Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is one of the most common red cell disorders in the world. The aim of this study was to investigate whether the G6PD Mahidol variant and haplotype 1311T/93C, which are prevalent in the Kachin ethnic population along the China-Myanmar border area, offer protection against Plasmodium vivax infection. Malaria was monitored in nine villages near the Laiza township, Kachin State, Myanmar, where 258 cases of uncomplicated P. vivax were identified in 2013–2017. From the same villages, 250 unrelated, malaria-free participants were recruited to serve as the control cohort. Quantitative enzyme activity analysis in 100 healthy individuals identified that both male hemizygotes and female heterozygotes of the G6PD Mahidol variant had on average ~40% lower enzyme activity relative to the wild-type individuals. Compared with the overall prevalence of 25.2% in the control cohort, the G6PD Mahidol variant had a significantly lower prevalence (7.0%) among the 258 vivax patients (P < 0.0001, χ2 test). Logistic regression analysis of G6PD genotypes stratified by sex showed that the individuals with the Mahidol 487A allele had dramatically reduced odds of having acute vivax malaria (adjusted odds ratio = 0.213 for male 487A hemizygotes, P < 0.0001, and 0.248 for female 487GA heterozygotes, P < 0.001). Furthermore, both 487A hemizygous male and 487GA heterozygous female patients had significantly lower asexual parasitemias than the wild-type patients, suggesting a potential effect on alleviating disease severity. In contrast, the silent mutation haplotype 1311T/93C was highly prevalent (49.6%) in the study population, but it was not associated with altered G6PD enzymatic activities nor did it seem to provide protection against vivax infection or disease severity. Taken together, this study provided evidence that the Mahidol G>A mutation offers protection against P. vivax infection and potentially reduces disease severity in a Kachin population.
Keywords: G6PD, Mahidol variant, haplotype 1311T/93C, malaria, Plasmodium vivax, Kachin population
1. Introduction
Malaria, caused by Plasmodium parasite infection, is a major global public health problem. In humans, the invasion and replication of the parasite within the red blood cells (RBCs) are associated with the morbidity and mortality of the disease. Consequently, changes in RBCs are major mechanisms of host resistance to malaria. Through its long history of co-evolution with the human host, the malaria parasites have acted as a vital evolutionary selection force to shape the contemporary human genome (Lopez et al., 2010). Several erythrocyte disorders including hemoglobin C (HbC), HbS, α and β thalassemia, and glucose-6-phosphate dehydrogenase (G6PD) deficiency are protective against malaria, and they are identified as the main representations in adaptation to malaria selection by large-scale case-control analyses (Luzzatto and Seneca, 2014; Min-Oo and Gros, 2005; van Zwieten et al., 2014). These disorders reduce parasite invasions of the RBCs and impair parasite growth in the erythrocyte.
G6PD deficiency is the most common enzymopathy worldwide, with more than 400 million affected individuals (Beutler, 1994; Cappellini and Fiorelli, 2008). There are up to 217 described G6PD gene mutations within its coding region, and their effects on the stability and catalytic efficiency of the enzyme vary greatly (Gomez-Manzo et al., 2016). As a result, the clinical manifestation of the G6PD variants ranges from very mild with almost no symptoms to severe acute hemolytic anemia (AHA). AHA in G6PD deficient individuals can also be induced by ingesting drugs or food causing increased oxidative stress, such as the antimalarial drugs primaquine (Brito-Sousa et al., 2019; Chu et al., 2018) and tafenoquine (Chu and Freedman, 2019). The distribution of G6PD deficient variants vary geographically (Howes et al., 2013), but they have extensive overlap with the distribution of malaria, suggesting they might offer protective advantages against malaria. Although there is strong evidence for the protective effect of the African variant (A-) against P. falciparum infection and cerebral malaria (Bienzle et al., 1972; Guindo et al., 2007; Ruwende et al., 1995; Shah et al., 2016), the pictures for other G6PD variants are less clear and evidence remains circumstantial (Howes et al., 2012). Moreover, since G6PD deficiency is X-linked, G6PD deficient male hemizygotes and female homozygotes will express the full defect. However, female heterozygotes will have a mixture of G6PD normal and G6PD deficient RBCs due to random X-chromosome inactivation (Chu et al., 2018). Thus, the protective effect of G6PD against malaria may vary between male and female populations. For example, for a number of G6PD alleles in Tanzania, only female heterozygotes are protected from severe falciparum malaria (Manjurano et al., 2015). Further, a recent meta-analysis indicated that the negative association of G6PD deficiency with uncomplicated malaria was evident in Africa but not in Asia, and in the heterozygous females but not in the homozygous females or hemizygous males (Mbanefo et al., 2017).
The Greater Mekong region (GMS) comprising Cambodia, Laos, Myanmar, Thailand, Vietnam and Yunnan province in China was historically a hyperendemic region for P. falciparum and P. vivax. As the GMS countries are moving towards malaria elimination aiming to achieve this goal by 2030, there has been a major change of malaria epidemiology with increased proportions of P. vivax malaria (Cui et al., 2018). In the GMS, the standard treatment for uncomplicated P. vivax malaria remains as chloroquine as the schizontocide and primaquine for radical cure. In Kachin State of northeast Myanmar, primaquine is administered to P. vivax patients without checking their G6PD status or supervision, which has been associated with AHA in a vivax patient (Chen et al., 2017). The G6PD Mahidol 487G>A variant is the most common in the GMS (Howes et al., 2013), and its frequency in the Kachin (Jingpo) population near the China-Myanmar border reached ~20% (Li et al., 2015). Although this variant is a Class III mutation and normally has moderate enzyme activity (30 – 60% relative activity) (Yoshida et al., 1971), recent studies showed that individuals carrying this mutation could have severe G6PD deficient phenotypes (Bancone et al., 2014; Deng et al., 2017). Although the Mahidol variant does not seem to offer protection against infections by either P. falciparum or P. vivax in Thailand, it was associated with reduced P. vivax parasite density, suggesting that P. vivax couldbe a driving force behind the selective advantage conferred by this mutation (Louicharoen et al., 2009). P. vivax parasites has a preferential tropism towards reticulocytes (Kanjee et al., 2018). Interestingly, a recent ex vivo study showed that Mahidol CD71+ reticulocytes were enzymatically normal and could sustain normal growth and development of P. vivax parasites even in severely G6PD deficient samples (Bancone et al., 2017). This suggests that the protective effect of the Mahidol variant against vivax parasitemia may be attributable to other factors rather than intrinsic barriers in the reticulocytes.
Another G6PD polymorphism that reached high prevalence in the Kachin population is the haplotype 1311T/93C (Li et al., 2015). 1311C>T is a silent mutation in exon 11 of the G6PD gene, which is prevalent in worldwide populations. 1311C>T was often observed in association with another polymorphism 93T>C in intron 11, making up the haplotype 1311T/93C (Nuchprayoon et al., 2008). This haplotype has high prevalence in many Asian countries. Its frequency reached 32% in Pakistan (Moiz et al., 2009), 14% in Saudi Arabia (Kurdi-Haidar et al., 1990), 44% in Iran (Mortazavi et al., 1997), and 83.3% in the Negrito population of Malaysia (Amini and Ismail, 2013). Some individuals with the haplotype 1311T/93C show significantly reduced levels of G6PD (Jiang et al., 2006). This haplotype, together with the 3’ UTR mutation rs1050757G, was associated with reduced G6PD activity to 10–60% of normal activity in a Malaysian population, whereas some individuals with this genotype present normal G6PD activity (Amini and Ismail, 2013). Therefore, the current study aimed to determine whether the G6PD Mahidol variant and the 1311T/93C haplotype could offer protection against vivax malaria in a Kachin population located at the China-Myanmar border.
2. Materials and methods
2.1. Study subjects and sample collection
This study was conducted in nine villages of the Laiza township, Kachin State, Myanmar, where malaria is endemic and G6PD deficiency is prevalent (Li et al., 2013; Li et al., 2015). All residents in these villages are of the Kachin ethnicity. The study was approved by the Institutional Review Boards of Kunming Medical University and Kachin Bureau of Health. During 2013–2017, patients with acute P. vivax malaria from these villages attending the clinics and township hospital were recruited to study after obtaining written informed consent (assent for minors). For comparison, unrelated healthy villagers were recruited to match the P. vivax patients for age and sex to serve as the healthy control group. These control subjects were malaria-negative by microscopy at the time of recruitment and did not have a malaria history in the previous two years. For the 100 randomly-chosen, healthy villagers, 0.5 ml of venous blood were collected in an EDTA tube for quantitative analysis of G6PD activity. For all healthy participants and malaria patients, approximately 100 μl of finger-prick blood were spotted on Whatman™ 3 filter paper, dried and used for genotyping purpose.
2.2. Malaria diagnosis and quantification of parasites
For the P. vivax cases, thin and thick blood smears were prepared and examined under a light microscope at 100 × under an oil immersion lens by two microscopists with more than five years of experience in malaria diagnosis, who were trained at regional malaria diagnosis workshops organized by the World Health Organization (WHO) and met the WHO competence level 1 criteria. Densities of P. vivax asexual stages and gametocytes were estimated by counting against 500 white blood cells (WBCs). If the numbers between the two microscopists differed by >20%, they were further evaluated by another senior microscopist to reconcile the discrepancy. Parasite densities were calculated assuming a WBC count of 6000/μl of blood (Liu et al., 2016).
2.3. G6PD enzyme activity assay
Blood samples in 100 randomly-chosen, healthy villagers were analyzed using a quantitative G6PD assay (Trinity Biotech, St. Louis, MO, USA) according to the manufacturer’s instructions. The result for each subject was normalized to the subject’s hemoglobin (Hb) concentration (U/g Hb). For this assay, the cut-off value for G6PD deficiency was set at 4.5 U/g Hb (Deng et al., 2017), with those having G6PD activity higher than this value considered normal.
2.4. DNA extraction, molecular identification of malaria infection and genotyping of G6PD variants
Genomic DNA was extracted from dried blood spots with the TaKaRa Genomic DNA Kit (TaKaRa Biotechnology, Beijing, China) according to the manufacturer’s instructions. P. vivax mono-infection was further verified by PCR targeting the 18S rRNA genes (Li et al., 2014). Eleven common G6PD variants including Mahidol 487G>A, Kaiping 1388G>A, Canton 1376G>T, Gaohe 95A>G, Chinese-4 392G>T, Viangchan 871G>A, Chinese-5 1024C>T, Union 1360C>T, Coimbra 592C>T, and the silent mutations 1311C>T and IVS11nt93T>C, were genotyped in all participators using the SNaPshot assay described previously (Zhang et al., 2015).
2.5. Statistical analysis
Differences in G6PD enzyme activity were compared using the t-test and differences in asexual- and sexual-stage parasite densities were compared using the Mann-Whitney U test (if two groups) and Kruskal-Wallis test (if more than 2 groups). Gametocyte and G6PD deficiency gene carriage rates were compared using the χ2 test. Assuming a binomial error distribution, a logistic regression model was used to estimate the adjusted odd ratios (AORs) and 95% confidence intervals (CIs) for contracting vivax malaria, using the wild-type (WT) G6PD as the reference. We used the additive (trend) model, where male and female genotypes were coded as 0/2 and 0/1/2, respectively. For AORs, gender and age were included as a potential confounding factor in a multivariate regression analysis (Clayton, 2008). Linkage disequilibrium (LD) analysis was carried out by SHEsis (http://analysis.bio-x.cn/myAnalysis.php) (Shi and He, 2005). Statistical analysis was done using the SPSS 21.0 and GraphPad Prism 6.0 statistical software; statistical significance level was set at α = 0.05.
2.6. Sample size calculation
Our earlier study determined that the proportion of G6PD deficient individuals in this Kachin population is ~20% (Li et al., 2015). For this case-control comparison, if the proportion G6PD deficient individuals in the malaria patient group is assumed to be 20% under the null hypothesis and 4.76% under the alternative hypothesis, for α = 0.05 and 90% power, the sample sizes for both the control and vivax patient groups would be 89.
To determine the sample size for achieving sufficient power in logistic regression analysis of AOR, we used a 10:1 ratio of cases to predictors, as proposed by Peduzzi and colleagues (Peduzzi et al., 1996). This theory suggests that the sample number (n) should be ten times the number of predictors (k, in our case, k = 1 for males and k = 3 for females) after considering the proportion of patients (p): n = 10k/p. Accordingly, the sample number would be (10 × 1/0.57 = 17) for males and (10 × 3/0.57 = 71) for females.
3. Results
3.1. G6PD enzyme activity and genotypes in healthy population
We first wanted to determine whether the G6PD Mahidol mutation is correlated with reduced G6PD activity. For this purpose, we randomly selected 100 unrelated healthy subjects from the study villages and quantitatively assessed their G6PD activity. The mean G6PD enzyme activities in the overall healthy population, as well as in the male and female populations were 5.77, 5.91, and 5.64 U/g Hb, respectively (Table S1). If the cutoff value of 4.5 U/g Hb is used, 24 participants had G6PD enzyme activity below this value and were considered G6PD deficient. Of those, six (four males and two females) had G6PD enzyme activity below 1.0 U/g Hb and were considered to have severe G6PD deficiency. If 30% of the male median (5.77 U/g Hb) was used as the cutoff value (1.71 U/g Hb), four males and four females had G6PD enzyme activities below this value.
Genotyping of the G6PD gene in these malaria-free, healthy individuals using the SNaPshot assay revealed that 22% carried the G6PD Mahidol 487 G>A variant (Supplemental Table 1). In the 49 healthy males, the Mahidol variant occurred at 22.4%, whereas in the 51 healthy females, 21.6% carried the 487A allele (17.6% as heterozygous and 3.9% as homozygous). Individuals with the Mahidol variant in both male hemizygotes (mean = 2.77 U/g Hb, 95% CI: 1.58 – 3.96) and female heterozygotes (mean = 2.91 U/g Hb, 95% CI: 2.32 – 3.49) had on average ~40% lower enzyme activity relative to the WT individuals (mean = ~5.7 U/g Hb) (P < 0.001, t-test) (Supplemental Table 1).
In this healthy cohort, 35% carried the synonymous mutation haplotype 1311T/93C. In males, 1311C/93T (WT) and 1311T/93C (mutant) were absolutely linked and occurred at 75.5% and 24.5%, respectively. In females, 52.9% were WT (1311CC/93TT), while 41.2% and 3.9% carried heterozygous and homozygous mutations, respectively (Supplemental Table 1). Only one female subject carried the 1311CC/93CT allele. In both males and females, the different 1311T/93C alleles did not have significant effect on G6PD enzyme activity (P > 0.05, t-test).
3.2. G6PD genotypes in healthy villagers and patients with acute P. vivax infections
A total of 258 acute P. vivax patients were identified in 2013–2017 from the nine villages located in the Laiza township of northeast Myanmar (Table 1). The P. vivax patients were relatively young (mean age 24.2 years, range 2–79 years) and male-biased (64.0%). To compare whether G6PD mutations could confer protection against vivax malaria, 250 healthy, malaria-free participants (62% males, ages 2–75 years) were recruited from the same villages to serve as the control group. The control participants and vivax patients were approximately matched in sex (P = 0.783, χ2 test) and age (P = 0.327, Mann-Whitney U test). Both control participants and vivax patients were genotyped for G6PD variants using the SNaPshot assay. For the 250 healthy control group, four different G6PD variants were identified. The Mahidol variant was the predominant mutation, accounting for 27.2%, and other G6PDvariants were found in five subjecting, who carried the Kaiping 1388G>A (1.2%), Viangchan 871G>A (0.4%), and Chinese-4 392G>T (0.4%) variants. In the 258 vivax patients, however, the Mahidol variant only occurred at a frequency of 7.0% (Table 1), which was significantly lower than that in the control group (P < 0.0001, χ2 test). Other less frequent G6PD variants identified in four vivax patients included the Viangchan 871G>A (0.8%), Chinese-4 392G>T (0.4%), and Chinese-5 1024C>T (0.4%).
Table 1.
Characteristics of the healthy villagers and P. vivax patients of a rural Kachin population.
| Characteristic | Healthy villagers | Vivax patients | P value |
|---|---|---|---|
| Number of participants (% males) | 250 (62.4%) | 258 (64.0%) | 0.783* |
| Age (years) (mean/range) | 25.55 (2–75) | 24.16(2–79) | 0.327# |
| G6PD Mahidol carrier [N (%)] | 68 (27.2%) | 18 (7.0%) | <0.0001* |
| G6PD haplotype 1311T/93C | 129 (51.6%) | 112 (43.4%) | 0.079* |
Chi-square test
Mann-Whitney’s U test.
In contrast to the skewed distribution of the Mahidol 487 G>A in the control group and vivax patients, the frequencies of the 1311T/93C haplotype in healthy villagers and P. vivax patients were not significantly different (P = 0.079, χ2 test) (Table 1). In the male populations, 1311T/93C occurred at 46.2% and 35.4% in the control group and vivax patients, respectively (Table 2). In healthy, malaria-free females, the heterozygous and homozygous mutants occurred at 43.6% and 17.0%, respectively, whereas in female vivax patients, these mutants occurred at 40.7 % and 18.7%, respectively (Table 2).
Table 2.
Frequencies of the G6PD alleles in healthy villagers and P. vivax patients and protective effect of mutant alleles§.
| Polymorphic sites | Sex | Allele | Healthy villagers | Vivax patients | AOR (95% CI)# | P* | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| 487G>A | Male | G | 121 | 80.1 | 155 | 95.1 | 1 | |
| A | 30 | 19.9 | 8 | 4.9 | 0.213 (0.093–0.487) | <0.0001 | ||
| Female | GG | 61 | 64.9 | 81 | 89.0 | 1 | ||
| GA | 28 | 29.8 | 10 | 11.0 | 0.248 (0.110–0.561) | <0.001 | ||
| AA | 5 | 5.3 | 0 | 0 | 0.049 (0.002–1.187) | 0.064 | ||
| GA+AA | 33 | 35.1 | 10 | 11.0 | 0.223 (0.104–0.479) | <0.0001 | ||
| 1311C>T | Male | C | 81 | 53.6 | 105 | 64.4 | 1 | |
| T | 70 | 46.4 | 58 | 35.6 | 0.677 (0.425–1.079) | 0.101 | ||
| Female | CC | 37 | 39.4 | 37 | 40.7 | 1 | ||
| CT | 41 | 43.6 | 37 | 40.7 | 0.777 (0.393–1.538) | 0.469 | ||
| TT | 16 | 17.0 | 17 | 18.7 | 1.264 (0.493–3.241) | 0.626 | ||
| CT + TT | 57 | 60.6 | 54 | 59.3 | 0.993 (0.645–1.529) | 0.974 | ||
For the Mahidol variant analysis, other minor mutations (five from healthy villagers and four from vivax patients) were excluded. For the 1311C>T analysis, 2 from the healthy villagers and 3 from malaria patients were excluded from logistic model because of lack of linkage between 1311C and 93T or between 1311T and 93C.
Adjusted odds ratio (AOR) and 95% confidence interval (95% CI) was based on comparison with the reference group (values shown as 1).
Statistical comparison was done by logistic regression.
In the healthy population, LD analysis showed that the two G6PD mutations 1311C>T and 93T>C were in significant LD (r2 = 0.978). However, both 1311C>T and the 93T>C mutation were in LD with the Mahidol 487G>A mutation. LD analysis conducted on vivax malaria patients showed similar results. Only the G6PD mutations 1311C>T and 93T>C were in significant LD (r2 = 0.956).
3.3. G6PD Mahidol variant reduced risks of P. vivax infection
Logistic regression analysis was performed to determine whether the G6PD Mahidol variant is associated with lower risks of P. vivax infection. Assuming all subjects in the study villages were equally exposed to P. vivax infections, the Mahidol 487 G>A would offer significant protection against acute vivax infection (AOR = 0.3723, 95% CI: 0.256 – 0.542, P < 0.0001). To evaluate the protective effect of different G6PD genotypes, healthy villagers and vivax patients were stratified into the male and female populations (Table 2). In males, the G6PD Mahidol 487A allele offered significant protection against acute vivax infection (AOR = 0.213, 95% CI: 0.093 – 0.487, P < 0.0001). Similar protection was also observed in the Mahidol 487GA heterozygotes in females (AOR = 0.213, 95% CI: 0.093 – 0.487, P < 0.0001). For the 487AA homozygote females, the protective effect of the mutation was marginally significant (AOR = 0.049, 95% CI: 0.002 – 1.187, P = 0.064), although the number of subjects in this category was too small to allow for a robust analysis. In contrast, the G6PD 1311T/93C haplotype did not offer noticeable protection against acute vivax malaria (AOR = 0.958, 95% CI: 0.771 – 1.190, P = 0.696).
3.4. G6PD Mahidol variant reduced parasitemia during P. vivax infection
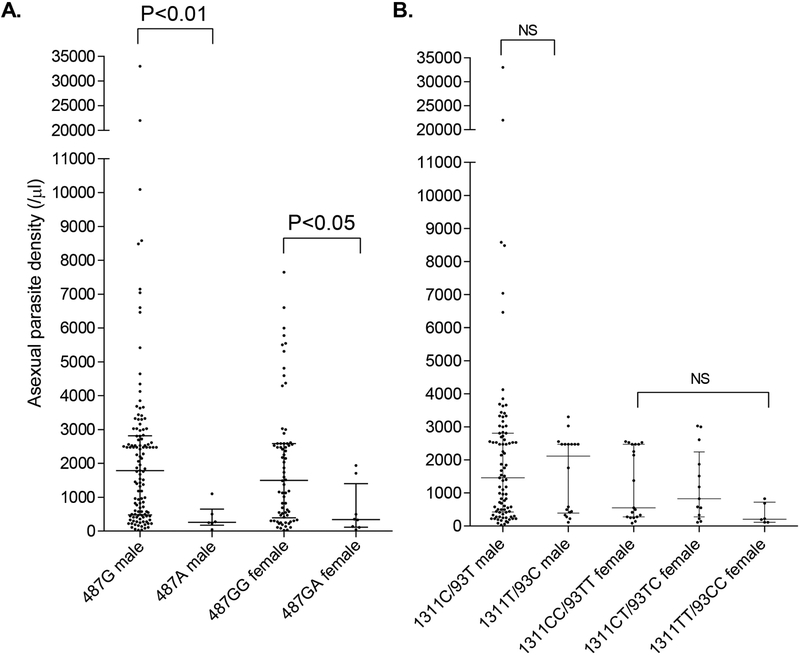
We compared parasite densities between vivax patients with different G6PD genotypes. The results showed that G6PD Mahidol 487 G>A in both hemizygous males and heterozygous females was associated with significantly lower asexual-stage parasite densities than the WT (P < 0.05, Mann-Whitney U test, Fig. 1A). If asexual-stage parasite density was considered a proxy for disease severity, this result suggested that G6PD Mahidol 487 G>A could reduce disease severity of vivax malaria. In contrast, the different 1311/93 haplotypes in either males or females did not show significant influences on asexual parasite density (Fig. 1B).
Fig. 1.
Comparison of asexual-stage parasite density among different G6PD alleles. (A) Comparison between 487G (WT) and 487A male vivax patients, and between 487GG (WT) and 487GA vivax patients. (B) Comparison among different 1311/93 haplotypes in both male and female vivax patients. NS, not significant. Comparison was done using the Mann-Whitney U test.
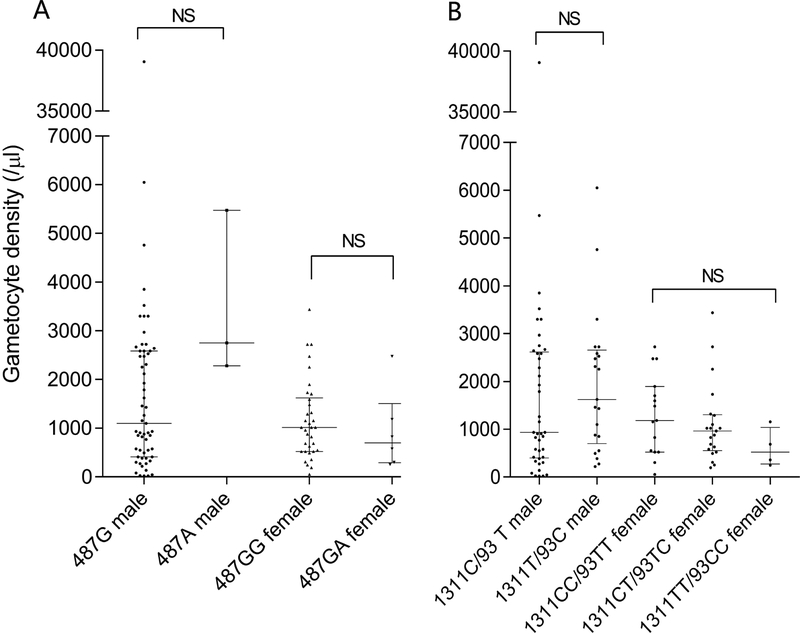
Next, we tested whether gametocyte density was different among P. vivax patients with different G6PD genotypes. In the 258 P. vivax cases, gametocytes were detected in 103 (39.9%) patients. In the 103 gametocytemic and 155 non-gametocytemic P. vivax patients, 9 patients in each group carried the G6PD 487G>A (p =0.456, χ2 test), suggesting that 487G>A did not affect the presence of gametocytes in vivax patients. We further compared the gametocyte density among different groups of G6PD genotypes. Our results showed that neither the G6PD 487G>A variant nor the different silent 1311/93 haplotypes had evident effect on gametocyte density in these patients (Fig. 2)
Fig. 2.
Comparison of gametocyte density among different G6PD alleles. (A) Comparison between 487G (WT) and 487A male vivax patients, and between 487GG (WT) and 487GA vivax patients. (B) Comparison among different 1311/93 haplotypes in both male and female vivax patients. NS, not significant. Data were compared using the Mann-Whitney U test and Kruskal-Wallis test.
4. Discussion
Malaria is among the strongest known evolutionary selection force in the recent history of the human genome, and the various red cell disorders are known to confer protection from malaria (Taylor and Fairhurst, 2014). G6PD deficiency is highly prevalent in many ethnic groups residing in malaria-endemic Africa, Asia and the Mediterranean area, where it confers different degrees of protection against the malignant malaria parasite P. falciparum. A recent comprehensive analysis of the African A- G6PD deficiency allele demonstrated that male hemizygotes and female homozygotes have increased risk of severe malarial anemia, whereas the male hemizygotes and female heterozygotes have reduced risk of cerebral malaria (Network, 2014). Such a small overall effect on the risk of severe malaria from P. falciparum infection conferred by G6PD deficiency suggests the possibility of a lesser role of P. falciparum in the selection of G6PD deficiency, but this may point to P. vivax as the candidate for the selection force. Historically, the China-Myanmar border area has been a malaria hyperendemic area where both P. falciparum and P. vivax were rampant, and there is a high incidence of RBC disorders such as thalassemias and G6PD deficiency (Tay et al., 1987). It is thus interesting to determine the evolutionary history of the G6PD mutant alleles and to test the malaria selection theory in the Kachin population (Louicharoen et al., 2009).
Our study showed that G6PD Mahidol 487G>A was associated with protection against P. vivax infection in both male homozygotes and female heterozygotes. This protective effect was not observed in the earlier study conducted in a Karen population at the Thailand-Myanmar border (Louicharoen et al., 2009). Although this difference may result from the study of different human populations, the use of a drastically increased sample size in our study may have allowed us to detect this protective effect. In this earlier study, the Mahidol variant was found to reduce P. vivax asexual parasitemia (Louicharoen et al., 2009), a result also confirmed in our study in the Kachin population. Given that the gametocyto genesis process is considered the parasite’s response to stress conditions, we speculated that growth of the parasite in G6PD deficient RBCs may favor the production of gametocytes. However, we did not observe any differences in either the presence of gametocytes or gametocytemia between the Mahidol variant and wild-type groups.
Oxidative stress produced by G6PD deficiency may be the main reason of resistance against malaria (Kosower and Kosower, 1970). G6PD enzyme activity is directly related to malaria susceptibility (Santana et al., 2013). Although the Mahidol variant is considered a Class III mutation, supposedly only resulting in a moderate level of reduction of enzyme activity, quantitative measurement showed that individuals carrying this mutation could show a severe G6PD deficiency phenotype. It seems logical that the protection mechanism might be related to the accelerated progression of the G6PD deficient RBCs towards senescence, which might be prohibitory for parasite development in infected RBCs (Kosower and Kosower, 1970). However, this hypothesis is undermined by recent finding showing that young reticulocytes, the targets of P. vivax invasion, had normal in G6PD activity and P. vivax assumed normal development during ex vivo culture in Mahidol variant reticulocytes (Bancone et al., 2017). Although the reason for this seemingly contradictory finding is not clear, the protective mechanism of the Mahidol variant against P. vivax in vivo might differ from that under in vitro culture conditions and could involve additional factors such as host immune responses, as has been speculated. Nonetheless, it is conceivable that preferential targeting and destruction of G6PD-normal reticulocytes by P. vivax in G6PD-deficient patients would further lower the overall G6PD activity in the RBCs and render the patients more vulnerable to AHA.
We confirmed the high prevalence of the 1311T/93C haplotype in the study population. Although these silent mutations were associated with moderately reduced G6PD enzyme activity in a Malaysian population (Amini and Ismail, 2013), we did not find any noticeable effect of this haplotype on G6PD enzyme activity, which is consistent with findings from elsewhere (Cikes et al., 2004; Moiz et al., 2009). It has been reported that 31.0% of the G6PD Mediterranean and 13.8% of the G6PD Aures variant were linked to haplotype 1311T/93C (Al-Jaouni et al., 2011), but our results showed that the Mahidol variant and the 1311T/93C haplotype were in linkage equilibrium in the Kachin population. Consistent with the lack of effect on G6PD enzyme activity, we did not find an association of the 1311T/93C haplotype with altered responses to vivax infection.
In summary, this study, conducted in Kachin villages along the China-Myanmar border, identified protection of the G6PD Mahidol variant provided protection against uncomplicated P. vivax infection and a higher parasite load, suggesting that P. vivax malaria might be the driving force for G6PD deficiency in other Mahidol variant prevalent areas of the GMS. In contrast, the haplotype 1311T/93C, which also reached high prevalence in the study population, did not seem to offer protection against vivax infection. These silent mutations might have evolved independently, their high frequencies may be explained by random genetic drift and hitch-hiking effect with other mutations in the G6PD gene.
Supplementary Material
Highlights.
The G6PD Mahidol 487 G>A reached a prevalence of >20% in the Kachin population
The G6PD Mahidol variant was associated with protection against P. vivax infections
The G6PD Mahidol variant was also associated with lower P. vivax parasite density
The G6PD 1311T/93C haplotype reached ~50% in the study population
The G6PD 1311T/93C haplotype did not offer protection against P. vivax infection.
Acknowledgments
We would like to thank our field staff Ms. Zadong Kong and Nam San for helping with parasite counting. YH was supported by National Natural Science Foundation of China (#31760308, # 31260264), and Research Innovation Fund for Graduate Students of Kunming Medical University (J11415015138). LC was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH U19AI089672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare that they have no competing interests.
References
- Al-Jaouni SK, Jarullah J, Azhar E, Moradkhani K, 2011. Molecular characterization of glucose-6-phosphate dehydrogenase deficiency in Jeddah, Kingdom of Saudi Arabia. BMC research notes 4, 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini F, Ismail E, 2013. 3′-UTR variations and G6PD deficiency. Journal of human genetics 58, 189–194. [DOI] [PubMed] [Google Scholar]
- Bancone G, Chu CS, Somsakchaicharoen R, Chowwiwat N, Parker DM, Charunwatthana P, White NJ, Nosten FH, 2014. Characterization of G6PD genotypes and phenotypes on the northwestern Thailand-Myanmar border. PloS one 9, e116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancone G, Malleret B, Suwanarusk R, Chowwiwat N, Chu CS, McGready R, Renia L, Nosten F, Russell B, 2017. Asian G6PD-Mahidol Reticulocytes Sustain Normal Plasmodium Vivax Development. The Journal of infectious diseases 216, 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E, 1994. G6PD deficiency. Blood 84, 3613–3636. [PubMed] [Google Scholar]
- Bienzle U, Ayeni O, Lucas AO, Luzzatto L, 1972. Glucose-6-phosphate dehydrogenase and malaria. Greater resistance of females heterozygous for enzyme deficiency and of males with non-deficient variant. Lancet 1, 107–110. [DOI] [PubMed] [Google Scholar]
- Brito-Sousa JD, Santos TC, Avalos S, Fontecha G, Melo GC, Val F, Siqueira AM, Alecrim GC, Bassat Q, Lacerda MVG, Monteiro WM, 2019. Clinical Spectrum of Primaquine-induced Hemolysis in G6PD Deficiency: A Nine-Year Hospitalization-Based Study from the Brazilian Amazon. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Fiorelli G, 2008. Glucose-6-phosphate dehydrogenase deficiency. Lancet 371, 64–74. [DOI] [PubMed] [Google Scholar]
- Chen X, He Y, Miao Y, Yang Z, Cui L, 2017. A young man with severe acute haemolytic anaemia. BMJ 359, j4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CS, Bancone G, Nosten F, White NJ, Luzzatto L, 2018. Primaquine-induced haemolysis in females heterozygous for G6PD deficiency. Malaria journal 17, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CS, Freedman DO, 2019. Tafenoquine and G6PD: a primer for clinicians. Journal of travel medicine 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikes V, Abaza I, Krzelj V, Terzic IM, Tafra R, Trlaja A, Marusic E, Terzic J, 2004. Prevalence of factor V Leiden and G6PD 1311 silent mutations in Dalmatian population. Archives of medical research 35, 546–548. [DOI] [PubMed] [Google Scholar]
- Clayton D, 2008. Testing for association on the X chromosome. Biostatistics (Oxford, England) 9, 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Cao Y, Kaewkungwal J, Khamsiriwatchara A, Lawpoolsri S, Soe TN, Kyaw MK, Sattabongkot J, 2018. Malaria Elimination in the Greater Mekong Subregion: Challenges and Prospects, in: Manguin S, Dev V (Eds.), Towards Malaria Elimination: A Leap Forward. IntechOpen, pp. 179–200. [Google Scholar]
- Deng Z, Yang F, Bai Y, He L, Li Q, Wu Y, Luo L, Li H, Ma L, Yang Z, He Y, Cui L, 2017. Co-inheritance of glucose-6-phosphate dehydrogenase deficiency mutations and hemoglobin E in a Kachin population in a malaria-endemic region of Southeast Asia. PloS one 12, e0177917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Manzo S, Marcial-Quino J, Vanoye-Carlo A, Serrano-Posada H, Ortega-Cuellar D, Gonzalez-Valdez A, Castillo-Rodriguez RA, Hernandez-Ochoa B, Sierra-Palacios E, Rodriguez-Bustamante E, Arreguin-Espinosa R, 2016. Glucose-6-Phosphate Dehydrogenase: Update and Analysis of New Mutations around the World. International journal of molecular sciences 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindo A, Fairhurst RM, Doumbo OK, Wellems TE, Diallo DA, 2007. X-linked G6PD deficiency protects hemizygous males but not heterozygous females against severe malaria. PLoS medicine 4, e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes RE, Dewi M, Piel FB, Monteiro WM, Battle KE, Messina JP, Sakuntabhai A, Satyagraha AW, Williams TN, Baird JK, Hay SI, 2013. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malaria journal 12, 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes RE, Piel FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, Hogg MM, Battle KE, Padilla CD, Baird JK, Hay SI, 2012. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS medicine 9, e1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Yu G, Liu P, Geng Q, Chen L, Lin Q, Ren X, Ye W, He Y, Guo Y, Duan S, Wen J, Li H, Qi Y, Jiang C, Zheng Y, Liu C, Si E, Zhang Q, Tian Q, Du C, 2006. Structure and function of glucose-6-phosphate dehydrogenase-deficient variants in Chinese population. Human genetics 119, 463–478. [DOI] [PubMed] [Google Scholar]
- Kanjee U, Rangel GW, Clark MA, Duraisingh MT, 2018. Molecular and cellular interactions defining the tropism of Plasmodium vivax for reticulocytes. Current opinion in microbiology 46, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower NS, Kosower EM, 1970. Molecular basis for selective advantage of glucose-6-phosphate-dehydrogenase-deficient individuals exposed to malaria. Lancet 2, 1343–1344. [DOI] [PubMed] [Google Scholar]
- Kurdi-Haidar B, Mason PJ, Berrebi A, Ankra-Badu G, al-Ali A, Oppenheim A, Luzzatto L, 1990. Origin and spread of the glucose-6-phosphate dehydrogenase variant (G6PD-Mediterranean) in the Middle East. American journal of human genetics 47, 1013–1019. [PMC free article] [PubMed] [Google Scholar]
- Li N, Parker DM, Yang Z, Fan Q, Zhou G, Ai G, Duan J, Lee MC, Yan G, Matthews SA, Cui L, Wang Y, 2013. Risk factors associated with slide positivity among febrile patients in a conflict zone of north-eastern Myanmar along the China-Myanmar border. Malaria journal 12, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhao Z, Wang Y, Xing H, Parker DM, Yang Z, Baum E, Li W, Sattabongkot J, Sirichaisinthop J, Li S, Yan G, Cui L, Fan Q, 2014. Nested PCR detection of malaria directly using blood filter paper samples from epidemiological surveys. Malaria journal 13, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yang F, Liu R, Luo L, Yang Y, Zhang L, Liu H, Zhang W, Fan Z, Yang Z, Cui L, He Y, 2015. Prevalence and molecular characterization of glucose-6-phosphate dehydrogenase deficiency at the China-Myanmar border. PloS one 10, e0134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Feng G, Zeng W, Li X, Bai Y, Deng S, Ruan Y, Morris J, Li S, Yang Z, Cui L, 2016. A more appropriate white blood cell count for estimating malaria parasite density in Plasmodium vivax patients in northeastern Myanmar. Acta tropica 156, 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C, Saravia C, Gomez A, Hoebeke J, Patarroyo MA, 2010. Mechanisms of genetically-based resistance to malaria. Gene 467, 1–12. [DOI] [PubMed] [Google Scholar]
- Louicharoen C, Patin E, Paul R, Nuchprayoon I, Witoonpanich B, Peerapittayamongkol C, Casademont I, Sura T, Laird NM, Singhasivanon P, Quintana-Murci L, Sakuntabhai A, 2009. Positively selected G6PD-Mahidol mutation reduces Plasmodium vivax density in Southeast Asians. Science 326, 1546–1549. [DOI] [PubMed] [Google Scholar]
- Luzzatto L, Seneca E, 2014. G6PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. British journal of haematology 164, 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjurano A, Sepulveda N, Nadjm B, Mtove G, Wangai H, Maxwell C, Olomi R, Reyburn H, Riley EM, Drakeley CJ, Clark TG, 2015. African glucose-6-phosphate dehydrogenase alleles associated with protection from severe malaria in heterozygous females in Tanzania. PLoS genetics 11, e1004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbanefo EC, Ahmed AM, Titouna A, Elmaraezy A, Trang NT, Phuoc Long N, Hoang Anh N, Diem Nghi T, The Hung B, Van Hieu M, Ky Anh N, Huy NT, Hirayama K, 2017. Association of glucose-6-phosphate dehydrogenase deficiency and malaria: a systematic review and meta-analysis. Scientific reports 7, 45963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min-Oo G, Gros P, 2005. Erythrocyte variants and the nature of their malaria protective effect. Cellular microbiology 7, 753–763. [DOI] [PubMed] [Google Scholar]
- Moiz B, Nasir A, Moatter T, Naqvi ZA, Khurshid M, 2009. Population study of 1311 C/T polymorphism of Glucose 6 Phosphate Dehydrogenase gene in Pakistan - an analysis of 715 X-chromosomes. BMC genetics 10, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi Y, Chopra R, Gordon-Smith EC, Rutherford TR, 1997. Frequency of the G6PD nt 1311 C/T polymorphism in English and Iranian populations: relevance to studies of X chromosome inactivation. Journal of medical genetics 34, 1028–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Network MGE, 2014. Reappraisal of known malaria resistance loci in a large multicenter study. Nature genetics 46, 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuchprayoon I, Louicharoen C, Charoenvej W, 2008. Glucose-6-phosphate dehydrogenase mutations in Mon and Burmese of southern Myanmar. Journal of human genetics 53, 48–54. [DOI] [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR, 1996. A simulation study of the number of events per variable in logistic regression analysis. Journal of clinical epidemiology 49, 1373–1379. [DOI] [PubMed] [Google Scholar]
- Ruwende C, Khoo SC, Snow RW, Yates SN, Kwiatkowski D, Gupta S, Warn P, Allsopp CE, Gilbert SC, Peschu N, et al. , 1995. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature 376, 246–249. [DOI] [PubMed] [Google Scholar]
- Santana MS, Monteiro WM, Siqueira AM, Costa MF, Sampaio V, Lacerda MV, Alecrim MG, 2013. Glucose-6-phosphate dehydrogenase deficient variants are associated with reduced susceptibility to malaria in the Brazilian Amazon. Transactions of the Royal Society of Tropical Medicine and Hygiene 107, 301–306. [DOI] [PubMed] [Google Scholar]
- Shah SS, Rockett KA, Jallow M, Sisay-Joof F, Bojang KA, Pinder M, Jeffreys A, Craik R, Hubbart C, Wellems TE, Kwiatkowski DP, 2016. Heterogeneous alleles comprising G6PD deficiency trait in West Africa exert contrasting effects on two major clinical presentations of severe malaria. Malaria journal 15, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YY, He L, 2005. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell research 15, 97–98. [DOI] [PubMed] [Google Scholar]
- Tay AH, Lam SK, Quak SH, Teo J, Raman GV, Wong HB, 1987. Glucose-6-phosphate-dehydrogenase deficiency and thalassaemia traits in some South-East Asian populations. The Journal of the Singapore Paediatric Society 29 Suppl 1, 113–116. [PubMed] [Google Scholar]
- Taylor SM, Fairhurst RM, 2014. Malaria parasites and red cell variants: when a house is not a home. Current opinion in hematology 21, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zwieten R, Verhoeven AJ, Roos D, 2014. Inborn defects in the antioxidant systems of human red blood cells. Free radical biology & medicine 67, 377–386. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Beutler E, Motulsky AG, 1971. Human glucose-6-phosphate dehydrogenase variants. Bulletin of the World Health Organization 45, 243–253. [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang Y, Liu R, Li Q, Yang F, Ma L, Liu H, Chen X, Yang Z, Cui L, He Y, 2015. A multiplex method for detection of glucose-6-phosphate dehydrogenase (G6PD) gene mutations. International journal of laboratory hematology 37, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.