Abstract
Objective:
Dissemination of pre-Exposure Prophylaxis (PrEP) is a priority for reducing new HIV infections, especially among vulnerable populations. However, there are limited data available on PrEP discontinuation following initiation, an important component of the PrEP cascade.
Design:
Patients receiving PrEP within the San Francisco Department of Public Health Primary Care Clinics (SFPCC) are included in a PrEP registry if they received a PrEP prescription, were not receiving post-exposure prophylaxis, and not known to be HIV positive.
Methods:
We calculated PrEP discontinuation for patients initiating PrEP at any time from January 2012 to July 2017 and evaluated their association with demographic and risk variables using Cox regression analysis.
Results:
Overall, 348 patients received PrEP over the evaluation period. The majority (84%) were men, and the cohort was racially/ethnically diverse. The median duration of PrEP use was 8.3 months. In adjusted analysis, PrEP discontinuation was lower among older patients (aHR 0.89; 95% CI: 0.80–0.99; p=0.03); but higher among Black patients (compared with White patients) (aHR 1.87; 95 %CI: 1.27–2.74; p=0.001), patients who inject drugs (aHR 4.80; 95%CI 2.66–8.67; p<0.001), and transgender women who have sex with men (compared with men who have sex with men) (aHR 1.94; 95%CI: 1.36–2.77; p<0.001).
Conclusion:
Age, racial/ethnic, and risk category disparities in PrEP discontinuation were identified among patients in a public-health funded primary care setting. Further efforts are needed to understand and address PrEP discontinuation among priority populations to maximize the preventive impact of PrEP, and reverse HIV-related disparities at a population level.
Keywords: PrEP discontinuation, racial/ethnic disparities
Introduction
Pre-exposure prophylaxis has been shown to have high efficacy in HIV prevention in several clinical trials and clinical cohorts.[1–7] In the US, although PrEP uptake is increasing each year, uptake has been lower among Black and Latino patients than White patients in demonstration projects, clinical cohorts, and national reviews of pharmacy records.[7–11] Similar disparities in adherence rates and PrEP discontinuation were observed in the US PrEP Demo project.[12]
Continued PrEP use over time is an important step in the PrEP continuum, but there are limited data about PrEP discontinuation rates, particularly within primary care settings with diverse patient populations. In a Kaiser study of 972 patients who initiated PrEP, approximately 30% discontinued PrEP during the 3 year study follow-up period.[13] In this clinical cohort, the risk of discontinuation was highest among women and those with a history of alcohol use. Similar discontinuation rates were noted in another clinical cohort of PrEP users within a San Francisco sexual health clinic.[14] In an LGBT focused health center in Chicago, only 43% were still in care after 12 months, with uninsured patients less likely retained in care.[15] However, these cohorts were in clinical settings focused on PrEP, and not necessarily reflective of patients in general primary care practice. Furthermore, both cohorts were nearly all men and approximately 70% White, not reflecting the racial/ethnic demographics of new HIV diagnoses in the US.[16] As PrEP dissemination expands, more data are needed on implementation within primary care settings with diverse patient populations.[17, 18]
In this study, we assessed PrEP discontinuation rates among individuals who initiated PrEP within the San Francisco Department of Public Health Primary Care Clinics (SFPCC), an integrated safety-net primary care network that provides care for uninsured and publically-insured patients within San Francisco. We evaluated PrEP use and factors associated with PrEP discontinuation among patients who initiated PrEP from 2012–2017.
Methods
Patients receiving PrEP within the SFPCC were initiated on PrEP by their primary care providers within one of 14 primary care clinics. PrEP initiation, prescription refills (filled through the patient’s preferred commercial or community pharmacy), adherence counseling and follow-up visits, and laboratory testing was determined by the prescribing clinician. Clinical PrEP guidelines were developed in 2015 to support PrEP implementation including recommendation for quarterly laboratory testing, and clinic follow-up, and management of adverse events. Standard patient retention practices varied between clinics, and included follow-up phone outreach for patients with missed visits, but not proactive outreach for those with overdue visits or laboratory testing. All of the patients receiving PrEP are included in a registry to monitor PrEP use and related outcomes. The study was reviewed and approved by the University of California, San Francisco Institutional Review Board.
Patients were included in this study if they received PrEP at one of 14 SFPCC clinics and met the following criteria based on medical record review: 1) verified to have been provided a PrEP prescription by a clinician; 2) were not receiving post-exposure prophylaxis; and 3) were not known to be HIV infected through surveillance reporting or SFDPH laboratory testing. Patients initiating PrEP from January 1st, 2012 through December 31st, 2016, with follow-up through July 2017 were included in this analysis. We obtained dates of PrEP initiation and discontinuation, age and race/ethnicity, insurance and housing status, self-reported substance use, mental health disorders, visit data, and PrEP prescription data via electronic health record abstraction. Race/ethnicity was reported as a single categorical variable based upon the availability of the data within the electronic health record. PrEP indication was collected through chart review and classified into the following exclusive categories: sero-different relationship, men who have sex with men (MSM), people who inject drugs (PWID), transgender women who sex with men (TGWSM), or high-risk heterosexual.
Mental health diagnoses recorded for patients included anxiety, depression, post-traumatic stress disorder (PTSD), schizophrenia. and bipolar disorder; the latter two were considered serious mental illness (SMI). Data were captured on stimulant use (any amphetamine or cocaine/crack use mentioned by a medical provider) and heavy alcohol use (mention of heavy or disordered alcohol use by a medical provider) if it was reported in the medical chart. Patients with documentation from a provider of homelessness or unstable housing were classified as having housing instability.
PrEP panel management and patient navigation support were initiated at four of the 14 SFPCC clinics evaluated in this study starting in November of 2015. The primary care-based PrEP navigators within SFPCC were placed within four clinics: two youth clinics, an HIV care clinic which expanded to provide PrEP, and an LGBT clinic with embedded youth and transgender clinic services. The PrEP navigators provided ongoing support and active outreach for patients who initiated PrEP and referrals to case management and other services as needed at two of the clinics, while pharmacists provided panel management (use of clinic registries to monitor patients who were due for visits, laboratory testing, or refills) without active outreach at the others. The navigators and/or the pharmacists at these clinics proactively reached out to patients on PrEP for upcoming (or overdue) clinical or laboratory visits, and supported patients to schedule and attend these visits. They also used clinic registries of patients who initiated PrEP to provide insurance support, adherence counseling, and laboratory and visit adherence support through in-person and telephone visits. Periods of PrEP use beginning after 11/1/2015 among patients at the four participating clinics were considered covered by panel management and patient navigation support. Patients in clinics without patient navigation received follow-up per provider and clinic procedures.
PrEP discontinuation was defined as either a documented discontinuation by a medical provider or a PrEP prescription gap of >90 days. Patients who were lost to follow-up were considered as discontinuing PrEP. Follow-up for discontinuation was censored at the end of July 2017. Kaplan-Meier plots were used to describe time from the beginning of each period of PrEP use to the first discontinuation, both overall and by race/ethnicity. Cox models with robust standard errors were used to assess correlates of earlier discontinuation. A total of 444 PrEP use periods among 348 patients were considered in the analysis reflecting that patients who discontinued PrEP could restart PrEP. All analyses were implemented using Stata Version 15.1 (Stata Corp, College Station, TX).
Results
Overall, 348 patients initiated PrEP during the observation period and had complete data for this analysis (Table 1). The majority were male sex at birth (84%), and the sample was ethnically/racially diverse with 39% White, 27% Latinx, and 12% Black patients. MSM comprised the most common PrEP indication (66%), although TGWSM accounted for 13%, 16% were in a sero-different relationship, and 4.9% were identified as high-risk heterosexual. Only two patients had a PrEP indication of PWID. Nearly one quarter of the sample had at least one gap in PrEP use after initiation, and the most common reason was being lost to follow-up (46%). Other reasons included missed visits or laboratory testing (44%), medication cost (12%), and decreased HIV risk (10%); there was no documented reason for 16% of gaps. More than three-fourths of the patients accessing PrEP had public insurance (Medicaid or Medicare) and 14% were uninsured; 12% had documented housing instability by a provider. Nearly half of the patients had a provider-documented mental health diagnosis, including 10% who had an SMI. Access to patient navigation services was available for approximately one quarter of the PrEP follow-up time in this analysis.
Table 1:
Demographic, patient, clinic, and provider characteristics of patients initiating Pre-exposure prophylaxis (PrEP) within the San Francisco Department of Public Health primary care clinics.
| Variable | N (%) N=348 |
|---|---|
| Patient | |
| Birth Sex | |
| Male | 293 (84) |
| Female | 55 (16) |
| Age (median, IQR) | 35 (28–45) |
| Race/Ethnicity | |
| White | 135 (39) |
| Black | 42 (12) |
| Latino | 93 (27) |
| Asian | 28 (8) |
| Other | 50 (14) |
| PrEP Indication | |
| Men who have sex with men | 230 (66) |
| Sero-different couple | 55 (16) |
| Transgender women who have sex with men | 44 (13) |
| Injection Drug Use | 2 (1) |
| High-risk heterosexual | 17 (5) |
| Insurance | |
| Private | 27 (8) |
| Public (Medicaid or Medicare) | 273 (78) |
| Uninsured | 48 (14) |
| Housing instability | 43 (12) |
| Mental Health Diagnosis1 | |
| None | 183 (53) |
| Anxiety | 31 (9) |
| Depression | 77 (22) |
| Post-traumatic Stress Disorder | 23 (7) |
| Severe mental illness (Bipolar or Schizophrenia) | 34 (10) |
| Heavy Alcohol Use1 | 61 (18) |
| Illicit substance use (heroin, cocaine, or meth)1 | 55 (16) |
| Clinic and Provider | |
| PrEP Panel Management or patient navigation | 1142 (26) |
| Number of PrEP patients seen by provider | |
| 1 | 66 (19) |
| 2–5 | 116 (33) |
| >5 | 169 (48) |
| PrEP Initiation and Persistence | |
| PrEP Initiation Year | |
| 2012–2014 | 66 (19 |
| 2015 | 114 (33) |
| 2016 | 164 (47) |
| Number of gaps in PrEP use3 | |
| 0 | 264 (76) |
| 1 | 72 (21) |
| 2 | 12 (3) |
Based on provider assessment as documented in the patient medical record.
Of 444 PrEP use periods
Gaps were defined as interruptions of ≥90 days in PrEP use.
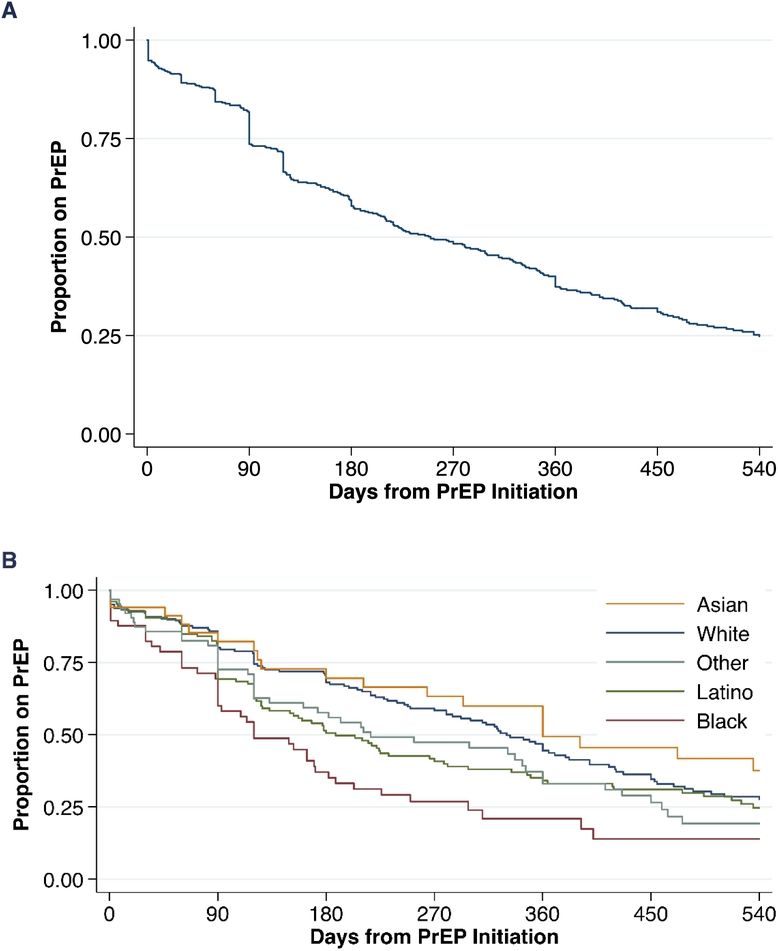
The median duration of PrEP use before discontinuation was 250 days (8.3 months) (Table 2 and Figure 1A). Black patients had much shorter durations of PrEP use compared with White patients (120 days vs 330 days) (Table 2 and Figure 1B). In adjusted analysis, older age was associated with a lower risk of having a gap in PrEP [adjusted Hazard Ratio (aHR) per decade of age 0.89; 95% Confidence Interval (CI): 0.80–0.99; p=0.03] (Table 3). Compared with White patients, Black patients were the only racial/ethnic group with a significantly higher risk of discontinuing PrEP (aHR 1.87; 95 %CI: 1.27–2.74; p=0.001). Patients who started PrEP with an indication of PWID (aHR 4.80; 95%CI 2.66–8.67, p<0.001), or who were TGWSM (aHR 1.94; 95%CI: 1.36–2.77; p<0.001) were more likely to discontinue PrEP compared with MSM. Patients who reported a history of illicit drug use were also more likely discontinue PrEP (aHR 1.55; 95% CI: 1.18–2.02; p=0.001) compared with patients with no history of illicit drug use. Panel management and PrEP navigation was not significantly associated with fewer PrEP discontinuations (aHR 1.23; 95% CI: 0.88–1.72; p=0.23).
Table 2:
Median days of PrEP use prior to discontinuation by demographics, PrEP indication, substance use, and PrEP panel management or patient navigation within San Francisco Department of Public Health primary care clinics.*
| Variable | Median Days (95% Confidence Interval) |
|---|---|
| Overall | 250 (210 – 310) |
| Age | |
| <25 | 90 (82 – 222) |
| 25–39 | 226 (188 – 292) |
| 40–54 | 360 (231 – 391) |
| 55+ | 382 (179 – 450) |
| Race/Ethnicity | |
| White | 330 (280 – 379) |
| Black | 120 (90 – 171) |
| Latino | 188 (127 – 270) |
| Asian | 360 (211 – --) |
| Other | 217 (120 – 346) |
| PrEP Indication | |
| Men who have sex with men | 292 (222 – 347) |
| Sero-different couple | 331 (183 – 391) |
| Transgender women who have sex with men | 120 (69 – 178) |
| Injection Drug Use | 30 (30 – --) |
| High-risk heterosexual | 350 (85 – --) |
| Illicit substance use | |
| Yes | 178 (120 – 239) |
| No | 285 (221 – 343) |
| PrEP Panel Management or patient navigation | |
| Yes | 161 (114 – 222) |
| No | 298 (228 – 347) |
Discontinuation was defined as either a documentation of discontinuation by a medical provider or a PrEP prescription gap of >90 days.
Figure 1:
Overall Pre-exposure prophylaxis use over time (A) and by race/ethnicity (B) within San Francisco Department of Public Health primary care clinics.
Table 3:
Unadjusted and adjusted analysis of variables associated with discontinuations in Pre-exposure Prophylaxis use within San Francisco Department of Public Health primary care clinics.*
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Variable | Hazard Ratio | p-value | Hazard Ratio | p-value |
| Patient | ||||
| Birth Sex | ||||
| Male | (ref) | - | ||
| Female | 1.18 (0.86–1.62) | 0.30 | - | |
| Age (per 10 years) | 0.85 (0.77–0.95) | 0.004 | 0.89 (0.80–0.99) | 0.03 |
| Race/Ethnicity | ||||
| White | (ref) | (ref) | ||
| Black | 2.00 (1.38–2.89) | <0.001 | 1.87 (1.27–2.74) | 0.001 |
| Latino | 1.26 (0.94–1.70) | 0.12 | 1.08 (0.79–1.48) | 0.62 |
| Asian | 0.80 (0.53–1.21) | 0.30 | 0.73 (0.40–1.18) | 0.20 |
| Other | 1.31 (0.92–1.85) | 0.13 | 1.20 (0.84–1.72) | 0.32 |
| PrEP Indication | ||||
| Men who have sex with men | (ref) | (ref) | ||
| Sero-different couple | 1.02 (0.76–1.36) | 0.92 | 0.92 (0.68–1.25) | 0.60 |
| Transgender women who have sex with men | 2.04 (1.44–2.91) | <0.001 | 1.94 (1.36–2.77) | <0.001 |
| Injection Drug Use | 5.21 (2.55–10.6) | <0.001 | 4.80 (2.66–8.67) | <0.001 |
| High-risk heterosexual | 1.14 (0.61–2.14) | 0.68 | 0.84 (0.40–1.74) | 0.64 |
| Insurance | ||||
| Private | (ref) | - | ||
| Public (Medicaid or Medicare) | 0.99 (0.66–1.48) | 0.95 | - | |
| Uninsured | 1.18 (0.73–1.92) | 0.50 | - | |
| Housing instability | 1.16 (0.83–1.60) | 0.39 | - | |
| Mental Health Diagnosis1 | ||||
| None | (ref) | - | ||
| Anxiety | 0.92 (0.59–1.44) | 0.73 | - | |
| Depression | 0.97 (0.73–1.28) | 0.81 | - | |
| Post-traumatic Stress Disorder | 0.94 (0.57–1.54) | 0.79 | - | |
| Severe mental illness (Bipolar or Schizophrenia) | 1.38 (0.96–1.98) | 0.08 | - | |
| Heavy Alcohol Use1 | 1.07 (0.81–1.43) | 0.62 | - | |
| Illicit substance use (heroin, cocaine, or meth)1 | 1.59 (1.23–2.05) | <0.001 | 1.55 (1.18–2.02) | 0.001 |
| Clinic and Provider | ||||
| PrEP Panel Management or patient navigation | 1.48 (1.14–1.92) | 0.003 | 1.23 (0.88–1.72) | 0.23 |
| Number of patients seen by provider | ||||
| 1 | (ref) | (ref) | ||
| 2–4 | 1.08 (0.74–1.56) | 0.69 | 1.00 (0.68–1.47) | 0.93 |
| >4 | 1.35 (0.98–1.86) | 0.07 | 1.05 (0.74–1.50) | 0.77 |
| PrEP Initiation and Persistence | ||||
| PrEP Initiation Year | ||||
| 2012–2014 | (ref) | (ref) | ||
| 2015 | 1.35 (0.99–1.84) | 0.06 | 1.15 (0.83–1.59) | 0.40 |
| 2016 | 1.63 (1.19–2.24) | 0.003 | 1.19 (0.81–1.75) | 0.36 |
Discontinuation was defined as either a documentation of discontinuation by a medical provider or a PrEP prescription gap of >90 days. PrEP discontinuation was evaluated using repeated measures.
Based on provider assessment as documented in the patient medical record.
Discussion
In this diverse cohort of primary care-based patients with multiple concomitant challenges such as housing instability, substance use and mental illness, PrEP discontinuation rates were higher than have been reported in research-based and specialty PrEP programs. PrEP discontinuation was higher for younger, Black, substance using, and TGWSM patients who are under-represented in published PrEP demonstration projects, but over-represented among new HIV diagnoses in the US. This is the first report demonstrating racial/ethnic differences in PrEP discontinuation among diverse patients in a safety-net primary care setting, where PrEP expansion has been a major focus for implementation. In our analysis, PrEP panel management with patient navigation was not associated with lower rates of PrEP discontinuation.
Although lower PrEP uptake and persistence has been previously reported among Black patients and youth in other PrEP demonstration projects and clinical settings in the US, these observations were all made in sexual health or specialty clinics.[3, 19, 20] In the US PrEP Demonstration project, conducted at two sexual health clinics and a LGBT community health center, PrEP retention was also noted to be lower among Black compared with White participants during the 12 months of follow-up.[12] Structural and social barriers such as stigma, medical mistrust, and perceived racism have been identified as important barriers for PrEP uptake, and likely impact PrEP continuation as well.[21, 22] Even after adjusting for important individual factors in our analysis, disparities in PrEP discontinuations persist indicating social and structural barriers must better addressed to support Black patients continuing PrEP.
PWID had a high risk of PrEP discontinuation in this study, although only two patients were started on PrEP for this indication. PWID risk is a PrEP indication based on the Centers for Disease Control and Prevention PrEP guidelines; however there is little implementation data with this risk group. Our findings are similar to the data on lower HIV care engagement among PWID, suggesting that PWID need additional support across the HIV care and prevention continuum.[23] Leveraging the experience from HIV treatment and prevention programs, integrating PrEP retention support into syringe access services, offering it in combination with methadone maintenance programs, or buprenorphine (in a primary care setting); and providing support for housing and other social needs have the potential to improve outcomes.[24] Similarly, TGWSM also had a high of risk of PrEP discontinuation among our cohort of patients. Many of the prior clinical trials and demonstration projects enrolled a low proportion of trans women, limiting the ability to understand factors associated with PrEP uptake and discontinuation in this population. Community concerns such as lack of targeting of PrEP for trans women, and the potential for drug-drug interactions with feminizing hormones, have been identified as potential barriers to PrEP uptake, while integration of PrEP within gender-affirming healthcare settings is a potential facilitator for PrEP engagement.[25–27] These factors may also impact PrEP retention and discontinuation
Our analysis demonstrated that clinics with PrEP panel management or patient navigation did not have lower rates of PrEP discontinuations. Panel management, with associated registries, have been used as part of chronic disease management for several conditions including diabetes and cardiovascular disease.[28] While the use of registries as part of quality improvement for diabetes management has been shown to be useful, one study suggests that impact is limited without attention to specific clinical actions.[29] For example, in one study, improvements were made in diabetes screening, but without improvements in glycemic or blood pressure control. In the SFPCC, panel management was variably implemented within the different clinical settings by either patient navigators or a pharmacist, and further research into components of PrEP management that may lead to improved outcomes (eg., outreach to patients who missed appointments or follow-up laboratory testing) is needed. It is also possible that underlying systemic challenges at these clinics (e.g., not having integrated laboratory services or not having the ability to make follow-up appointments while in clinic), may also have contributed to the lack of improvement seen.
This analysis has several limitations. The number of patients accessing PrEP was relatively small in this clinical dataset compared with other clinical PrEP programs.[12–14] This may be because of patients’ ability to access sexual health services, including PrEP, through local municipal STD or a community-based sexual health clinic in San Francisco, allowing them to bypass receiving PrEP from their primary care provider. However, the population of PrEP patients in this study is more diverse (racially/ethnically and by PrEP indication) than reported in many other clinical settings, and is more reflective of the populations most at risk for HIV in the US. Another limitation was the use of electronic medical record review to identify patient cofactors. Because of variabilities in ascertainment and documentation by medical providers, reporting of these certain variables may be incomplete. Furthermore, a substantial proportion of patients were lost to follow-up over the study period. The causes for this lack of follow-up are likely variable and may include insurance change, relocation, seeking care elsewhere, and disengagement in care, although these reasons were rarely documented in the medical record. Because all patients lost to follow-up were classified as having discontinued PrEP, we may have biased our findings toward higher estimates of PrEP discontinuation if, in fact, patients continued PrEP outside of the SFPCC. Finally, we assumed that patients initiating PrEP were at ongoing risk for HIV throughout the evaluation period. Through the medical record review, we were not able to assess if PrEP discontinuations were secondary to decreases in HIV risk, and therefore appropriate discontinuations. SFPCC clinical guidelines encouraged providers to discuss HIV risk factors and assess ongoing need for PrEP through shared decision-making. However, many patients discontinued PrEP without consulting their providers and objective assessments of HIV risk were not always available or documented in the medical record.
We found that PrEP discontinuation was high among a diverse primary care-based cohort of patients receiving PrEP, with significant disparities among populations disproportionally impacted by HIV. Additional data are needed to understand patient, provider, and structural factors associated with PrEP discontinuation and interventions to support PrEP use for patients at risk for HIV.
Funding:
This study was supported by a supplement to National Institutes of Health R01MH109320 (PI: Buchbinder), and K23MH104116 (PI: Scott)
Contributor Information
Hyman M. SCOTT, Bridge HIV, San Francisco Department of Public Health
Matthew SPINELLI, University of California, San Francisco.
Eric VITTINGHOFF, Department of Epidemiology and Biostatistics, University of California, San Francisco.
Alicia MOREHEAD-GEE, National Clinician Scholars Program at University of California, Los Angeles.
Anne HIROZAWA, San Francisco Department of Public Health.
Catherine JAMES, San Francisco Department of Public Health.
Hali HAMMER, San Francisco Department of Public Health.
Albert LIU, Bridge HIV, San Francisco Department of Public Health.
Monica GANDHI, Department of Medicine, University of California, San Francisco.
Susan BUCHBINDER, Bridge HIV, San Francisco Department of Public Health.
References:
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, et al. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin Infect Dis 2015; 61(10):1601–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina JM, Charreau I, Spire B, Cotte L, Chas J, Capitant C, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV 2017; 4(9):e402–e410. [DOI] [PubMed] [Google Scholar]
- 6.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med 2015; 373(23):2237–2246. [DOI] [PubMed] [Google Scholar]
- 7.Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med 2016; 176(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus JL, Hurley LB, Hare CB, Silverberg MJ, Volk JE. Disparities in Uptake of HIV Preexposure Prophylaxis in a Large Integrated Health Care System. Am J Public Health 2016; 106(10):e2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush S, Ng L, Magnuson D, Piontkowsky D, Giler RM. Significant Uptake of Truvada for Pre-Exposure Prophylaxis (PrEP) Utilization in the US in Late 2014–1Q2015. IAPC Prevention. June 2015. Abstract#74. In. [Google Scholar]
- 10.Giler R, Magnson D, Trevor H, Bush S, Rawlings K, McCallister S. Changes in truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) utilization in the United States: (2012–2016). IAS 2017. Abstract#1614. In. [Google Scholar]
- 11.Siegler AJ, Mouhanna F, Giler RM, Weiss K, Pembleton E, Guest J, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doblecki-Lewis S, Liu AY, Feaster DJ, Cohen SE, Elion R, Bacon O, et al. Patterns and Correlates of Participant Retention in a Multi-City Pre-Exposure Prophylaxis Demonstration Project. J Acquir Immune Defic Syndr 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus JL, Hurley LB, Hare CB, Nguyen DP, Phengrasamy T, Silverberg MJ, et al. Preexposure Prophylaxis for HIV Prevention in a Large Integrated Health Care System: Adherence, Renal Safety, and Discontinuation. J Acquir Immune Defic Syndr 2016; 73(5):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, Carrico A. HIV Pre-exposure Prophylaxis (PrEP) Uptake and Retention Among Men Who Have Sex with Men in a Community-Based Sexual Health Clinic. AIDS Behav 2018; 22(4):1096–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rusie LK, Orengo C, Burrell D, Ramachandran A, Houlberg M, Keglovitz K, et al. Preexposure Prophylaxis Initiation and Retention in Care Over 5 Years, 2012–2017: Are Quarterly Visits Too Much? Clin Infect Dis 2018; 67(2):283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. HIV Surveillance Report, 2017; vol. 29 http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published November 2018 2018. [Google Scholar]
- 17.Krakower D, Ware N, Mitty JA, Maloney K, Mayer KH. HIV providers’ perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav 2014; 18(9):1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krakower DS, Mimiaga MJ, Rosenberger JG, Novak DS, Mitty JA, White JM, et al. Limited Awareness and Low Immediate Uptake of Pre-Exposure Prophylaxis among Men Who Have Sex with Men Using an Internet Social Networking Site. PLoS One 2012; 7(3):e33119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu AY, Cohen SE, Vittinghoff E, et al. Pre-exposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Internal Medicine 2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolle CP, Rosenberg ES, Siegler AJ, Sanchez TH, Luisi N, Weiss K, et al. Challenges in Translating PrEP Interest Into Uptake in an Observational Study of Young Black MSM. J Acquir Immune Defic Syndr 2017; 76(3):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cahill S, Taylor SW, Elsesser SA, Mena L, Hickson D, Mayer KH. Stigma, medical mistrust, and perceived racism may affect PrEP awareness and uptake in black compared to white gay and bisexual men in Jackson, Mississippi and Boston, Massachusetts. AIDS Care 2017; 29(11):1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baral S, Logie CH, Grosso A, Wirtz AL, Beyrer C. Modified social ecological model: a tool to guide the assessment of the risks and risk contexts of HIV epidemics. BMC Public Health 2013; 13:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liappis AP, Laake AM, Delman M. Active injection drug-abuse offsets healthcare engagement in HIV-infected patients. AIDS Behav 2015; 19(1):81–84. [DOI] [PubMed] [Google Scholar]
- 24.Baral SD, Stromdahl S, Beyrer C. The potential uses of preexposure prophylaxis for HIV prevention among people who inject drugs. Curr Opin HIV AIDS 2012; 7(6):563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson EC, Jin H, Liu A, Raymond HF. Knowledge, Indications and Willingness to Take Pre-Exposure Prophylaxis among Transwomen in San Francisco, 2013. PLoS One 2015; 10(6):e0128971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sevelius JM, Deutsch MB, Grant R. The future of PrEP among transgender women: the critical role of gender affirmation in research and clinical practices. J Int AIDS Soc 2016; 19(7(Suppl 6)):21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sevelius JM, Keatley J, Calma N, Arnold E. ‘I am not a man’: Trans-specific barriers and facilitators to PrEP acceptability among transgender women. Glob Public Health 2016; 11(7–8):1060–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner BJ, Parish-Johnson JA, Liang Y, Jeffers T, Arismendez SV, Poursani R. Implementation of the Chronic Care Model to Reduce Disparities in Hypertension Control: Benefits Take Time. J Gen Intern Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Connor PJ, Desai J, Solberg LI, Reger LA, Crain AL, Asche SE, et al. Randomized trial of quality improvement intervention to improve diabetes care in primary care settings. Diabetes Care 2005; 28(8):1890–1897. [DOI] [PubMed] [Google Scholar]