Hashimoto et al., 2008; Ishikawa et al., 2004).
GABA local circuit neurons are a heterogeneous population of cells that differ in their electrophysiological properties, axonal targets and expression of calcium binding proteins and neuropeptides (Makram et al., 2004). The majority of local circuit neurons can be grouped into three non-overlapping populations: cells that express the calcium binding protein parvalbumin (PV), cells that express the neuropeptide somatostatin (SS), and cells that express the calcium binding protein calretinin (CR) (Kawaguchi and Kubota 1997). Of these different populations, the PV class of local circuit neurons appears to be particularly affected in the PFC of schizophrenia patients (Dienel and Lewis 2018; Steullet et al., 2017; Toker, 2018). Albeit, recent reports suggest that also somatostatin interneurons could be affected (Alherz et al 2017). It has been reported that expression of PV, but not other calcium binding proteins, is reduced in the prefrontal cortex in schizophrenia (Lewis et al., 2005). Moreover, dual in situ hybridization studies indicated that the decrease in GAD-67 mRNA levels seen in the PFC of schizophrenia patients may be due to a selective reduction in PV+ local circuit neurons (Makram et al., 2004).
We (Jentsch et al., 2009) and others (Chen et al., 2008) have demonstrated that dysbindin-1 plays a critical role in glutamate release and have proposed a mechanism underlying the role of this protein in synaptic vesicle trafficking; however, there is a dearth of information regarding the role of dysbindin-1 in GABAergic transmission.
Using in vitro whole cell clamp recordings, Western blots and immunohistochemistry in the prelimbic and infralimbic PFC, we report here that dysbindin-1 deficient mice exhibit a significant decrease in the frequency of spontaneous and miniature inhibitory postsynaptic currents (sIPSCs and mIPSCs), significant decrease in the amplitude of mIPSCs and significant decreases in PV staining and protein level. These results suggest that loss of dysbindin-1 affects GABAergic transmission at pre- and post-synaptic level and decreases parvalbumin markers.
Methods:
Animals
We used mice that had been backcrossed to the C57Bl/6J background (Jackson Laboratories, Bar Harbor, Maine). All animals were genotyped as previously described (Jentsch et al., 2009). Male mice were used in the electrophysiological and immunohistochemistry experiments described here; all subjects were 45-60 days of age at the time of study. All experimental protocols were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee.
Electrophysiology
Brain slices (300 μm) were prepared from dysbindin-1 wild-type (dys +/+), HET (dys +/−) and MUT mice (dys −/−) mice. Subjects were anesthetized with isoflurane (Abbott Laboratories). The brain was removed, and coronal slices containing the infralimbic and prelimbic PFC were cut at 300 μm thickness in ice-cold high-sucrose solution containing (in mM): sucrose, 200; KCl, 1.9; Na2HPO4, 1.2; NaHCO3, 33; MgCl2, 6; CaCl2, 0.5; D-Glucose, 10; ascorbic acid, 0.4. Slices were incubated at 33°C for at least 1 h before recordings; the incubation medium contained (in mM): NaCl, 125; KCl, 2.5; NaH2PO4,1.25; NaHCO3, 25; MgCl, 4, CaCl, 1, D-Glucose, 10; sucrose, 15; ascorbic acid, 0.4, aerated with 5%CO2/95%O2. After incubation, slices were transferred to a submerged chamber and superfused with oxygenated artificial cerebrospinal fluid (aCSF) (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 2.0 CaCl2, 1.3 MgCl2, 10 D-Glucose and 0.4 ascorbic acid at room temperature. Recordings were made using a Multiclamp 700B amplifier (Axon Instruments, CA), connected to a computer running Windows XP and Axograph X software. All recordings were obtained from pyramidal neurons or fast spiking interneurons FSI) in layers V or VI of the prelimbic or infralimbic cortex, identified using infrared-differential interference contrast optics and video-microscopy.
Voltage clamp: When recording in pyramidal neurons, CNQX (10 μM) and dl-APV (50 μM) was included in the perfusion solution to block glutamate receptors. For voltage-clamp recordings, electrodes (3-7 MΩ resistance in situ) were filled with a solution containing (in mM): 135 CsCl, 10 HEPES, 2 MgCl2, 1 EGTA, 4 NaCl, 2 Na-ATP, 0.3 tris-GTP, 1 QX-314, 10 phosphocreatine; 285 mOsmols. Series resistances (10-20 MΩ), and input resistances were continually monitored throughout the experiment via a −1 mV (100 ms) hyperpolarizing pulse. Pyramidal neurons were clamped at −80 mV. Miniature IPSCs (mIPSCs) were measured after adding 1 μM TTX to the buffer solution. For current clamp recordings, an internal solution based on k+ gluconate was used containing in mM (125 K+ gluconate, 10 HEPES, 20 KCl, 4 ATP-Mg2+, 0.3 GTP-Na+ and 14 phosphocreatine.
Immunohistochemistry
For immunohistochemistry, male wild-type (dys +/+), dysbindin-1 HET (dys +/−) and MUT mice (dys −/−) mice (PN 38-43) were anesthetized with isofluorane and perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were post fixed in 4% paraformaldehyde for 1 hour before being transferred to 30% sucrose in PB for > 48 hours. Following this cryoprotection, coronal sections (40-50 μm) were taken through the brain using a freezing sledge microtome. Sections were collected into PB and transferred to a cryoprotectant solution and stored at −20°C until use.
Different series of this tissue were processed for immunohistochemistry using primary antisera directed against parvalbumin (1:10000). Tissue was processed concurrently in order to Peroxidase (ABC) method utilizing Vector VIP as a chromagen.
The density of immunostaining within the medial PFC was compared using a Nikon Eclipse E-600 light microscope equipped with a video camera and coupled to Scion Image analysis software. For each section, a rectangle was drawn across the cortical layers, spanning from the pial surface to the underlying white matter. The average light transmittance was determined for the entire cortical thickness as well as for the superficial and deep halves of the cortical thickness. Transmittance in the underlying white matter was used as a measurement of background staining and was subtracted from the transmittance within the cortex to obtain a mean optical density of staining. Results were averaged from 3-4 sections for each animal and comparisons were made between average transmittance values between littermates.
Preparation of Synaptosomes
Infralimbic and prelimbic PFC (referred as PFC) tissue from three animals/genotype (unless otherwise indicated) was pooled together to make N=1 in each group. The tissue was homogenized with 10 strokes in a Potter homogenizer holding 5 ml of ice-cold isolation buffer containing 320 nM sucrose, 1 mM Na-EDTA, 10 mM Tris-HEPES (pH 7.4) and a protease inhibitor cocktail (Sigma, catalog # P8340). The homogenates were centrifuged at 600 g for 10 min to obtain a pellet fraction (P1) enriched in cell debris, intact cells and nuclei. The post-nuclear fraction (S1) was collected and centrifuged for 15 min at 9,200 g. The pellet was collected and washed by resuspension in Krebs buffer containing 125 mM NaCl, 5 mM KCl, 0.1 mM MgCl2, 1 mM CaCl2, 10 mM D-Glucose and 10 mM HEPES-NAOH, pH 7.4. After washing, the P2 pellet was resuspended in Krebs buffer, and protein concentration was determined by a Bradford assay (Bio Rad). The pellet was a crude synaptosomal fraction.
Isolated synaptosomes were lysed in 0.1 M phosphate buffer (pH 7.2) containing 0.1% sodium dodecyl sulfate, 1% IGEPal, 1% protease, and 1% phosphatase inhibitor cocktails (Sigma), and centrifuged at 14,000g for 10 min at 4°C. Aliquots of supernatant (40-50 μg of protein) were separated on NuPAGE 4-12% Bis-Tris gels and 3-8% Tris-Acetate gels (Invitrogen Inc., Carlsbad, CA) and transferred onto nitrocellulose membranes, immunoblotted overnight at 4°C.Horseradish peroxidase-conjugated secondary antibodies at a concentration of 1:5,000 (Abcam) were applied, and detection performed using chemiluminescence (Pierce Biotechnology Inc., Rockford, IL). The immunoblotting experiments were performed four times and were quantitatively analyzed using software (Imaging Station, Carestream Health, Inc, Rochester, NY).
Statistics
Protein analysis: Groups were compared using analysis of variance plus Student-Newman-Keuls post hoc test. Data shown are mean ± SEM. Numbers of animals were 4 per group. Significance was set as p<0.05.When only two groups were compared, Student-t-test were used (p<0.05). ANOVA tests with a p<0.05 were used to compare changes in electrophysiological responses and imunnohistochemistry labeling.
Results
Inhibitory Postsynaptic potentials
In order to investigate the effects of loss of dysbindin-1 in inhibitory responses occurring in the prefrontal cortex, we measured the amplitude, frequency and kinetic of sIPSCs and mIPSCs in pyramidal neurons as well as assessed excitatory inputs into FSPV+ interneurons.
sIPSCs
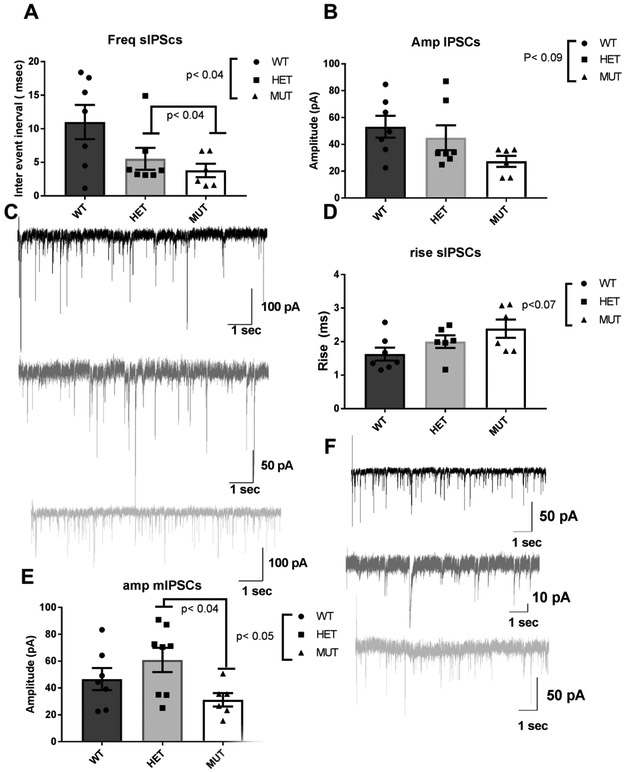
Dysbindin-1 −/− mice exhibit a statistically significant decrease in the sIPSCs interval inter event and it is genotype-related [Fig 1A : dys +/+: 11.0 ± 2.5 Hz (n=7): dys +/−: 5.5 ± 1.6 Hz (n=6) and dys −/−: 3.7 ± 1.0 Hz (n=6), ANOVA F (2,17) = 3.87, p< 0.04].The amplitude of sIPSCs also shows a genotype-related trend towards decrease [Fig 1B: dys +/+: 53.1 ± 8.2 pA (n=7) ; dys +/−: 44.9 ± 9.3 pA (n=6) ; dys −/−: 27.3 ± 4.2 pA (n=6), ANOVA F (2,17) = 2.73, p< 0.09].
Fig 1.
Synaptic effects of loss of dysbindin-1. A) Loss of dysbindin-1 elicits a significant genotype-related decrease in interval inter events of sIPSCs recorded in pyramidal neurons located in deep layers of the PFC [ANOVA F(2,14)=3.8, p< 0.04 and pair wise comparisons show a significant difference between dys+/− and dys−/− (p< 0.4)]. B) The amplitude of sIPSCs shows a genotype-related trend for decreases C) Representative traces of sIPSCs for dys+/+, dys+/− and dys−/− mice. D) Rise of sIPSCs shows a genotype-related trend for increase, E) Analysis of mIPSCs shows a significant genotype-related decrease in the amplitude [ANOVA F(2,18)=3.35, p< 0.05] and pair wise comparison show that dys −/− mice have a significant decrease compared with dys+/− mice (p<0.04). F) Representative traces of mIPSCs. Note the difference in scales.
Analysis of the sIPSCs kinetics shows that there is a genotype-related trend for increase in the rise time of the sIPSCs: dys +/+ 1.6 ± 0.1 msec (n=7); dys +/−: 2.0 ± 0.1 msec (n=6) ; dys −/−: 2.3 ± 0.2 msec ANOVA F(2,16)=3.08, p<0.07(Fig 1D). The decay time did not show statistical differences between the genotypes (dys +/+: 5.6 ± 1.5 msec; dys +/−: 6.4 ± 0.9 msec; dys −/−: 6.8 ± 1.0 msec), neither the charge transfer (dys +/+: 271.0 ± 131.2 pA/mV; dys−/+: 231.2 ± 56.6 pA/mV; dys−/−: 149.6 ± 25.1 pA/mV).
mIPSCs
Assessment of mIPSCs shows that the interval inter event is not affected by the mice genotype [dys+/+: 9.1 ± 3.1 Hz; dys+/−: 5.6 ± 1.4 Hz; dys−/− 5.7 ± 1.5 Hz], however, the amplitude of the mIPSCs shows a significant decrease [Fig 1 E: dys+/+: 46.6 ± 8.3 pA; dys +/− :60.9 ± 9.1 pA; dys −/−: 31.2 ± 5.0 pA, ANOVA F(2,18)=3.35 p< 0.05, and multiple comparisons how a significant difference between dys +/− and dys −/− (p< 0.04), Fig 1E] . When the kinetics of the mIPSCs were analyzed, no significant differences were found.
In summary, dysbindin-1 deletions decrease the frequency of sIPSCs and increase their rise time. When mIPSCs were assessed significant decreases in amplitude were found, suggesting that deficiencies in dysbindin-1are affecting pre- and postsynaptic GABAergic spontaneous and quantal transmission, but not quantal release.
Excitatory Postsynaptic Potentials
We have previously described decreases in amplitude and frequency of mini excitatory postsynaptic potentials (mEPSCs) recorded in pyramidal neurons of dys −/+ and dys −/− mice (Jentsch et al., 2009). In order to assess the effects of dysbindin-1 deletions on glutamate inputs impinging specifically into putative PV+ interneurons, we recorded fast spiking interneurons (FS) and assessed s and mEPSCs in the three genotypes. We did not find any statistical difference in any of the measures assessed for either sEPSCs or mEPSCs (for results see table 1 and 2). So, a difference with the data reported for pyramidal neurons, dysbindin-1 deletions do not seem to affect glutamate inputs into the FS interneurons.
Table 1.
Assessment of sEPSCs in FSPV+ interneurons (X ± SE).
| Dys +/+ (n=6) | Dys +/− (n=5) | Dys −/− (n=10) | |
|---|---|---|---|
| Interval inter event (Hz) | 4.7 ± 2.2 | 10.4 ± 4.8 | 9.2 ± 3.6 |
| Amplitude (pA) | 20.9 ± 3.1 | 25.5 ± 5.0 | 29.6 ± 5.5 |
| Rise (msec) | 2.3 ± 0.6 | 1.2 ± 0.4 | 1.5 ± 0.3 |
| Decay (msec) | 2.2 ± 0.6 | 1.1 ± 0.4 | 2.1 ± 0.5 |
| AUC (pA/mV) | 47.5 ± 12.5 | 25.7 ± 5.4 | 45.4 ± 9.8 |
| CV | 0.55 ± 0.09 | 0.48 ±0.1 | 0.58 ± 0.06 |
| 0.58 |
Table 2.
Assessment of mEPSCS in FSPV+ interneurons (X±SE).
| Dys +/+ (n= 6) | Dys +/− (n=5) | Dys −/− (n=10) | |
|---|---|---|---|
| Interval inter event (Hz) | 4.1 ± 1.7 | 6.9 ± 3.1 | 10.3 ± 2.7 |
| Amplitude (pA) | 19.1 ± 3.5 | 25.5 ± 4.2 | 26.0 ± 3.5 |
| Rise (msec) | 2.6 ± 0.7 | 1.3 ± 0.3 | 1.2 ± 0.3 |
| Decay (msec) | 2.6 ± 2.0 | 1.6 ± 0.4 | 1.5 ± 0.5 |
| AUC (pAms) | 73.1 ± 41.0 | 37.5 ± 8.0 | 26.6 ± 4.9 |
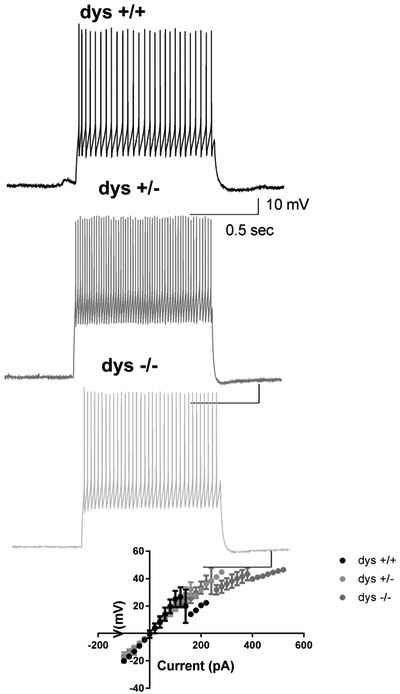
When intrinsic properties of FS interneurons were assessed, we did not find significant changes in excitability, input resistance, rehobase current or spike threshold (Fig. 2 and Table 3). These results suggest that loss of dysbindin-1 doesn’t affect basic properties of FS interneurons.
Fig 2.
Loss of dysbindin-1 does not affect basic properties of fast spiking interneurons, A) Representative traces of excitability recorded in fast spiking interneurons of dys +/+, dys +/− and dys −/− mice. B) I/V curve showing that loss of dysbindin-1 protein does not affect the input resistance of fast spiking interneurons.
Table 3.
Intrinsic properties of FSPV+ interneurons (X ± SE).
| Dys +/+ (n=3) | Dys +/− (n=3) | Dys −/− (n=9) | |
|---|---|---|---|
| Input Resistance (MOhms) | 234.4 ± 31.7 | 249.3 ± 64.0 | 241.3 ± 25.2 |
| Rheobase Current (pA) | 185.0 ± 37.0 | 160.0 ± 70.5 | 203.3 ± 42.2 |
| Spike Threshold (mV) | 43.9 ± 4.7 | 43.3 ± 3.8 | 40.8 ± 1.2 |
Immunohistochemistry
There is consistent evidence of changes in the circuitry of the prefrontal cortex in schizophrenia. Significant changes in GABAergic elements have been observed, for example, a selective decrease in the expression of parvalbumin, a calcium binding protein characteristic of fast-spiking interneurons, has been observed in the PFC of schizophrenia patients (Lewis et al., 2005).
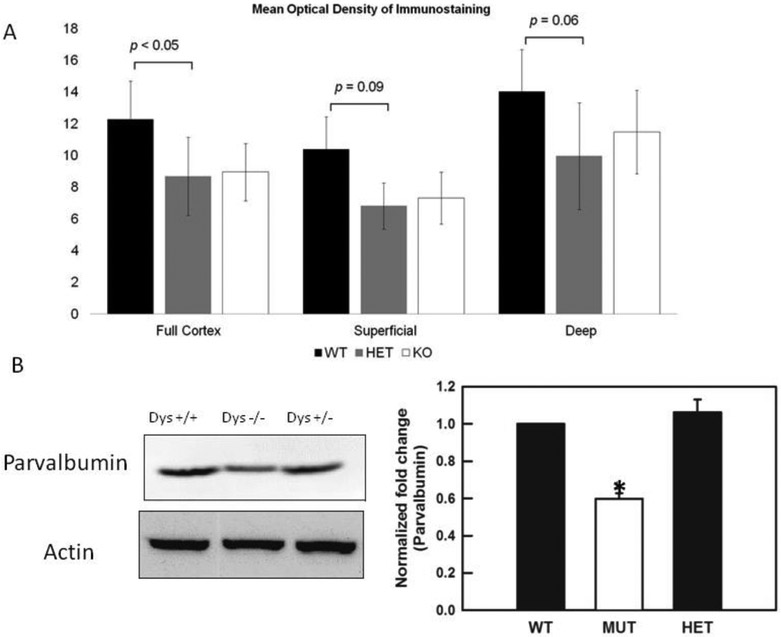
We found that the expression of parvalbumin shows a trend for decreased in both the dysbindin-1 +/− and −/− mice as compared to the dys +/+ littermates when measured directly in deep layers of the PFC as mean optical density [dys +/+: 14.3 ± 3.0; dys −/+ 10 ± 4.0; dys −/− 11.5 ± 2.5, layers V-VI, ANOVA F (1,6)= 4.0, p< 0.06, Fig 3A], or when measured in the full cortex [dys +/+: 12.3 ± 3.0; dys −/+ 8.3 ± 2.0; dys −/− 9.0 ± 2.0, ANOVA F(1,6)= 3.3 p< 0.05, Fig 3A], but not when only superficial layers of the PFC were examined (Fig. 3A).
Fig 3.
Expression of parvalbumin in dys +/+, dys +/− and dys −/− mice. A) Measures of mean optical density show that parvalbumin levels are significantly decrease in dys +/− and dys −/− mice when the full PFC was assessed [ANOVA F(1,6)=3.3 p< 0.05] , however, when the PFC was subdivided in superficial layers (I-IV) and deep layers (V-VI) , superficial layers do not show significant differences, but deep layers show a trend for significance [ANOVA F(1,6)= 4.0p< 0.06]. B) Western blots show that parvalbumin is significantly decreased in tissue from dys−/− mice [ANOVA F (1,6)= 181.1, p<0.010].
Western blots measuring protein levels of parvalbumin were also preformed, and it was found that indeed, dys −/− mice have significant lower levels of parvalbumin [ANOVA F (1,6)= 181.1, p<0.010, Fig. 3B].
In summary our immunohistochemistry and Western blot studies show that, similarly to what have been reported in post mortem tissue of schizophrenia patients, dysbindin-1 deficient mice exhibit deceases in the levels of parvalbumin.
Discussion
Dysbindin-1 is expressed fairly ubiquitously throughout the brain where it is found in both pre- and postsynaptic neuronal elements (Camargo et al., 2007; Kumamoto et al., 2006, Talbot et al., 2004) including glutamatergic, GABAergic and dopaminergic terminals. The role of dysbindin-1 in glutamate neurotransmission in the PFC has been explored by us (Jentsch et al., 2009) and others (Chen et al., 2008) and it has been shown that dysbindin-1 deficient mice show decreases in glutamate release, deficits in synaptic vesicle priming (Saggu et al., 2013), decreases in probability of release (Chen et al 2008; Saggu et al., 2013) and deficits in working memory (Carr et al., 2013 ; Papaleo et al., 2014; Scheggia et al., 2018). However, the effects of dysbindin-1 deletions in GABAergic transmission in the PFC have been less studied.
Here we report that dysbindin-1 deletions elicit a significant decrease in the frequency of sIPSCs, a trend for decrease in the amplitude of sIPSCs, significant decreases in the amplitude (but not frequency) of mIPSCs as well as decreases in the protein levels of parvalbumin. Similar findings have been reported previously in the PFC (Yuang et al., 2016) and the hippocampus (Larimore et al., 2017), and opposite results were found in the amygdala (Huang et al., 2019). Changes in frequency of IPSCs generally are taken to indicate a change in release probability, whereas changes in amplitude suggest an alteration in postsynaptic receptor sensitivity or conductance. Dysbindin-1 deletions have been associated with deficits in the synaptic vesicle dynamics of glutamate (Chen et al., 2008; Dickman and Davis, 2009; Dickman et al., 2012; Gokhale et al., 2015, 2016; Mullin et al., 2015; Saggu et al., 2013), and our results suggest that loss of dysbindin-1 is also affecting the synaptic vesicle dynamics in GABAergic neurons. This suggestion is supported by the data showing that whereas frequency of sIPSCs (mediated by action potentials) is decreased in dysbindin-1 mice, frequency of mIPSCs that are mediated by random fusion of presynaptic vesicles is not affected in dys −/−mice.
We also found significant decreases in the amplitude of mIPSCs in dys −/−. These results suggest that dysbindin-1 deletions may elicit alterations in postsynaptic GABAA receptors. Furthermore, we found an increase in the rise time of the sIPSCs in dysbindin-1 deficient mice and changes in the time-to-rise of sIPSCs suggest an alteration in the current flowing through postsynaptic neurotransmitter receptors and, therefore, are indicative of a postsynaptic change. Thus, taking together the data from the frequency, amplitude and kinetics of s and mIPSCs, our results suggest that GABA transmission is severely compromised in the PFC of dysbindin-1 deficient animals.
Correct GABA signaling is extremely important for the functioning of the cortical networks. Previous experiments by our group (Seamans et al., 2003) and others (Gonzales-Burgos and Lewis, 2008) have demonstrated that GABAergic interneurons play a critical role in maintaining cortical network activity, and Rao and colleagues (2000) have shown that activity of GABA neurons in the PFC is essential for normal working memory. Moreover, parvalbumin positive (PV+) GABA interneurons are essential for the generation of gamma oscillations (Ferando and Mody, 2015; Huang et al., 2016; Lasztóczi and Klausberger 2014; Lozano-Soldevilla et al., 2014) . Gamma oscillations underlie working memory and enhance information processing (Womelsdorf et al., 2007). Studies in post mortem tissue of schizophrenia patients have shown alteration in GABA markers (Woo et al., 1998; Lewis et al., 2005) and in vivo studies have shown that schizophrenia patients exhibit deficits in gamma oscillations when performing working memory tasks (Lee et al., 2003; Spencer et al., 2003; Whittington and Traub 2003), thus, it follows, that deficits in GABA transmission will compromise the generation of gamma oscillations and thus performance in working memory tasks, and indeed, dysbindin-1 knockout mice show deficits in this cognitive domain (Karlsgodt et al., 2011; Papaleo et al., 2012,2014; Scheggia et al., 2018).
In a previous study, Ji and colleagues (2009) showed that dys−/− mice exhibit a decrease in inhibitory inputs to pyramidal neurons in layer V of PFC. In their results Ji et al report decreases in intrinsic excitability of putative FSPV+ interneurons as well as significant decreases in frequency and amplitude of sIPSCs. In our experiments we replicated the changes in frequency and amplitude of sIPSC in dys −/− mice, however, we didn’t find decreases in the frequency or amplitude of excitatory activity impinging into FSPV+ interneurons. One possible explanation is that Ji and colleagues performed their recordings in neuronal cultures, and our experiments are preformed in brain slices. Obviously, in neuronal cultures, the normal connectivity that is vital for synaptic transmission is altered.
The immunohistochemical and Western blot experiments show that the levels of the calcium binding protein parvalbumin are decreased in the PFC of dysbindin-1 deficient mice, however a difference with the electrophysiological data, we did not find genotype-related decreases. Both dys −/+ and dys −/− expressed similar levels of reduction. A possible explanation for these differences may reside in the big variability show in the measures of optical density. Furthermore, the methods used for quantifying PV may not be able to detect subtle differences.
Reports using post-mortem tissue from schizophrenia patients have shown that the expression of PV but not other calcium binding proteins was reduced in the prefrontal cortex (Hashimoto et al., 2008). PV is a calcium-binding protein marker for a class of fast-spiking GABAergic neurons. It is debated whether reduced PV in postmortem studies is caused by a reduction in cell number or merely loss of protein expression. In either case, reduced PV immunoreactivity suggests disruption of an important source of local circuit inhibition (Lewis et al., 2005; Zhang and Reynolds,2002)
An important factor when discussing the role of dysbindin-1 in GABAergic transmission is the regional differences in the penetrance of the deletion. Larimore and colleagues (2017) have shown that the most penetrant GABAergic molecular phenotypes occur in the hippocampus when compared with the PFC and Huang and colleagues (2019) showed that in the amygdala, opposite to what has been reported in the hippocampus and to our present result, deletions of dysbindin-1 enhanced GABAergic transmission. Thus, it is possible that deletion of dysbindin-1 elicits different results depending on the brain region and/or the penetrance of the deletion.
Numerous reports have shown that, in the PFC, dysbindin-1 mutant mice show decreases in glutamate release (Chen et al., 2008;Jentsch et al., 2009; Papaleo and Weinberger, 2011; Saggu et al.., 2013; Wirth et al., 2012), decreases in expression of DAD2R and decreases in GABAergic transmission (Ji et al., 2009; Yuang et al., 2016; present results). All these neurotransmitter systems have been implicated in the patophysiology of schizophrenia (Carlsson et al., 2001;Coyle 2017; Grace 2016; Lewis and Moghaddam, 2006; Winterer and Weinberger, 2004; Papaleo et al., 2012). So, how mutations in dysbindin-1could be regulating these three different neurotransmitter systems? A plausible explanation could be the role of dysbindin-1 in the BLOC-1 complex. Dysbindin-1 is part of the Biogenesis of Lysosome-related Organelle Complex 1 (BLOC-1 complex) (Starcevic and Dell'Angelica, 2004) which is compromised by 8 proteins (dysbindin, snapin, muted, pallidin, cappuccino and BLOS 1–3). This complex has been related to multiple cellular functions including synaptic vesicle dynamics, stabilization of the t-SNARE complex (Larimore et al., 2011; Mullin et al., 2011, Newell-Litwa et al., 2009, 2010) and intracellular protein trafficking involving lysosomes and related organelles (Ghiani and Dell’Angelica, 2011) and several authors (Ghiani et al., 2010; Starcevic and Dell'Angelica, 2004) have demonstrated that loss of dysbindin-1 is accompanied by loss of all the BLOC-1 members. Thus, deletion of dysbindin-1 could affect synaptic vesicle dynamics, including their tethering to the presynaptic terminals and therefore the release of classical neurotransmitters. Furthermore, it has been shown that membrane proteins that traffic from endosomes to lysosomes, as the DAD2 R does, are particularly affected by the loss of dysbindin-1 (Ghiani and Dell’Angelica, 2011), thus resulting in reduced expression of D2R in the mutant mice. So, deletions of dysbindin-1 could potentially affect GABA and glutamate release and decrease expression of D2R. Thus, dysbindin-1 reductions may represent a direct link to the disruption of these three signaling systems.
Conclusions:
Our experiments suggest that deficits in dysbindin-1 elicit decreases in presynaptic GABAergic transmission, perhaps due to the role of dysbindin-1 in synaptic vesicle dynamics (Saggu et al., 2013) and affect GABAergic receptors perhaps due to the postsynaptic role of dysbindin-1 in the tethering of receptors. The disruption in excitatory/inhibitory balance produced by deletions in dysbindin-1 may underlie the deficits in gamma oscillations described in schizophrenia patients (Lee et al., 2003; Spencer et al., 2003; Whittington and Traub 2003) and thus contribute to the cognitive deficits show by the dysbindin-1 mutant mice.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
COI: The authors do not have conflict of interest
References:
- Alherz F, Alherz M, Almusawi H.(2017) NMDAR hypofunction and somatostatin-expressing GABAergic interneurons and receptors: A newly identified correlation and its effects in schizophrenia. Schizophr Res Cogn. 9;8:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Huang HS.(2006) Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 52(2):293–304. [DOI] [PubMed] [Google Scholar]
- Baek JH, Kim JS, Ryu S, Oh S, Noh J, Lee WK, Park T, Lee YS, Lee D, Kwon JS, Hong KS (2012) . Association of genetic variations in DTNBP1 with cognitive function in schizophrenia patients and healthy subjects. Am J Med Genet B Neuropsychiatr Genet.;159B(7):841–9 [DOI] [PubMed] [Google Scholar]
- Bast T, Pezze M, McGarrity S.(2017) Cognitive deficits caused by prefrontal cortical and hippocampal neural disinhibition. Br J Pharmacol.;174(19):3211–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Funke B, Bates JA, Lencz T, Kucherlapati R, Malhotra AK.(2007) DTNBP1 genotype influences cognitive decline in schizophrenia. Schizophr Res.;89(1-3):169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ.(2007) Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia Mol Psychiatry. 12(1):74–86 [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML.(2001) Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 41:237–60 [DOI] [PubMed] [Google Scholar]
- Carlson GC, Talbot K, Halene TB, Gandal MJ, Kazi HA, Schlosser L, Phung QH, Gur RE, Arnold SE, Siegel SJ.(2011) Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci U S A. 108(43): E962–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Jenkins KA, Weinberger DR, Papaleo F. (2013) Loss of dysbindin-1 in mice impairs reward-based operant learning by increasing impulsive and compulsive behavior. Behav Brain Res. 15;241:173–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, Guo N, Huang HP, Xiong W, Zheng H, Zuo PL, Zhang CX, Li W, Zhou Z. (2008) DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol.; 181 (5):791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Hashimoto R, Hattori S, Yohda M, Lipska B, Weinberger DR, Kunugi H (2006). Effect of antipsychotic drugs on DISC1 and dysbindin expression in mouse frontal cortex and hippocampus. J Neural Transm. 113(9): 1337–46. [DOI] [PubMed] [Google Scholar]
- Coyle JT. (2017) Schizophrenia: Basic and Clinical. Adv Neurobiol.; 15:255–280 [DOI] [PubMed] [Google Scholar]
- Cox MM, Tucker AM, Tang J, Talbot K, Richer DC, Yeh L, Arnold SE.(2009) Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav.;8(4):390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel SJ, Lewis DA (2018). Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiol Dis. 22. pii: S0969-9961 (18)30199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Davis GW. (2009) The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science. 20;326(5956):1127–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Tong A, Davis GW. (2012) Snapin is critical for presynaptic homeostatic plasticity. J Neurosci. 20;32(25):8716–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferando I, Mody I. (2015) In vitro gamma oscillations following partial and complete ablation of δ subunit-containing GABAA receptors from parvalbumin interneurons. Neuropharmacology.;88:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell'Angelica EC.(2010) The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry.;15(2): 115, 204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Dell'Angelica EC. (2011) Dysbindin-containing complexes and their proposed functions in brain: from zero to (too) many in a decade. ASN Neuro. 27;3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. (2016) Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 17(8):524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glen WB Jr., Horowitz B, Carlson GC, Cannon TD, Talbot K, Jentsch JD and Lavin A (2014) Impaired hippocampal synaptic plasticity and contextual fear conditioning in dysbindin deficient mice. Hippocampus. 24(2):204–13. doi: 10.1002/hipo.22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale A, Vrailas-Mortimer A, Larimore J, Comstra HS, Zlatic SA, Werner E, Manvich DF, Iuvone PM, Weinshenker D, Faundez V.(2015) Neuronal copper homeostasis susceptibility by genetic defects in dysbindin, a schizophrenia susceptibility factor. Hum Mol Genet. 1;24(19):5512–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale A, Hartwig C, Freeman AH, Das R, Zlatic SA, Vistein R, Burch A, Carrot G, Lewis AF, Nelms S, Dickman DK, Puthenveedu MA, Cox DN, Faundez V.(2016) The Proteome of BLOC-1 Genetic Defects Identifies the Arp2/3 Actin Polymerization Complex to Function Downstream of the Schizophrenia Susceptibility Factor Dysbindin at the Synapse. J Neurosci. 7;36(49):12393–12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. (2008) GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull.;34(5):944–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. (2011) GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast.:723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, Ragland JD, Carter CS (2019). Memory and cognition in schizophrenia. Mol Psychiatry. 24(5):633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Avilés JG, Morris HM, Volk DW, Mirnics (2008) Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 13(2):147–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser J, Koenigs M.(2018) The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol Psychiatry. 15;83(8):638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yoon K, Ko H, Jiao S, Ito W, Wu JY, Yung WH, Lu B, Morozov A (2016) . 5-HT3a Receptors Modulate Hippocampal Gamma Oscillations by Regulating Synchrony of Parvalbumin-Positive Interneurons. Cereb Cortex.;26(2):576–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CCY, Muszynski KJ, Bolshakov VY, Balu DT (2019) . Deletion of Dtnbp1 in mice impairs threat memory consolidation and is associated with enhanced inhibitory drive in the amygdala. Transl Psychiatry. 9;9(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Mizukami K, Iwakiri M, Hidaka S, Asada T. (2004) GABAA receptor gamma subunits in the prefrontal cortex of patients with schizophrenia and bipolar disorder. Neuroreport. 6;15(11):1809–12. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Trantham-Davidson H, Jairl C, Tinsley M, Cannon TD, Lavin A. (2009). Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology. 34(12):2601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia JM, Hu Z, Nordman J, Li Z. (2014) The schizophrenia susceptibility gene dysbindin regulates dendritic spine dynamics. J Neurosci. 8;34(41):13725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, Lu B (2009). Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci U S A.;106(46): 19593–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Robleto K, Trantham-Davidson H, Jairl C, Cannon TD, Lavin A, Jentsch JD (2011) Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance Biol Psychiatry. 1;69(1):28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. (1997) GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex.;7(6):476–86 [DOI] [PubMed] [Google Scholar]
- Kumamoto N, Matsuzaki S, Inoue K, Hattori T, Shimizu S, Hashimoto R, Yamatodani A, Katayama T, Tohyama M. (2006) Hyperactivation of midbrain dopaminergic system in schizophrenia could be attributed to the down-regulation of dysbindin. Biochem Biophys Res Commun. 345(2):904–9 [DOI] [PubMed] [Google Scholar]
- Larimore J, Tornieri K, Ryder PV, Gokhale A, Zlatic SA, Craige B, Lee JD, Talbot K, Pare JF, Smith Y, Faundez V. (2011) The schizophrenia susceptibility factor dysbindin and its associated complex sort cargoes from cell bodies to the synapse. Mol Biol Cell.;22(24):4854–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimore J, Zlatic SA, Arnold M, Singleton KS, Cross R, Rudolph H, Bruegge MV, Sweetman A, Garza C, Whisnant E, Faundez V. (2017) Dysbindin Deficiency Modifies the Expression of GABA Neuron and Ion Permeation Transcripts in the Developing Hippocampus. Front Genet. 10;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasztóoczi B, Klausberger T. (2014) Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron. 5;81(5):1126–1139. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Haig A, Gordon E. (2003) "Gamma (40 Hz) phase synchronicity" and symptom dimensions in schizophrenia. Cogn Neuropsychiatry.;8(1):57–71. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW.(2005) Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 6(4):312–24. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. (2006) Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol.; 63(10):1372–6. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW.(2012) Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35(1):57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. (2014) Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol.;26:22–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Soldevilla D, ter Huurne N, Cools R, Jensen O (2014) . GABAergic modulation of visual gamma and alpha oscillations and its consequences for working memory performance. Curr Biol. 15;24(24):2878–87 [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. (2004) Interneurons of the neocortical inhibitory system. Nat Rev Neurosci.;5(10):793–807 [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. (2000) Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 28(1):53–67. [DOI] [PubMed] [Google Scholar]
- Mullin AP, Gokhale A, Larimore J, Faundez V.(2011) Cell biology of the BLOC-1 complex subunit dysbindin, a schizophrenia susceptibility gene. Mol Neurobiol.;44(1):53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin AP, Sadanandappa MK, Ma W, Dickman DK, VijayRaghavan K, Ramaswami M, Sanyal S, Faundez V. (2015) Gene dosage in the dysbindin schizophrenia susceptibility network differentially affect synaptic function and plasticity. J Neurosci 13;35(19):7643–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Litwa K, Salazar G, Smith Y, Faundez V.(2009) Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol Biol Cell.;20(5): 1441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Litwa K, Chintala S, Jenkins S, Pare JF, McGaha L, Smith Y, Faundez V. (2010) Hermansky-Pudlak protein complexes, AP-3 and BLOC-1, differentially regulate presynaptic composition in the striatum and hippocampus. J Neurosci. 20;30(3):820–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Weinberger DR. (2011) Dysbindin and Schizophrenia: it's dopamine and glutamate all over again. Biol Psychiatry. 1;69(1):2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN, Weinberger DR. (2012)Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol Psychiatry. 17(1):85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Burdick MC, Callicott JH, Weinberger DR. (2014) Epistatic interaction between COMT and DTNBP1 modulates prefrontal function in mice and in humans. Mol Psychiatry.;19(3):311–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. (2000) Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci.;20(1):485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggu S, Cannon TD, Jentsch JD, Lavin A (2013). Potential molecular mechanisms for decreased synaptic glutamate release in dysbindin-1 mutant mice Schizophr Res. 146(1-3):254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Nogueira L, Lavin A. (2003) Synaptic basis of persistent activity in prefrontal cortex in vivo and in organotypic cultures. Cereb Cortex 13(11):1242–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggia D, Mastrogiacomo R, Mereu M, Sannino S, Straub RE, Armando M, Managò F, Guadagna S, Piras F, Zhang F, Kleinman JE, Hyde TM, Kaalund SS, Pontillo M, Orso G, Caltagirone C, Borrelli E, De Luca MA, Vicari S, Weinberger DR, Spalletta G, Papaleo F. Publisher Correction: (2018) Variations in Dysbindin-1 are associated with cognitive response to antipsychotic drug treatment. Nat Commun. 29;9(1):3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW (2003) . Abnormal neural synchrony in schizophrenia. J Neurosci.;23(19):7407–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcevic M, Dell'Angelica EC.(2004) Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1). J Biol Chem. 2;279(27):28393–401 [DOI] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Coyle J, Didriksen M, Gill K, Grace AA, Hensch TK, LaMantia AS, Lindemann L, Maynard TM, Meyer U, Morishita H, O'Donnell P, Puhl M, Cuenod M, Do KQ. (2017) Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry.;22(7):936–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, et al. (2004): Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest 113:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, LeGros RP, Louneva N, Yeh L, Cohen JW, Hahn CG, et al. (2009): Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Hum Mol Genet 18:3851–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker L, Mancarci BO, Tripathy S, Pavlidis P. (2018) Transcriptomic Evidence for Alterations in Astrocytes and Parvalbumin Interneurons in Subjects With Bipolar Disorder and Schizophrenia. Biol Psychiatry. 1;84(11):787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Gomez N, Mata I, Perez-Iglesias R, Rodriguez-Sanchez JM, Ayesa R, Fatjo-Vilas M, Crespo-Facorro B. (2015) Dysbindin gene variability is associated with cognitive abnormalities in first-episode non-affective psychosis. Cogn Neuropsychiatry.;20(2):144–56 [DOI] [PubMed] [Google Scholar]
- Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, et al. (2004): Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry 61:544–555. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Rothmond DA, Hyde TM, Kleinman JE, Straub RE (2008): Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res 98:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. (2004) Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci.;27(11):683–90. [DOI] [PubMed] [Google Scholar]
- Wirth C, Schubert F, Lautenschlager M, Brühl R, Kläar A, Majic T, Lang UE, Ehrlich A, Winterer G, Sander T, Schouler-Ocak M, Gallinat J. (2012) DTNBP1 (dysbindin) gene variants: in vivo evidence for effects on hippocampal glutamate status. Curr Pharm Biotechnol.;13(8):1513–21 [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. (2003) Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 26(12):676–82. [DOI] [PubMed] [Google Scholar]
- Wolf C, Jackson MC, Kissling C, Thome J, Linden DE. (2011) Dysbindin-1 genotype effects on emotional working memory. Mol Psychiatry.;16(2):145–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. (2007) Modulation of neuronal interactions through neuronal synchronization. Science. 15;316(5831):1609–12. [DOI] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA.(1998) A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 95(9):5341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Yang F, Xiao Y, Tan S, Husain N, Ren M, Hu Z, Martinowich K, Ng JS, Kim PJ, Han W, Nagata KI, Weinberger DR, Je HS (2016) . Regulation of Brain-Derived Neurotrophic Factor Exocytosis and Gamma-Aminobutyric Acidergic Interneuron Synapse by the Schizophrenia Susceptibility Gene Dysbindin-1. Biol Psychiatry. 15;80(4):312–322 [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. (2002). A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 1;55(1-2): 1–10. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Burdick KE, Lencz T, Malhotra AK. (2010) Meta-analysis of genetic variation in DTNBP1 and general cognitive ability. Biol Psychiatry. 15;68(12):1126–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkstok JR, de Wilde O, van Amelsvoort TA, Tanck MW, Baas F, Linszen DH (2007) . Association between the DTNBP1 gene and intelligence: a case-control study in young patients with schizophrenia and related disorders and unaffected siblings. Behav Brain Funct;. 203:19. [DOI] [PMC free article] [PubMed] [Google Scholar]