Abstract
Objective
To investigate cellular sources of miRNAs within adipose tissue, the impact of obesity on miRNA expression, and examine targets of miRNAs.
Methods
miRNA expression by qPCR was examined in adipocytes, adipose tissue macrophages (ATM), and peripheral blood mononuclear cells (PBMC) from normal weight and individuals with obesity. Differentiated 3T3-L1 adipocytes were co-cultured with macrophages, and 3T3-L1 and differentiated human mesenchymal stem cells were transfected with miR-155 with PPARγ and GLUT4 abundance measured via Western blot analysis.
Results
Abundance of miR-155 and miR-210 was increased in ATM of subjects with obesity by 6.7-fold and 2.9-fold (p = 0.002 and p = 0.013, respectively). miR-130b expression was increased 1.8-fold in the ATM and 4.3-fold in adipocytes from subjects with obesity (p = 0.007 and p = 0.02, respectively). PPARγ mRNA expression decreased 32% (p=0.044) in adipocytes from individuals with obesity. In 3T3L1 cells exposed to macrophages, PPARγ expression decreased 99.4% (p=0.02). PPARγ protein content declined 75% (p=0.001) in 3T3L1 cells transfected with a miR-155. GLUT4 protein was reduced by 55% (p=0.021) in differentiated human mesenchymal stem cells exposed to miR-155.
Conclusions
Adipose tissue miRNAs are influenced in a cell-type specific fashion by obesity, with macrophage miR-155 potentially impacting neighboring adipocytes.
Keywords: MicroRNA, Obesity, Inflammation, Macrophage
Introduction
The prevalence of obesity has increased rapidly in the last century, and now ranks as a major cause of morbidity and mortality in the industrialized world.(1) The increase in obesity is accompanied by comorbidities such as hypertension, dyslipidemia, and type 2 diabetes (T2DM), all risk factors for cardiovascular disease. A study of 1 million adults followed over 14 years showed obesity was strongly associated with an increased risk of all-cause and cardiovascular mortality.(2)
Obesity leads to a state of low-grade chronic inflammation, with the adipose tissue macrophage being a key element. Macrophages increase in the adipose tissue of individuals with obesity, being responsible for the increased cytokine production and low-grade inflammation. Because inflammation is linked to the morbidities that accompany obesity (8), delineation of adipocyte-macrophage interactions would lead to a better understanding of the pathogenesis of obesity-induced disease.
Adipose tissue secretes biologically active molecules that yield systemic effects on target tissues. Recently, microRNAs (miRNA) have been found to be secreted by adipose tissue and thus are potentially involved in such intercellular communication.(9) These small, non-coding RNAs bind to complementary sequences within the 3’ untranslated region of messenger RNAs (mRNAs) and thereby modulate protein production, typically degrading or repressing translation of targeted mRNAs.(10–15) They have been shown to function in multiple biologic processes, including adipocyte differentiation (16), and recent work has examined the effects of obesity on miRNA expression.
Ortega et al identified several miRNAs that were differentially expressed in subcutaneous adipose tissue from individuals with obesity compared to those of normal weight (17); however, the adipose tissue was not separated into its constituent cell types prior to analysis so the contribution of individual cell types (i.e., the macrophages versus the adipocytes) could not be discerned. The purpose of this study was to determine the impact of obesity on the expression of select miRNA species by human adipocytes, adipose tissue macrophages (ATM) and peripheral blood mononuclear cells (PBMC, the circulating macrophage precursor), as well as examine potential targets of the differentially expressed miRNAs. We chose to focus initially on miR-155 because of its role in macrophage recruitment into adipose tissue as well as evidence linking it to insulin resistance.(9, 18) Six additional miRNAs known to be expressed by adipose tissue or impact adipogenesis were also examined. We hypothesized that the patterns of miRNA expression would differ between the adipocytes and the ATM, reflecting distinct biological roles, and that the milieu within the adipose tissue would result in different miRNA expression by ATM as compared to PBMCs.
Methods
Participants
Equal numbers of males and females within each group of any racial or ethnic background, ages of 18–25, were recruited from the local community using IRB-approved approaches. Persons of normal weight (N=10) and persons with obesity (N=6) were tested. Normal weight was defined as BMI 18–24.9 kg/m2 and obesity as BMI greater than 30 kg/m2. Subjects were excluded if they were pregnant, had medical conditions or used medications, known to impact metabolic function that would obscure the interpretation of results. Examples include diabetes, cardiovascular, endocrine, organ dysfunction, hyperlipidemia, or chronic debilitating disease. Informed consent was obtained in accordance with the guidelines of the University of Oklahoma Health Sciences Center IRB, who approved the study, prior to the conduct of any procedures. A routine medical history and physical examination was performed by a physician (JBT) to assure suitability for the study.
Anthropometric measures
Height (using a standard stadiometer), weight, body mass index (BMI), and blood pressure (using an appropriate sized cuff, based on arm circumference and American Heart Association cuff size guidelines) were measured. Lean mass and fat content were measured using dual energy X-ray absorptiometry (DXA, Lunar iDXA, GE Healthcare, Fairfield, CT).
Biochemical analyses
A fasting blood sample was collected and serum and plasma were separated by centrifugation and stored at −80°C until analysis. Plasma glucose was measured by the glucose oxidase method (YSI 2300 STAT Plus, Yellow Springs, OH). Serum insulin was measured by chemiluminescent ELISA (ALPCO, Salem, NH). Glucose and insulin values were used to estimate insulin resistance using the HOMA-IR model.(19) Serum triglycerides, and total and HDL cholesterol were measured by colorimetric assay (Wako Life Sciences, Inc., Mountain View, CA). Serum IL-6 and TNF-α were measured using chemiluminescent ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol, with the exception that the primary incubation for the IL-6 ELISA occurred overnight at 4°C. C Reactive Protein (CRP) was assayed by ELISA (Abcam, Cambridge, MA).
Fat Biopsies and isolation of adipocytes and macrophages from fat biopsies
Subcutaneous adipose biopsies were obtained from the abdomen of subjects using a biopsy needle and syringe after administration of lidocaine for local anesthesia. The adipose tissue was weighed, minced, then treated with collagenase A from Clostridium histolyticum (300 Units/mL in PBS with 2% BSA, Roche Diagnostics, Indianapolis, IN) to separate the stromal vascular portion from the adipocytes. The tissue was shaken at 200–300 rpm for 40 min at 37°C. The cells were then filtered through mesh and centrifuged for 5 min at 500 × g. The floating cells were collected as the adipocyte fraction. 10μL of the cell suspension was stained with trypan blue and loaded on a standard hemocytometer for analysis by microscopy using a Nikon TE2000-E equipped with a Photometrics Cool Snap ES digital camera. Cells were counted in each of the four outside squares of the hemocytometer. Metamorph software (Molecular Devices) was used to assess cell characteristics and calculate cell surface area and volume. Cells less than 500μm2 in area with a shape factor of 0.9 were considered as an adipocyte.
The pelleted cells were incubated with anti-CD14 microbeads (Miltenyi Biotec, Auburn, CA) to enrich the macrophage population. CD14 antigen belongs to the LPS receptor complex and is strongly expressed on monocytes and macrophages (20). The cells were then applied to a magnetic column to remove any cells not presenting the CD14 antigen. Bound cells (i.e., CD14+ cells) were then flushed from the column and stained with both anti-CD14 antibody conjugated to fluorescein isothiocyanate and anti-CD31 antibody conjugated to phycoerythrin. CD31, also known as platelet endothelial cell adhesion molecule or PECAM-1, is expressed on macrophages.(21) The cells were then sorted using the Influx cell sorter (BD Biosciences, San Jose, CA) in the Flow Cytometry Core Lab. The cells expressing both CD14 and CD31 were collected as the adipose tissue macrophages (ATM) and RNA was isolated from the cells and stored at −80°C until analysis. The purity of the ATM prior to sorting for CD14 and CD 31 positive cells was 40–85%. A representative plot has been included in Supplemental Figure 1A.
Collection and enrichment of peripheral blood mononuclear cells
Whole peripheral blood was drawn into anticoagulant-containing vacutainers. To isolate mononuclear cells, 25 mL of the whole blood was diluted four-fold with PBS containing EDTA, layered over Ficoll-Paque PREMIUM (1.077 g/mL; GE Healthcare Bio-Sciences, Pittsburgh, PA) and separated by density gradient centrifugation according to the protocol from Miltenyi Biotec. The buffy coat was transferred to a new tube and cells positive for both CD14 and CD31 were isolated as described above. The purity of the PBMC prior to sorting for CD14 and CD31 positive cells was 95% or greater. A representative plot has been included in Supplemental Figure 1B. The purified mononuclear cells were used for subsequent experiments.
RNA isolation
Total RNA was isolated from cells using the Qiagen miRNeasy Kit according to the manufacturer’s protocol. Quantity of RNA isolated was determined using the NanoDrop ND-1000 (Thermo Scientific, Waltham, MA). For miRNA, reverse transcription was done on equal amounts of total RNA (10 ng/sample) using the TaqMan microRNA Reverse Transcription Kit (Life Technologies, Grand Island, NY) and a pool of miRNA-specific RT primers. For mRNA, reverse transcription was done using equal amounts of total adipocyte RNA (2 μg) and the SuperScript VILO cDNA Synthesis Kit (Invitrogen, Life Technologies, Grand Island, NY) according to the manufacturer’s instructions. The quantity of ATM RNA was insufficient for further analysis.
Total RNA was isolated from equal volumes of serum using the Qiagen miRNeasy Serum/Plasma Advanced Kit according to the manufacturer’s directions. During RNA isolation, a known quantity of the C. elegans miR-39–3p miRNA mimic was added to each sample as a spike-in control. For miRNA, reverse transcription was done as described for cells, except that equal starting volumes, rather than equal masses, of RNA were used in each reaction. Equal volumes were used as no endogenous small, non-coding RNA has been reproducibly established for use as a control in serum, and normalizing to volume allowed the miRNA to be examined similar to a circulating hormone or cytokine.
Qpcr
For miRNA, qPCR was performed in triplicate using TaqMan microRNA Assays and TaqMan Universal Master Mix II, no UNG (Life Technologies) on the Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Valencia, CA). Seven miRNA species (miR-30a, miR-125b, miR-130b, miR-155, miR-199a, miR-210, and miR-214) were selected from the literature as differentially expressed in adipose tissue or impacting adipogenesis.(18, 22–28) See Table S1 for sequence information. Data were normalized to the endogenous control U6 snRNA or the exogenous control cel-miR-39–3p for cells or serum, respectively. Similarly, qPCR for adipocyte mRNA was performed in triplicate using TaqMan Gene Expression Assays and Universal Master Mix II, no UNG. Expression of PPARG and SLC2A4 (GLUT4), targets of both miR-130b and miR-155, were measured in the adipocytes. Expression of IL-6 was also measured as a marker of inflammation with the adipocytes. Gene expression data was normalized to GAPDH. Relative expression for both miRNA and mRNA was calculated using the 2−ΔΔCt method (29).
Co-Culture of murine 3T3-L1 adipocytes and RAW264.7 macrophages
Fully differentiated 3T3-L1 adipocytes were trypsinized and plated into 24-well dishes at a density of 150,000 cells/well. Cell culture inserts with 8μm pore size (Millipore Sigma, Burlington, MA) were seeded with RAW264.7 macrophages (50,000 cells/insert). Adipocytes (lower chamber) were incubated in the absence or presence of macrophages (upper chamber) for 48 hours. After co-culture, the conditioned media were removed; macrophage-containing inserts were removed and all wells and inserts were washed twice with DPBS. After thoroughly aspirating the DPBS from the inserts and the wells, cells were lysed with 2× Laemmeli sample buffer, scraped and transferred to microcentrifuge tubes, and heated at 95°C for 5 min in preparation for Western blotting.
Transfection of 3T3-L1 adipocyte
Fully differentiated 3T3-L1 adipocytes were transiently transfected with either a fluorescently labeled control miRNA mimic with no known mRNA targets or mmu-miR-155–5p mimic using DharmaFECT Duo Transfection Reagent (Dharmacon, Inc., Chicago, IL). Briefly, adipocytes were trypsinized, counted, and plated at a density of 150,000 cells/well on 24-well dishes already containing the miRNA-transfection reagent complex. After 48 hours, the cells were washed twice with DPBS to remove media and transfection complexes and then lysed with 2× Laemmeli sample buffer and prepared for Western blotting as described.
Human Mesenchymal Stem Cell Differentiation and Transfection
Human mesenchymal stem cells were isolated from umbilical cord Wharton’s jelly from healthy women at delivery according to “explant method”, and subjected to adipogenic differentiation as previously described(30). Briefly, adipogenesis was induced with 3 cycles of adipogenic induction medium (low-glucose DMEM with 5%FBS, 1 μM dexamethasone, 200 μM indomethacin, 500 M IBMX, and 170 pM insulin) and maintenance medium (low-glucose DMEM with 5% FBS and 170 pM insulin) each for 3 days. Adipogenic-differentiated cells were trypsinized, counted, and replated for transfection in 12 well dishes at a density of 1e5 cells/well with either control miRNA mimic with no known mRNA targets or mmu-miR-155–5p mimic (Dharmacon, Inc., Chicago, IL) in a similar fashion to the 3T3L1 adipocyte transfection as above.
Western Blot Analysis
Equal volumes (15 μl) of protein lysates were separated on AnykD polyacrylamide gels (Bio-Rad, Hercules, CA) under reducing, denaturing conditions and transferred to polyvinylidene fluoride (PVDF) membrane. After transfer, the membrane was blocked with BSA (5% in TBST) and then incubated with antibodies specific for PPARγ protein (Cell Signaling Technology, Danvers, MA), GLUT4 (abcam, Cambridge, MA) and beta-actin (Santa Cruz Biotechnology, Dallas, TX). The proteins of interest were detected by enhanced chemiluminescence (Pierce, Rockford, IL) and analyzed by imaging densitometry. Actin was used as a loading control for all experiments and results were adjust accordingly.
Statistical analysis
Phenotypic and biologic characteristics were evaluated by Student’s t-test unless they were not normally distributed in which case Independent Samples Mann-Whitney U test was used. Gene and miRNA expression data were not normally distributed so Independent Samples Mann-Whitney U test was used to determine differences in expression between the groups with normal weight and the groups with obesity. Protein abundance was compared using Student’s t-test. miRNA abundance was assessed by 2-way ANOVA with post-hoc Tukey HSD. The significance level was set at p < 0.05.
Results
Participant groups differ only in adiposity and adipose macrophage number
The subjects studied were of similar age with equal distribution of males and females between the group with normal weight and group with obesity. The only significant differences between the groups were weight, BMI, and fat mass. (Table 1) There was no difference in serum levels of inflammatory markers, IL-6, CRP or TNF-α. The cellular characteristics of the adipose biopsies were also similar between the two groups. (Table 2)
Table 1.
Participant Characteristics
| Normal Weight | Obese | |
|---|---|---|
| Female/Male | 5/5 | 3/3 |
| Age (years) | 22.8 +/− 1.14 | 23.2 +/− 1.17 |
| Weight (kg)* | 66.4 +/− 9.19 | 107.7 +/− 14.52 |
| Height (cm) | 170.8 +/− 9.45 | 172.7 +/− 7.82 |
| Systolic Blood Pressure | +/− 11 | +/− 9 |
| Diastolic Blood Pressure | +/− 8 | +/− 7 |
| BMI* | 22.7 +/− 1.13 | 36.2 +/− 3.18 |
| Lean Mass (kg) | 46.1 +/− 11.37 | 57.23 +/− 13.35 |
| Fat Mass (kg)* | 17.8 +/− 5.78 | 47.2 +/− 7.79 |
| Large Artery Elasticity | 18.2 +/− 3.01 | 18.4 +/− 2.14 |
| Small Artery Elasticity | 7.4 +/− 2.12 | 9.8 +/− 3.94 |
| Glucose (mg/dL) | 80.7 +/− 6.5 | 82.1 +/− 7.2 |
| Insulin (uU/mL) | 10.4 +/− 4.9 | 14.5 +/− 5.2 |
| HOMA-IR | 2.1 +/−1.0 | 3.0 +/− 1.2 |
| Cholesterol (mg/dL) | 130.4 +/− 24.0 | 119.5 +/− 14.6 |
| HDL- Cholesterol (mg/dL) | 54.1 +/− 12.5 | 42.9 +/− 10.1 |
| Triglycerides (mg/dL) | 80.5 +/−34.8 | 72.1 +/− 33.0 |
| IL-6 (pg/mL) | 1.6 +/− 1.1 | 1.9 +/− 0.9 |
| C-Reactive Protein (mcg/mL) | 2.0 +/− 3.8 | 2.5 +/− 4.3 |
| TNF-α (pg/ml) | 4.7 +/−1.0 | 5.0 +/− 1.0 |
Average +/− SD for each measure.
Indicates a p-value of less than 0.05
Table 2.
Cellular Characteristics
| Normal Weight | Obese | |
|---|---|---|
| Biopsy Mass (grams) | 1.29 +/−0.60 | 1.89 +/− 1.08 |
| Cell Count ATM (105 cells)* | 6.15 +/− 4.96 | 7.43 +/− 1.72 |
| Cell Count PBMC (106 cells) | 2.61 +/− 0.71 | 2.76 +/− 1.64 |
| Adipocyte Area (μm2) | 222.29 +/− 76.29 | 226.22 +/− 23.18 |
| Adipocyte Volume (μm3) | 475.13 +/− 214.29 | 456.13 +/− 106.57 |
Average +/− SD for each measure.
Indicates a p-value of less than 0.05. ATM, adipose tissue macrophage; PBMC, purified peripheral blood mononuclear cell.
Selected miRNAs are expressed by adipocytes and ATMs and influenced by obesity
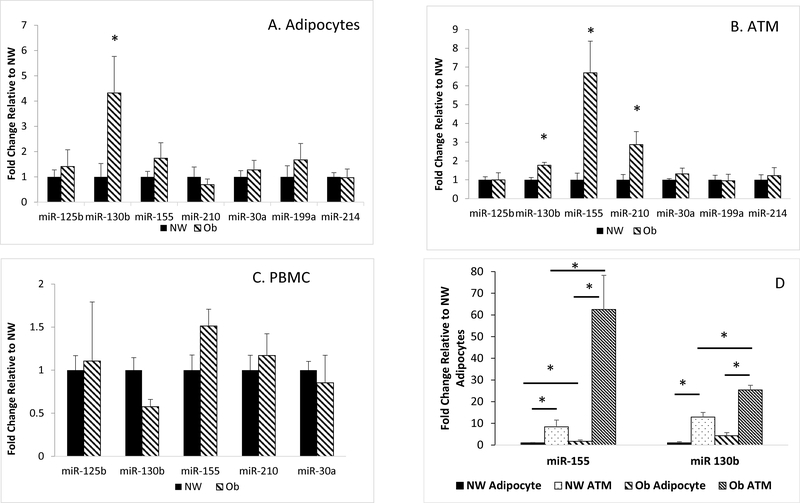
All seven miRNA species were detected within the adipocytes and ATM, while only five species were detectable in the purified peripheral blood mononuclear cells (PBMC). miR-214 was the most abundant in the adipocyte (p<0.001) across the entire cohort. Within the ATM across the cohort, miR-214 and miR-155 were the most abundant (p<0.001 for each miRNA and p=0.191 between miR-214 and miR-155). miR-155 was the most abundant in the PBMC of the entire cohort (p<0.001) (Table 3). miR-199a and miR-214 were of very low abundance in PBMC (Ct > 37). Within adipocytes, miR-130b abundance was increased by 4-fold (p=0.03) in the group with obesity over the group with normal weight (Figure 1A). In ATM, miR-155, miR-130b, and miR-210 were increased in the group with obesity compared to the group of normal weight by 6.7 (p=0.002), 1.8 (p=0.007), and 2.9-fold (p=0.013) respectively (Figure 1B). There was no effect of obesity within the PBMC (Figure 1C). In comparing the abundance of miRNAs between the adipocytes and ATM, the expression of miR-155 and miR-130b were higher in the ATM as compared to adipocytes in both the group of normal weight (8.4-fold and 12.9-fold respectively, p<0.001) and in the group with obesity (62.5-fold and 25.4-fold respectively, p<0.001) (Figure 1D). There was no difference in miR-210 expression between the adipocyte and the ATM in either the group with group of normal weight or the group with obesity.
Table 3.
Ct Values across Cell Types
| miR-125b | miR-130b | miR-155 | miR-199a | miR-210 | miR-30a | miR-214 | U6 snRNA/ cel-miR39–3p | |
|---|---|---|---|---|---|---|---|---|
| Adipose | 27.4+/−1.4 | 31.3+/−1.1 | 36.7+/−1.6 | 32.6+/−1.3 | 29.0+/−1.8 | 29.5+/−1.5 | 23.8+/−1.9* | 23.8+/−2.4 |
| ATM | 24.7+/−1.3 | 26.9+/−1.3 | 22.8+/−2.0* | 27.9+/−1.5 | 28.9+/−1.9 | 25.3+/−1.2 | 21.5+/−1.4* | 24.0+/−1.4 |
| PBMC | 32.2+/−1.2 | 28.4+/−1.7 | 22.8+/−1.6* | ND | 30.0+/−1.7 | 29.7+/−0.9 | ND | 25.9+/−1.3 |
| Serum | ND | 31.1+/−0.8 | 31.6+/−0.9 | ND | 32.6+/−1.0 | ND | ND | 20.1+/−0.8 |
Each Ct value is the average +/− SD for the entire cohort (n = 16). Higher number reflects a lower abundance.
Indicates a p-value of less than 0.05 by ANOVA within each cell type. ATM, adipose tissue macrophage; PBMC, purified peripheral blood mononuclear cell; ND, not determined due to Ct >37. U6 was used as endogenous control for Adipose, ATM and PMBC, while cel-miR-39–3p was used as a spike-in control for the serum.
Figure 1.
A. miRNA expression in the adipocytes. * = p<0.05. B. miRNA expression in the adipose tissue macrophages (ATM). * = p<0.05. C. miRNA expression in purified peripheral blood mononuclear cells (PBMC). D. Comparison of miRNA expression between the adipocyte and ATM. * = p<0.05. Normal weight group (NW). Group with obesity (Ob). N=10 in the NW group and N=6 in Ob group. Ct were normalized to the endogenous control U6 snRNA. Fold change is relative to NW in each miRNA.
miR-155 and miR-130b were increased in the circulation of individuals with obesity
Of the differentially expressed miRNAs within the ATM and adipocytes, miR-155 and miR-130b demonstrated increased expression in the serum of group with obesity as compared to the group of normal weight. miR-155 was increased 2.1-fold (p=0.002) and miR-130b as increased 1.6-fold (p=0.007) in the group with obesity. (Figure S2)
The abundance of select mRNA targets of miR-155 and miR-130b are reduced in adipocytes
Within the adipocytes, expression of PPARG, a target of miR-155 and miR-130b, was decreased by 32% (p=0.023). Additionally, SLC2A4 (GLUT4), a target of PPARG, was also decreased in the adipocytes by 53% (p=0.031). There was no difference in IL-6 expression between the group with normal weight and the group with obesity. (Figure 2).
Figure 2.
mRNA expression in adipocytes from individuals with normal weight and obesity. PPARG and GLUT4 expression were decreased in the adipocytes of the subjects with obesity. * = p<0.05. IL-6 expression was not different between the two groups. Normal weight group (NW). Group with obesity (Ob). N=10 in the NW group and N=6 in Ob group. Ct were normalized GAPDH. Fold change is relative to NW in each miRNA.
PPARγ expression is decreased in 3T3-L1 adipocytes exposed to macrophages
In co-culture experiments, 3T3-L1 adipocytes (murine cell line) exposed to RAW264.7 macrophages (murine cell line) demonstrated a decrease in PPARγ1 (Upper band) expression of 99% (p=0.02) and a trend toward a decrease in PPARγ2 (Lower band) by 95% (p=0.07). (Figure 3A–C) In the supernatant collected from the co-culture experiment, miR-155 abundance was not significantly different in the supernatant from 3T3-L1 adipocytes as compared to the macrophages (p=0.29); however, in the co-culture the miR-155 abundance trended 1.9-fold higher relative to the macrophage (p=0.06), but increased 3.7-fold relative to the 3T3-L1 (p=0.006). (Figure 3D).
Figure 3.
PPARγ expression in 3T3-L1 exposed to macrophages. PPARγ1 (Panel A) and PPARγ−2 (Panel B) adjusted for actin. L1 refers to the 3T3-L1 adipocytes (murine cell line) alone, Macro refers to macrophages (RAW264.7, murine cell line) alone, and Macro+L1 refers to the co-culture conditions. Western blot of PPARγ−1 and 2 is demonstrated in Panel C. miR155 abundance in supernatant from 3T3-L1 exposed to macrophage co-culture as compared to supernatant from 3T3-L1 alone. *p<0.05 (Panel D). Each experiment was done in triplicate. Fold change is relative to 3T3-L1 adipocytes.
PPARγ protein expression is decreased in 3T3-L1 adipocytes transfected with miR-155
In the fully differentiated 3T3-L1 adipocytes (murine cell line) transfected with miR-155, PPARγ1 (upper band) expression was decreased by 75% (p=0.001) relative to the 3T3-L1 adipocytes transfected with the control miRNA mimic. PPARγ2 (lower band) expression was decreased 58% (p=0.007) relative to the 3T3-L1 adipocytes transfected with the control miRNA mimic. GLUT4, a downstream target of PPARγ, abundance was decreased 54% (p=0.024) relative to the 3T3-L1 adipocyte transfected with the control miRNA mimic. (Figure 4)
Figure 4.
PPARγ expression in 3T3-L1 adipocytes (murine cell line) exposed to miR-155. PPARγ1 (Panel A), PPARγ2 (Panel B) and GLUT4 (Panel C) adjusted for actin. NTC refers to the No Template Control, anti-155 to the cells transfected with the anti-miR155 mimic, and miR155 to the cells transfected with the mimic of miR-155. *p<0.05. Western blot for PPARγ and actin is demonstrated in Panel D. Western blot for GLUT4 and actin is demonstrated in Panel E. Each experiment was done in triplicate. Fold changes is relative to the 3T3-L1 adipocytes transfected with NTC miRNA mimic.
GLUT4 protein expression is decreased in differentiated human mesenchymal stem cells transfected with miR-155
In the mesenchymal human stem cells differentiated down an adipose lineage, GLUT4 protein abundance was decreased by 55% (p=0.021) relative to the cells transfected with the control miRNA mimic. (Figure 5)
Figure 5.
GLUT4 expression in differentiated human mesenchymal stem cells exposed to miR155. GLUT 4 (Panel A) adjusted for actin. NTC refers to the No Template Control and miR-155 to the cells transfected with the mimic of miR-155. *p<0.05. Western blot for GLUT4 and actin is demonstrated in Panel B. Each experiment was done in triplicate. Fold changes is relative to the differentiated human mesenchymal stem cell transfected with NTC miRNA mimic.
Discussion
We have demonstrated that miRNA expression differs among cell types within adipose tissue of humans and that the response to obesity varies according to cell type. Specifically, miR-155–5p and miR-130b-3p are more abundant in ATM compared to the adipocytes. Macrophages isolated from individuals with obesity show increased expression of miRs 155, 130b, and 210 whereas in the adipocyte only miR-130b is affected by obesity. In contrast, miRNA expression within the purified PBMC differed from the related ATM as miRs 199a and 214 were not detected, and there was no effect of obesity. In circulation, miR-155 and miR-130b were both increased in the subjects with obesity compared to subjects with normal weight.
We also provide evidence supporting a role for macrophage-derived miR-155 as a regulator of PPARG and SLC2A4 (GLUT4) in subcutaneous fat. PPARG mRNA expression was decreased in adipocytes from subjects with obesity, and PPARγ and GLUT4 protein abundance was decreased when cultured adipocytes were exposed to macrophages, an effect that was duplicated by transfection with miR-155 in both a mouse and human model. From this we conclude that obesity alters miRNA expression in a cell type-specific manner, and that miRNAs secreted from the macrophage, such as miR-155, likely impact adipocyte metabolism.
miR-155 is of particular interest because of its participation in inflammatory processes. Karkeni and colleagues examined the role of miR-155 in adipose depot inflammation. Similar to our findings, they reported an increase in miR-155 abundance in the subcutaneous adipose tissue of humans with obesity, although they did not separate adipocytes from ATMs.(18) They also provided strong evidence that TNFα, via NFkB, is a major stimulus for miR-155 production by adipocytes which in turn, regulates PPARγ.(18) These data are consistent with our finding of reduced PPARG mRNA in adipocytes from the individuals with obesity.
The expression of the selected miRNAs and the impact of obesity was very different when purified PBMCs were examined, suggesting that the environment of the mononuclear cells is key to miRNA expression. PBMCs contain monocytes that differentiate into macrophages which infiltrate adipose tissue.(31) These macrophages then respond to the local environment becoming either “proinflammatory” or “anti-inflammatory”.(32) In the obese state, the pro-inflammatory, activated M1 macrophages populate adipose tissue.(33–35) As miR-155 is involved in macrophages activation (36), being upregulated in M1 macrophages (37), our finding of increased abundance of miR-155 in the ATM from the group with obesity is consistent with the purported role for activation and recruitment of M1 macrophages in fat depots. Interestingly, it is likely that the M1 macrophages numbers are increased in the group with obesity despite no increase in circulating markers of inflammation, IL-6, CRP, or TNF-α or an increase of IL-6 within the adipocytes. This raises the possibility that increased ATM expression of miR-155 may be an early molecular event portending a future inflammatory state before systemic inflammation is present.
Recently, Ying and colleagues have begun to elucidate a role for macrophage-derived miR-155 as a regulator of carbohydrate metabolism. In experiments performed primarily in mice, the investigators have shown that miR-155 is secreted from ATMs in exosomes, with an increased abundance in exosomes derived from mice with obesity.(9) They also found that exosomes from mice with obesity impaired insulin signaling in 3T3-L1 adipocytes and L6 myocytes because miR-155 reduced Pparg expression which, in turn, reduced Slc2A4 (Glut4).(9) While the initial effect of miR-155 on PPARγ may improve insulin sensitivity, the overwhelming impact on insulin sensitivity through decreased GLUT4 abundance and macrophage activation appeared to outweigh any beneficial effect. They concluded that ATMs secrete exosomes bearing miRNAs which function as paracrine or endocrine modulators of insulin action and overall glucose homeostasis.
Our findings provide strong evidence that what was observed in the murine model and postulated with regard to miR-155 by Ying et al (9) also occurs in humans. We found increased expression of miR-155 by ATM from human individuals with obesity, abundant secretion of miR-155 by a murine macrophage cell line, the expected effects of miR-155 on PPARγ and GLUT4 in both the human and murine model, and increased abundance of miR-155 in the circulation in humans with obesity. While we cannot prove in the human subject the origin of the miR-155 in circulation, the murine model suggests that it is secreted by the ATM.
Lee and colleagues found that the levels of miR-130b were higher in preadipocytes, but decreased with differentiation (38) and miR-130b was decreased in women with obesity.(38) This is different from our results, but could be attributable to the age difference in cohorts. Participants in the present study were younger, and it has been shown that preadipocytes’ ability to replicate and differentiate decreases with age, (39) which may explain the difference in miR-130b expression.
Giardina and colleagues have demonstrated that weight loss decreases miR-210 expression within adipose tissue (22). In considering our findings, Giardina looked at whole adipose tissue, while we separated ATM from adipocytes. It is possible that the miR-210 was predominantly coming from the ATM and that ATM number may decrease with weight loss.
The present study has several strengths. This was the first study to examine the expression of miRNA within the cellular components that comprise adipose tissue in humans. Additionally, the cohort with obesity examined was relatively healthy and had not yet developed significant insulin resistance, dyslipidemia, or other cardiometabolic risk factors. Nevertheless, distinct changes in miRNA expression were observed, thus a very early event resulting from obesity and not the result of secondary changes. The putative mRNA targets of the differentially expressed miRNAs were also examined and showed the expected changes in expression. Additionally, we were able to replicate the in vivo findings in vitro.
A few limitations are also identified. The biopsied population is small. It is possible the other differences in gene expression would be observed with a larger sample size; however, the miRNA expression was substantially different between the two groups despite very little difference in their biochemical makeup. We did not collect protein, so it was not possible to measure protein abundance of the target genes. We did not have enough RNA to measure gene expression in the adipose tissue macrophages nor was protein collected from these specimens either; however, the in vitro experiments support what was seen in vivo. Also, only CD14+ and CD31+ was used to define ATM. These markers are also present on granulocytes so it is possible that some granulocytes may have been included in this fraction. Even with these cells included, the miR-155 abundance was significantly increased in the individuals with obesity. Lastly, in the present analysis, only miR-155 was explored in-depth in the in vitro models as we did not fully explore the roles of miR-130b and miR-210. Such examination of these miRNAs was beyond the scope of this project, but will serve as an area of exploration in future studies.
Conclusion
In summary, we have shown that miRNA expression within the adipose tissue is cell-type specific and influenced by obesity. Increases in both miR-155 and miR-130b expression by the macrophage may be released in exosomes that are taken up by the adipocyte, resulting in decreased expression of their targets including PPARG and GLUT4. We have also demonstrated through in vitro experiments that miR-155 from the macrophage influences adipocyte function by affecting of PPARG. Future work is needed to define the mechanisms that impact the differential expression of miRNAs within ATM of adipose tissue as well as the impact of the differentially expressed miRNAs on their local and systemic targets.
Supplementary Material
What is known about this subject.
miR-155 is increased in adipose tissue from individuals with obesity
Obesity leads to inflammation with the adipose tissue macrophage being a key mediatory of the inflammatory process
Study Importance Questions.
miRNAs are differentially expressed in subcutaneous adipose tissue from individuals with obesity compared to those of normal weight; however, the constituent cell types (i.e., the macrophages versus the adipocytes) that contribute the miRNA has not been elucidated
miRNA expression differs among cell types within adipose tissue of humans and the response to obesity varies according to cell-type, specifically miR-155 and miR-130b are more abundant in adipose tissue macrophages compared to the adipocytes
PPARγ mRNA was decreased in adipocytes of individuals with obesity, and the PPARγ protein abundance was decreased in cultured adipocytes exposed to macrophages, an effect that was duplicated by transfection with miR-155
Acknowledgments
We wish to thank our subjects for participation in the study.
Funding: NIH, NIDDK 5K23DK106533, 5K08DK115797, CMRI Metabolic Research Program, Harold Hamm Diabetes Center and University of Oklahoma Health Sciences Center Department of Pediatric Diabetes/Endocrinology.
Abbreviations
- T2DM
type 2 diabetes mellitus
- MiRNA
microRNA
- PPARγ
peroxisome proliferator-activated receptor gamma
- ATM
adipose tissue macrophage
- PBMC
peripheral blood mononuclear cell
Footnotes
Disclosure: The authors have not conflict so interest.
References
- 1.Skilton MR, Celermajer DS. Endothelial dysfunction and arterial abnormalities in childhood obesity. International Journal of Obesity. 2006;30(7):1041–9. [DOI] [PubMed] [Google Scholar]
- 2.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Endocrinol Metab Clin North Am. 2008;37(3):663–84. [DOI] [PubMed] [Google Scholar]
- 3.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 4.Chan CC, Damen M, Alarcon PC, Sanchez-Gurmaches J, Divanovic S. Inflammation and Immunity: From an Adipocyte’s Perspective. J Interferon Cytokine Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unamuno X, Gomez-Ambrosi J, Rodriguez A, Becerril S, Fruhbeck G, Catalan V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48(9):e12997. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(2):461S–5S. [DOI] [PubMed] [Google Scholar]
- 7.Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155(4):407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Comprehensive Physiology. 2018;9(1):1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell. 2017;171(2):372–84 e12. [DOI] [PubMed] [Google Scholar]
- 10.Andreasen D, Fog JU, Biggs W, Salomon J, Dahslveen IK, Baker A, et al. Improved microRNA quantification in total RNA from clinical samples. Methods (Duluth). 2010;50(4):S6–9. [DOI] [PubMed] [Google Scholar]
- 11.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107(5):677–84. [DOI] [PubMed] [Google Scholar]
- 12.Kloting N, Berthold S, Kovacs P, Schon MR, Fasshauer M, Ruschke K, et al. MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS ONE [Electronic Resource]. 2009;4(3):e4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Ruan K. MicroRNA detection by microarray. Anal Bioanal Chem. 2009;394(4):1117–24. [DOI] [PubMed] [Google Scholar]
- 14.Ono K MicroRNA links obesity and impaired glucose metabolism. Cell Res. 2011;21(6):864–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107(6):810–7. [DOI] [PubMed] [Google Scholar]
- 16.Heneghan HM, Miller N, Kerin MJ. Role of microRNAs in obesity and the metabolic syndrome. Obesity Reviews. 2010;11(5):354–61. [DOI] [PubMed] [Google Scholar]
- 17.Ortega FJ, Moreno-Navarrete JM, Pardo G, Sabater M, Hummel M, Ferrer A, et al. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS One. 2010;5(2):e9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karkeni E, Astier J, Tourniaire F, El Abed M, Romier B, Gouranton E, et al. Obesity-associated Inflammation Induces microRNA-155 Expression in Adipocytes and Adipose Tissue: Outcome on Adipocyte Function. J Clin Endocrinol Metab. 2016;101(4):1615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergman RN, Phillips SP, Cobelli C. Phsysiologic evaluation of factors controlling glucose tolerance in man. J Clin Invest. 1981;68:1456–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamani F, Zare Shahneh F, Aghebati-Maleki L, Baradaran B. Induction of CD14 Expression and Differentiation to Monocytes or Mature Macrophages in Promyelocytic Cell Lines: New Approach. Advanced pharmaceutical bulletin. 2013;3(2):329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53(5):1285–92. [DOI] [PubMed] [Google Scholar]
- 22.Giardina S, Hernandez-Alonso P, Salas-Salvado J, Rabassa-Soler A, Bullo M. Modulation of Human Subcutaneous Adipose Tissue microRNA Profile Associated with Changes in Adiposity-Related Parameters. Molecular nutrition & food research. 2017. [DOI] [PubMed] [Google Scholar]
- 23.Giroud M, Pisani DF, Karbiener M, Barquissau V, Ghandour RA, Tews D, et al. miR-125b affects mitochondrial biogenesis and impairs brite adipocyte formation and function. Molecular metabolism. 2016;5(8):615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He H, Sun D, Zeng Y, Wang R, Zhu W, Cao S, et al. A Systems Genetics Approach Identified GPD1L and its Molecular Mechanism for Obesity in Human Adipose Tissue. Scientific reports. 2017;7(1):1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu F, Xu P, Sun B, Xiao Z. Differences in the MicroRNA profiles of subcutaneous adipose-derived stem cells and omental adipose-derived stem cells. Gene. 2017;625:55–63. [DOI] [PubMed] [Google Scholar]
- 26.Wang KH, Kao AP, Singh S, Yu SL, Kao LP, Tsai ZY, et al. Comparative expression profiles of mRNAs and microRNAs among human mesenchymal stem cells derived from breast, face, and abdominal adipose tissues. The Kaohsiung journal of medical sciences. 2010;26(3):113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaragosi LE, Wdziekonski B, Brigand KL, Villageois P, Mari B, Waldmann R, et al. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome biology. 2011;12(7):R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, Zhou Z, Wang J, Li S. MiR-130b promotes obesity associated adipose tissue inflammation and insulin resistance in diabetes mice through alleviating M2 macrophage polarization via repression of PPAR-gamma. Immunol Lett. 2016;180:1–8. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 30.Boyle KE, Patinkin ZW, Shapiro AL, Baker PR 2nd, Dabelea D, Friedman JE. Mesenchymal Stem Cells From Infants Born to Obese Mothers Exhibit Greater Potential for Adipogenesis: The Healthy Start BabyBUMP Project. Diabetes. 2016;65(3):647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bories G, Caiazzo R, Derudas B, Copin C, Raverdy V, Pigeyre M, et al. Impaired alternative macrophage differentiation of peripheral blood mononuclear cells from obese subjects. Diab Vasc Dis Res. 2012;9(3):189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59(5):879–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B. Macrophage polarization and meta-inflammation. Translational research : the journal of laboratory and clinical medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castoldi A, Naffah de Souza C, Camara NO, Moraes-Vieira PM. The Macrophage Switch in Obesity Development. Frontiers in immunology. 2015;6:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. [DOI] [PubMed] [Google Scholar]
- 36.Squadrito ML, Etzrodt M, De Palma M, Pittet MJ. MicroRNA-mediated control of macrophages and its implications for cancer. Trends in immunology. 2013;34(7):350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jablonski KA, Gaudet AD, Amici SA, Popovich PG, Guerau-de-Arellano M. Control of the Inflammatory Macrophage Transcriptional Signature by miR-155. PLoS One. 2016;11(7):e0159724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim MM, Srikantan S, et al. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol. 2011;31(4):626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.