Abstract
Neonates often develop poor immunity against intracellular pathogens. Since CD8+ T cells are essential for eliminating infectious agents, it is crucial to understand why they behave differently in early life. Previous studies in mice have demonstrated that neonatal CD8+ T cells fail to form memory because of an intrinsic propensity to differentiate into short-lived effectors. However, the underlying mechanisms remain undefined. We now show that neonatal CD8+ T cells exhibit higher glycolytic activity than adult CD8+ T cells after infection, which may be due to age-related differences in Lin28b expression. Importantly, when glycolysis is pharmacologically inhibited, the impaired formation of neonatal memory CD8+ T cells can be restored. Collectively, these data suggest that neonatal CD8+ T cells are inherently biased toward undergoing glycolytic metabolism after infection, which compromises their ability to develop into memory CD8+ T cells in early life.
Introduction
Infections remain a major cause of neonatal morbidity and mortality. Repeated infections with the same intracellular pathogen (RSV, Rhinovirus) are common in early life (1), indicating a reduced capacity to develop neonatal memory CD8+ T cells. However, the basic mechanisms that prevent neonates from generating robust memory CD8+ T cell responses are poorly understood, and the lack of this knowledge has limited our ability to develop more rationale strategies to enhance immunity in early life.
During infection, naïve antigen-specific CD8+ T cells undergo massive clonal expansion and differentiate into effector CD8+ T cells capable of eliminating infected cells (2–4). Once the infection has been controlled, the majority (~90–95%) of CD8+ T cells undergo apoptosis. However, a small percentage (~5–10%) of cells survive and differentiate into long-lived memory cells, protecting the host against reinfection. Recent studies have demonstrated that neonatal CD8+ T cells are intrinsically defective at forming memory CD8+ T cells (5–7). Surprisingly, this impairment is not due to a lack of responsiveness or proliferation, but rather to an inherent propensity of neonatal CD8+ T cells to rapidly become terminally differentiated, thereby losing their potential to transition into the long-lived memory pool (7).
The propensity for CD8+ T cells to become terminally differentiated in early life relates to the unique properties of the hematopoietic stem cells (HSCs) from which neonatal CD8+ T cells are derived (8). Whereas neonatal CD8+ T cells are produced from highly proliferative fetal HSCs that originate in the liver (9, 10), adult CD8+ T cells are generated from more quiescent adult HSCs in the bone marrow (9, 11). Notably, fetal HSCs use distinct metabolic pathways compared to adult HSCs, which are largely regulated by Lin28b (12–14). Lin28b is a classic oncofetal gene, which creates a metabolic program conducive for rapid cell growth in fetal life as well as in aggressive cancers (15). Whether this metabolic program is retained in neonatal CD8+ T cells and alters their fate during infection remains an open question.
In adults, CD8+ T cells undergo extensive changes in their metabolic properties throughout the course of infection. Following activation, naïve CD8+ T cells switch their glucose metabolism from oxidative phosphorylation to aerobic glycolysis, similar to cancer cells (16–18). This phenomenon (known as the Warburg effect) is required to mobilize sufficient amounts of proteins, nucleic acids, lipids, and carbohydrates to undergo massive clonal expansion (19) and acquire effector functions (20). Once the infection has been cleared, effector CD8+ T cells must decrease anabolic activity to become a more quiescent memory cell. This is accomplished by switching from glycolysis back to fatty acid oxidation (21–23). In this study, we investigated whether age-related differences in metabolic programming underlie the impaired development of neonatal memory CD8+ T cells.
Materials and Methods
Mice.
B6-Ly5.2/Cr mice were purchased from Charles River Laboratories. TCR transgenic mice specific for the HSV-1 glycoprotein gB498–505 peptide SSIEFARL (gBT-I mice, (24)) were provided by Janko Nikolich-Zugich (University of Arizona, Tucson, AZ) and crossed with Thy1.1 or C57BL/6 mice purchased from Jackson Laboratories. Lin28b transgenic mice (25) driven under the CD2 promoter were provided by Leonid Pobezinsky (University of Massachusetts) and crossed with gBT-I mice. Neonatal and adult gBT-I animals of both sexes were used at 5–7 days and 2–4 months old, respectively. Mice were housed under specific pathogen-free conditions at Cornell University College of Veterinary Medicine, accredited by the Assessment and Accreditation of Laboratory Animal Care.
In vitro T cell stimulation assays.
Splenic CD8+ T cells were isolated from gBT-I mice by positive immunomagnetic selection (Miltenyi Biotec), stimulated with plate-bound anti-CD3 (5 ug/ml) and anti-CD28 (20 ug/ml), and cultured in complete media supplemented with 10 U/ml of IL-2 (Biolegend) for 48 hours. For glycolytic inhibition experiments, cells were cultured with media supplemented with 0.3 mM of 2-Deoxy-D-glucuse (2-DG) (Sigma-Aldrich).
Dual adoptive transfer experiments.
gBT-I splenic neonatal and adult CD8+ T cells were enriched by positive immunomagnetic selection (Miltenyi Biotec). Combined cells were suspended at 5×105 cells per ml of PBS and 100 ul injected i.v. into adult B6-CD45.1 recipient mice. The next day, recipient mice were infected (5×103 CFU, i.v.) with wild-type Listeria monocytogenes stably expressing the gB-8p peptide (denoted LM-gB), as previously described (6).
Antibodies and flow cytofluorimetric analysis.
Antibodies were purchased from eBioscience, Biolegend, Invitrogen, or BD Biosciences and concentrations were used as recommended by the manufacturer. Flow cytofluorimetric data were acquired using DiVa software from an LSRII equipped with 4 lasers (BD Biosciences). Analysis was performed with FlowJo (Tree Star, Ashland, OR).
Cell sorting.
To perform metabolic flux analysis, donor CD8+ T cells were recovered from recipient mice by positive immunomagnetic selection (Miltenyi Biotec) and subsequently labeled with antibodies against CD8 (53–6.7), CD4 (Gk1.5), CD45.1 (A20), CD45.2 (104), and Thy1.1 (OX-7). For experiments that isolated True Naïve (TN) and Virtual Memory (VM) populations, antibodies against CD122 (Tm-b1) and CD44 (IM7) were used. For experiments that isolated the short-lived effector cell (SLEC) population, antibodies against KLRG1 (2F1) and CD127 (A7R34) were used. The cells were then sorted to >95% purity on a fluorescence-activated cell sorting (FACS) Aria III (BD Biosciences).
Metabolic bioassays.
To measure oxygen consumption rates (OCR) and extra-cellular acidification rates (ECAR), 2.5 – 3×105 sorted CD8+ T cells were plated in buffer-free media containing 2 mM glutamine and 1 mM sodium pyruvate. 25mM glucose was additionally supplemented to measure OCR. Using an XFp Extracellular Flux Analyzer (Agilent Seahorse, Santa Clara, CA), OCR was measured following the addition of 1 uM oligomycin, 1 μM FCCP, and 0.5 uM rotenone/antimycin A, and ECAR was measured following the addition of 10 mM glucose, 1 μM oligomycin, and 50 μM 2-Deoxy-D-glucose (2DG).
Quantitative PCR.
RNA was isolated using Trizol (Life Technologies), and cDNA was generated with the iScript Reverse Transcription Supermix kit (BioRad). Real-time RT-PCR was performed on an Applied Biosystems 7900HT with primers that have been previously described (26). Gene expression was calculated relative to Actb.
Metabolomics.
Metabolite extraction from activated neonatal and adult CD8+ T cells were performed exactly as described in a previous study (27). An LC-MS (QE-MS, Thermo Scientific) was used for metabolite profiling, and the relative abundance of each metabolite was calculated using the commercially available software Sieve 2.2 (Thermo Scientific) according to previously described procedures (27).
Statistical analysis.
Statistical analysis was performed using Prism (GraphPad Software, Inc, La Jolla, CA). Error bars represent standard error of the mean and are representative of biological replicates within an experiment. Significance was determined by Student t test or a two-way ANOVA followed by a Tukey post-hoc test as indicated in the figure legends. p values <0.05 were considered significant.
Results and Discussion:
Neonatal CD8+ T cells exhibit higher glycolytic metabolism after in vitro stimulation
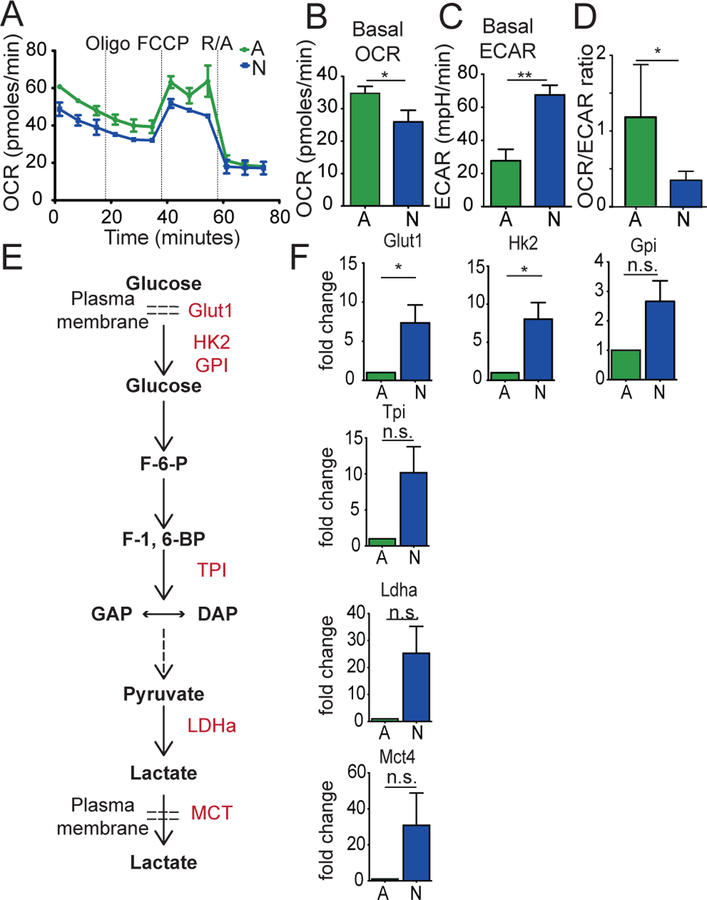
Previous studies have demonstrated that neonatal T cells from mice (6–8, 28, 29) and humans (7, 30, 31) are inherently more reactive than their adult counterparts after in vitro stimulation. To understand the metabolic changes that occur during this process, we activated CD8+ T cells from neonatal and adult TCR transgenic mice (gBT-I mice) and used the Seahorse Extracellular flux analyzer to compare their bioenergetics profiles. After 2 days of stimulation via the TCR and CD28, we found that the basal oxygen consumption rate (OCR), an indicator of oxidative phosphorylation (OXPHOS), was higher in adult cells (Fig. 1A,B). In contrast, neonatal CD8+ T cells exhibited a higher basal extracellular acidification rate (ECAR) (Fig. 1C), which is used as a readout for aerobic glycolysis. Overall, neonatal CD8+ T cells possessed a lower OCR/ECAR ratio (Fig. 1D), suggesting that CD8+ T cells in early life preferentially use glycolytic metabolism after stimulation.
Figure 1. Neonatal CD8+ T cells exhibit higher glycolytic metabolism after in vitro stimulation.
(A) OCR measurements of adult and neonatal CD8+ T cells at 48hr after α-CD3 and α-CD28 activation. (B) Basal OCR, (C) Basal ECAR, and (D) Basal OCR/ECAR ratios in adult and neonatal CD8+ T cells at 48hr after α-CD3 and α-CD28 activation. (E) Pathway of critical proteins in glycolysis. (F) Fold change in mRNA expression of proteins 8 hours post-stimulation using qPCR. Data representative of two independent experiments with 3–5 biological replicates/group. * p<0.05, ** p<0.005 by unpaired student t-test.
To extend our extracellular flux data, we performed qPCR and compared the expression levels of genes in the glycolysis pathway in neonatal and adult CD8+ T cells at 8 hrs post-stimulation. We found that most of the key genes involved in glycolysis were highly upregulated in neonatal CD8+ T cells after activation (Fig. 1F). We also used liquid chromatography/high-resolution mass spectrometry to determine how glycolytic metabolites are altered in different-aged CD8+ T cells at 18 hrs after stimulation. In line with our qPCR data, neonatal CD8+ T cells produced higher amounts of metabolites involved in the glycolysis pathway (e.g., pyruvate, lactate) than adults (Supplemental Fig. 1). Collectively, these findings demonstrate that neonatal CD8+ T cells undergo augmented glycolysis following TCR activation.
Neonatal CD8+ T cells possess an inherent propensity to undergo glycolytic metabolism following infection
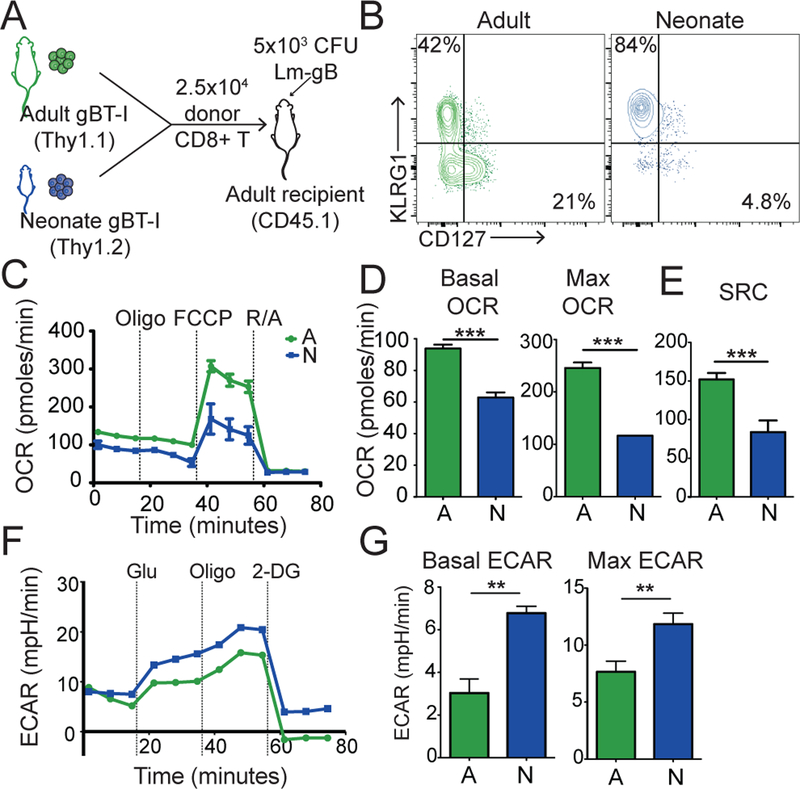
We next assessed the metabolic changes that occur in neonatal and adult CD8+ T cells after infection in vivo. Our strategy was to co-transfer the same number of neonatal (Thy1.2) and adult (Thy1.1) gBT-I TCR transgenic cells into congenic recipients (Ly5.2 mice), which were subsequently infected with a recombinant Listeria monocytogenes expressing the gB peptide (LM-gB) (Fig. 2A). By comparing equal numbers of monoclonal neonatal and adult CD8+ T cells in the same environment, we were able to focus on cell-intrinsic differences in metabolic reprogramming. Consistent with our earlier work (6), neonatal CD8+ T cells preferentially gave rise to short-lived effector cells (SLECs, KLRG1+, CD127-), whereas adult CD8+ T cells formed more memory precursor cells (MPECs, KLRG1-, CD127+) after infection (Fig. 2B). To compare their metabolic profiles, we used FACS to isolate neonatal and adult donor cells at the peak of infection (7 dpi) and performed extracellular flux analysis under basal conditions and after drug-induced stress. Neonatal donor CD8+ T cells exhibited a lower basal and maximum OCR and a higher basal and maximum ECAR compared to their adult counterparts (Fig. 2C–D). Moreover, neonatal CD8+ T cells had significantly lower levels of spare respiratory capacity (SRC) (Fig. 2E), which has previously been shown to be critical for the development of memory CD8+ T cells (32). These results suggest that neonatal CD8+ T cells fail to transition into the long-lived memory pool because of an inability to undergo oxidative metabolism in response to increased stress.
Figure 2. Neonatal CD8+ T cells possess an inherent propensity to undergo glycolytic metabolism following infection.
(A) The experimental design to examine the metabolic programs of neonatal and adult CD8+ T cells during infection. (B) Representative contour plots of KLRG1 and CD127 expression at 7 dpi (C) OCR measurements, (D) Basal and max OCR, and (E) SRC values in adult and neonatal CD8+ T cells during a Mitochondrial Stress Test at 7 dpi (F) ECAR measurements and (G) Basal and max ECAR values of adult and neonatal CD8+ T cells during a Glycolysis Stress Test at 7 dpi. Data representative of 2 independent experiments with three biological replicates/group. ** p<0.005, *** p<0.0005 by unpaired student t-test.
To better understand why neonatal CD8+ T cells use different metabolic programs than adults after infection, we next performed a series of experiments to control for phenotypic differences that are present before and after infection. First, we considered that age-related changes in metabolic reprogramming might be due to differences in the proportion of naïve and virtual memory cells in the starting pool. Indeed, we previously found that neonatal CD8+ T cells are comprised of nearly twice as many virtual memory cells (antigen-inexperienced cells with a memory phenotype) as adults prior to infection (8). To control for these initial phenotypic differences, we repeated our co-transfer experiments with sorted populations of CD44loCD122lo true naïve (TN) and CD44hiCD122hi virtual memory (VM) donor cells from neonatal and adult gBT-I mice and directly compared their metabolic profiles at the peak of infection (Supplemental Fig 2a–b). Regardless of the initial phenotype, the neonatal donor cells (both TN and VM) utilize an elevated glycolytic program compared to their adult counterparts (Supplemental Fig 2c–g). These data suggest that age-related changes in metabolic reprogramming cannot be simply explained by the different phenotypes of cells that are present prior to infection.
Second, we tested the possibility that metabolic changes observed in neonatal and adult effector CD8+ T cells are due to phenotypic differences that are present at the peak of the response. For example, the lower levels of OCR and SRC observed in neonatal cells could be due to a higher proportion of SLECs in the bulk population, rather than a difference in metabolic programming in the responding cells. To exam this possibility, we repeated our co-transfer experiment. However, this time we sorted out an equivalent phenotypic subset (SLECs) from neonatal and adult donor cells at the peak of the response (7 dpi) and directly compared their metabolic profiles (Supplemental Fig. 2h). The adult SLECs still showed higher rates of basal and maximum OCR and exhibited a larger SRC compared to neonatal SLECs (Supplemental Fig. 2i–k). In contrast, the neonatal SLECs displayed higher basal and maximum ECAR (Supplemental Fig. 2l–m), which is consistent with the overall metabolic programs observed in the bulk neonatal and adult populations. Taken together, these data suggest that age-related differences in metabolic reprogramming of CD8+ T cells are due to inherent changes at the individual cell level, rather than shifts in the representation of naïve or effector subsets in the mixed population.
Lin28b drives a more glycolytic metabolic program in CD8+ T cells
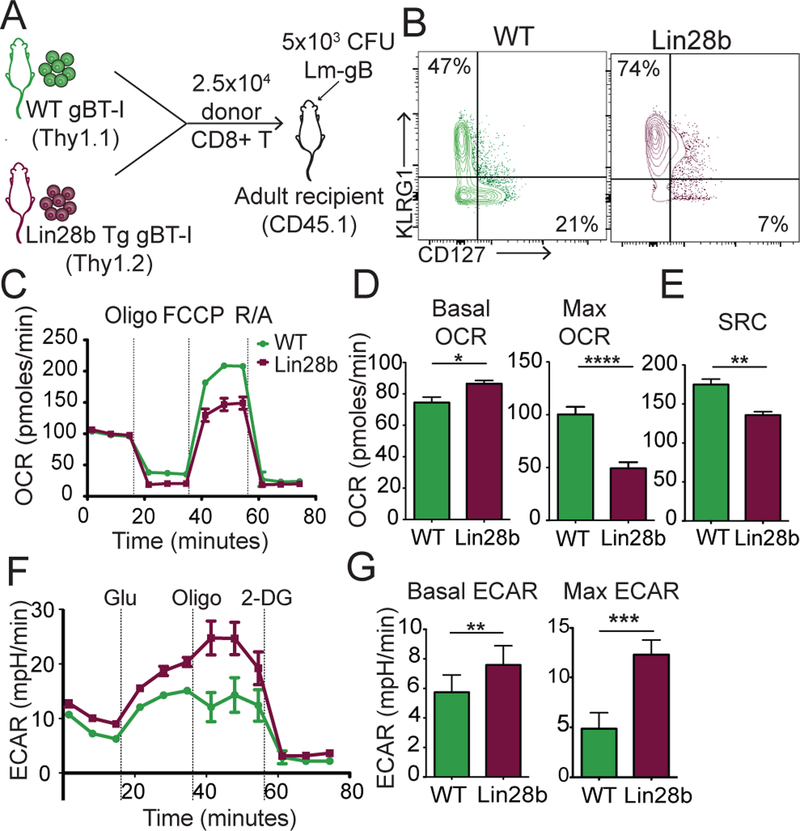
Another key question is, how are neonatal CD8+ T cells programmed to use glycolysis after infection? We previously showed that neonatal and adult CD8+ T cells adopt different fates during infection because they are derived from distinct progenitor populations, which are specified, at least in part, by their differential expression of Lin28b (8). Interestingly, Lin28b is preferentially expressed in fetal HSCs and was recently shown to be a major positive regulator of glucose metabolism (14). Thus, we hypothesized that metabolic changes between neonatal and adult CD8+ T cells could be attributed to developmental-related differences in Lin28b expression. To test this, we crossed gBT-I mice with Lin28b transgenic (Lin28b Tg) mice to generate a source of adult donor CD8+ T cells that expressed Lin28b. We then co-transferred an equal number of WT adult (Thy1.1) and Lin28b Tg adult (Thy1.2) donor CD8+ T cells into the same recipient mice (Ly5.2) and asked whether forced expression of Lin28b in adult CD8+ T cells drives a more ‘neonatal-like’ metabolic program after infection with LM-gB (Fig. 3A). Consistent with our previous work, we found that Lin28b Tg adult donor cells preferentially became SLECs during infection, similar to neonatal CD8+ T cells (Fig. 3B). At 7 dpi, we sorted out WT and Lin28b Tg donor CD8+ T cells and compared their metabolic profiles via extracellular flux analysis. Induction of Lin28b in adult CD8+ T cells resulted in lower rates of cellular respiration and a decrease in SRC (Fig. 3C–E), levels similar to those observed in neonatal donor CD8+ T cells (Fig. 2). The Lin28b Tg cells also displayed higher rates of glycolytic metabolism (Fig. 3F,G), suggesting that Lin28b promotes rapid differentiation of CD8+ T cells in early life by enhancing glucose metabolism.
Figure 3. Lin28b drives a more glycolytic metabolic program in CD8+ T cells.
(A) The experimental design to examine the metabolic programs of WT adult and Lin28b Tg adult CD8+ T cells during infection. (B) Representative contour plot of KLRG1 and CD127 expression at 7 dpi (C) OCR measurements, (D) Basal and max OCR, (E) SRC values in adult and neonatal CD8+ T cells at 7 dpi from a mitochondrial stress test (F) ECAR measurements, (G) Basal and max ECAR values in WT and Lin28b tg CD8+ T cells from a glycolysis stress test at 7 dpi. Data representative of 2 independent experiments with 3 biological replicates/group. * p<0.05, ** p<0.005 by unpaired student t-test
Inhibiting glycolytic metabolism restores the formation of neonatal memory CD8+ T cells
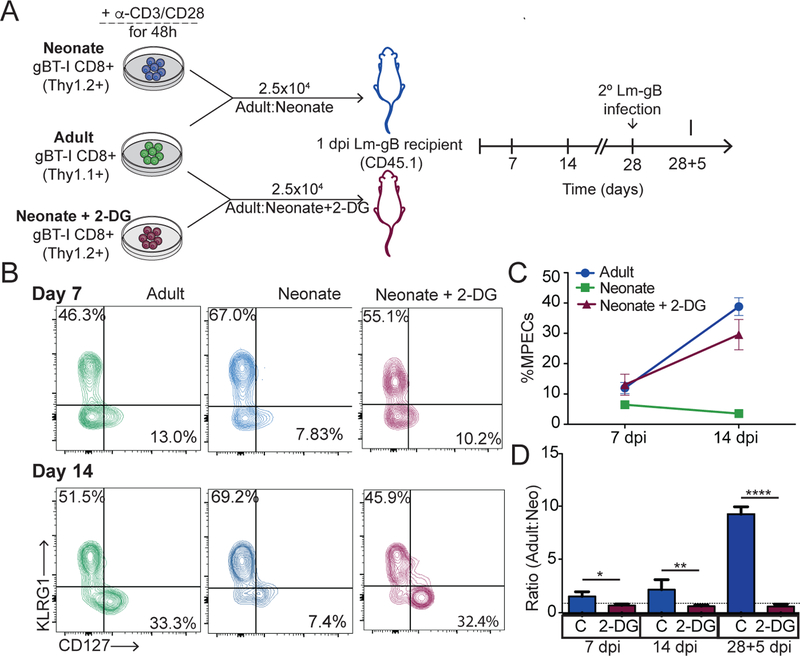
Our data thus far indicates that increased glycolysis in neonatal CD8+ T cells is associated with more rapid effector cell differentiation. However, whether changes in glucose metabolism are ultimately responsible for altering the fate of neonatal CD8+ T cells during infection is unclear. Recent studies have demonstrated that CD8+ T cells with high glycolytic metabolism are short-lived and quickly die after infection, whereas those with low glycolytic metabolism persist and transition into the long-lived memory pool (33). Thus, we hypothesized that neonatal CD8+ T cells are impaired at forming memory because they undergo a more pronounced glycolytic flux after antigenic stimulation. To test this hypothesis, we designed an experiment to limit glucose metabolism in neonatal CD8+ T cells before priming and examined changes in their ability to respond to infection. Our strategy involved stimulating neonatal and adult CD8+ T cells in vitro, in the presence or absence of a competitive inhibitor for glucose (2-DG), and then co-transferring adult donor CD8+ T cells with either neonatal (2-DG treated) or neonatal (control) donor cells into an LM-gB infected-matched recipient (Fig. 4A). At various times after infection, we examined the phenotype of each donor population and found that inhibiting glycolysis in neonatal cells resulted in significantly more MPECs and fewer SLECs (Fig. 4B,C). In fact, the neonatal cells treated with 2-DG exhibited a phenotype comparable to the adult donor CD8+ T cells, indicating that a sizeable portion of 2-DG treated cells were able to transition into the memory stage (Fig. 4B,C). Strikingly, 2-DG treatment also enabled neonatal CD8+ T cells to survive during contraction and mount a more proliferative recall response following reinfection with LM-gB, similar to adult donor CD8+ T cells (Fig. 4D). Thus, limiting entry into glycolysis is sufficient to limit terminal differentiation and promote the development of neonatal memory CD8+ T cells.
Figure 4. Inhibiting glycolytic metabolism restores the formation of neonatal memory CD8+ T cells.
(A) Schematic of the experimental design: Adult and neonatal CD8+ T cells were stimulated with α-CD3 and α-CD28 in the presence of a vehicle control or 0.3mM of 2-DG for 48h. 2.5×104 activated adult (thy1.1) and neonatal (thy1.2) cells were then co-transferred I.V. into congenic WT recipients (CD45.1) that were infected with 5X103 CFU of LM-gB the previous day. Recipients were rechallenged with 5×104 CFU of LM-gB at 28 dpi. Phenotype of donor cells were assessed through bleeds at indicated timepoints. (B) Representative contour plots of KLRG1 and CD127 expression at 7 dpi and 14 dpi (C) Percent of adult, neonatal, and 2-DG neonatal cells that are MPECs at 7 and 14dpi (D) Representative contour plots of donor populations at 14 dpi (E) Ratio of adult and neonatal donor populations with respective to time. Data representative of 3 independent experiments with 4–6 biological replicates/group. * p<0.05, ** p<0.005, **** p<0.00005 by unpaired student t-test.
In summary, these findings demonstrate that neonatal and adult CD8+ T cells are intrinsically different and respond to antigen stimulation with dissimilar metabolic programs, leading them to adopt different fates. Higher glycolytic flux biases neonatal CD8+ T cells to become short-lived effectors at the expense of forming memory cells. Perhaps counterintuitively, reducing glycolytic metabolism during priming can enhance the survival of neonatal memory CD8+ T cells. These findings suggest that many of the intrinsic differences between neonatal and adult CD8+ T cells can be attributed to age-related changes in metabolic reprogramming and demonstrate that neonatal memory T cells responses can be therapeutically enhanced.
Although the underlying basis for why neonatal and adult CD8+ T cells use distinct metabolic programs requires further investigation, our findings suggest that neonatal CD8+ T cells exhibit a different metabolic program than adults because they are derived from fetal-liver HSCs. Thus, it would be interesting to examine the metabolic programs in other fetal-derived lymphocytes to see if they also have a higher glycolytic flux than their adult counterparts. It is also interesting to speculate that Lin28b serves as a metabolic rheostat, balancing the need to generate different amounts of effector and memory cells at various stages of life. In the future, it will be important to investigate how the different metabolic states in neonatal and adult CD8+ T cells influence their ability to give rise to different subsets of memory cells (e.g., tissue resident memory, central memory, and effector memory). Such studies could provide us with novel strategies to fine tune the development of memory CD8+ T cells during critical stages of development.
Supplementary Material
Key points.
Neonatal CD8+ T cells exhibit a bias for glycolytic metabolism after infection
Higher glycolytic activity in neonatal CD8+ T cells relates to Lin28b expression
Inhibiting glycolysis rescues the formation of neonatal memory CD8+ T cells
Acknowledgments
This work was supported by National Institute of Health awards R01AI105265 and R01AI110613 (to B.D.R, from the National Institute of Allergy and Infectious Disease), and a T32 training grant from the National Institute of Biomedical Imaging and Bioengineering (to C.T., award 1T32EB023860).
References
- 1.Chang J, and Braciale TJ. 2002. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med 8: 54–60. [DOI] [PubMed] [Google Scholar]
- 2.Butz EA, and Bevan MJ. 1998. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doherty PC 1998. The numbers game for virus-specific CD8+ T cells. Science 280: 227. [DOI] [PubMed] [Google Scholar]
- 4.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, and Ahmed R. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8: 177–187. [DOI] [PubMed] [Google Scholar]
- 5.Wissink EM, Smith NL, Spektor R, Rudd BD, and Grimson A. 2015. MicroRNAs and Their Targets Are Differentially Regulated in Adult and Neonatal Mouse CD8+ T Cells. Genetics 201: 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith NL, Wissink E, Wang J, Pinello JF, Davenport MP, Grimson A, and Rudd BD. 2014. Rapid proliferation and differentiation impairs the development of memory CD8+ T cells in early life. J Immunol 193: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynaldi A, Smith NL, Schlub TE, Venturi V, Rudd BD, and Davenport MP. 2016. Modeling the dynamics of neonatal CD8(+) T-cell responses. Immunol Cell Biol 94: 838–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Wissink EM, Watson NB, Smith NL, Grimson A, and Rudd BD. 2016. Fetal and adult progenitors give rise to unique populations of CD8+ T cells. Blood 128: 3073–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jotereau F, Heuze F, Salomon-Vie V, and Gascan H. 1987. Cell kinetics in the fetal mouse thymus: precursor cell input, proliferation, and emigration. J Immunol 138: 1026–1030. [PubMed] [Google Scholar]
- 10.Owen JJ, and Ritter MA. 1969. Tissue interaction in the development of thymus lymphocytes. J Exp Med 129: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foss DL, Donskoy E, and Goldschneider I. 2001. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med 193: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinoda G, Shyh-Chang N, Soysa TY, Zhu H, Seligson MT, Shah SP, Abo-Sido N, Yabuuchi A, Hagan JP, Gregory RI, Asara JM, Cantley LC, Moss EG, and Daley GQ. 2013. Fetal deficiency of lin28 programs life-long aberrations in growth and glucose metabolism. Stem Cells 31: 1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shyh-Chang N, and Daley GQ. 2013. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell 12: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Consortium D, Investigators M, Altshuler D, and Daley GQ. 2011. The Lin28/let-7 axis regulates glucose metabolism. Cell 147: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton JE, and Gregory RI. 2012. How does Lin28 let-7 control development and disease? Trends Cell Biol 22: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krauss S, Brand MD, and Buttgereit F. 2001. Signaling takes a breath--new quantitative perspectives on bioenergetics and signal transduction. Immunity 15: 497–502. [DOI] [PubMed] [Google Scholar]
- 17.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, and Thompson CB. 2000. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Molecular cell 6: 683–692. [DOI] [PubMed] [Google Scholar]
- 18.Loos JA, and Roos D. 1973. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. 3. Stimulation by tuberculin and allogenic cells. Experimental cell research 79: 136–142. [PubMed] [Google Scholar]
- 19.Warburg O 1956. On respiratory impairment in cancer cells. Science 124: 269–270. [PubMed] [Google Scholar]
- 20.Chang CH, Curtis JD, Maggi LB Jr., Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, and Pearce EL. 2013. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153: 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, and Choi Y. 2009. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, and Ahmed R. 2009. mTOR regulates memory CD8 T-cell differentiation. Nature 460: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Windt GJ, and Pearce EL. 2012. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev 249: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller SN, Heath W, McLain JD, Carbone FR, and Jones CM. 2002. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol Cell Biol 80: 156–163. [DOI] [PubMed] [Google Scholar]
- 25.Pobezinsky LA, Etzensperger R, Jeurling S, Alag A, Kadakia T, McCaughtry TM, Kimura MY, Sharrow SO, Guinter TI, Feigenbaum L, and Singer A. 2015. Let-7 microRNAs target the lineage-specific transcription factor PLZF to regulate terminal NKT cell differentiation and effector function. Nat Immunol 16: 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, and Green DR. 2011. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35: 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Ser Z, and Locasale JW. 2014. Development and quantitative evaluation of a high-resolution metabolomics technology. Anal Chem 86: 2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith NL, Patel RK, Reynaldi A, Grenier JK, Wang J, Watson NB, Nzingha K, Yee Mon KJ, Peng SA, Grimson A, Davenport MP, and Rudd BD. 2018. Developmental Origin Governs CD8(+) T Cell Fate Decisions during Infection. Cell 174: 117–130 e114. [DOI] [PubMed] [Google Scholar]
- 29.Adkins B, Williamson T, Guevara P, and Bu Y. 2003. Murine neonatal lymphocytes show rapid early cell cycle entry and cell division. J Immunol 170: 4548–4556. [DOI] [PubMed] [Google Scholar]
- 30.Palin AC, Ramachandran V, Acharya S, and Lewis DB. 2013. Human neonatal naive CD4+ T cells have enhanced activation-dependent signaling regulated by the microRNA miR-181a. J Immunol 190: 2682–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schonland SO, Zimmer JK, Lopez-Benitez CM, Widmann T, Ramin KD, Goronzy JJ, and Weyand CM. 2003. Homeostatic control of T-cell generation in neonates. Blood 102: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 32.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, and Pearce EL. 2012. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, Mohney RP, Klebanoff CA, Lal A, Finkel T, Restifo NP, and Gattinoni L. 2013. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest 123: 4479–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.