Abstract
Rationale
Alcohol consumption in adolescents and emerging adults is a significant issue. However, our understanding of the topography of alcohol use within drinking episodes in this population is at a nascent stage.
Objectives
This study characterized rate of alcohol consumption in the daily lives of problem drinkers ages 16–24 years (N = 75). We examined whether AUD symptoms and the presence of peers, factors relevant to alcohol consumption in youth, were associated with rate of consumption.
Methods
Ecological momentary assessment (EMA) was used (Nobservations = 799). Rate of consumption was defined as change in estimated blood alcohol concentration (eBAC) relative to the start of the drinking episode. Piecewise multi-level modeling was used to test hypotheses. As a comparison, we examined whether indicators of quantity and frequency (Q-F) were associated with AUD symptoms and presence of peers.
Results
For all participants, eBAC increased sharply early in the episode, then plateaued. Participants with more AUD symptoms or who were in the presence of peers had significantly steeper increases in eBAC over the early part of the episode. Participants with more AUD symptoms were also more likely to engage in binge-like behavior. For Q-F, only peak eBAC and peak number of standard drinks were associated with AUD symptoms, and not presence of peers.
Conclusions
Findings highlight the value of rate of consumption as an indicator of use in youth, one sensitive to the influence of relevant person-level and situational factors. Intervention efforts may benefit from targeting the speed at which youth drink.
Keywords: alcohol use disorder, youth, rate of consumption, peers, ecological momentary assesment
Alcohol is the most commonly used substance by adolescents and emerging adults in the United States. Two out of every three high school students report lifetime alcohol use, and nearly twenty-percent of youth report binge drinking in the past two weeks (Johnston et al., 2018). This high frequency of consumption in youth has significant societal impacts, including low academic achievement, sexual and physical victimization, suicide, and preventable death (Hingson & White, 2014; U.S. Department of Health and Human Services, 2007). Early alcohol use also confers liability for lifetime struggles with addiction (Marshall, 2014; SAMHSA, 2016). Although research has identified factors associated with general drinking patterns among youth (e.g., Colder et al., 2010; Nash et al., 2005), our understanding of the topography of alcohol use within drinking episodes is at a nascent stage. Individual drinking episodes, though small in isolation, are necessary building blocks for the development of alcohol problems. Thus, improving our understanding of how youth drink may suggest means of reducing risky behavior and preventing negative consequences.
Problems arise not only from how much but also how quickly individuals consume alcohol. Drinking at faster rates accelerates the rise in blood alcohol concentration (BAC) levels (Leeman et al., 2010; Li et al., 2007), produces greater subjective intoxication and stimulation in adults (Carpenter et al., 2017; Conrod et al., 1997; Conrod et al., 2001; Martin, Balaban, & McBurney, 2006; Martin & Earleywine, 1990), and yields more pronounced behavioral impairments independent of drinking quantity (Bernosky-Smith et al., 2012; Connors & Maisto, 1979; Fillmore & Vogel-Sprott, 1998; Goodwin et al., 1969; Jones & Vega, 1972; Moskowitz & Burns, 1976; Perry et al., 2006; Ryback, 1970). Thus, there is compelling evidence that rate of consumption is an important indicator of alcohol use. This is further supported by evidence that protective behavioral strategies aimed at slowing or spacing out drinks (i.e., reducing the rate of consumption) reduce the risk of negative consequences from alcohol use (e.g., Pearson, 2013). Despite this, few studies have examined rate of consumption during adolescence and emerging adulthood. This is an important gap because youth are socially and neurologically programmed to take risks (Steinberg, 2004, 2007, 2008), which may make them prone to drinking at accelerated rates and experiencing the consequences thereof. Further, because of their often smaller size, adolescents may experience sharper increases in BAC levels than adults given similar quantities of alcohol (e.g., Donovan, 2009).
In line with their propensity toward risky behavior, binge drinking is prevalent among youth (Johnston et al., 2018; Patrick & Schulenberg, 2014). Binge drinking, defined as consumption that results in BAC levels of .080g% within a 2-hour period (NIAAA, 2004; Siqueira & Smith, 2015), is a special case of accelerated consumption rate, one with specific time and intoxication thresholds. Although the prevalence of binge drinking among youth supports the contention that they are prone to drinking at faster rates, existing research is epidemiological in nature and limited to this special case. This leaves a significant gap in our understanding of within-episode drinking patterns among youth.
Beyond the importance of characterizing rate of consumption in youth, there is a need to understand what person-level or situational factors are associated with drinking rate. If rate of consumption is meaningful, then it should be elevated in people and contexts associated with alcohol-related risk. One potential person-level factor is alcohol use disorder (AUD). Research using ecological momentary assessment (EMA) suggests that adults with AUD or low alcohol sensitivity drink faster than comparisons without AUD or with higher sensitivity (Carpenter et al., 2017; Simons, Wills, Emery, & Marks, 2015; Trela et al., 2016). These findings suggest that a high rate of consumption may represent a possible instantiation of AUD in daily life. That is, while consumption is necessary for the development of AUD, the development of AUD may, in a recursive process, similarly influence drinking behavior. There is a need for research that examines how AUD is expressed in behavior.
One potential situational factor associated with drinking rate is the presence of peers. Drinking among youth often occurs around peers (Chassin et al., 2009; Hawkins et al., 1992; Schulenberg et al., 2012), and peer group influence is one of the leading predictors of binge drinking among youth (Andrews et al., 2002; Coleman & Cater, 2005). Thus, it may be that youth drink faster in the presence of peers than when they are alone. However, no previous work has examined the association of peers and rate of consumption.
In the present study, we leveraged EMA methods to characterize within-episode variation in rate of alcohol consumption among a clinical sample of adolescent and emerging adult drinkers. EMA makes it possible for participants to report their drinking as it occurs, minimizing recall bias (Shiffman et al., 2008). Reports are automatically time-stamped and the resulting temporal resolution is highly beneficial for accurately assessing rate of consumption. EMA also facilitates the calculation of estimated blood alcohol concentration (eBAC; Hustad & Carey, 2005; Mathews & Miller, 1979) values. By incorporating information in addition to quantity consumed, eBAC offers a measure of consumption informed by the pharmacokinetics of alcohol. EMA is also valuable for assessing drinking among individuals under the legal drinking age, for whom laboratory administration of alcohol is prohibited. Yet, few studies have used EMA to directly assess alcohol consumption in youth. We sought to fill this gap.
First, we modeled the within-episode drinking topography among youth by identifying the functional form of the trajectory of rising and falling eBAC levels using piecewise multilevel modeling (MLM). Second, we tested the effect of moderators, hypothesizing that youth with more AUD symptoms or who were in the presence of peers would drink at faster rates. Based on previous work (Carpenter et al., 2017), we expected most drinking to occur early in the episode and specifically hypothesized that differences would be most apparent then. Third, we examined whether the effect of moderators was specific to rate of consumption, or if similar effects would also be observed for indices of quantity (Q), the amount of alcohol consumed, and frequency (F), the number of drinking episodes (Greenfield & Kerr, 2008). Q-F are more commonly examined measures of consumption and it is important to determine whether rate of consumption provides unique information in comparison.
We additionally examined whether AUD symptoms and the presence of peers were associated with the probability of engaging in binge-like behavior, defined as reaching an eBAC of .080g% or higher within the first two hours of a drinking episode. Binge-like behavior provided a complementary indicator of elevated rate of consumption. By definition, binge drinking involves a relatively intense form of elevated rate of consumption, one that has been studied extensively in both youth and adults (e.g., Courtney & Polich, 2009). To our knowledge, no EMA studies have examined whether youth with more severe AUD or who are in the presence of peers are more likely to binge drink in their daily lives.
Finally, although not a primary focus of the current paper, we examined, in supplemental analyses, the association of rate of consumption with subjective response to alcohol in terms of subjective stimulation, sedation, and high. We also examined whether these associations were moderated by AUD symptoms or the presence of peers. This was done to provide a comparison in youth to previous EMA work that found that faster consumption rate was associated with stimulation, but not sedation, in adults (Carpenter et al., 2017). Treloar and Miranda (2017) previously analyzed the association of subjective response and AUD symptoms in the data used in the current study. However, they examined the interaction of AUD symptoms and drinking, compared to nondrinking reports, and did not consider rate of consumption.
Method
Participants
Seventy-five participants (36 Female, 48%), ages 16 to 24 years, were recruited from the community for a clinical trial of the effects of a medication plus a psychosocial platform on alcohol use (NCT01641445). This age range was chosen because neuro-maturation extends into the mid-twenties (Giedd, 2004). Data were culled from a pre-randomization, pre-medication EMA period of approximately one week. Participants were not instructed to reduce or otherwise alter substance use patterns during this period. Selection criteria required participants to drink at least twice weekly in the past 30 days and express interest in reducing their alcohol use, and, thus, were seeking treatment. Participants could not meet criteria for a current DSM-IV-TR Axis I disorder other than alcohol, cannabis, nicotine, or disruptive behavior disorders, endorse current suicidal ideation or psychotic symptoms, or take medications or have medical conditions for which the study medication would be contraindicated. Females were ineligible if they were pregnant or nursing. Mean age for the sample was 20.9 (SD = 2.0) years, 29 participants (39.7%) were under 21. The majority was White (77.1%), Black (12.9%), or Asian (4.3%), and 18.7% were of Hispanic ethnicity.
Procedure
The Brown University Institutional Review Board approved all procedures. Written informed consent or assent was obtained from 18- to 24-year-olds and minors, respectively, and parents of minors provided written permission. Following an initial phone screen, volunteers underwent an in-person screening and, if eligible, were trained to complete EMA reports using a smartphone (Omnia; Samsung Electronics, Ridgefield Park, NJ). Smartphones loaded with software developed for this study were provided to participants. Participants were compensated $5 per day for complying with the EMA protocol (defined as interacting with the smartphone).
EMA reports were completed in participants’ usual settings. Participants entered data by tapping directly on the smartphone screen. The EMA protocol included a combination of device-prompted reports and self-initiated reports completed upon waking (i.e., morning reports) and before and after consumption of standard alcoholic drinks (see Treloar & Miranda, 2017). Participants responded to device-delivered audible prompts several times per day. Device-delivered reports were delivered randomly within 3-hour time blocks and were suspended by self-initiated drink reports.
Participants completed begin- and end-drink reports to assess within-episode drinking topography. For the present analyses, these were the only reports used. Participants were instructed to initiate a begin-drink report when they were about to consume an alcoholic beverage. Participants initiated end-drink reports when they finished consuming that drink. Participants were asked to complete up to three begin- and end-drink report pairs. After the first 26 participants, we expanded this to have participants make a drink report for every drink they consumed.1 Participants reported the total amount of alcohol consumed (in oz.) and the type of beverage. Thus, it was possible for participants to report more or less than one standard drink in an end-drink report. Reasons for doing so may have included if a participant consumed a drink that was smaller than a standard drink, or if a participant forgot or otherwise did not have a chance to complete the end-drink report until later in the episode.
Training in the EMA protocol included a demonstration by research staff using the smartphone, accompanied by a graphic manual with age-appropriate and detailed instructions. The manual included pictures of standard drinks for several beverage types (i.e., beer, flavored malt beverage, malt liquor, straight liquor, fortified wine, mixed drink, wine) to facilitate consistent reporting of begin- and end-drink reports. Participants also received a wallet card with brief instructions regarding the protocol and the definition of standard drinks. The EMA program itself included safeguards to promote consistent reporting of drinks. Device-delivered (e.g., random) prompts queried whether participants were currently drinking, and if so, automatically initiated a begin-drink report.
Measures
Alcohol use disorder
AUD symptoms were assessed with the Kiddie Schedule for Affective Disorders for School-Age Children (KSADS; Kaufman et al., 1997), a clinician-administered interview based on DSM-IV-TR criteria. Interviewers were trained in diagnostic assessment and had high interrater reliability for AUD diagnoses (kappa’s > 0.90). To more closely match DSM-5 AUD, we did not include the “recurrent legal problems” criterion.
Alcohol consumption
All begin-drink reports assessed: (1) whether or not the participant had already started consuming their drink, (2) how many min ago they started drinking (if the answer to (1) was “yes”), and (3) substances other than alcohol used in the last 3 hours/since the last drink. All end-drink reports assessed: (1) how many min ago they finished last drink, (2) beverage type, with options of “Beer,” “Malt Liquor (Colt 45, etc.),” “Liquor (straight or mixed),” “Wine (Merlot, etc.),” “Wine Cooler (Boone’s, etc.),” and “Fortified Wine (Mad Dog, 20/20, etc.),” (3) ounces of alcohol consumed (participants were instructed to include only ounces of liquor for a mixed drink), and (4) substances other than alcohol used since the last drink.
Estimated BAC levels
Momentary eBAC values were computed from each begin- and end-drink report using a formula well-suited for ad lib drinking (Hustad & Carey, 2005; Mathews & Miller, 1979). The formula calculates eBAC values from gender, weight, the average population rate for metabolizing alcohol, time elapsed in hours (per EMA timestamps),2 and cumulative number of standard drinks consumed. Standard drinks were calculated by participant reports of ounces and type of alcohol consumed. By adjusting for gender, weight, metabolism, and time, eBAC provides a more precise estimate of consumption than number of drinks alone. Previous work has established the reliability and validity of eBAC, with eBAC correlated at .500 to .600 with BAC derived from venous and breath samples (Hustad & Carey, 2005).
Presence of peers
At the first begin-drink report of an episode, participants recorded the presence of peers using multiple checkboxes that included friends, boy/girlfriend, and adults such as parents and teachers. Categories of friends and boy/girlfriend were combined to form an episode-level “presence of peers” variable (0=peers not present; 1=peer(s) present). For 7 drinking episodes, participants only completed end-drink reports and, thus, information on presence of peers was not available. Consequently, these 7 episodes (nobservations = 10) were excluded from analyses involving presence of peers.
Subjective response to alcohol
In supplemental analyses, we examined the association of rate of consumption with subjective stimulation, sedation, and high. Subjective response was assessed at the first begin-drink report and the first three end-drink reports of a drinking episode. Participants responded to the following: “How [adjective] do you feel right now?” on a 0 (“not at all”) to 10 (“extremely”) scale. Subjective states included energized, excited, sedated, sluggish, and high. Energized and excited were combined to create a stimulation indicator and sedated and sluggish were combined to create a sedation indicator. These items were based on items from the Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993) and used in prior EMA research with adults and adolescents (e.g., Miranda et al., 2016; Miranda, Monti et al., 2014; Miranda, Ray et al., 2014).
Analytic approach
Data preparation
Drinking observations (i.e., begin- and end-drink reports) were nested within drink episodes, which were nested within individuals. Drink episodes were defined as the period from the start of the first begin- drink report to either the final drink-related report of that study day or when estimated BAC returned to .000g%, whichever occurred first. Any drinking that occurred after estimated BAC returned to .000g% was considered to be part of a new drink episode. To avoid potential carryover effects, all secondary drink episodes were removed from the data (nepisodes = 22; nobservations = 107). Thus, episodes were synonymous with days. Additionally, in rare cases, the eBAC formula produced an extreme and unlikely value (e.g., values associated with coma and death; nobservations = 11). These values were winsorized to .250%, and results did not differ when extreme estimated BAC values were not winsorized. Finally, within episode, time of observations ranged from 0 (the initial begin-drink report) to 526 min (M = 74, SD = 99). However, few observations occurred after 300 min (5 hours; nobservations = 35), and we therefore censored these reports.
Modeling strategy
MLM with restricted maximum likelihood estimation using the PROC MIXED procedure in SAS® 9.4 (SAS, 2014) accounted for the nesting of the EMA data, uneven spacing of observations across episodes and persons, and varying numbers of reports per episode and person. Unless noted otherwise, models had three levels (moment, episode, and person) and included random intercepts at the episode- and person-level. We also initially included episode-level random slopes for time (i.e., slope-60 and slope-300, see below). However, including random slopes did not affect the results and they were, therefore, dropped for parsimony. All models also included age (sample centered), whether participants reported only the first 3 drinks vs. all drinks (report number), day of study (determined by participant sleep schedule), day of week, and hour of day as covariates. The primary outcome was change in eBAC, a clinically interpretable metric with intrinsic meaning. The unstandardized regression weights for models including eBAC as an outcome can be meaningfully interpreted as effect sizes (Baguley, 2009).
Examining the association of rate of consumption required building a multi-level model able to parsimoniously model the nonlinear rise and fall of estimated BAC estimates over the course of the drinking episode (Jones, Wigmore, & House., 2006). Therefore, we first conducted a nonparametric loess regression with a smoothing window corresponding to 25% overlap in the adjacent windows (Cleveland & Devlin, 1988), graphing eBAC across time (in min for ease of presentation). Loess regression has advantages over MLM in visualizing raw data, as it makes fewer assumptions about the form the data takes. The disadvantage of loess regression is that it is substantially more cumbersome to test for group differences. Therefore, our purpose in using loess regression was as a visual aid to help in determining an optimal MLM specification.
Based on the visual inspection of the loess regression, we then fit a piecewise MLM to the data in order to characterize rate of consumption in the sample and to test hypotheses. The piecewise MLM allowed us to specify nodes in order to estimate multiple linear slopes for time. By estimating multiple slopes, we were able to more accurately model change in eBAC over the episode. Estimated BAC was the DV, while slopes for time elapsed and covariates were the IVs. To examine the role of our moderators, two additional piecewise MLMs were conducted, one with AUD symptoms and the other with presence of peers interacted with the slopes for time elapsed. As a result, we tested whether individuals with more AUD symptoms or who were in the presence of peers had a faster rate of consumption (defined as change in eBAC relative to the start of the episode) over the course of the episode. By estimating multiple slopes, we were additionally able to examine moderation within the episode.
For comparison analyses examining indices of Q-F, we specified models separately examining AUD symptoms and presence of peers with the following DVs: 1.) momentary eBAC level, 2.) person-level peak eBAC, 3.) number of standard drinks, 4.) person-level peak number of standard drinks, 5.) the number of episodes per person over the EMA monitoring period, and 6.) the number of standard drinks per episode. These indices represent a range of possible ways to capture quantity and/or frequency at both the momentary and person-level. We examined drinking indices in terms of momentary and person-peak eBAC to provide a close comparison to rate of consumption (which was based on eBAC). We also examined standard drinks as the more typically used metric for assessing quantity (Greenfield & Kerr, 2008). The number of episodes per person was a measure of frequency, and the number of drinks per episode was a measure of average volume (i.e., Q-F) over the EMA period. For models with momentary and episode-level DVs, we specified MLMs, and for person-level DVs, which did not involve nesting, we specified linear regression models.
To examine differences in the propensity to engage in binge-like behavior, we conducted two multi-level logistic models (PROC GLIMMIX) at the episode-level, one with AUD symptoms, and the other with presence of peers as the IVs. A random intercept was estimated at the person-level. The DV was whether or not participants reached an estimated BAC of .080g% or higher at any point within the first 2 hours of an episode.
Results
Preliminary analyses
Mean number of AUD symptoms endorsed was 3.4 (SD = 2.2). In terms of AUD severity (No AUD: 0–1 symptoms, Mild: 2–3 symptoms, Moderate: 4–5 symptoms, Severe: 6+ symptoms), 17 participants (22.7%) did not meet for AUD, 25 (33.3%) met for Mild, 19 (25.3%) met for Moderate, and 14 (18.7%) met for Severe AUD. Peers were present in 148 (64.4%) of the drinking episodes. There were 237 total drinking episodes. The final data included 799 observations between 0 and 300 min (M = 57, SD = 71) into the drinking episode. There were 393 begin-drink and 406 end-drink reports.
Rate of consumption
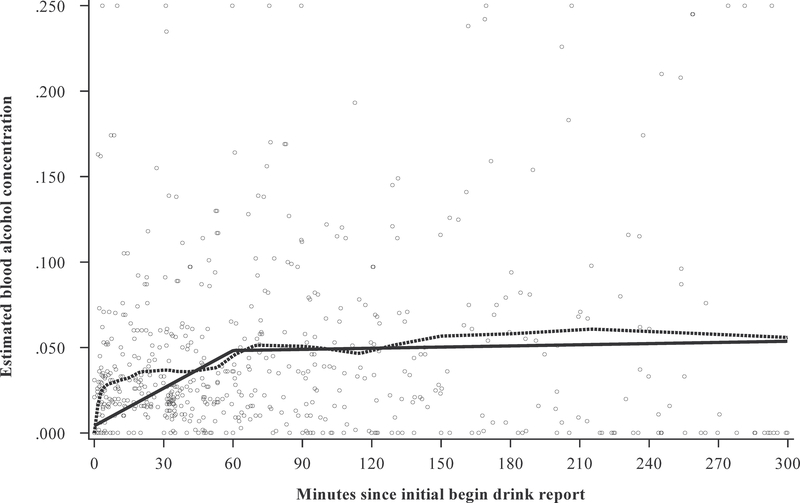
We first examined the within-episode functional form of rate of consumption over the drinking episode. Visual inspection of the loess regression fits revealed that, across all participants, there was a sharp initial increase in eBAC, which continued to rise with an initial peak shortly after the episode began at approximately 60 min. After 60 min, eBAC plateaued (Figure 1).
Fig. 1.
Estimated blood alcohol concentration (eBAC) over time. The dotted line represents the loess regression and the solid line represents the model fit from the piecewise multi-level model (each “O” represents a moment-level report)
We next fit the piecewise MLM for eBAC over time. In the interest of parsimony, we choose to specify a single node, with the result of creating two regression splines. The decision for where to place this node was made with careful consideration, taking into account multiple potential variations. Placing the node too early might restrict the initial spline to a small number of first drink reports, limiting variability in eBAC. In contrast, placing the node too late might inappropriately dilute the initial increase in eBAC evident early in the episode. We therefore adopted an empirical approach and conducted models with nodes specified at multiple time points (30, 45, 60, and 75 min). The pattern of eBAC change proved robust and fit did not vary greatly across these models. Additionally, results for the moderator analyses, presented below, did not differ across these models. We ultimately chose to specify a node at 60 min. This node had the advantage of not being too early or too late in the episode, being at a local peak in eBAC, and dividing the data roughly in half, with most reports (65.1%) occurring at or before 60 min.
Thus, we estimated two linear slopes, or regression splines, each representing change in eBAC over time, with the first slope representing change in eBAC over the first 60 min (slope-60), over which the majority of increase in eBAC took place, and the second from min 61 to min 300 (slope-300).3 Importantly, this latter slope was relative to slope-60. That is, the estimate for slope-300 represented a deviation from slope-60. These two slopes increased our ability to capture nonlinearity in the data, while maintaining parsimony.
Figure 1 presents the overall trajectory of eBAC across the drinking episode for all participants. Both the results of the loess regression and the piecewise MLM fits for the sample are displayed, as well as the raw eBAC estimates, plotted as a function of time since the first begin-drink report. As can be seen, the MLM fits parallel those from the loess regression and, thus, the piecewise MLM appears able to capture variation in eBAC change over the drinking episode. Moreover, it does so in a parsimonious way, with the estimation of a single node at 60 min. For the piecewise MLM, the effects for slope-60 (b = .044, 95% CI = [.036, .052], p < .001) and slope-300 (b = −.043, 95% CI = [−.054, .033], p < .001) were significant, indicating that, across participants, eBAC increased steeply over the first 60 min of the drinking episode and then continued to increase somewhat, but at a significantly slower rate than over the first 60 min.
Moderator analyses
To examine moderation, two models were conducted, one with AUD symptoms and the other with presence of peers added, along with their interactions with slope-60 and slope-300. Thus, these models tested the effects of each moderator on change in eBAC over the first 60 min, relative to the start of the episode, and from min 61 to min 300, relative to the first 60 min.
AUD symptoms
We first examined the association of AUD symptoms and change in eBAC (rate of consumption). Results of the model are presented in Table 1. There were no main effects for AUD symptoms,4 slope-60, or slope-300. There were, however, interactions for slope-60 and AUD symptoms (b = .009, 95% CI = [.006, .013], p < .001), and for slope-300 and AUD symptoms (b = −.012, 95% CI = [−.017, −.007], p < .001). The positive interaction for slope-60 indicates that participants with more AUD symptoms had significantly faster rates of consumption over the first 60 min of the episode. The negative interaction for slope-300 indicates that, relative to slope-60, participants with more AUD symptoms had greater attenuation of their increase over the remainder of the drinking episode. As slope-300 was relative to slope-60, this negative estimate does not necessarily indicate a decline in eBAC.
Table 1.
Parameter estimates for multi-level model of change in estimated blood alcohol concentration (eBAC) over time by alcohol use disorder symptoms.
| Est. | 95% CI | t | |
|---|---|---|---|
| Intercept | .032 | [.015, .049] | 3.70*** |
| AUD symptoms | −.001 | [−.004, .001] | −1.04 |
| Slope-60 | .011 | [−.003, .025] | 1.50 |
| Slope-300 | −.002 | [−.022, .017] | −0.23 |
| AUD symptoms x slope-60 | .009 | [.006, .013] | 5.33*** |
| AUD symptoms x slope-300 | −.012 | [−.017, −.007] | −4.91*** |
| Study Day | .0002 | [−.001, .002] | 0.29 |
| Weekday (Sat. is reference) | |||
| Sunday | −.011 | [−.025, .002] | −1.67 |
| Monday | −.011 | [−.026, .003] | −1.52 |
| Tuesday | −.023 | [−.039, −.008] | −2.95** |
| Wednesday | −.017 | [−.032, −.002] | −2.30* |
| Thursday | −.016 | [−.031, −.002] | −2.19* |
| Friday | −.017 | [−.029, −.006] | −3.03** |
| Hour of day | −.0004 | [−.001, .0001] | −1.43 |
| Age | −.003 | [−.005, −.0005] | −2.38* |
| Report number | −.006 | [−.016, .005] | −1.06 |
Note. N = 75 individuals, 799 observations used. Degrees of freedom were calculated using the Kenward-Roger approximation. Covariates in the model consisted of age (centered), whether participants made only 3 begin-/end-drink report pairs vs. unlimited drink reports (report number), day of study, weekday, and hour of day. AUD = alcohol use disorder.
p < .05.
p < .01.
p < .001.
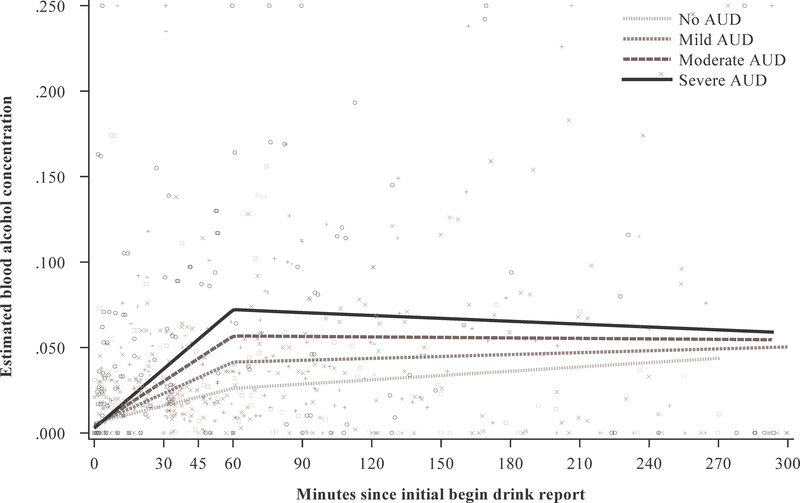
To better visualize these interactions, we plotted change in eBAC over time by AUD severity levels (i.e., no AUD, mild AUD, moderate AUD, and severe AUD; Figure 2). As can be seen in the figure, change in eBAC over the first 60 min was greater in participants with more AUD symptoms. After min 60, change in eBAC largely plateaued for all participants. However, the attenuation effect was larger in participants with more AUD symptoms, while eBAC continued to increase somewhat in the no AUD and mild AUD groups.
Fig. 2.
Estimated blood alcohol concentration (eBAC) over time by alcohol use disorder severity. (Squares represent the No AUD, “X’s the Mild AUD, “+”s the Moderate AUD, and “O”s the Severe AUD group)
Presence of peers
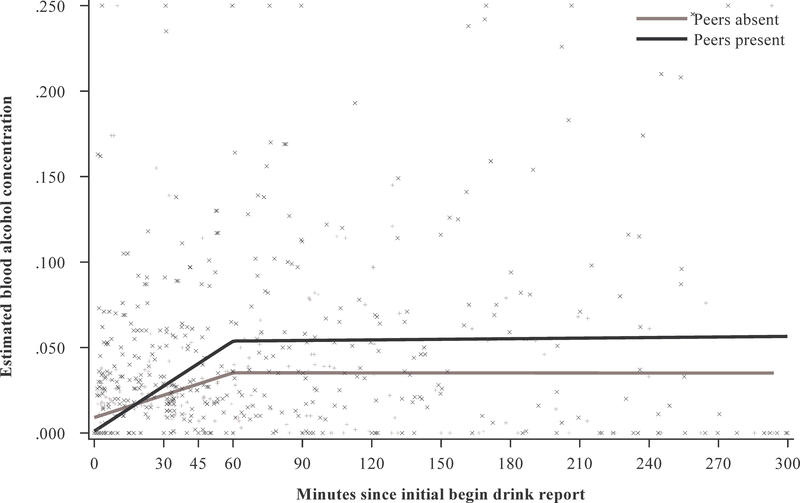
Table 2 presents the results of the model examining the effect of peers. There was no main effect for presence of peers, but there were main effects for slope-60 (b = .027, 95% CI = [.013, .041], p < .001) and slope-300 (b = −.028, 95% CI = [−.047, −.010], p = .002), indicating that eBAC increased steeply over the first 60 min, and then plateaued over the remainder of the drinking episode. These main effects must be understood in the context of significant interactions for the presence of peers and slope-60 (b = .025, 95% CI = [.009, .042], p = .003), and the presence of peers and slope-300 (b = −.024, 95% CI = [−.046, −.002], p = .032). As seen in Figure 3, participants drank significantly faster over the initial 60 min of the episode when they were in the presence of peers than when they were not. Over the remainder of the episode, change in eBAC was largely attenuated for both groups, and slightly more so for participants in the presence of peers.
Table 2.
Parameter estimates for multi-level model of change in estimated blood alcohol concentration (eBAC) over time by presence (vs. absence) of peers.
| Est. | 95% CI | t | |
|---|---|---|---|
| Intercept | .033 | [.017, .050] | 3.93*** |
| Presence of peers | −.011 | [−.023, .0002] | −1.93 |
| Slope-60 | .027 | [.013, .041] | 3.81*** |
| Slope-300 | −.028 | [−.047, −.010] | −3.04** |
| Peers x slope-60 | .025 | [.009, .042] | 3.01** |
| Peers x slope-300 | −.024 | [−.046, −.002] | −2.15* |
| Study Day | .001 | [−.001, .002] | 0.73 |
| Weekday (Sat. is reference) | |||
| Sunday | −.010 | [−.024, .003] | −1.51 |
| Monday | −.010 | [−.024, .005] | −1.28 |
| Tuesday | −.024 | [−.040, −.009] | −3.07** |
| Wednesday | −.020 | [−.035, −.006] | −2.75** |
| Thursday | −.015 | [−.029, .000] | −1.98* |
| Friday | −.016 | [−.027, −.005] | −2.76** |
| Hour of day | .000 | [−.001, .000] | −1.54 |
| Age | −.003 | [−.006, −.001] | −2.53* |
| Report number | −.006 | [−.017, .005] | −1.06 |
Note. N = 75 individuals, 789 observations used. Degrees of freedom were calculated using the Kenward-Roger approximation. Covariates in the model consisted of age (centered), whether participants made only 3 begin-/end-drink report pairs vs. unlimited drink reports (report number), day of study, weekday, and hour of day.
p < .05.
p < .01.
p < .001.
Fig. 3.
Estimated blood alcohol concentration (eBAC) over time by presence of peers. (“+”s represent moments with peers absent, and “X”s with peers present)
Gender and age
We tested two additional MLMs examining whether either gender or age were significant moderators of rate of consumption. There were no associations for gender, either as a main effect (b = .002, 95% CI = [−.010, .014], p = .747) or interacted with slope-60 (b = .006, 95% CI = [.010, .022], p = .450) or slope-300 (b = −.012, 95% CI = [−.033, .009], p = .257). Similarly, there were no associations for age, either as a main effect (b = −.001, 95% CI = [−.004, −.002], p = .426) or interacted with slope-60 (b = −.002, 95% CI = [−.006, .002], p = .324) or slope-300 (b = .002, 95% CI = [−.008, .003], p = .406).
Quantity and frequency
We next sought to examine the specificity of associations to rate of consumption by examining whether AUD symptoms and the presence of peers were associated with indices of Q-F (i.e., momentary eBAC level, person-level peak eBAC, momentary number of standard drinks, person-level peak number of standard drinks, the number of episodes per person, and the number of drinks per episode). Across all drink episode moments, average eBAC was .034g% (SD = .049 g%). Neither AUD symptoms (b = .002, 95% CI = [−.0004, .004], p = .103), nor the presence of peers (b = −.005, 95% CI = [−.005, .015], p = .313) were associated with momentary eBAC level. Person-level average peak eBAC was .080g% (SD = .073g%). AUD symptoms (b = .009, 95% CI = [.001, .016], p = .023), but not presence of peers (b = −.001, 95% CI = [−.053, .050], p = .995), was associated with peak eBAC. Across all drink episode moments, average number of standard drinks consumed was 1.49 drinks. Neither AUD symptoms (b = 0.03, 95% CI = [−0.01, 0.07], p = .127), nor the presence of peers (b = 0.03, 95% CI = [−0.16, 0.22], p = .730) were associated with momentary number of standard drinks. Person-level average peak number of standard drinks was 4.20 drinks. AUD symptoms (b = 0.36, 95% CI = [0.01, 0.71], p = .046), but not presence of peers (b = −0.16, 95% CI = [−2.55, 2.24], p = .900), was associated with peak number of standard drinks. Participants drank on 42.4% of the total study days. Neither AUD symptoms (b = 0.06, 95% CI = [−0.11, 0.22], p = .503), nor the presence of peers (b = 0.62, 95% CI = [−1.74, 0.50], p = .271) were associated with the number of drinking episodes per person. The average number of drinks per episode was 2.54 drinks (SD = 2.56). Neither AUD symptoms (b = 0.16, 95% CI = [−0.02, 0.33], p = .075), nor the presence of peers (b = 0.38, 95% CI = [−0.34, 1.11], p = .300) were associated with the number of drinks per episode.
Binge drinking
As an alternative, and more frequently used, index that incorporates consumption rate, we examined whether AUD symptoms and presence of peers were associated with greater probability of engaging in binge-like behavior (i.e., achieving an eBAC of .080g% in the first two hours of an episode). Of the 237 drinking episodes, 11.39% (n = 27) qualified as a binge episode. Results from the multi-level logistic models are presented in Table 3. AUD symptoms were associated with a greater likelihood of a binge episode (OR = 1.42, 95% CI = [1.11, 1.82], p = .007). In contrast, presence of peers was not associated with the likelihood of a binge episode.5
Table 3.
Parameter estimates for multi-level logistic models examining the associations of alcohol use disorder symptoms and the presence of peers and probability of a binge episode.
| IV: AUD symptoms | IV: Presence of peers | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Intercept | 0.04 | [0.003, 0.43] | 0.14 | [0.01, 1.48] |
| AUD symptoms | 1.42 | [1.11, 1.82]** | ||
| Presence of peers | 1.25 | [0.47, 3.35] | ||
| Study Day | 1.09 | [0.93, 1.28] | 1.10 | [0.94, 1.29] |
| Weekday (Sat. is reference) | ||||
| Sunday | 0.87 | [0.21, 3.54] | 0.80 | [0.21, 3.04] |
| Monday | 0.33 | [0.07, 1.48] | 0.42 | [0.10, 1.81] |
| Tuesday | 0.13 | [0.01, 1.18] | 0.14 | [0.02, 1.18] |
| Wednesday | 0.11 | [0.01, 0.97]* | 0.12 | [0.01, 0.96]* |
| Thursday | 0.20 | [0.04, 1.07] | 0.24 | [0.05, 1.25] |
| Friday | 0.35 | [0.10, 1.31] | 0.43 | [0.13, 1.45] |
| Hour of day episode began | 1.06 | [0.96, 1.17] | 1.04 | [0.94, 1.14] |
| Age | 0.96 | [0.74, 1.24] | 0.91 | [0.71, 1.16] |
| Report number | 0.37 | [0.13, 1.10] | 0.52 | [0.18, 1.49] |
Note. N = 75 individuals, 237 (AUD symptoms), 230 (presence of peers) drinking episodes used. Covariates in the model consisted of age (centered), whether participants made only 3 begin-/end-drink report pairs vs. unlimited drink reports (report number), day of study, weekday, and hour of day episode began. AUD = alcohol use disorder.
p < .05.
p < .01.
p < .001.
Supplemental analyses: Subjective response
Finally, we examined the association of subjective response and rate of consumption. For these analyses, we separately considered subjective stimulation, sedation, and high as DVs. The primary IVs were eBAC level and change in eBAC. Change in eBAC was calculated by subtracting the eBAC value at the previous time point from the current time point. Effects for eBAC level and change were scaled to represent the amount of change in the dependent variable for an increase in eBAC of .010%. As subjective response items were only assessed at the first begin-drink report and the first three end-drink reports, these analyses included only 592 observations.
In main effects models, neither eBAC level nor change in eBAC were associated with subjective stimulation, sedation, or high (Table S1). There were statistical trends indicating that faster rate of consumption was nonsignificantly associated with reduced stimulation (b = −0.08, 95% CI = [−0.17, 0.003], p = .058) and greater sedation (b = 0.08, 95% CI = [−0.01, 0.16], p = .079). For models examining interactions with AUD symptoms (Table S2), there were no significant interactions with eBAC level or change predicting stimulation. However, AUD symptoms significantly interacted with change in eBAC to predict both sedation (b = 0.04, 95% CI = [0.002, 0.08], p = .041) and high (b = 0.06, 95% CI = [0.02, 0.11], p = .007). As seen in Figures S1 and S2, individuals with more AUD symptoms reported both more sedation and high when drinking at faster rates. For models examining interactions with presence of peers (Table S3), there were no interactions with eBAC level or change for stimulation, sedation, or high. There were main effects for presence of peers, such that being in the presence of peers was associated with greater stimulation (b = 0.68, 95% CI = [0.19, 1.18], p = .041) and reduced sedation (b = −0.55, 95% CI = [−1.01, −0.09], p = .018) regardless of alcohol consumption.
Discussion
This study leveraged EMA methods to characterize the within-episode drinking topography of a clinical sample of adolescent and emerging adult drinkers in their natural environment. Youth consumed alcohol at faster rates early in drinking episodes. Specifically, eBACs rose sharply during the first 60 minutes of drinking episodes, and then plateaued. This initial steep increase in eBAC suggests the early part of drinking episodes may be an important target for intervention efforts aimed toward attenuating some of the harmful effects of alcohol consumption. Slowing initial intake and preventing sharp increases in BAC may mitigate some of the adverse effects of alcohol use, given evidence that elevated rate of consumption is associated with negative consequences (Bernosky-Smith et al., 2012; Connors & Maisto, 1979; Fillmore & Vogel-Sprott, 1998; Goodwin et al., 1969; Jones & Vega, 1972; Moskowitz & Burns, 1976; Perry et al., 2006; Ryback, 1970). This is in line with research showing that increasing protective behavioral strategies (e.g., spacing drinks over time) can reduce negative consequences of alcohol (e.g., Pearson, 2013).
This study is one of the few to investigate alcohol consumption in youth in near real-time in their natural environments. Individual events of consumption, while small by themselves, are the building blocks out of which addiction is formed. There is, thus, a need to better understand the nature of consumption as it occurs in daily life. This is especially true in adolescents and emerging adults, where it may be possible to interrupt the accumulation of drinking episodes before they reach critical mass and have long-term consequences.
Consistent with our hypothesis, the steep increase early in the episode was exacerbated among participants with greater numbers of AUD symptoms. Over the first 60 min, each additional AUD symptom was associated with a .009g% increase in eBAC. Thus, the average difference in eBAC between participants with zero and nine AUD symptoms was more than the legal drinking limit for driving (.081g%). This finding is consistent with prior EMA work that found adults with AUD drank faster than their non-AUD counterparts (Carpenter et al., 2017; Simons et al., 2015). It may be that individuals with more AUD symptoms drink faster to maximize positive effects, or, perhaps, to counteract tolerance. Results add to mounting evidence that rate of consumption is an important behavioral correlate of AUD, one that captures the expression of AUD in daily life.
Participants also consumed alcohol at faster rates when in the presence of peers. Despite the well-documented influence of peers on the drinking habits of youth, this is the first study to examine the influence of peers on rate of consumption. The presence of peers was associated with a .025g% increase in eBAC over their absence. Given the importance of peers in the drinking behavior of youth (e.g., Chassin, Hussong, & Beltran, 2009), it is, perhaps, somewhat surprising that participants were not among peers at an even higher percentage of episodes than they were. It may be that the observed percentage of episodes with peers reflects the severity of AUD present in the sample, as individuals with more severe AUD may be more likely to drink by themselves. However, we are not aware of any EMA studies that report how often youth drank in the presence versus absence of peers, making it difficult know whether the present findings are out of the norm. Multiple reasons exist for why rate of consumption early in drinking episodes might increase in the presence of peers. For example, peers might exert pressure to drink faster explicitly (e.g., through drinking games), or implicitly (e.g., perceived need to “keep pace”). Individuals may also be more likely to be with peers in contexts in which alcohol use is encouraged (e.g., bars). Individuals may also increase their consumption in an effort to decrease social anxiety. Individuals likely experience a variety of these and other influences when in the presence of peers. Intervention efforts that focus on understanding what may lead an individual to drink faster around peers may be able to counteract influences and help bring about reductions in drinking speed.
Notably, AUD symptoms and the presence of peers were largely not associated with indices of Q-F (momentary eBAC level, momentary number of standard drinks, number of episodes per person, number of standard drinks per episode). These indices were selected to correspond with typical Q-F indices in epidemiological work (Greenfield & Kerr, 2008). However, adjustments were made to fit the nature of EMA data. Specifically, the current study was over a much shorter time period than retrospective studies that are more typically used to assess Q-F. In particular, we assessed consumption in terms of eBAC, which is not often calculated in epidemiological studies given that it is difficult to retrospectively obtain accurate estimates of the length of drinking episodes. In terms of Q-F, we therefore examined indices based on both eBAC, to provide comparisons to rate of consumption, and standard drinks, which represent more typical assessments of quantity. Results did not differ between the eBAC and standard drink assessments. Overall, participants did not consume particularly large quantities of alcohol on average. There was an association of greater AUD severity, but not the presence of peers, and higher person-level peak eBAC (and person-level peak number of standard drinks). These two effects, however, do not provide information on what occurred within drinking episodes or when peak consumption occurred. These findings align with prior work that shows an imperfect relationship between AUD and Q-F in both youth and adults (Lane & Sher, 2015; Lee et al., 2011; Lewinsohn et al., 1996). This includes work that examined alcohol consumption in terms of eBAC values (e.g., Carpenter et al., 2017). Additionally, AUD requires no minimum Q-F threshold for the disorder and only two of the eleven AUD criteria imply increased Q-F (i.e., consuming larger quantities or over a longer period of time than intended and tolerance; Leeman et al., 2010). Findings from the present study suggest there is something unique about quantity and time (i.e., rate), compared to the indices of Q-F examined in this study, in regard to AUD symptoms and presence of peers. Our findings suggest the importance of large and sudden shifts in consumption (i.e., sharp increases in eBAC), which rate of consumption captures. Rate may be an additional, complementary indicator of consumption to more typical Q-F measures.
In addition to the association with rate of consumption, AUD symptoms, but not the presence of peers, were associated with a greater probability of engaging in binge-like behavior. Binge drinking, in essence, represents a relatively intense example of elevated consumption rate, with specific thresholds regarding time and quantity, and is associated with numerous negative effects. The association with AUD symptoms thus provides additional support that youth with more AUD symptoms tend to drink at significantly faster rates. It should be noted, however, that differences in consumption rate for AUD severity emerged well within the first two hours of the drinking episode, and, thus, before a binge could be said to have occurred. In this way, binge drinking is less sensitive to specific inter-episode changes than change in eBAC. It is also less sensitive, as a dichotomous variable, to degrees of consumption rate. This may explain why the presence of peers was associated with elevated consumption rate, but not probability of a binge.
There is reason to believe that consumption rate may be an especially important indicator in youth. Drinking fast is inherently risky and may reflect aspects of impulsivity (e.g., sensation seeking),6 and adolescence and emerging adulthood are developmental periods associated with heightened impulsivity and risk-taking behavior (Steinberg, 2004, 2007, 2008). Adolescents, in particular, may be prone to experiencing sharp increases in eBAC, due to their often smaller size compared to adults (e.g., Donovan, 2009). Moreover, many participants were under the legal drinking age and may have faced restrictions in terms of obtaining and using alcohol. Drinking at an elevated rate may be a way for youth to get the most “bang for their buck” given these restrictions. In the present study we did not find any association for age (or gender) and rate of consumption, but we also examined a relatively restricted range of ages. Although there was a main effect for age in models where it was included as a covariate (Tables 1 and 2), there were no main effects or interactions with time (i.e., slope-60, slope-300) in the model specifically examining age. Thus, while the present results provide important information about the speed at which youth drink in their daily lives, there is a need for more work on this topic. In particular, future work should examine rate of consumption in youth compared to older adults.
Supplemental analyses examined the association of rate of consumption with subjective response to alcohol. These analyses were not a primary focus, but meant to provide complementary information to Carpenter et al. (2017) in youth. Greater change in eBAC (i.e., faster rate of consumption) was nonsignificantly associated with reduced stimulation and increased sedation. Additionally, participants with more AUD symptoms reported significantly increased sedation and high following greater increases in eBAC. There were no interactions with the presence of peers. These findings are inconsistent with Carpenter et al. (2017), who found that greater change in eBAC was associated with greater stimulation and unrelated to sedation. However, Carpenter et al. (2017) was conducted in adults and, although few studies have examined subjective response to alcohol in youth, those that have suggest differences in youth compared to adults. Specifically, greater eBAC levels are associated with decreased stimulation (Miranda et al., 2014; Treloar et al., 2017), and increased sedation and high in youth (Miranda et al., 2014). These findings are largely consistent with the present results. Treloar and Miranda (2017), analyzing the same data as the current study, found that youth reported greater sedation, though not high, at end-drink reports, and that youth with more AUD symptoms reported more stimulation at end-drink reports. However, they examined alcohol use dichotomously and not the amount or rate of consumption. Thus, their finding for stimulation may reflect a general hypersensitivity to stimulation in youth (Spear, 2011), that does not increase further with more consumption. Thus, as the amount and, in individuals with more AUD symptoms, rate of consumption increase, stimulation may decrease, and sedation and high may increase. However, this is largely speculative and additional research is needed to understand the association of consumption and subjective response in youth.
Despite these new and important findings, it is necessary to consider several limitations of this study. First, while intensively longitudinal, we examined consumption over a brief period of time (i.e., approximately one week). Second, participants were interested in reducing their alcohol use, and future research should examine whether findings generalize to individuals not seeking treatment. Third, AUD symptoms were assessed cross-sectionally and, thus, the present study did not test causal relationships between AUD symptoms and rate of consumption. We conceptualize there being a recursive relationship between momentary alcohol consumption and AUD. Individuals must first drink in order to develop AUD, and AUD, in turn, influences drinking behavior. In this study, we were primarily interested in the latter part of this relationship. Future longitudinal work is needed to examine the causal nature of the relationship between consumption rate and factors like AUD and presence of peers. Fourth, we relied on participants to self-report drinking. EMA-based self-report of alcohol consumption correlates well with transdermal assessment of use, a more objective measure (Simons et al., 2015), which indicates that participants are adherent in reporting their alcohol use. Nevertheless, future EMA work should leverage advances in technology that offer the potential to capture BAC objectively in real-time. Fifth, while eBAC values were calculated using a valid and reliable formula that accounted for multiple factors relevant to absorption (Hustad & Carey, 2005), the formula remains an estimate and did not account for all possible factors (e.g., food intake, metabolic tolerance). Sixth, the current study did not examine the effects or consequences of elevated consumption rate. Future work should examine the outcomes of elevated consumption rate in youth in daily life. Seventh, participants only reported broadly on the categories of individuals they were with during drinking episodes (e.g., friends, boyfriend/girlfriend). Future work should collect additional information (e.g., gender; Thrul et al., 2017) in order to explore potential moderators of the association for rate of consumption and presence of peers. Finally, the majority of drink reports occurred early in the episode (64.8% within the first 60 min) and, thus, it makes sense that moderators had their largest influence in this part of the episode. Differences tended to decrease following this, but, as there were fewer reports made overall as time from initial drink increased, we are less confident about these decreases and they should be interpreted with caution. It is unclear whether there were fewer drink reports later in the episodes because few episodes lasted that long, or whether participants became less likely to report drinks late in the episode (e.g., due to fatigue or intoxication).
On balance, we examined rate of consumption in a population that may be prone to drinking fast. Our EMA sampling strategy, with participants recording when they began and ended each drink, enabled the calculation of change in eBAC with good temporal resolution. Calculating eBAC better accounted for the pharmacokinetics of alcohol consumption than relying solely on the number of standard drinks. Our analytical approach accommodated a complicated data structure in our statistical model and efficiently characterized drinking patterns over time. The findings suggest that rate of consumption is a meaningful indicator of alcohol use.
In conclusion, the present study examined the pattern of alcohol consumption in the daily life of youth interested in reducing their alcohol use. Examining the topography of alcohol use within drinking episodes, we found that eBAC increased steeply over the first 60 min of the drinking episode, and then plateaued. Rate of consumption in the first part of the episode was further elevated in youth who met for more AUD symptoms and when youth drank in the presence of peers. Youth with more AUD symptoms were also more likely to engage in binge-like behavior. In contrast, indices of Q-F were largely unrelated to AUD symptoms or the presence of peers, with the exception of person-level peak eBAC and peak number of standard drinks with AUD symptoms. The findings highlight the importance of rate of consumption as a measure of alcohol use, one that may be particularly sensitive to the influence of person-level and situational factors. The findings may potentially inform intervention efforts, suggesting that there may be value developing strategies to help youth to slow their use.
Supplementary Material
Acknowledgments
The National Institute of Alcohol Abuse and Alcoholism (AA007850, PI: Miranda; AA024808, PI: Treloar; AA007459, PI: Monti; L30AA027041: PI, Emery) and the National Institute on Drug Abuse (DA016184, PI: Rohsenow) supported this research.
Footnotes
Conflict of interest statement: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Drink reports beyond the third begin-/end-drink report pair were infrequent in the sample, with 98 such reports (12.3% of prompts). Excluding the 26 participants who could not make more than three report pairs did not affect the results, except that the interaction for presence of peers and slope-300 became nonsignificant.
For the first drink of an episode, if participants reported having already started their drink, the number of min ago that participants began drinking was added to time elapsed and included in the calculation of eBAC.
In addition to the piecewise MLM, we considered using a polynomial model (i.e., quadratic, cubic). However, the fit of the piecewise model (AIC: −2,809.6; BIC: −2,815.6) was superior in terms of fit (with smaller values [i.e., more negative] being better) to that of both the quadratic (AIC: −2,787.0; BIC: −2,793.0) and cubic models (AIC: −2,793.0; BIC: −2,793.0). We additionally favored a piecewise MLM over a polynomial model because the resulting regression splines from the piecewise MLM could be interacted with moderators (AUD symptoms and presence of peers), thus facilitating our goal of examining the effect of these moderators on rate of consumption within the drinking episode. The piecewise model, which had linear slopes, was also superior to piecewise models with quadratic (AIC: −2,799.6; BIC: −2,805.6) and cubic (AIC: −2,794.6; BIC: −2,800.6) slopes.
Viewing Figure 2, there may appear to be a main effect for AUD symptoms, as, for example, individuals with severe AUD have the highest eBACs for much of the episode. However, it should be noted that the bulk of prompts occurred early in the episode, before substantial differences in eBAC emerged. Thus, taking into account the entirety of the episode, there was no main effect. This also applies below to the presence of peers.
We additionally conducted these analyses without a time specifier for binge-like behavior (i.e., whether participants achieved an eBAC of .080g% at any point in the episode). The number of binge episodes increased to 33. However, results for AUD symptoms and presence of peers did not change.
While drinking fast is a risky behavior, there are situations (e.g., pre-gaming) in which it may be planned in advance. Future work should examine possible differences in planned vs. unplanned fast consumption.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Andrews JA, Tildesley E, Hops H, & Li F (2002) The influence of peers on young adult substance use. Health Psychology 21:349. [DOI] [PubMed] [Google Scholar]
- Baguley T (2009) Standardized or simple effect size: What should be reported? British Journal of Psychology 100:603–617. [DOI] [PubMed] [Google Scholar]
- Bernosky-Smith KA, Aston ER, & Liguori A (2012) Rapid drinking is associated with increases in driving- related risk- taking. Human Psychopharmacology: Clinical and Experimental 27:622–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RW, Trela CJ, Lane SP, Wood PK, Piasecki TM, & Trull TJ (2017) Elevated rate of alcohol consumption in borderline personality disorder patients in daily life. Psychopharmacology 234:3395–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Hussong A, Beltran I Adolescent substance use In: Lerner R, Steinberg L, editors. Handbook of Adolescent Psychology. Vol. 1 Hoboken, NJ: Wiley; 2009. pp. 723–763. [Google Scholar]
- Cleveland WS, & Devlin SJ (1988) Locally weighted regression: an approach to regression analysis by local fitting. Journal of the American Statistical Association 83:596–610. [Google Scholar]
- Colder CR, Chassin L, Lee MR, & Villalta IK (2010) Developmental perspectives: Affect and adolescent substance use In Kassel JD (Ed.), Substance abuse and emotion. (pp. 109–135). Washington, DC US: American Psychological Association. [Google Scholar]
- Coleman L, & Cater S (2005) Underage ‘binge’drinking: A qualitative study into motivations and outcomes. Drugs: Education, Prevention And Policy 12:125–136. [Google Scholar]
- Connors GJ, & Maisto SA (1979) Effects of alcohol, instructions, and consumption rate on affect and physiological sensations. Psychopharmacology 62:261–266. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, & Pihl RO (2001) Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology 157:20–30. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO, & Mankowski S (1997) Biphasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcoholism: Clinical and Experimental Research 21:140–149. [PubMed] [Google Scholar]
- Courtney KE, & Polich J. (2009). Binge drinking in young adults: Data, definitions, and determinants. Psychological Bulletin, 135, 142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan JE (2009) Estimated blood alcohol concentrations for child and adolescent drinking and their implications for screening instruments Pediatrics 123:e975–e981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M (1998) Behavioral impairment under alcohol: cognitive and pharmacokinetic factors Alcoholism: Clinical and Experimental Research 22:1476–1482 [PubMed] [Google Scholar]
- Giedd JN (2004) Structural magnetic resonance imaging of the adolescent brain Annals of the New York Academy of Sciences 1021:77–85 [DOI] [PubMed] [Google Scholar]
- Goodwin DW, Crane JB, Guze SB (1969) Alcoholic” blackouts”: A review and clinical study of 100 alcoholics American Journal of Psychiatry 126:191–198 [DOI] [PubMed] [Google Scholar]
- Greenfield TK, Kerr WC (2008) Alcohol measurement methodology in epidemiology: recent advances and opportunities Addiction 103:1082–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY (1992) Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: Implications for substance abuse prevention Psychological bulletin 112:64. [DOI] [PubMed] [Google Scholar]
- Hingson R, White A (2014) New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review Journal of studies on alcohol and drugs 75:158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustad JT, Carey KB (2005) Using calculations to estimate blood alcohol concentrations for naturally occurring drinking episodes: A validity study Journal of Studies on Alcohol 66:130–138 [DOI] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME (2018) Monitoring the Future national survey results on drug use, 1975–2017: Overview, key findings on adolescent drug use [Google Scholar]
- Jones AW, Wigmore J, House C (2006) The course of the blood-alcohol curve after consumption of large amounts of alcohol under realistic conditions Canadian Society of Forensic Science Journal 39:125–140 [Google Scholar]
- Jones BM, Vega A (1972) Cognitive performance measured on the ascending and descending limb of the blood alcohol curve Psychopharmacologia 23:99–114 [DOI] [PubMed] [Google Scholar]
- Kaufman J et al. (1997) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data Journal of the American Academy of Child & Adolescent Psychiatry 36:980–988 [DOI] [PubMed] [Google Scholar]
- Lane SP, Sher KJ (2015) Limits of current approaches to diagnosis severity based on criterion counts: an example with DSM-5 alcohol use disorder Clinical Psychological Science 3:819–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-T, Rose JS, Engel-Rebitzer E, Selya A, Dierker L (2011) Alcohol dependence symptoms among recent onset adolescent drinkers Addictive behaviors 36:1160–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O’Malley SS (2010) Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addiction biology 15:109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, & Seeley JR (1996) Alcohol consumption in high school adolescents: frequency of use and dimensional structure of associated problems. Addiction 91:375–390 [DOI] [PubMed] [Google Scholar]
- Li TK, Hewitt BG, Grant BF (2007) The Alcohol Dependence Syndrome, 30 years later: A commentary Addiction 102:1522–1530 [DOI] [PubMed] [Google Scholar]
- Marshall EJ (2014) Adolescent alcohol use: risks and consequences Alcohol and Alcoholism 49:160–164 [DOI] [PubMed] [Google Scholar]
- Martin CS, Balaban CD, McBurney DH (2006) Tonic and phasic processes in the acute effects of alcohol Experimental and clinical psychopharmacology 14:209. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M (1990) Ascending and descending rates of change in blood alcohol concentrations and subjective intoxication ratings Journal of Substance Abuse 2:345–352 [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM (1993) Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res 17:140–146 [DOI] [PubMed] [Google Scholar]
- Matthews DB, Miller WR (1979) Estimating blood alcohol concentration: Two computer programs and their applications in therapy and research Addictive Behaviors 4:55–60 [DOI] [PubMed] [Google Scholar]
- Miranda R Jr, MacKillop J, Treloar H, Blanchard A, Tidey JW, Swift RM, … & Monti PM (2016) Biobehavioral mechanisms of topiramate’s effects on alcohol use: an investigation pairing laboratory and ecological momentary assessments. Addiction Biology, 21:171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R Jr, Monti PM, Ray L, Treloar HR, Reynolds EK, Ramirez J,... & Blanchard A (2014) Characterizing subjective responses to alcohol among adolescent problem drinkers. Journal of Abnormal Psychology 123:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Ray L, Blanchard A, Reynolds EK, Monti PM, Chun T,… & Ramirez J (2014) Effects of naltrexone on adolescent alcohol cue reactivity and sensitivity: an initial randomized trial. Addiction Biology 19:941–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz H, Burns M (1976) Effects of rate of drinking on human performance Journal of Studies on Alcohol 37:598–605 [DOI] [PubMed] [Google Scholar]
- Nash SG, McQueen A, Bray JH (2005) Pathways to adolescent alcohol use: Family environment, peer influence, and parental expectations Journal of Adolescent Health 37:19–28 [DOI] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism (2004) NIAAA council approves definition of binge drinking. NIAAA Newsletter, 3, 3. [Google Scholar]
- Patrick ME, Schulenberg JE (2014) Prevalence and predictors of adolescent alcohol use and binge drinking in the United States Alcohol Research: Current Reviews 35:193. [PMC free article] [PubMed] [Google Scholar]
- Pearson MR (2013) Use of alcohol protective behavioral strategies among college students: A critical review Clinical Psychology Review 33:1025–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PJ et al. (2006) The association of alcohol- induced blackouts and grayouts to blood alcohol concentrations Journal of Forensic Sciences 51:896–899 [DOI] [PubMed] [Google Scholar]
- Ryback RS (1970) Alcohol amnesia: Observations in seven drinking inpatient alcoholics Quarterly Journal of Studies on Alcohol [PubMed] [Google Scholar]
- SAS Institute (2014) SAS/STAT 9.4 user’s guide. Cary: Author. [Google Scholar]
- Shiffman S, Stone AA, Hufford MR (2008) Ecological momentary assessment. Ann Rev Clin Psychol 4:1–32 [DOI] [PubMed] [Google Scholar]
- Siqueira L, Smith VC, & Committee on Substance Abuse (2015). Binge drinking. Pediatrics peds-2015. [DOI] [PubMed] [Google Scholar]
- Simons JS, Wills TA, Emery NN, Marks RM (2015) Quantifying alcohol consumption: self-report, transdermal assessment, and prediction of dependence symptoms Addictive Behaviors 50:205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2011) Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child Development Perspectives 5:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2004) Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences 1021:51–58 [DOI] [PubMed] [Google Scholar]
- Steinberg L (2007) Risk taking in adolescence: New perspectives from brain and behavioral science Current Directions in Psychological Science 16:55–59 [Google Scholar]
- Steinberg L (2008) A social neuroscience perspective on adolescent risk-taking Developmental Review 28:78–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2016) Results from the 2015 National Survey on Drug Use and Health: National Findings; Rockville, MD. [PubMed] [Google Scholar]
- Treloar H, Celio MA, Lisman SA, Miranda R Jr, Spear LP (2017). Subjective alcohol responses in a cross-sectional, field-based study of adolescents and young adults: Effects of age, drinking level, and dependence/consequences. Drug and alcohol dependence 170:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela CJ, Piasecki TM, Bartholow BD, Heath AC, Sher KJ (2016) The natural expression of individual differences in self-reported level of response to alcohol during ecologically assessed drinking episodes Psychopharmacology 233:2185–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar H, Miranda R Jr (2017) Craving and acute effects of alcohol in youths’ daily lives: Associations with alcohol use disorder severity Experimental and clinical psychopharmacology 25:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrul J, Labhart F, & Kuntsche E (2017) Drinking with mixed- gender groups is associated with heavy weekend drinking among young adults. Addiction 112:432–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2007) U.S. Department of Health and Human Services, Office of the Surgeon General. The Surgeon General’s Call to Action To Prevent and Reduce Underage Drinking: A Guide to Action for Educators. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.