Abstract
To determine whether old B cells have the same capacity to switch isotypes as young cells, we purified splenic follicular, marginal zone, and age-associated B cell subsets from C57BL/6 mice. Cells were stimulated in culture with interleukin 4 and either lipopolysaccharide or anti-CD40, and switching to IgG1 was measured by flow cytometry of surface immunoglobulin. The results show that switching was robust in follicular and marginal zone B cells from old mice and was comparable to their young counterparts. However, age-associated B cells from old mice switched poorly relative to the other subsets. Expression of activation-induced deaminase, which initiates switching, was quantified by qPCR of mRNA, and it was equal between young and old follicular B cells. Thus, in this ex vivo system, the follicular and marginal zone cells from young and old mice behaved similarly, showing that the molecular machinery to perform switching is intact in old B cells.
Keywords: Age, Mice, B cell subsets, Ex vivo stimulation, Class switch recombination, Activation-induced deaminase
1. Introduction
Two hallmarks that define antibodies are somatic hypermutation (SHM) and class switch recombination (CSR). The impact of age on SHM has been widely documented in studies from mice and humans, and demonstrates that mutated antibodies persist in old animals, which are likely generated by memory B cells after lifelong contact with environmental and self-antigens [1, 2]. However, specificity for the immunizing antigen decreases with age [3], which may be due to changes in the composition of B cell subsets. Three major B cell populations are found in spleens of mice: follicular (FO), marginal zone (MZ), and age-associated B cells (ABC) [4, 5]. These populations undergo shifts in numbers with age, such that FO cells decline and ABCs increase, whereas MZ cells remain constant. We have previously shown that the repertoires of rearranged VH and Vκ genes in these subsets from old mice are diverse and overlap in gene usage [6]. However, although gene usage was similar, there were significant differences in SHM of VH and Vκ genes. FO cells had the lowest frequency (3 X 10−3 mutations/nucleotide), which is consistent with their naïve status. MZ cells had a two-fold higher frequency (6 X 10−3 mutations/nucleotide), suggesting that the cells encountered microbial antigens during circulation. ABCs had a four-fold increase (13 X 10−3 mutations/nucleotide), indicating that they had contacted antigen. The heterogeneous gene usage and high SHM frequency by ABCs predicts that these polyclonal cells arise primarily in response to diverse endogenous antigens during the life of an individual.
The impact of age on CSR is more controversial. Studies in spleens from immunized old mice suggest that total IgG is not reduced [7], whereas other reports of old splenic B cells stimulated in culture show that IgG is diminished [8]. Both SHM and CSR are initiated by the activation-induced deaminase (AID) protein, which deaminates cytosine to uracil, and the U:G mismatch in DNA is recognized by base excision repair and mismatch repair pathways [9–11]. These repair pathways produce DNA strand breaks, which are handled differently during SHM and CSR [12]. In brief, SHM uses low-fidelity DNA polymerases for mutations, and CSR recruits DNA recombination proteins for isotype switching. To systematically compare CSR, we measured switching to IgG1 by FO and MZ B cells from young and old mice after stimulation with IL4 and either lipopolysaccharide (LPS) or anti-CD40 in culture.
2. B cell populations change with age
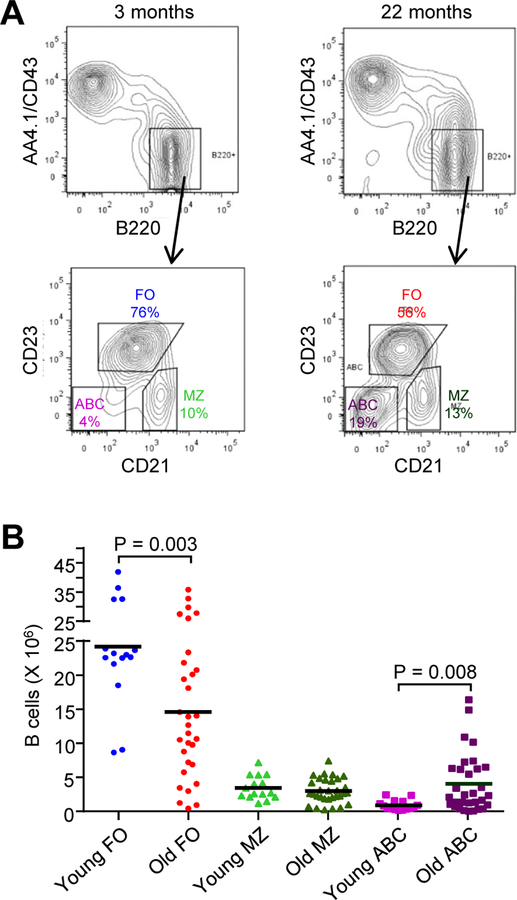
Profound changes in the composition of B cell subsets occur with age, which may contribute to the decline in immune status. To measure the frequency of FO, MZ, and ABC populations in young (3–5 months) and old (18–24 months) female C57BL/6 mice [4], spleen cells were stained with antibodies to AA4.1 and CD43 to remove transitional B cells, and with B220 to select mature B cells. B220+ cells were then stained with antibodies for CD21 and CD23 to distinguish the three subsets (Fig. 1A). FO cells were CD21lo CD23+, MZ cells were CD21+CD23-, and ABCs were CD21- CD23-. An analysis of 18–33 mice for each population showed a range in the number of B cells, particularly for FO cells (Fig. 1B). There was a significant decrease in FO B cells from old mice compared to young mice, with an average of 25 X106 cells from young mice versus 15 X 106 cells from old mice (P = 0.003). MZ B cells were constant with age, with an average of 3 X 106 cells from young and old mice. ABCs were low in young mice, at 1 X 106 cells, and significantly higher in old mice, at 5 X 106 cells (P = 0.008), although the range was wide. Thus, old mice have fewer naïve FO B cells than their young counterparts, and more antigen-experienced ABCs. The MZ B cell population was surprisingly constant, perhaps from chronic stimulation by endogenous antigens. These numbers are important when considering immune responses in old mice, which are generally diminished. Quantitatively, there are fewer FO B cells available to respond to immunization, and more ABCs, which overall are refractory to BCR stimulation [4] and may not generate a recall response. Therefore, splenic B cells from old mice may be compromised in their ability to mount a robust immune response.
Fig. 1.
Frequency of FO, MZ, and ABC subsets in young and old C57BL/6 female mice. Flow cytometry was performed on splenocytes from young (3–5 months) and old (18–24 months) mice by staining with antibodies against AA4.1, CD43, B220, CD21, and CD23, and gating on live, mature B220+ cells. (A) Representative chromatograms are shown. Numbers represent percentage of cells in the indicated populations. (B) Number of B cells in each subset from young and old mice. Each symbol represents a mouse, with the mean number of each B cell population shown with a bar (n = 18–33 mice for each subset and age). P values were determined using an unpaired, equal variance Student t test.
3. Old FO and MZ B cells undergo CSR with similar efficiencies as young cells
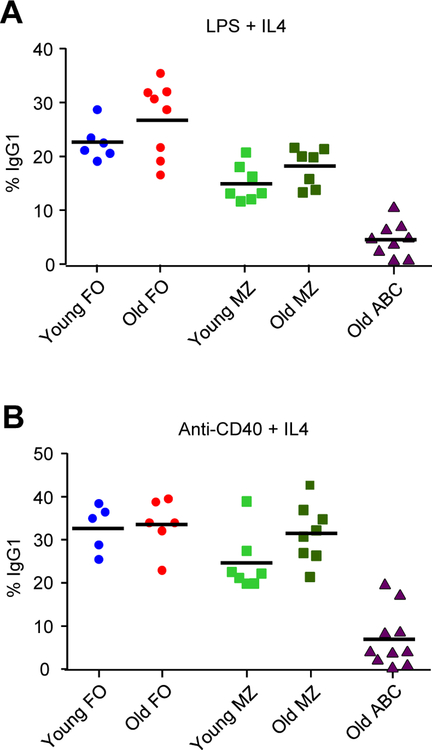
The gold standard to determine if the molecular machinery is working for CSR is to stimulate splenic B cells in culture with either LPS or anti-CD40, which signal through the TLR4 and CD40 receptors, respectively. These compounds drive most B cells to proliferate, express AID, and switch isotypes [13, 14]. When IL4 is added, the cells produce IgG1 on the surface after 3–4 days in culture. As shown in Fig. 2, young and old FO and MZ B cells produced comparable amounts of IgG1 as measured by flow cytometry for surface immunoglobulin when stimulated with IL4 and either LPS (Fig. 2A) or anti-CD40 (Fig. 2B). The results show that old FO and MZ B cells can respond to external stimuli as well as their young counterparts during ex vivo conditions. In marked contrast, the old ABC population switched poorly relative to FO and MZ cells. We were not able to test young ABCs because there were too few cells in this subset (Fig. 1B).
Fig. 2.
CSR to IgG1 in B cell subsets stimulated ex vivo. Cells were isolated as shown in Fig. 1 and stimulated for 4 days. Cells were then stained with anti-IgG1 for flow cytometry analysis [26]. (A) Cells stimulated with 5 µg/ml LPS and 5 ng/ml IL4. (B) Cells stimulated with 2.5 µg/ml anti-CD40 and 5 ng/ml IL4. Each symbol represents a mouse, with the mean number of switched cells per population indicated with a bar (n = 5–10 mice for each subset and age).
4. AID expression is comparable in young and old FO B cells during CSR
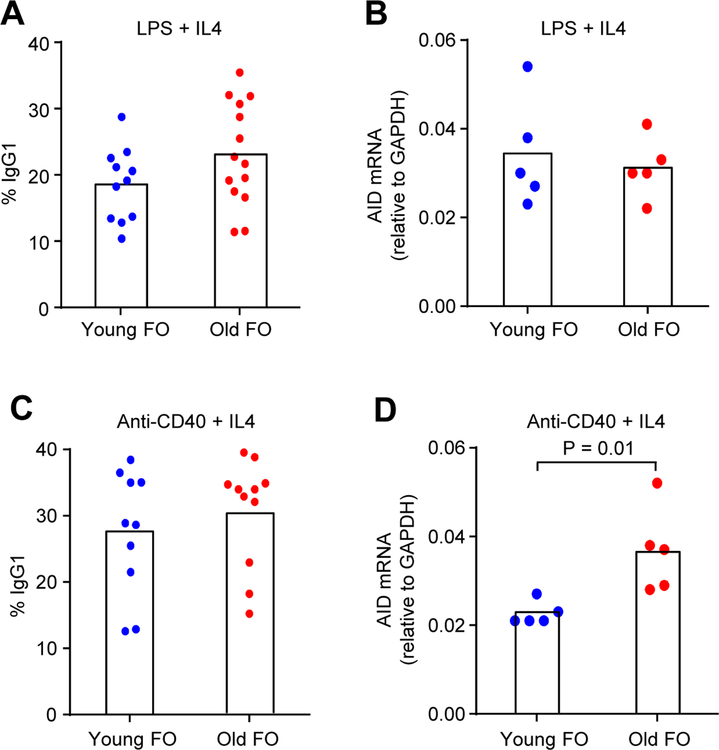
We then looked at expression of AID which initiates CSR. As measured by IgG1 and qPCR of AID transcripts after 3 days of stimulation, the amount of switching and AID was similar between young and old FO B cells when stimulated with LPS and IL4 (Fig. 3A and B). When stimulated with anti-CD40 and IL4, switching was the same in young and old cells, but AID levels were somewhat higher in cells from old mice (Fig. 3C and D). Thus, AID expression and the downstream readout of IgG1 expression were comparable in both young and old FO B cells.
Fig. 3.
IgG1 and AID expression in young and old FO cells after 3 days stimulation. AID transcripts were measured by qPCR [26] and normalized to GAPDH. (A) and (B), stimulated with LPS and IL4. (C) and (D), stimulated with anti-CD40 and IL4. Each symbol represents a mouse, with the mean number of switched cells and AID expression indicated by a column (n = 5–14 mice for each age). Significance was determined by a two-tailed Student t test.
5. Discussion
We demonstrate robust switching to IgG1 in FO and MZ B cell subsets from young and old C57BL/6 mice during ex vivo stimulation with IL4 and either LPS or anti-CD40. Our results differ from reports in the literature, notably from Blomberg and colleagues, who describe reduced CSR and AID after LPS and anti-CD40 stimulation in B cells from old mice compared to young mice [8, 15–17]. Their studies were performed in BALB/c mice; however, we also examined young and old FO B cells from BALB/cJ mice, and the frequency of CSR was unchanged, and it was similar to cells from C57BL/6 mice (data not shown). Differences in experimental techniques to isolate B cells may explain the discordant data. Frasca et al [8] isolated total splenic B cells with anti-B220, would include the ABC population in old mice. As shown in Fig. 2, this subset does not switch effectively, and the presence of ABC cells may dampen the overall CSR response. In our studies reported here, splenic FO B cells were isolated using positive selection with anti-CD23, and MZ B cells were purified with anti-CD21.
6. Conclusion
The results presented here emphasize the need to purify B cell subsets for comparative studies, since FO, MZ, and ABC subsets have different antigenic histories, as documented by the varying frequencies of SHM [6, 18]. Old mice have increased ABC cells, which do not respond well to LPS or anti-CD40, which could lower overall CSR frequencies. Furthermore, the established dogma that old animals are compromised in antibody responses [19–22] can be due a number of factors that affect B cell stimulation, such as decreased amounts of antigen, cytokines, and CD40 ligand on T cells [23–25]. Nonetheless, it is apparent that old FO and MZ B cells have the intrinsic capability to undergo vigorous CSR. These results indicate that the molecular machinery to perform switching, which includes AID and proteins involved in nonhomologous end-joining, is intact with age.
Highlights.
Old murine B cells undergo robust class switch recombination with LPS or anti-CD40
Follicular B cells from young and old mice switch to IgG1 with equal frequencies
Expression of activation-induced deaminase is similar in young and old B cells
Molecular machinery for switching is intact in old follicular and marginal zone cells
Acknowledgements
All animal protocols were reviewed and approved by the Animal Care and Use Committee of the National Institute on Aging. This work was supported entirely through the Intramural Research Program at the National Institutes of Health, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Williams GT, Jolly CJ, Kohler J, Neuberger MS, The contribution of somatic hypermutation to the diversity of serum immunoglobulin: dramatic increase with age, Immunity, 13 (2000) 409–417. [DOI] [PubMed] [Google Scholar]
- [2].Rosner K, Winter DB, Kasmer C, Skovgaard GL, Tarone RE, Bohr VA, Gearhart PJ, Impact of age on hypermutation of immunoglobulin variable genes in humans, J Clin Immunol, 21 (2001) 102–115. [DOI] [PubMed] [Google Scholar]
- [3].Wu YC, Kipling D, Dunn-Walters DK, Age-Related Changes in Human Peripheral Blood IGH Repertoire Following Vaccination, Frontiers in immunology, 3 (2012) 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP, A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice, Blood, 118 (2011) 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P, Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity, Blood, 118 (2011) 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Knode L.M. Russell, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, Ford ML, Tobias JW, Cancro MP, Gearhart PJ, Age-Associated B Cells Express a Diverse Repertoire of VH and Vkappa Genes with Somatic Hypermutation, J Immunol, 198 (2017) 1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Han S, Yang K, Ozen Z, Peng W, Marinova E, Kelsoe G, Zheng B, Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice, J Immunol, 170 (2003) 1267–1273. [DOI] [PubMed] [Google Scholar]
- [8].Frasca D, Van der Put E, Riley RL, Blomberg BB, Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase, J Immunol, 172 (2004) 2155–2162. [DOI] [PubMed] [Google Scholar]
- [9].Maul RW, Gearhart PJ, AID and somatic hypermutation, Adv Immunol, 105 (2010) 159–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saribasak H, Gearhart PJ, Does DNA repair occur during somatic hypermutation?, Semin Immunol, 24 (2012) 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zanotti KJ, Gearhart PJ, Antibody diversification caused by disrupted mismatch repair and promiscuous DNA polymerases, DNA repair, 38 (2016) 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zanotti KJ, Maul RW, Yang W, Gearhart PJ, DNA Breaks in Ig V Regions Are Predominantly Single Stranded and Are Generated by UNG and MSH6 DNA Repair Pathways, J Immunol, 202 (2019) 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hodgkin PD, Lee JH, Lyons AB, B cell differentiation and isotype switching is related to division cycle number, J Exp Med, 184 (1996) 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rush J, Liu M, Odegard V, Unniraman S, Schatz D, Expression of activation-induced cytidine deaminase is regulated by cell division, providing a mechanistic basis for division-linked class switch recombination, Proc Natl Acad Sci USA, 102 (2005) 13242–13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frasca D, Riley RL, Blomberg BB, Aging murine B cells have decreased class switch induced by anti-CD40 or BAFF, Experimental gerontology, 42 (2007) 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Frasca D, Landin AM, Alvarez JP, Blackshear PJ, Riley RL, Blomberg BB, Tristetraprolin, a negative regulator of mRNA stability, is increased in old B cells and is involved in the degradation of E47 mRNA, J Immunol, 179 (2007) 918–927. [DOI] [PubMed] [Google Scholar]
- [17].Frasca D, Landin AM, Riley RL, Blomberg BB, Mechanisms for decreased function of B cells in aged mice and humans, J Immunol, 180 (2008) 2741–2746. [DOI] [PubMed] [Google Scholar]
- [18].Johnson SA, Rozzo SJ, Cambier JC, Aging-dependent exclusion of antigen-inexperienced cells from the peripheral B cell repertoire, J Immunol, 168 (2002) 5014–5023. [DOI] [PubMed] [Google Scholar]
- [19].Zheng B, Han S, Takahashi Y, Kelsoe G, Immunosenescence and germinal center reaction, Immunol Rev, 160 (1997) 63–77. [DOI] [PubMed] [Google Scholar]
- [20].Kline GH, Hayden TA, Klinman NR, B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation, J Immunol, 162 (1999) 3342–3349. [PubMed] [Google Scholar]
- [21].Miller JP, Allman D, The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors, J Immunol, 171 (2003) 2326–2330. [DOI] [PubMed] [Google Scholar]
- [22].Cancro MP, B cells and aging: gauging the interplay of generative, selective, and homeostatic events, Immunol Rev, 205 (2005) 48–59. [DOI] [PubMed] [Google Scholar]
- [23].Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L, Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses, J Exp Med, 200 (2004) 1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang W, Brahmakshatriya V, Swain SL, CD4 T cell defects in the aged: causes, consequences and strategies to circumvent, Experimental gerontology, 54 (2014) 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lefebvre JS, Masters AR, Hopkins JW, Haynes L, Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses, Sci Rep, 6 (2016) 25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zanotti KJ, Maul RW, Castiblanco DP, Yang W, Choi YJ, Fox JT, Myung K, Saribasak H, Gearhart PJ, ATAD5 deficiency decreases B cell division and Igh recombination, J Immunol, 194 (2015) 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]