Abstract
Objective:
Oral tenofovir-based pre-exposure prophylaxis (PrEP) is an important tool for prevention of new HIV infections, which also reduces subclinical HSV-2 shedding and symptomatic lesions in HIV-negative, HSV-2-seropositive individuals. However, the impact of PrEP on mucosal immunity has not been examined in detail.
Design:
Here we evaluate paired genital tissue and systemic immune profiles to characterize the immunological effects of PrEP in HIV-negative, HSV-2-seropositive African women sexually exposed to HIV.
Methods:
We compared local and systemic innate and T-cell characteristics in samples collected during PrEP usage and two months after PrEP discontinuation.
Results:
We found that frequencies of cervical CCR5+CD4+ cells, regulatory T-cells, and tissue macrophages were significantly reduced during PrEP use compared to after PrEP discontinuation. In contrast, peripheral blood CD4+ and CD8+ T-cells expressing markers of activation and trafficking were increased during PrEP usage.
Conclusions:
Together, our data are consistent with PrEP altering immunity differentially in the female genital tract compared to circulation in HSV-2+ women. Further study including comparison to HSV-2 negative women is needed to define the overall impact and mechanisms underlying these effects. These results point to the critical need to study the human mucosal compartment to characterize immune responses to mucosal infections.
Keywords: Cellular immunity, Activation, CD4, CD8, Women, Prevention of sexual transmission, Herpesvirus
INTRODUCTION
Despite significant advancement in our understanding of the immune system, most knowledge comes from studies using model systems, such as mice, or by investigation of human peripheral blood cells (PBMC). However, most human infections of public health importance are transmitted across a mucosal surface; thus, we must define the unique immune responses at these locations so that we can better prevent infection at these sites of first exposure. HIV is an example of a pathogen that most commonly infects the host across a mucosal surface, and there is an urgent need to provide protection to young women who are at disproportionate risk of acquiring new HIV infections[1]. Importantly, infection with genital herpes simplex virus type 2 (HSV-2) increases the risk of HIV acquisition by 2–3 fold[2]. HSV-2 infection is extremely prevalent, with estimated rates of HSV-2 sero-positivity in women in regions such as South Africa, Kenya, and Uganda as high as 70%[3–5]. There is strong correlative epidemiological evidence for the synergistic effects of HIV with HSV-2[6, 7], and the underlying immune mechanisms that predispose HSV-2+ women to HIV infection include increased numbers and persistence of mucosal CD4+ T cells and CCR5+ HIV target cells in the skin of HSV-2+ individuals[8–11], altered DC activity[12, 13], and changes in the local cytokine milleu[13–15]. However, the lack of an effective HSV-2 vaccine[16], and partial suppression of HSV-2 reactivation with current antivirals[17], highlight the urgency to better characterize the mucosal immune landscape in HSV-2+ individuals to determine if mucosal immunity plays a role in HIV susceptibility in HSV-2+ persons.
Oral tenofovir-based pre-exposure prophylaxis (PrEP) has been demonstrated to be efficacious at preventing new HIV infections in high-risk individuals[18–21], and is now recommended by many public health agencies for individuals at high risk of exposure to HIV infection. However, there are limited data on if PrEP has an impact on mucosal immunity. Interestingly, data from human and rodent cell cultures have revealed both pro-inflammatory[22–24] and anti-inflammatory[24–26] effects of tenofovir in vitro, whereas both topical tenofovir[27–29] and oral PrEP[30] have been reported to promote peripheral immune quiescence. This is of relevance given that immune quiescence, or reduced immune activation, has been associated with protection from HIV acquisition[31–36]. Moreover, cervical HIV-neutralizing IgA is increased in women on-PrEP, and in women with higher HIV exposure[37], revealing that both PrEP and HIV exposure can alter mucosal immune responses. Thus, a more detailed analysis of mucosal immunity during PrEP usage and HIV exposure is needed. Finally, consistent use of oral PrEP has been shown to modestly decrease HSV-2 shedding and lesion rates in HIV-negative, HSV-seropositive women, as well as the quantity of virus shed[38]. Thus, we hypothesized that PrEP could additionally alter mucosal immune cells in HSV-seropositive women through modulation of viral shedding and associated pathology, thereby necessitating concordant modifications in immunity to respond to viral reactivations.
Here, we profiled mucosal and circulating immunity in HSV-2+ women who took PrEP due to sexual exposure to HIV-1, and longitudinally re-assessed these characteristics two months after discontinuation of PrEP. Our findings suggest that the effect of PrEP on mucosal immunity in HSV-2+ women may be distinct from its effect on systemic immunity.
METHODS
Study participants.
Partners PrEP was a randomized, placebo-controlled clinical trial of oral tenofovir or tenofovir/emtricitabine versus placebo conducted among 4758 heterosexual HIV serodiscordant couples (Clinicaltrials.gov)[18]. Two sites, Kampala, Uganda and Thika, Kenya were identified where all women enrolled in the parent Partners PrEP Study were offered enrollment into a genital sampling substudy to enable characterization of genital mucosal immune phenotypes longitudinally after PrEP usage compared to PrEP discontinuation. This involved collection of additional samples at the visit when study drug was stopped (participants had been taking study drug for 24–36 months) and/or a visit 2 months after study drug was discontinued. A total of 90 women had cervical, vaginal, and serum samples all collected at one or both of these visits. Mucosal and blood samples were collected at the same time points. Biopsies were randomly sampled from the ectocervix. Of these 90 women, 20 women had been randomized to placebo (all had samples collected at a single visit) and 70 to PrEP (N=22 with samples collected at both visits, N=32 and N=38 with samples collected at one or the other visit). For this analysis, we selected women who were HSV-2+ and taking PrEP. Some of the samples could be matched to longitudinally paired samples at post-PrEP timepoints whereas others were only cross-sectional (Table 1). The Partners PrEP Study was coordinated at the University of Washington with human subjects research oversight there and at participating collaborating Institutional Review Boards.
Table 1:
Participant characteristics by visit category
| Characteristic | Visit when sample collected | ||
|---|---|---|---|
| All PrEP-stop visits | All post-PrEP visits | Total with both visits | |
| Total participants N | 22 | 24 | |
| Cervical biopsy analyzed, N (%) | 15 (68.2%) | 20 (83.3%) | 7 |
| PBMCs analyzed, N (%) | 19 (86.4%) | 19 (79.2%) | 19 |
| Cervical Swab analyzed, N (%) | 16 (72.7%) | 16 (66.7%) | 16 |
| Vaginal Swab, N (%) | 16 (72.7%) | 16 (66.7%) | 16 |
| Serum, N (%) | 16 (72.7%) | 16 (66.7%) | 16 |
| Age, Mean (IQR) | 38 (35–39) | 35 (31.5–38.25) | 34 (30.5–37.5) |
| Kenyan site, N (%) | 11 (50) | 11 (45.8%) | 1 (14.3%) |
| Days since LMP*, Mean (IQR) | 103 (8–35) | 49 (10.75–24.5) | |
| HIV Exposure index**, Mean (IQR) | 2.041 (1.040–2.857) | 2.158 (0.861–3.174) | |
| Days since last sex, Mean (IQR) | 7 (2–14) | 6 (2–7) | |
| condom use reported at last sex, N (%) | 11 (84.6%) | 14 (93.3%) | |
| hormonal contraceptive use reported, N (%) | 6 (27.3%) | 8 (33.3%) | |
| Bacterial STI detected at enrollment, N (%) | 0 (0%) | 0 (0%) | |
| BV or intermediate vaginal flora, N (%) | 3 (27.3%) | 4 (17.4%) | |
LMP, self-reported last menstrual period
HIV exposure score, longitudinal exponential score developed to quantify epidemiologic factors contributing to HIV risk[60]
IHC and image analysis.
Cervical biopsies were prepared into formalin-fixed paraffin embedded (FFPE) blocks and sectioned into 4 μM sections. Slides were de-paraffinized and rehydrated using graded xylene and ethanol washes. Antigen-retrieval was then performed by heating to 95°C for 20min in Trilogy solution (Cell Marque). Slides were washed in TBST buffer, quenched in 3% hydrogen peroxide solution (Sigma-Aldrich), and then blocked using TSA blocking reagent (Life Technologies). Primary antibodies included: CCR5 (clone MC5, a kind gift from Dr. Mack[39]), CD4 (Abcam, Ab133616), CD68 (Dako PG-M1), CD38 (Abcam, Ab183326), CD8 (Dako M7103), and Foxp3 (Abcam, Ab20034). Doubly-stained slides were stained sequentially where the first antibody was incubated at room temperature for 1hr, washed, detected using PowerVision poly-HRP reagent (Leica), and amplified with tyramide-488 solution for 10min. Slides were then subjected to a second round of antigen-retrieval to strip residual antibodies and complexes and incubated overnight with the second primary antibody. Detection protocols were repeated as above and second antibodies were amplified using tyramide-594 solution. Staining controls which were devoid of primary antibodies were subjected to the same process. Finally, nuclei were detected using DAPI and coverslips were mounted with prolong diamond anti-fade reagent (Invitrogen). Slides were imaged on an Aperio Scanscope FL (Leica) and un-manipulated whole slide images were analyzed using HALO software (Indica Labs) by blinded researchers.
Flow cytometry.
Cryopreserved PBMC samples were thawed and stained for flow cytometry and/or intracellular cytokine staining as previously described[40, 41]. Antibodies (Supplemental Digital Content 1) were purchased from BD, Biolegend, eBioscience, Beckman Coulter, and Invitrogen. Data analysis was performed in a blinded fashion using Flowjo v9.9 (BD). For multifunctional T-cell analysis, Boolean gating was applied in Flowjo v9.9 (BD), and data was exported to Pestle v1.8 for processing and import into SPICE v5.0[42].
Cytokine detection.
Cytokine/chemokine levels in previously frozen cervical or vaginal swabs and serum were measured using Miliplex MAP multiplex kits (Human Cytokine/Chemokine panels I-VI from EMD Millipore) using the overnight protocol and analyzed on the BioPlex-200 (Bio-Rad). Human cytokine/chemokine panels I and II were identical with the exception of RANTES which was removed from panel II. Samples with values below the lower detection limit (MinDC + 2SD) were assigned the value of half the lower detection limit in pg/ml, (MinDC + 2SD)/2.
Statistics.
Investigators were blinded to PrEP status and demographic information. Samples were labeled with a barcode, which was later translated to patient ID, date, PrEP status, and other demographic information etc. All the data was analyzed prior to being unblinded to PrEP status. All immunofluorescence data were compared between PrEP and post-PrEP visits using rank-based regression[43], adjusting for menstrual phase, recent sex, hormonal contraceptive use, and BV (Supplemental Digital Content 2). Because BV was only available at a subset of visits, we conservatively only adjusted for BV if doing so reduced the difference between groups. If BV adjustment increased the difference, we did not adjust, in order to include all visits in the analysis. Rank-based regression was performed using R version 3.3.3 and R package “Rfit”. Matched longitudinal immunofluorescence data were analyzed via Wilcoxon matched-pairs signed rank test using GraphPad Prism software version 7.0d. For immunofluorescence comparisons, p≤0.05 was considered statistically significant. Multiple comparisons corrections for flow cytometry and cytokine data were performed using the two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 5%.
RESULTS
PrEP usage confers a reduction in the density of cervical CD4+ and CD8+ cells
To examine the effects of PrEP usage on immunity in HSV-2+ women, we used matched PBMC and genital mucosal samples from a subset of women enrolled in the Partners PrEP Study (Table 1). Cervical biopsies and swabs plus PBMC were collected from select women at the study visit when PrEP was discontinued in the Partners PrEP Study, and at a post-drug discontinuation study visit 2 months later (Post-PrEP visit). We quantified total CD4+ and CD8+ cells present in cervical biopsies by immunofluorescence microscopy and digital image analysis and found the overall frequencies of both cervical CD4+ and CD8+ cells were lower in women on-PrEP compared to 2 months after PrEP discontinuation. We quantified these CD4+ and CD8+ cells in both an inclusive cross-sectional analysis, and in a subset of the total biopsies which could be longitudinally matched to a repeat visit of the same individual after PrEP cessation, with both approaches resulting in similar outcomes (Figure 1a–b). In addition, the numbers of CD4+ and CD8+ cells in the cervix increased or trended toward increase after PrEP discontinuation (Figure 1c–d).
Figure 1. PrEP discontinuation alters mucosal immune cell abundance and phenotype.
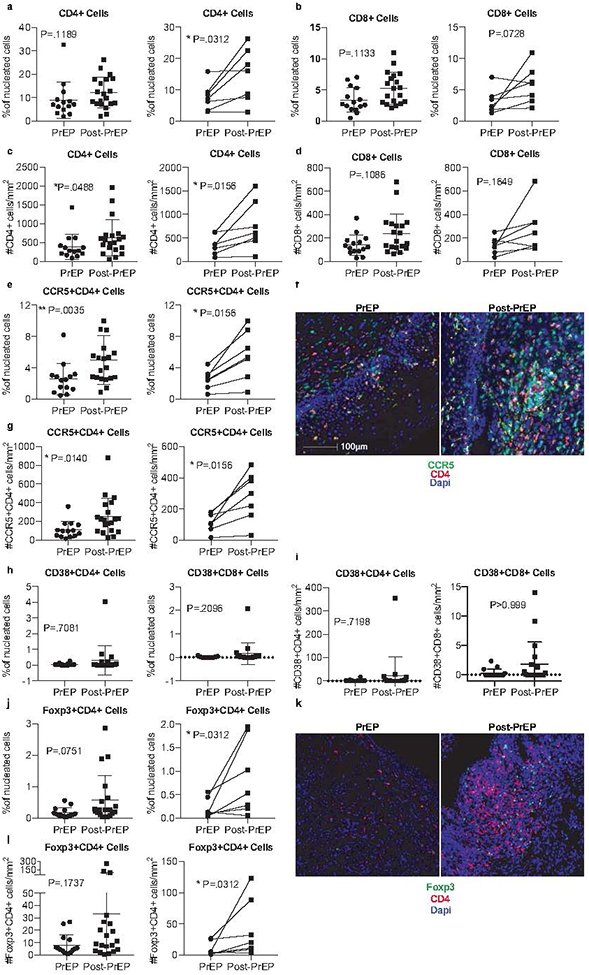
Formalin-fixed paraffin-embedded (FFPE) cervical biopsies were stained for CD4+ cells (a,c), CD8+ cells (b, d), CCR5+CD4+ cells (e, g), CD38+CD4+ or CD38+CD8+ cells (h, i), and Foxp3+CD4+ Tregs (j, l). The percentage of positive cells as frequency of total nucleated cells in whole tissue scans was determined from unmanipulated images (representative images shown in f and k). Additionally, we report the number of cells per mm2 of tissue. Results from a cross-sectional analysis or longitudinally matched pairs are shown, with lines connecting matched pairs. Cross sectional analyses are adjusted for menstrual phase, recent sex, and hormonal contraceptive use. %CD4+ cells and %CD38+CD4+ were also adjusted for BV. Thirty-five cervical biopsies were available for cross-sectional analysis: 15 women had collection at the PrEP stop visit, while 20 women had collection 2 months after PrEP; 7 of these women had paired samples at both timepoints.
Given our finding of increased CD4+ and CD8+ cells in the cervix in the post-PrEP sample, we next assessed the frequencies of cervical CCR5+CD4+ HIV target-cells and found that discontinuation of PrEP correlates with a statistically significant increase in the frequency of CCR5+CD4+ cells within the cervix (Figure 1e–f). In contrast, we found that there was no difference in the frequency or number of activated CD38+CD4+ or CD38+CD8+ cervical cells at the PrEP or post-PrEP timepoints (Figure 1h–i).
Tissue Treg frequencies rise after PrEP discontinuation
Regulatory T-cells (Tregs) traffic to sites of inflammation and serve an essential role during primary HSV-2 infection to elicit early immune cell responses, while controlling inflammation[44, 45]. Moreover, several studies have suggested that an increased frequency of peripheral Tregs may be associated with protection from HIV acquisition by establishing a state of immune quiescence[40, 46]. Thus, given that we observed an increase in CD4+ and CD8+ cell infiltration into the cervix following PrEP discontinuation, we next quantified the frequency and number of cervical tissue Tregs. We found a low frequency and number of Foxp3+CD4+ Tregs within cervical tissues during PrEP usage, followed by an increase in the frequency and number of Tregs in the mucosa post-PrEP (Figure 1j–l). In sum, we find concurrent increases in CD4+, CD8+, and Treg cells within the cervical mucosa post-PrEP, with the Tregs potentially following T-cells to restrain possible T-cell-driven inflammation.
Cervical tissue macrophages are restrained during PrEP usage
To next examine the effect of PrEP on innate immunity and additional HIV target cells within the mucosa, we assessed CD68+ tissue macrophages, which are also susceptible to HIV infection. The frequency and number of total CD68+ cervical tissue macrophages were lower on-PrEP and became elevated post-PrEP (Figure 2a–b). Additionally, quantification of CCR5+CD68+ cervical tissue macrophages revealed a trend toward an increase in frequency and number of such cells post-PrEP (Figure 2c–e). Altogether, our analysis of cellular immunity within the cervical tissues in the context of PrEP use compared to post-PrEP revealed an increased abundance of both macrophages as well as CD4+ and CD8+ cells in the cervical mucosa upon PrEP discontinuation.
Figure 2. PrEP discontinuation is associated with an increase in total and CCR5+ macrophages in cervical tissue.
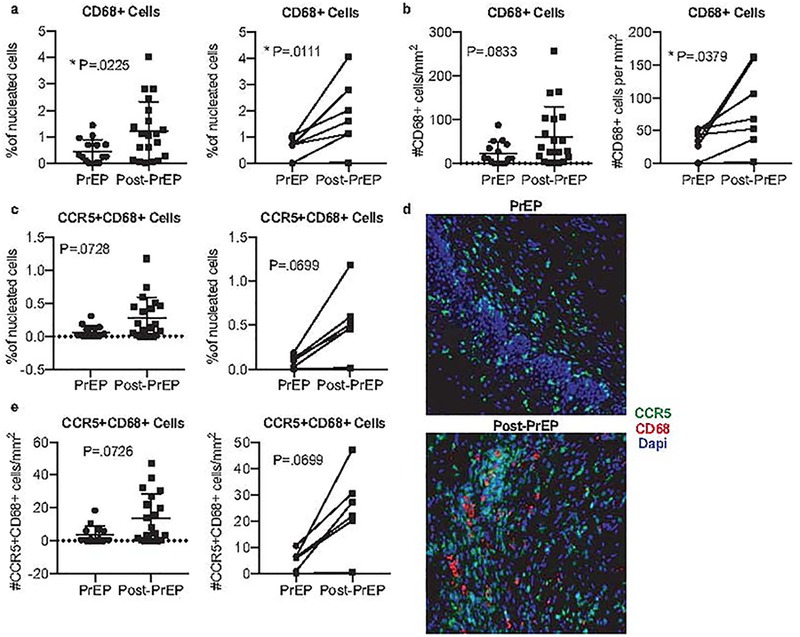
Formalin-fixed paraffin-embedded (FFPE) cervical biopsies were stained for CD68+ macrophages (a-b) or CCR5+ CD68+ macrophages (c, e). The percentage of positive cells as frequency of total nucleated cells in whole tissue scans was determined from unmanipulated images (representative images shown d). Additionally, we report the number of cells per mm2 of tissue. Results from a cross-sectional analysis or longitudinally matched pairs are shown, with lines connecting matched pairs. Cross sectional analyses are adjusted for menstrual phase, recent sex, and hormonal contraceptive use. Thirty-five cervical biopsies were available for cross-sectional analysis: 15 women had collection at the PrEP stop visit, while 20 women had collection 2 months after PrEP; 7 of these women had paired samples at both timepoints.
PrEP does not influence cervical and vaginal cytokine profiles
We next performed a comprehensive analysis of 28 cytokines and chemokines within the vagina and cervix to determine the impact of PrEP usage on the soluble milieu within the genital mucosa. After correcting for multiple comparisons, we found that oral PrEP usage had no statistically significant impact on the expression of cervical or vaginal cytokines (Supplemental Digital Content 3). Our assessment of cervical and vaginal cytokines is consistent with data regarding cytokine expression during tenofovir gel usage[47], as Masson et al found no differences between placebo or tenofovir gel in cytokine concentrations analyzed from cervicovaginal lavage (CVL) samples[47].
Discontinuation of PrEP induces a quiescent circulating T-cell immune signature.
Contrary to our findings in the mucosa, we found that the frequency of activated peripheral blood CD4+ T-cells was significantly decreased after PrEP discontinuation. Specifically, we observed an elevated frequency of both circulating CCR5+ and CXCR4+CD4+ T-cells during PrEP compared to post-PrEP (Figure 3a–b). Consistent with a pro-inflammatory state, we also observed significant increases in CD38, Ki-67, and PD-1-expressing CD4+ T-cells during PrEP compared to post-PrEP (Figure 3c–e), and an elevation in the frequency of circulating Tregs during PrEP usage compared to post-PrEP, although when corrected for multiple comparisons, this difference was no longer statistically significant (Figure 3f).
Figure 3. Circulating T-cell phenotypes but not cytokine production potential changes after discontinuation of oral PrEP.
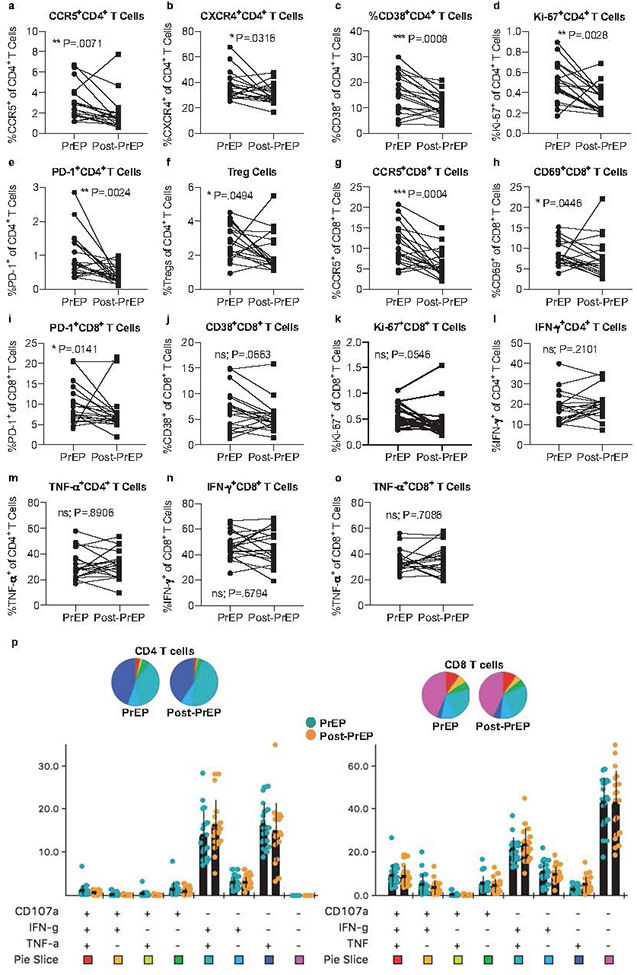
Cryopreserved PBMCs were thawed and prepared for analysis by flow cytometry. Live singlets were gated on forward and side scatter, then CD3 positivity. The frequencies of CCR5+ (a), CXCR4+ (b), CD38+ (c), Ki-67+ (d), and PD-1+ (e) CD4+ T-cells and Tregs (f) are shown as matched pairs during PrEP and post-PrEP timepoints. Tregs were defined as Foxp3+CD25hiCD127lo cells and are represented as a frequency of total CD4+ T-cells (f). The frequencies of CCR5+ (g), CD69+ (h), PD-1 (i), CD38+ (j), and Ki-67+ (k) CD8+ T-cells are similarly shown as matched pairs during PrEP and post-PrEP timepoints. For intracellular cytokine staining, thawed PBMCs were stimulated with PMA and ionomycin for 6 hours, then stained for a panel of cytokines and analyzed via flow cytometry. Live singlets were gated on forward and side scatter, then CD3 positivity and downstream markers of interest. The frequencies of IFN-γ+ (l) or TNF-α+ (m) CD4+ T-cells, or IFN-γ+ (n) or TNF-α+ (o) CD8+ T-cells were enumerated. The frequencies of multifunctional CD4 (left) or CD8 (right) T-cells were assessed by SPICE[42] analysis (p). Nineteen longitudinally-matched pairs of PBMCs were analyzed.
Similar to blood CD4+ T cells, there was a significant reduction in the frequency of circulating CD8+ T-cells expressing CCR5, CD69, and PD-1 post-PrEP (Fig 3g–i), as well as trends toward decreased frequencies of CD38 and Ki-67 (Figure 3j and k). However, after multiple comparisons corrections, the only significant result remaining among CD8+ T-cells was in CCR5+CD8+ T-cells. In sum, mucosal immune responses are distinct from those within circulation, demonstrating that circulating immunity is not a good proxy for genital immunity.
PrEP usage does not impact circulating T-cell cytokine production
Because we observed several differences in the expression patterns of CD4+ and CD8+ T-cell phenotypes, we next assessed their respective functionality. We found that the ability of both CD4+ and CD8+ T-cells to produce cytokines including IFN-γ and TNF-α upon polyclonal stimulation remained robust both during PrEP use and post-PrEP (Figure 3l–o). Moreover, we assessed the polyfunctionality of these T-cells, and found that peripheral T-cells during PrEP use produced multiple cytokines comparably to after PrEP discontinuation (Figure 3p). Therefore, whereas we observed alterations in the frequencies of T-cells expressing trafficking, activation, and proliferation markers post-PrEP that may contribute to HIV susceptibility, we did not observe differences in their ex vivo cytokine production capacity.
PrEP usage promotes circulating innate immune cell quiescence.
Next, we assessed the innate immune cell compartment in circulating blood during PrEP compared to post-PrEP. We found that the frequency of peripheral myeloid DCs (mDCs), characterized as CD45+CD11c+HLA-DR+, was increased post-PrEP compared to on-PrEP (Figure 4a). Similarly, the frequency of circulating mDC expressing CCR5 or the co-stimulatory molecule CD80 was increased post-PrEP compared to on-PrEP (Figure 4b–c). We additionally assessed three fractions of peripheral monocytes based on their expression of CD14 and CD16[48] and found that the frequency of classic monocytes (CD14++CD16-) also increased post-PrEP, whereas intermediate (CD14++CD16+) and non-classic (CD14+CD16++) monocyte frequencies were stable during PrEP compared to post-PrEP (Figure 4d–f). The increase in classic monocytes mirrored that observed for tissue macrophages in the cervix (Figure 2). Thus, it appears that PrEP discontinuation is associated with an increase in the frequency and activation of certain subsets of innate immune cells in both the blood as well as the genital mucosal tissue.
Figure 4. Circulating antigen presenting cell frequency and phenotype differs between the on-PrEP and post-PrEP timepoints.
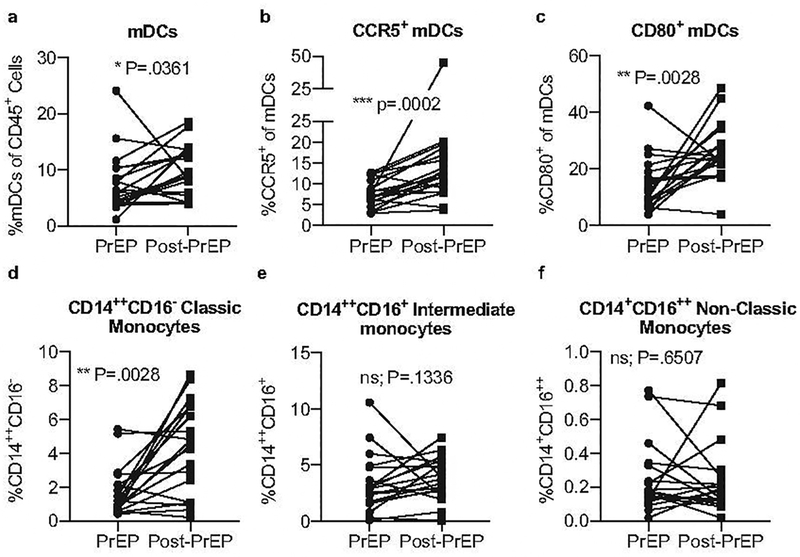
Cryopreserved PBMCs were stained for analysis by flow cytometry. Live singlets were gated on forward and side scatter, then CD45 positivity. The frequencies of total CD11c+HLA-DR+ mDCs (a), CCR5+ mDCs (b) and CD80+ mDCs (c) are shown. Monocytes were similarly gated from CD45+ cells. The frequency of classic CD14++CD16- (d), intermediate CD14++CD16+ (e), and non-classic CD14+CD16++ (f) monocytes is shown. Nineteen longitudinally-matched pairs of PBMCs were analyzed.
PrEP discontinuation does not affect serum cytokine or chemokine levels
Finally, we assessed serum cytokines during PrEP use and post-PrEP, as we did for genital secretions (Supplemental Digital Content 3). After correcting for multiple comparisons, there were no significant differences in serum cytokine or chemokine levels during PrEP usage compared to after PrEP discontinuation.
DISCUSSION
Tenofovir-based PrEP is comprised of reverse transcriptase inhibitors which act to impede HIV directly. However, it has been hypothesized that in addition to these direct anti-viral properties, PrEP may also have a chemo-vaccination effect. Specifically, data from studies of non-human primates suggested that by aborting infections, PrEP may allow for enhanced immune priming of HIV-specific immune responses in virus-exposed individuals[49–51]. We previously used samples from the Partners PrEP Study and found no differences in the response rate or magnitude of circulating HIV-specific T-cell responses in individuals on-PrEP compared to placebo, nor in the frequencies or phenotypes of circulating T-cells, NK cells, or APCs[41], suggesting that PrEP does not boost circulating HIV-specific immunity in humans. However, the HSV-2 status within the cohort was not used to stratify these results, nor did we previously examine these cells in the mucosa. Here, our analysis of matched cervical mucosa and peripheral blood samples from HSV-2+ women taking PrEP compared to 2 months post-PrEP revealed distinct differences in the effects on cellular immune markers in the mucosal versus systemic compartments. These results support the notion that mucosal sampling is critical to gain knowledge about immune events occurring at the sites of initial HIV exposures. Additionally, our results demonstrate for the first time that oral PrEP usage in HSV-2+ women alters mucosal immune activation. Specifically, PrEP discontinuation was associated with an increase in frequencies of CD4+ and CD8+ cells, which are likely to be T-cells, within the genital mucosa, as well as increases in abundance of CCR5+ CD4+ cells and CD68+ tissue macrophages. Given that these cells are potential targets of HIV infection, our results highlight the need for additional studies of mucosal immune changes in the context of PrEP usage to define the clinical significance of these results.
Our findings that mucosal T-cell abundance and phenotype is altered during oral PrEP use compared to post-PrEP could affect HIV susceptibility in two non-mutually exclusive ways. First, the CD8+ T-cells present within the cervical mucosa could be HIV-specific, and thus be licensed to kill HIV-infected cells, thereby conferring immune-mediated protection against viral spread. Alternatively, the CD8+ T-cells may be specific for other non-HIV antigens, but become activated in a TCR-independent bystander-mediated fashion, and so could be recruited into an inflammatory response against local HIV infections to thereby assist with controlling HIV spread. In either such instance, the usage of PrEP, associated with a reduced frequency of cervical CD8+ cells, could result in diminished immune-mediated protection against HIV infection. Conversely, the reduced abundance of CCR5-expressing CD4+ T-cells and macrophages during PrEP may provide benefit by reducing the likelihood of HIV:target-cell encounter. Thus, the increase in HIV target-cell abundance within the genital mucosa post-PrEP may have negative implications for HIV acquisition risk, especially by removing the protective antiviral effects of PrEP during a concurrent surge in local target-cell abundance. The increase in Treg frequency upon PrEP discontinuation may reflect a host response to control local tissue T-cell and macrophage activation. Of note, Tregs are known to respond to HSV-2 reactivation events, including asymptomatic shedding and lesions, and peak in number with the same kinetics as HSV-2 viral load[52]. In the context of HIV exposure of uninfected women, an increased frequency of circulating Tregs may help protect from HIV-acquisition[40], as reported in prior studies identifying the protective effect of immune quiescence [33, 46, 53].
We similarly find that CD68+ tissue macrophages, including those expressing CCR5, are maintained in a state of reduced activation during PrEP usage compared to post-PrEP. Potential roles for tissue macrophages in the context of infection in the cervix are multifactorial, including antigen presentation, cytokine production, and organization of memory lymphocyte clusters[54]. In the context of HSV-2 and PrEP use, we hypothesize that a low frequency of tissue macrophages, particularly those expressing CCR5, contributes to protection from HIV infection by reducing the abundance of HIV target-cells.
The mechanism whereby oral PrEP usage in HIV-exposed women leads to distinct alterations in mucosal and circulating immune phenotypes remains unclear. However, PrEP use in HSV-2+ women has been reported to reduce HSV-2 shedding and lesions[38]. A sustained reduction in shedding over time may result in a reduction in local mucosal immune activation, which could, in turn, provide a more immune quiescent genital mucosa that contributes to the protective efficacy of PrEP by reducing the HIV target-cell abundance within the genital mucosa. Similarly, discontinuation of PrEP may result in HSV-2 reactivation leading to increased mucosal immune activation, as it has been previously demonstrated that HSV-2 shedding contributes to immune cell infiltration and chronic inflammation within the genital tract[55, 56]. Notably, we also found PrEP discontinuation to be associated with reduced T-cell activation in circulation. It is not clear from our data if this reflects an indirect effect of mobilization of T-cell responses to the genital mucosa in response to HSV-2 reactivation, or a process that directly affects immune activation in systemic circulation.
Several limitations in study design and sample availability restrict our ability to test our hypothesis that PrEP affects mucosal immune activation through a reduction in HSV-2 shedding and/or lesions. First, mixed skin and genital tract swabs were not collected for HSV-2 detection as part of this clinical study, so we are unable to determine if discontinuation of PrEP correlated with a reduction in HSV-2 shedding rates or levels. While cervical biopsies could be used for detection of HSV particles by microscopy, intensive genital swabbing studies of HSV-2+ women have demonstrated the multifocal nature of HSV-2 shedding and reactivation[55, 56], and so assessment of HSV-2 shedding within a single mucosal site would likely not be a sufficient surrogate for the genital compartment. Additional samples from HSV-2- women are required to directly test this hypothesis with appropriate controls. Secondly, because we only have samples from 2 months after PrEP discontinuation, we cannot determine if the changes in local immunity are transient or more stable. Finally, different methods were used to examine the phenotype of immune cells in the mucosa versus the circulation (flow cytometry and microscopy, respectively), and so this could affect the comparisons between tissue sites. However, altogether, our data are consistent with the hypothesis that PrEP usage facilitates protective effects in the mucosa via providing direct antiviral activity, as well as by altering immune activation, perhaps by controlling HSV-2 virus activity.
Our data highlight the notion that immune cell responses in the periphery can be functionally distinct from the mucosa. This may be of importance given that we have focused on HSV-2+ women, where viral shedding events are localized to the genital tract in the absence of peripheral viremia. This led us to hypothesize that during periods of PrEP usage, HSV-2 viral shedding events may be reduced, ameliorating local genital tract inflammation including activated T-cells and macrophages. Moreover, resident memory T-cells and clusters of tissue macrophages serve as the dominant HSV-2 responsive local populations in the cervix, and have been shown to act independently from peripheral T-cells[57, 58]. Posavad et al have recently shown that up to 10% of cervical cells in asymptomatic HSV-2+ women are HSV-2-specific and mostly express TRM phenotypes, an effect that is not equivalent in matched blood samples[59]. Similarly, whereas Milman et al found similar enhanced Treg frequencies during HSV-2 viral reactivation events, these enhancements were not recapitulated in peripheral blood[52], and women who were at-risk for HIV acquisition due to genital inflammation did not similarly exhibit elevated inflammation in serum cytokine profiles[47]. Our data are consistent with the idea of tissue independency, and highlights the necessity in evaluating mucosal immune cells, particularly in the context of HSV-2 infection and HIV risk.
Supplementary Material
Supplemental Digital Content 1, “Supplement.docx”
Supplemental Digital Content 2, “Supplement.docx”
Supplemental Digital Content 3, “Supplement.docx”
Acknowledgments
We thank members of the Lund Lab and Dr. Florian Hladik for helpful discussions. We are grateful to the research staff and study participants in the Partners PrEP Study who made this study possible. Assistance with the rank-based regresstion analysis was provided by Corinne Mar and Katherine Thomas. Funding was provided by the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (R01 AI111738 to JRL, R01 AI141435 to JML, R01 AI131914 to JML and JRL and R01 AI096968 to JML and JMB) and by the Bill and Melinda Gates Foundation (grant OPP47674, to CC). The authors declare no conflicts of interest.
Disclosure. Funding was provided by the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (R01 AI111738 to JRL, R01 AI131914, to JML and JRL and R01 AI096968 to JML and JMB) and by Bill and Melinda Gates Foundation (grant OPP47674, to CC). The authors declare no conflicts of interest.
REFERENCES:
- 1.UNAIDS. The Gap Report. In; 2014. [Google Scholar]
- 2.Looker KJ, Elmes JAR, Gottlieb SL, Schiffer JT, Vickerman P, Turner KME, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis 2017; 17(12):1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffer JT, Corey L. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat Med 2013; 19(3):280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajagopal S, Magaret A, Mugo N, Wald A. Incidence of herpes simplex virus type 2 infections in Africa: a systematic review. Open Forum Infect Dis 2014; 1(2):ofu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015; 10(1):e114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20(1):73–83. [DOI] [PubMed] [Google Scholar]
- 7.Schiffer JT, Gottlieb SL. Biologic interactions between HSV-2 and HIV-1 and possible implications for HSV vaccine development. Vaccine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med 2009; 15(8):886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prodger JL, Gray R, Kigozi G, Nalugoda F, Galiwango R, Nehemiah K, et al. Impact of asymptomatic Herpes simplex virus-2 infection on T cell phenotype and function in the foreskin. AIDS 2012; 26(10):1319–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shannon B, Gajer P, Yi TJ, Ma B, Humphrys MS, Thomas-Pavanel J, et al. Distinct Effects of the Cervicovaginal Microbiota and Herpes Simplex Type 2 Infection on Female Genital Tract Immunology. J Infect Dis 2017; 215(9):1366–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shannon B, Yi TJ, Thomas-Pavanel J, Chieza L, Janakiram P, Saunders M, et al. Impact of asymptomatic herpes simplex virus type 2 infection on mucosal homing and immune cell subsets in the blood and female genital tract. J Immunol 2014; 192(11):5074–5082. [DOI] [PubMed] [Google Scholar]
- 12.Marsden V, Donaghy H, Bertram KM, Harman AN, Nasr N, Keoshkerian E, et al. Herpes simplex virus type 2-infected dendritic cells produce TNF-alpha, which enhances CCR5 expression and stimulates HIV production from adjacent infected cells. J Immunol 2015; 194(9):4438–4445. [DOI] [PubMed] [Google Scholar]
- 13.Stefanidou M, Ramos I, Mas Casullo V, Trepanier JB, Rosenbaum S, Fernandez-Sesma A, et al. Herpes simplex virus 2 (HSV-2) prevents dendritic cell maturation, induces apoptosis, and triggers release of proinflammatory cytokines: potential links to HSV-HIV synergy. J Virol 2013; 87(3):1443–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller MJ, Madan RP, Shust G, Carpenter CA, Torres NM, Cho S, et al. Changes in the soluble mucosal immune environment during genital herpes outbreaks. J Acquir Immune Defic Syndr 2012; 61(2):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesquita PMM, Preston-Hurlburt P, Keller MJ, Vudattu N, Espinoza L, Altrich M, et al. Role of Interleukin 32 in Human Immunodeficiency Virus Reactivation and Its Link to Human Immunodeficiency Virus-Herpes Simplex Virus Coinfection. J Infect Dis 2017; 215(4):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest 2011; 121(12):4600–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston C, Saracino M, Kuntz S, Magaret A, Selke S, Huang ML, et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet 2012; 379(9816):641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine 2012; 367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381(9883):2083–2090. [DOI] [PubMed] [Google Scholar]
- 20.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine 2010; 363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine 2012; 367(5):423–434. [DOI] [PubMed] [Google Scholar]
- 22.Zidek Z, Potmesil P, Holy A. Cytostatic activity of antiviral acyclic nucleoside phosphonates in rodent lymphocytes. Toxicol Appl Pharmacol 2003; 192(3):246–253. [DOI] [PubMed] [Google Scholar]
- 23.Melchjorsen J, Risor MW, Sogaard OS, O’Loughlin KL, Chow S, Paludan SR, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J Acquir Immune Defic Syndr 2011; 57(4):265–275. [DOI] [PubMed] [Google Scholar]
- 24.Zidek Z, Frankova D, Holy A. Activation by 9-(R)-[2-(phosphonomethoxy)propyl]adenine of chemokine (RANTES, macrophage inflammatory protein 1alpha) and cytokine (tumor necrosis factor alpha, interleukin-10 [IL-10], IL-1beta) production. Antimicrob Agents Chemother 2001; 45(12):3381–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostecka P, Holy A, Farghali H, Zidek Z, Kmonickova E. Differential effects of acyclic nucleoside phosphonates on nitric oxide and cytokines in rat hepatocytes and macrophages. Int Immunopharmacol 2012; 12(2):342–349. [DOI] [PubMed] [Google Scholar]
- 26.Zidek Z, Potmesil P, Kmoniekova E, Holy A. Immunobiological activity of N-[2-(phosphonomethoxy)alkyl] derivatives of N6-substituted adenines, and 2,6-diaminopurines. Eur J Pharmacol 2003; 475(1–3):149–159. [DOI] [PubMed] [Google Scholar]
- 27.McGowan I, Hoesley C, Cranston RD, Andrew P, Janocko L, Dai JY, et al. A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007). PloS one 2013; 8(4):e60147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vibholm L, Reinert LS, Sogaard OS, Paludan SR, Ostergaard L, Tolstrup M, et al. Antiviral and immunological effects of tenofovir microbicide in vaginal herpes simplex virus 2 infection. AIDS Res Hum Retroviruses 2012; 28(11):1404–1411. [DOI] [PubMed] [Google Scholar]
- 29.Hladik F, Burgener A, Ballweber L, Gottardo R, Vojtech L, Fourati S, et al. Mucosal effects of tenofovir 1% gel. Elife 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castillo-Mancilla JR, Meditz A, Wilson C, Zheng JH, Palmer BE, Lee EJ, et al. Reduced immune activation during tenofovir-emtricitabine therapy in HIV-negative individuals. J Acquir Immune Defic Syndr 2015; 68(5):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Begaud E, Chartier L, Marechal V, Ipero J, Leal J, Versmisse P, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology 2006; 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jennes W, Evertse D, Borget MY, Vuylsteke B, Maurice C, Nkengasong JN, et al. Suppressed cellular alloimmune responses in HIV-exposed seronegative female sex workers. Clin Exp Immunol 2006; 143(3):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaren PJ, Ball TB, Wachihi C, Jaoko W, Kelvin DJ, Danesh A, et al. HIV-exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+ T cell compartment and reduced expression of HIV-dependent host factors. J Infect Dis 2010; 202 Suppl 3:S339–344. [DOI] [PubMed] [Google Scholar]
- 34.Pancino G, Saez-Cirion A, Scott-Algara D, Paul P. Natural resistance to HIV infection: lessons learned from HIV-exposed uninfected individuals. J Infect Dis 2010; 202 Suppl 3:S345–350. [DOI] [PubMed] [Google Scholar]
- 35.Card CM, Ball TB, Fowke KR. Immune quiescence: a model of protection against HIV infection. Retrovirology 2013; 10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naranbhai V, Abdool Karim SS, Altfeld M, Samsunder N, Durgiah R, Sibeko S, et al. Innate immune activation enhances hiv acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis 2012; 206(7):993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lund JM, Broliden K, Pyra MN, Thomas KK, Donnell D, Irungu E, et al. HIV-1-Neutralizing IgA Detected in Genital Secretions of Highly HIV-1-Exposed Seronegative Women on Oral Preexposure Prophylaxis. J Virol 2016; 90(21):9855–9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bender Ignacio RA, Perti T, Magaret AS, Rajagopal S, Stevens CE, Huang ML, et al. Oral and Vaginal Tenofovir for Genital Herpes Simplex Virus Type 2 Shedding in Immunocompetent Women: A Double-Blind, Randomized, Cross-over Trial. J Infect Dis 2015; 212(12):1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segerer S, Mac KM, Regele H, Kerjaschki D, Schlondorff D. Expression of the C-C chemokine receptor 5 in human kidney diseases. Kidney Int 1999; 56(1):52–64. [DOI] [PubMed] [Google Scholar]
- 40.Pattacini L, Baeten JM, Thomas KK, Fluharty TR, Murnane PM, Donnell D, et al. Regulatory T-Cell Activity But Not Conventional HIV-Specific T-Cell Responses Are Associated With Protection From HIV-1 Infection. J Acquir Immune Defic Syndr 2016; 72(2):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pattacini L, Murnane PM, Baeten JM, Fluharty TR, Thomas KK, Bukusi E, et al. Antiretroviral Pre-Exposure Prophylaxis Does Not Enhance Immune Responses to HIV in Exposed but Uninfected Persons. J Infect Dis 2015; 211(12):1943–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011; 79(2):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hettmansperger TP, McKean JW. Robust Nonparametric Statistical Methods. 2nd ed: Chapman Hall, New York; 2011. [Google Scholar]
- 44.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science 2008; 320(5880):1220–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soerens AG, Da Costa A, Lund JM. Regulatory T cells are essential to promote proper CD4 T-cell priming upon mucosal infection. Mucosal immunology 2016; 9(6):1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, Fowke KR. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J Infect Dis 2009; 199(9):1318–1322. [DOI] [PubMed] [Google Scholar]
- 47.Masson L, Passmore JA, Liebenberg LJ, Werner L, Baxter C, Arnold KB, et al. Genital Inflammation and the Risk of HIV Acquisition in Women. Clin Infect Dis 2015; 61(2):260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120(23):4599–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cranage M, Sharpe S, Herrera C, Cope A, Dennis M, Berry N, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med 2008; 5(8):e157; discussion e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kersh EN, Adams DR, Youngpairoj AS, Luo W, Zheng Q, Cong ME, et al. T cell chemo-vaccination effects after repeated mucosal SHIV exposures and oral pre-exposure prophylaxis. PloS one 2011; 6(4):e19295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsegaye TS, Butler K, Luo W, Radzio J, Srinivasan P, Sharma S, et al. Repeated Vaginal SHIV Challenges in Macaques Receiving Oral or Topical Preexposure Prophylaxis Induce Virus-Specific T-Cell Responses. J Acquir Immune Defic Syndr 2015; 69(4):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milman N, Zhu J, Johnston C, Cheng A, Magaret A, Koelle DM, et al. In Situ Detection of Regulatory T Cells in Human Genital Herpes Simplex Virus Type 2 (HSV-2) Reactivation and Their Influence on Spontaneous HSV-2 Reactivation. J Infect Dis 2016; 214(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Songok EM, Luo M, Liang B, McLaren P, Kaefer N, Apidi W, et al. Microarray analysis of HIV resistant female sex workers reveal a gene expression signature pattern reminiscent of a lowered immune activation state. PLoS One 2012; 7(1):e30048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 2014; 346(6205):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnston C, Zhu J, Jing L, Laing KJ, McClurkan CM, Klock A, et al. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol 2014; 88(9):4921–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schiffer JT, Swan D, Al Sallaq R, Magaret A, Johnston C, Mark KE, et al. Rapid localized spread and immunologic containment define Herpes simplex virus-2 reactivation in the human genital tract. Elife 2013; 2:e00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beura LK, Mitchell JS, Thompson EA, Schenkel JM, Mohammed J, Wijeyesinghe S, et al. Intravital mucosal imaging of CD8(+) resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat Immunol 2018; 19(2):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol 2018; 19(2):183–191. [DOI] [PubMed] [Google Scholar]
- 59.Posavad CM, Zhao L, Dong L, Jin L, Stevens CE, Magaret AS, et al. Enrichment of herpes simplex virus type 2 (HSV-2) reactive mucosal T cells in the human female genital tract. Mucosal immunology 2017; 10(5):1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackelprang RD, Baeten JM, Donnell D, Celum C, Farquhar C, de Bruyn G, et al. Quantifying ongoing HIV-1 exposure in HIV-1-serodiscordant couples to identify individuals with potential host resistance to HIV-1. J Infect Dis 2012; 206(8):1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1, “Supplement.docx”
Supplemental Digital Content 2, “Supplement.docx”
Supplemental Digital Content 3, “Supplement.docx”


