Abstract
Background & Aims
Cardiovascular (CV) disease is the top cause of mortality in patients with nonalcoholic fatty liver disease (NAFLD). Female sex is protective against CV disease. We aimed to determine whether female sex remains a protective factor against CV disease (myocardial infarction, angina and stroke) in NAFLD.
Methods
We identified all adults diagnosed with NAFLD in Olmsted County, Minnesota between 1997 and 2014 and selected an age- and sex-matched (1:4) referent cohort from the general population. NAFLD was ascertained using a code-based algorithm with high validity tested by medical record review. The impact of female sex on incident CV events was examined using Cox proportional hazards regression analysis stratified by standard clinical risk factors.
Results
A total of 3,869 NAFLD and 15,209 age-and sex-matched referent subjects were identified. After a median follow-up time of 7 (range 1–20) years, 3,851 CV events were recorded. Female sex was protective for ischemic CV events in the general population (HR=0.71, 95%CI 0.62–0.80, p<.001), but the impact was significantly diminished among those with NAFLD (HR=0.90, 95%CI 0.74–1.08, p=.25), even after stratification by time-dependent CV risk factors and control for diagnostic testing (liver enzymes and ultrasound) during routine medical evaluations, as a surrogate of access to care. Among those with NAFLD, excess events were higher in women than men: CV disease (18% vs 9%) and mortality (9% vs 6%).
Conclusions
Women with NAFLD lose the CV protection conferred by the female sex and their risk is underestimated by current estimating methods in clinical practice.
Keywords: sex-based differences, ischemic events, NASH, cardiovascular risk
INTRODUCTION
General population studies have shown differences in the pathophysiology of cardiovascular disease (CVD) between men and women, supporting the concept of female hormonal protection in CVD1. For example, epidemiological data have indicated that coronary artery calcification on computed tomography occurs a decade later than in men2, 3 and that myocardial infarction among women occurs approximately eight years later than men 4. Therefore, sex is an important risk modifier included in commonly used models of CV risk estimation in clinical practice. In 2013 the American College of Cardiology and the American Heart Association endorsed the Pooled Cohort Equations (PCEs) for estimating 10-year risk of first hard atherosclerotic cardiovascular disease (ASCVD) event5. These are sex- and race-specific and include covariates such as of age, metabolic parameters and current smoking status. Because of a higher coefficient associated with male sex, the estimated 10-year risk in men is higher than that of women of similar age, race and covariates. The estimated risk impacts the level of preventative measures, which can vary between lifestyle counseling and initiation of primary prophylactic agents.
Several studies have demonstrated that CVD is the most common cause of death in nonalcoholic fatty liver disease (NAFLD), surpassing liver-related death 6, 7. The association between NAFLD, metabolic comorbidities and cardiovascular outcomes has been extensively explored8–11. In a recent population-based study from Olmsted County, MN we showed that NAFLD is an independent risk factor for incident metabolic comorbidities and death; the impact of NAFLD on CV events was significant only in subjects without metabolic comorbidities (RR=3.25, 95% CI=2.29–4.60), suggesting that the CV risk in NAFLD can be independent of comorbid dysmetabolic conditions. However, whether female sex remains a protective factor for CVD events in NAFLD has not been explored. Furthermore, the performance of CV risk-estimating equations has not been tested on special populations such as those with NAFLD. We hypothesized that compared to the general population patients with NAFLD have a higher metabolic burden which may reduce the protective role of female sex.
Using the community cohort of individuals diagnosed with NAFLD in Olmsted County, MN we explored the impact of sex as an effect modifier in CVD in reference to a general population cohort without a diagnosis of NAFLD. Specifically, we examined 1) the impact of female sex on CV risk and 2) the performance of Pooled Cohort ASCVD Risk Equations for estimating the 10-year risk of ischemic CV events in women versus men from the two groups.
METHODS
Study participants
We constructed a cohort of all adult individuals diagnosed with NAFLD in Olmsted County, MN between 1997 and 2014, using prospectively collected data in a medical-record linkage system, the Rochester Epidemiology Project (REP)12, 13. This is an electronic infrastructure that collates and indexes all the medical information, from all medical providers in this community. The system provides data on all encounters with the medical system, including administrative codes, access to medical records, including medical encounters, laboratories, imaging, medication prescriptions and procedures. NAFLD cases were identified using Hospital International Classification of Diseases Adapted (HICDA) codes, a system developed at Mayo Clinic for research diagnosis coding and adapted by REP in 1976: HICDA 05710421 (fatty liver), 05710431 (nonalcoholic steatohepatitis - NASH). Additionally, the International Classification of Diseases (ICD)-9 codes ICD 9-CM 571.5 (cirrhosis of the liver without mention of alcohol), 571.8 (other chronic nonalcoholic liver disease), 571.9 (unspecified chronic liver disease without mention of alcohol) were used. Subsequently, we searched for codes of diagnoses of exclusion for liver diseases of other causes (Supplementary Table 1). Subjects were ascertained as NAFLD cases if no codes for alternative liver disease etiology were identified prior to the index NAFLD diagnosis or during the following year. Individual chart review of a random sample of 10% of subjects (412 with NAFLD codes after exclusion of liver diseases of other causes and 131 with codes for liver diseases of other causes) was performed by a physician (A.M.A.) to determine the performance of the selection algorithm. A referent cohort without a diagnosis of NAFLD, individually matched by age and sex to the NAFLD subjects on the day of index NAFLD diagnosis (1 NAFLD:4 referent subjects), was identified from the general population using REP. Only participants with an active research authorization at the time of the data collection (2018) were included.
Primary cardiovascular outcomes were ischemic events, specifically myocardial infarction, angina, and stroke. Secondary outcomes included heart failure, atrial fibrillation/flutter and death. The CV outcomes were identified in the medical record linkage system using diagnostic codes which were previously validated in numerous studies of the Olmsted County population(Supplementary Table 2)14–17. Covariates of interest included body mass index (BMI), diabetes mellitus, hypertension, dyslipidemia, smoking and history of cardiovascular disease. Comorbidities were defined based on combinations of ICD 9-CM or HICDA codes (Supplementary Table 2), medications (Supplementary Table 3) and laboratory values, as follows: diabetes mellitus – diagnostic codes plus medications or laboratory values (fasting glucose ≥ 126 mg/dL or hemoglobin A1c ≥6.5%); dyslipidemia – diagnostic codes plus medications or laboratory values (LDL cholesterol >100 mg/dL or triglycerides >150 mg/dL); hypertension – diagnostic codes plus medications. The covariates were ascertained not only at the time of index NAFLD diagnosis/matching, but also as they occurred during the follow-up. The subjects were followed until death, migration out of Olmsted County or end-of-study (September 2018).
Statistical analysis
The absolute number of events and the age-stratified incidence rate of individual CV events per 1000 person-years were reported separately in men and women, among NAFLD and referent cohort. The rate was smoothed over age using Poisson regression and a cubic spline predictor. The impact of female sex on the risk of incident CV events was examined using Cox proportional hazards regression analysis stratified by personal history of CVD, BMI at the time of diagnosis/matching and time-dependent smoking, diabetes mellitus (DM), hypertension (HTN) and dyslipidemia (these covariates were included if present at index diagnosis or as they occurred subsequently during follow-up). Secondary analyses adjusting for time-dependent FIB-4 as a continuous variable (using all the time-points in which AST, ALT and platelets were tested during the follow-up) were performed to explore if the CV risk is related to liver disease severity. We used multi-state modeling18–20 to determine the proportion of men and women with NAFLD or controls who were in either of 4 states: 1) alive without any current or prior history of CVD; 2) alive with current of prior CVD, 3) dead with history of CVD; and 4) dead without history of CVD (Figure 1 of the Supplement, where each box is a state and each arrow represents a transition (rate) from one state to another, using the approach described in Putter et al.20). The multistate modeling approach is similar to competing risk analysis but with the advantage of exploring multiple states that an individual can unidirectionally transition towards the final state of death. The excess CVD and mortality between NAFLD and referent cohorts among women and men was derived from this model and reported by age.
To examine the performance of PCE in men and women with NAFLD compared to the referent cohort, predicted 10-year rates of primary ischemic CV events (composite of MI or stroke) were calculated using the sex- and race-specific equations, using age, total cholesterol, HDL cholesterol, treated or untreated systolic blood pressure, smoking status and diabetes as covariates. The PCEs performance was assessed using 2 parameters: discrimination (c-statistic) and calibration (standardized incidence ratio). Additional details on statistical modeling are provided in the Statistical Appendix section of the Supplement.
RESULTS
Performance of the NAFLD code-based identification algorithm
Of the 412 subjects identified as NAFLD by the code-based algorithm, 350 (85%) were true NAFLD after individual chart review. Of the 131 subjects who were excluded due to codes for other liver diseases, 114 (87%) were identified as true non-NAFLD after individual chart review (Supplementary Table 4). The algorithm performance parameters were as follows: sensitivity 95%, specificity 65%, positive predictive value 85%, negative predictive value 87%, and accuracy 85%.
The cohort consisted of 19,078 Olmsted County residents, of whom 3,869 had NAFLD and 15,209 were age- and sex-matched referent individuals without NAFLD. Median age at diagnosis/matching was 53 (IQR 43–64) years and 10,005 (52.4%) were women. Among those with NAFLD, women had a higher BMI, prevalence of diabetes and hypertension than men. This is in contrast to the trends noted among referents, in whom women had lower BMI and prevalence of metabolic comorbidities (Table 1). The median follow-up was 7 (range 1 to 20) years.
Table 1.
Demographic and clinical characteristics of NAFLD subjects compared to age- and sex-matched referent individuals from the general population†
| NAFLD cohort | Referent cohort | |||
|---|---|---|---|---|
| Women n=2,032 | Men n=1,837 | Women n=7,973 | Men n=7,236 | |
| Age-median (IQR) | 54 (44–64) | 51 (41–62) | 54 (44–64) | 52 (41–63) |
| Race/ ethnicity | ||||
| White | 1790 (88.1%) | 1614 (87.9%) | 6846 (85.9%) | 5861 (81.0%) |
| Black | 57 (2.8%) | 49 (2.7%) | 222 (2.8%) | 270 (3.7%) |
| Asian | 70 (3.4%) | 67 (3.6%) | 260 (3.3%) | 267 (3.7%) |
| Hawaiian/Pacific Islander | 8 (0.4%) | 3 (0.2%) | 16 (0.2%) | 12 (0.2%) |
| American Indian | 15 (0.7%) | 6 (0.3%) | 14 (0.2%) | 11 (0.2%) |
| Other/mixed | 85 (4.2%) | 79 (4.3%) | 211 (2.6%) | 229 (3.2%) |
| BMI- median (IQR) kg/m2 | 34 (29–40) | 32 (29–36) | 27 (23–32) | 28 (25–32) |
| Diabetes mellitus, n(%) | ||||
| at baseline | 629 (31.0%) | 496 (27.0%) | 639 (8.0%) | 794 (11.0%) |
| at last follow-up | 827 (40.7%) | 705 (38.4%) | 950 (11.9%) | 1136 (15.7%) |
| Hypertension, n(%) | ||||
| at baseline | 955 (47.0%) | 790 (43.0%) | 1855 (23.3%) | 1776 (24.5%) |
| at last follow-up | 1249 (61.5%) | 1085 (59.1%) | 2622 (32.9%) | 2548 (35.2%) |
| Dyslipidemia, n(%) | ||||
| at baseline | 1448 (71.3%) | 1321 (71.9%) | 3352 (42.0%) | 3086 (42.6%) |
| at last follow-up | 1606 (79.0%) | 1478 (80.5%) | 4127 (51.8%) | 3852 (53.2%) |
| Cardiovascular disease, n(%) | ||||
| at baseline | 487 (24.0%) | 453 (24.7%) | 1024 (12.8%) | 1270 (17.6%) |
| at last follow-up | 682 (33.6%) | 621 (33.8%) | 1527 (19.2%) | 1777 (24.6%) |
All p<.001 for the comparisons of NAFLD to the referent population
The protective role of female sex on CVD risk is nonsignificant in NAFLD
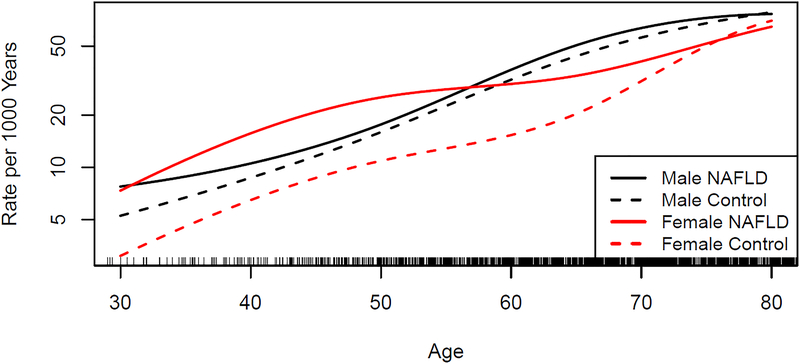
A total of 3,851 CV events occurred during the follow-up (Table 2). As seen in Figure 1, in the general population, incident ischemic CV events (MI, angina or stroke) increased with age but occurred at a lower rate in women compared to men. In contrast, the overall incidence of ischemic CV events in NAFLD women was similar to that of NAFLD men.
Table 2.
Number of incident cardiovascular events in NAFLD subjects and referent population during the study timeframe.
| Cardiovascular event | NAFLD cohort | Referent cohort | ||
|---|---|---|---|---|
| Men n=1,837 | Women n=2,032 | Men n=7,236 | Women n=7,973 | |
| Myocardial infarction | 64 | 59 | 216 | 131 |
| Angina/Ischemic heart disease | 112 | 120 | 315 | 233 |
| Stroke | 104 | 120 | 335 | 348 |
| Heart failure | 110 | 124 | 325 | 302 |
| Atrial fibrillation/flutter | 96 | 88 | 353 | 296 |
FIGURE 1.
Estimated incidence of ischemic cardiovascular events (myocardial infarction, angina or stroke) among women and men with nonalcoholic fatty liver disease compared to a referent cohort without NAFLD.
Female sex was associated with a lower risk of incident ischemic events among referent subjects without NAFLD (HR=0.72, 95% CI 0.65–0.80, p<.001; stratified by personal history of CVD) but among NAFLD individuals the impact was markedly diminished (HR=0.93, 95% CI 0.78–1.09, p=.36; stratified by personal history of CVD). In multivariate analysis stratified by BMI, personal history of CVD, time-dependent DM, HTN, dyslipidemia and smoking, female sex remained a protective factor for ischemic CV events in the referent group (HR=0.71, 95%CI 0.62–0.80, p<.001), but not in NAFLD (HR=0.90, 95%CI 0.74–1.08, p=.25). These findings did not change after further adjustment for liver disease severity by time-dependent FIB-4: HR=0.74 (0.63–0.86) in referents and 0.96 (0.77–1.18) in NAFLD individuals. The impact of female sex on the risk of individual types of CV events, including the primary and secondary outcomes of interest is shown in Table 3.
Table 3.
The impact of female sex on incident cardiovascular events in patients with NAFLD compared to age- and sex-matched referent individuals.
| Type of CV event | Hazard ratio of CV event (F/M)α 95% Confidence Interval |
|||
|---|---|---|---|---|
| Stratified by personal history of CVD | Stratified by personal history of CVD and CV risk factors∞ | |||
| NAFLD cohort | Referent cohort | NAFLD cohort | Referent cohort | |
| Ischemic events* | 0.93 0.78–1.09 |
0.72 0.65–0.80 |
0.90 0.74–1.08 |
0.71 0.62–0.80 |
| Myocardial infarction | 0.80 0.56–1.13 |
0.53 0.42–0.66 |
0.79 0.53–1.17 |
0.56 0.44–0.73 |
| Angina/ischemic heart disease | 0.96 0.75–1.24 |
0.65 0.55–0.78 |
0.89 0.67–1.19 |
0.65 0.53–0.79 |
| Stroke | 0.94 0.72–1.21 |
0.88 0.76–1.03 |
0.96 0.72–1.26 |
0.83 0.69–0.99 |
| Heart failure | 0.97 0.76–1.25 |
0.81 0.69–0.95 |
0.93 0.70–1.24 |
0.84 0.69–1.01 |
| Atrial fibrillation | 0.76 0.57–1.01 |
0.71 0.61–0.83 |
0.81 0.59–1.11 |
0.72 0.59–0.86 |
Composite of myocardial infarction, angina or stroke
Bold numbers represent statistically significant results (p<.005)
BMI, time-dependent smoking, diabetes mellitus, hypertension and dyslipidemia
Because the above results can be impacted by an ascertainment bias resulting from differential screening in women compared to men, we determined the proportion of men and women among NAFLD patients and referents who underwent blood testing for AST/ALT as part of routine medical care or abdominal ultrasound for any indication within 5 years before and after index date. A similar proportion of men and women underwent these tests in each of the 2 groups: among referents, 85% of women (6642 of 7816) and 82% of men (5749 of 7039); among NAFLD, 99% of women (2312 of 2328) and 99% of men (1957 of 1980). To correct for the possible ascertainment bias, we performed sensitivity analysis of the impact of female sex on CVD among those referents who underwent laboratory or ultrasound testing. The impact of female sex among the referents under medical surveillance was similar to that noted among all referents in the primary analysis (stratified HR=0.72, 95%CI 0.64–0.82). These data suggest that ascertainment bias due to preferential screening of women or unhealthy controls is not the likely cause of the main results.
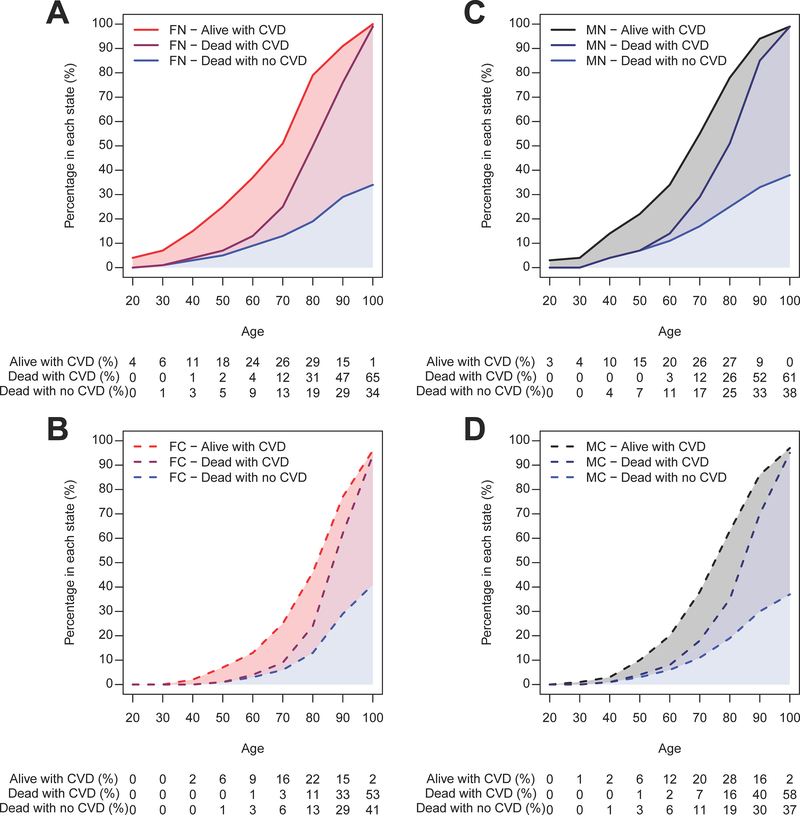
Women with NAFLD develop CV events at younger age than women without NAFLD
As seen in Figure 1, the incidence rate of CV events in women with NAFLD is higher than in referent women, especially at younger age. For example, the incidence rate of ischemic CV events for a 50 year-old woman with NAFLD is similar to that of a 68 year-old woman without NAFLD (30 events/1,000 person-years). Figure 2 illustrates the proportion of women and men, with and without NAFLD, who are in either of four states: alive without CVD, alive with CVD, dead with history of CVD and dead without history of CVD at different decades of age. Among women, those with NAFLD (panel A) are more likely to have CVD or to have died in reference to those without NAFLD (panel B), at any age. Among men, the differences in CVD and death between those with (panel C) and without NAFLD (panel D) are smaller. For example, in 60 year-old individuals, the excess CVD in NAFLD versus the referent cohort is 18% in women and 9% in men, while the excess overall mortality is 9% in women and 6% in men. In 70 year-old individuals, the excess CVD in NAFLD versus referent cohort is 19% in women and 11% in men, while the excess overall mortality is 16% in women and 11% in men (see Table 4 for excess outcomes by all ages).
FIGURE 2. Distribution of individuals among four states: dead without CVD, dead with CVD, alive with CVD and alive without CVD (remaining unshaded area) at different decades of age.
A. Female NAFLD. B. Female controls. C. Male NAFLD. D. Male controls.
The study subjects enter the model at their corresponding age at the index date of NAFLD diagnosis/matching and are followed longitudinally as they transition unidirectionally between the 4 states until the end of study. At each age, the proportion of individuals in each of the 4 states sum to 100%. The proportion of those in a state of CVD (myocardial infarction, angina or stroke) at each age can be calculated by adding the proportion of those alive with CVD and dead with CVD. Excess CVD in NAFLD compared to controls can be calculated by subtracting the proportion of controls with CVD from NAFLD subjects with CVD.
FN: female NAFLD; FC: female controls; MN: male NAFLD; MC: male controls; CVD: cardiovascular disease.
Table 4.
Excess cardiovascular disease and mortality in men and women with NAFLD compared to their non-NAFLD referent counterparts.
| Age | Cardiovascular disease | Death | ||
|---|---|---|---|---|
| Women (NAFLD vs matched referent) | Men (NAFLD vs matched referent) | Women (NAFLD vs matched referent) | Men (NAFLD vs matched referent) | |
| 30 | 6% | 3% | 1% | 0% |
| 40 | 10% | 8% | 4% | 3% |
| 50 | 14% | 8% | 6% | 3% |
| 60 | 18% | 9% | 9% | 6% |
| 70 | 19% | 11% | 16% | 11% |
| 80 | 27% | 9% | 26% | 16% |
| 90 | 14% | 5% | 14% | 15% |
| 100 | 11% | 1% | 5% | 4% |
Cardiovascular risk is underestimated in women with NAFLD
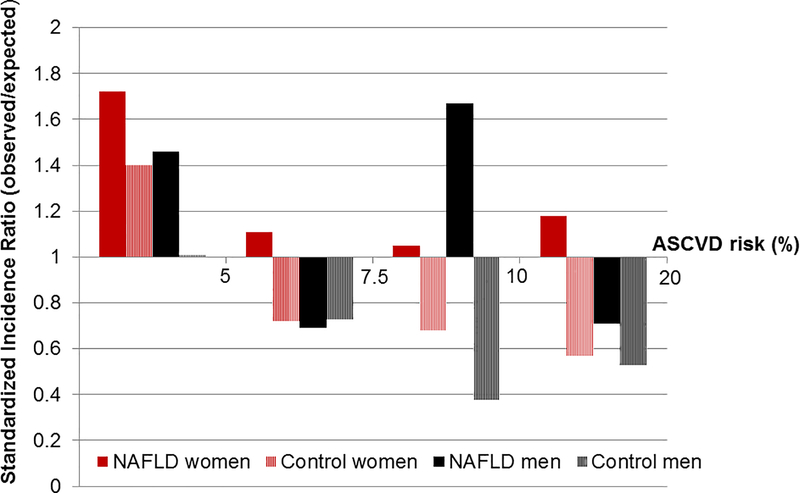
Given that the protective role of female sex on incident CV events is markedly diminished in NAFLD, we assessed the performance of the PCEs on the 10-year ASCVD risk prediction (myocardial infarction or stroke) in women versus men with NAFLD and referent subjects. The c-statistic in NAFLD women, NAFLD men, referent women and referent men was 0.71, 0.76, 0.71 and 0.70, respectively (Supplementary Table 5). However, the calibration was inferior in NAFLD women, in whom the rate of observed events was higher than expected (SIR>1) across all CV risk strata (Figure 3). In contrast, in NAFLD men, SIR>1 was noted in those with very low and intermediate (7.5–10%) ASCVD risk, while in the general population the SIR was <1 in most of the risk categories. Thus, the current PCEs consistently underestimate the 10-year risk of MI or stroke in NAFLD women, across all risk strata, while they overestimate the CV risk in those without NAFLD. In NAFLD men, the risk is underestimated in those with very-low and intermediate risk, and overestimated in those with low and high risk.
FIGURE 3. Performance of the Pooled Cohort Equations in estimating 10-year risk of myocardial infarction or stroke.
The Standardized Incidence Ratio (observed/expected rate of events) is illustrated in men and women with NAFLD compared to the general population, across strata of estimated risk (>1 represents underestimation, <1 represents overestimation of risk). The observed and predicted risks were plotted by clinical risk categories using cut points of 5%, 7.5% and 10%.
DISCUSSION
This study demonstrates that the female advantage in cardiovascular disease protection is lost in NAFLD subjects. Unlike women from the general population, who are 29% less likely to develop CVD than their male counterparts with similar cardiovascular risk factors, NAFLD women have a similar CVD risk to NAFLD men. Moreover, NAFLD women develop CVD at younger age than women from the general population. The excess CVD and mortality between those with and without NAFLD is much higher in women than men at all ages. In this community, current equations underestimate the 10-year ASCVD risk in NAFLD women while overestimating the risk in the general population. These findings have important clinical implications as they could impact counseling and adequate initiation of primary prevention methods with aspirin and statins in women with NAFLD.
Studies from the general population have shown that the prevalence of metabolic syndrome increases with age in a sex-specific manner: young and middle-aged women are protected due to sex- and gender-related factors, whereas a steep increase is noted starting after menopause due to changes in fat distribution and energy balance related to hormonal modifications 21–23. These differences in metabolic burden may account for the lower CVD in women from young and middle-age groups and the equalization of risk between sexes at older age. However, in patients with NAFLD, these differences in metabolic burden disappear, as women develop comorbidities at young age. Thus, the impact of protective hormonal factors may be diluted by the high metabolic burden, which may partially explain the lack of protective effect of female sex on cardiovascular disease.
Our findings that NAFLD and associated metabolic comorbidities have a “cardiovascular aging” effect of approximately 18 years in women are of significant public health importance. NAFLD women of young and middle-age have twice as many CV events as women of same age from the general population. Upward shifts in the incidence and prevalence of NAFLD in the past decades, more obvious in the young population, may result in a greater proportion of life lived with cardiovascular comorbidity and raise concern for future population-level burden of morbidity and mortality associated with NAFLD24. A high CVD burden results in less healthful years of life, poorer quality of life, and increased health care expenditures25. Although the relative increase in CV risk in NAFLD is higher in young women, the impact on public health escalates with the increase in age and absolute CV events. As seen in Table 4, the excess CVD in NAFLD increases progressively up to age 80 but is 2–3-fold higher in women than in men at all ages. The excess mortality in women with NAFLD arises from those who transition through a state of CVD, whereas the difference in mortality in those with NAFLD without CVD is similar in women and men.
Major guidelines recommend that decisions about aspirin, blood pressure, and statin therapy be determined from 10-year CVD risk estimates from the PCEs26,27. Their performance has been controversial because they overestimate risk among contemporary populations 28–33. Our data adds to the evidence that these equations overestimate the CV risk in men and women from the general population. However, the novelty of this work is that, in NAFLD, the PCEs underestimate CV risk in women, across all strata of CV risk. In subjects with an estimated ASCVD risk of ≥10%, in whom preventative measures are to be considered, the observed rate of CV events was lower than predicted in men and women from the general population as well as NAFLD men (suggesting that these groups may receive unnecessary prevention), whereas NAFLD women had a higher rate of CV events than predicted. In the group of subjects with an estimated ASCVD risk of 7.5–10%, in whom the net benefit of preventative therapy is smaller, but could be considered, NAFLD subjects had a higher rate of CV events than predicted. Underestimation of risk can impact preventable cardiovascular events. These findings suggest that NAFLD patients may benefit from initiation of statin and low-dose aspirin at a lower threshold and that shared-decision making should take into consideration NAFLD as an important comorbidity. Although the impact of NAFLD on CVD is largely mediated by metabolic comorbidities (which are included in the PCEs), NAFLD patients in general, and women in particular, may have different susceptibility to cardiovascular injury than the general population. Continued effort is needed to produce accurate risk assessment tools or other methods of CVD risk prediction such as coronary artery calcification34 for specific patient populations, such as that with NAFLD.
The strengths of this study include the large contemporary longitudinal dataset, with a wide age range, and the use of a matched cohort without NAFLD, representative of the population residing in this community. The medical record linkage infrastructure used in this study captures all medical events, including CVD, which occurred in this historical cohort and was documented by any provider; this is an ideal setting to study the natural history of NAFLD, because it does not rely on self-reporting and it minimizes event ascertainment bias by sex and referral bias. We collected hard clinical outcomes instead of surrogate markers of CV disease and we stratified by baseline as well as time-dependent metabolic risk factors, which is important to consider in NAFLD because it is a risk factor for such incident comorbidities. Although NAFLD was ascertained based on administrative codes, the algorithm used to exclude other causes of liver disease has high internal validity based on individual chart review. It is possible that a proportion of the general population had undiagnosed NAFLD and, if NAFLD is associated with increased CV risk, this sampling bias could have diminished the difference in the incidence of CV events between those with and without NAFLD. Thus, it is possible that after careful removal of undiagnosed NAFLD from the reference population, the relative risk of CV events would be even higher than our estimates. Although the risk of missed cases is generally high in large population-based cohorts due to lack of systematic screening in the community, data generated from cohorts who were identified as having NAFLD remain of critical importance nonetheless. We carefully explored if the differential impact of female sex on CVD in NAFLD versus referents was biased by differential intensity of diagnostic testing (serum liver enzymes and abdominal ultrasound, chosen as surrogates of medical surveillance that are also used in the conventional NAFLD diagnosis/screening algorithm) among genders; we demonstrated that these results are not the consequence of an imbalanced detection process related to differences in access to medical care between men and women among those with NAFLD or referents.
The limitations of this study include the predominantly white population, which limits generalizability to other races/ethnicities for conditions that have strong ethnic or socioeconomic determinants. However, the age and sex of Olmsted County residents are similar to those of the Upper Midwest and mortality rates are similar to the entire United States 35. Moreover, no single US community is fully representative of the entire United States, including the Framingham Heart Study which has provided critical evidence to CV disease epidemiology. The composite outcomes assessed by ASCVD did not include CV as a cause of death, as this could not be robustly identified from REP database. We did not assess the influence of menopausal status and therapies, in particular aspirin or statin use because prior large studies did not find a considerable impact of these variables on risk estimation32, 36.
In summary, this analysis of a large contemporary community cohort adds novel evidence that CVD associated with NAFLD has distinct consequences in women compared to men. These findings provide a critical perspective on the cardiovascular impact of NAFLD and challenge the current ASCVD risk-estimating methods which underserve women with NAFLD. As cardiovascular morbidity represents a significant burden in NAFLD, underestimating risk may have significant public health consequences, impacting half of the NAFLD population, or approximately 40 million women in the country.
Supplementary Material
SUPPLEMENTARY FIGURE 1. Schema of the multistate model used in the analysis. Each box represents a state and each arrow represents a transition (rate) from one state to another.
Study Highlights.
WHAT IS KNOWN
Female sex is protective against CV disease in the general population.
CVD is the top cause of mortality in patients with NAFLD.
WHAT IS NEW HERE
The female advantage in cardiovascular disease protection is lost in patients with NAFLD.
The excess CVD and mortality between NAFLD and controls is much higher in women than men at all ages.
The conventional risk-estimating equations underestimate the 10-year CVD risk in NAFLD women while overestimating the risk in the general population.
Acknowledgments
Funding: National Institute of Diabetes and Digestive and Kidney Diseases DK115594 (Alina M. Allen); American College of Gastroenterology Junior Faculty Award (Alina M. Allen). This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676.
The funding sources did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- CI
confidence interval
- CV
cardiovascular
- CVD
cardiovascular disease
- DM
diabetes mellitus
- HR
hazard ratio
- HICDA
Hospital International Classification of Diseases Adapted
- HTN
hypertension
- ICD
International Classification of Diseases
- NAFLD
nonalcoholic fatty liver disease
- PCEs
Pooled Cohort Equations
- REP
Rochester Epidemiology Project
- SIR
standardized incidence ratio
Footnotes
Potential Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Garcia M, Miller VM, Gulati M, et al. Focused Cardiovascular Care for Women: The Need and Role in Clinical Practice. Mayo Clin Proc 2016;91:226–40. [DOI] [PubMed] [Google Scholar]
- 2.Haberl R, Becker A, Leber A, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1,764 patients. J Am Coll Cardiol 2001;37:451–7. [DOI] [PubMed] [Google Scholar]
- 3.Devries S, Wolfkiel C, Fusman B, et al. Influence of age and gender on the presence of coronary calcium detected by ultrafast computed tomography. J Am Coll Cardiol 1995;25:76–82. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- 5.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149:389–97.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D, Kim WR, Kim HJ, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen AM, Therneau TM, Larson JJ, et al. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology 2018;67:1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ying I, Saposnik G, Vermeulen MJ, et al. Nonalcoholic fatty liver disease and acute ischemic stroke. Epidemiology 2011;22:129–30. [DOI] [PubMed] [Google Scholar]
- 10.Boddi M, Tarquini R, Chiostri M, et al. Nonalcoholic fatty liver in nondiabetic patients with acute coronary syndromes. Eur J Clin Invest 2013;43:429–38. [DOI] [PubMed] [Google Scholar]
- 11.Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138–1153. [DOI] [PubMed] [Google Scholar]
- 12.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roger VL, Killian JM, Weston SA, et al. Redefinition of myocardial infarction: prospective evaluation in the community. Circulation 2006;114:790–7. [DOI] [PubMed] [Google Scholar]
- 15.Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. Jama 2004;292:344–50. [DOI] [PubMed] [Google Scholar]
- 17.Weiss S, Sen I, Huang Y, et al. Cardiovascular morbidity and mortality after aortic dissection, intramural hematoma, and penetrating aortic ulcer. J Vasc Surg 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res 2002;11:91–115. [DOI] [PubMed] [Google Scholar]
- 19.Meira-Machado L, de Una-Alvarez J, Cadarso-Suarez C, et al. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res 2009;18:195–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–430. [DOI] [PubMed] [Google Scholar]
- 21.Razzouk L, Muntner P. Ethnic, gender, and age-related differences in patients with the metabolic syndrome. Curr Hypertens Rep 2009;11:127–32. [DOI] [PubMed] [Google Scholar]
- 22.Janssen I, Powell LH, Crawford S, et al. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med 2008;168:1568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovejoy JC, Champagne CM, de Jonge L, et al. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden CL, Carroll MD, Lawman HG, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA 2016;315:2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Writing Group M, Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 26.Force USPST, Bibbins-Domingo K, Grossman DC, et al. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016;316:1997–2007. [DOI] [PubMed] [Google Scholar]
- 27.Bibbins-Domingo K, Force USPST. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836–45. [DOI] [PubMed] [Google Scholar]
- 28.Kavousi M, Leening MJ, Nanchen D, et al. Comparison of application of the ACC/AHA guidelines, Adult Treatment Panel III guidelines, and European Society of Cardiology guidelines for cardiovascular disease prevention in a European cohort. Jama 2014;311:1416–23. [DOI] [PubMed] [Google Scholar]
- 29.DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015;162:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the Atherosclerotic Cardiovascular Risk Equation in a Large Contemporary, Multiethnic Population. J Am Coll Cardiol 2016;67:2118–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook NR, Ridker PM. Calibration of the Pooled Cohort Equations for Atherosclerotic Cardiovascular Disease: An Update. Ann Intern Med 2016;165:786–794. [DOI] [PubMed] [Google Scholar]
- 32.Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern Med 2014;174:1964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yadlowsky S, Hayward RA, Sussman JB, et al. CLinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Annals of Internal Medicine 2018. [DOI] [PubMed] [Google Scholar]
- 34.Greenland P, Blaha MJ, Budoff MJ, et al. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol 2018;72:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mora S, Wenger NK, Cook NR, et al. Evaluation of the pooled cohort risk equations for cardiovascular risk prediction in a multiethnic cohort from the women’s health initiative. JAMA Internal Medicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY FIGURE 1. Schema of the multistate model used in the analysis. Each box represents a state and each arrow represents a transition (rate) from one state to another.