Abstract
Understanding the health implications of human exposure to mixtures of chemical contaminants is aided by analytical methods that can screen for a broad range of both expected and unexpected compounds. We performed a proof-of-concept analysis combining human breast milk, a biomonitoring matrix for determining contaminant exposure to mothers and infants, with a non-targeted method based on comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS). A total of 172 presumably anthropogenic halogenated compounds and non-halogenated cyclic and aromatic compounds were tentatively identified in breast milk from San Diego, California through mass spectral database searches. Forty of the compounds were prioritized for confirmation based on halogenation or 100% frequency of detection, and the identities of 30 were verified using authentic standards. Thirty-four (85%) of the prioritized contaminants are not typically monitored in breast milk surveys, and 31 (77%) are regulated in at least one market worldwide, indicating breast milk may be a useful biomonitoring matrix for non-targeted analysis and the assessment of human exposure to future emerging or undiscovered contaminants.
Keywords: Organic contaminants, human breast milk, non-targeted analysis, GC×GC/TOF-MS, biomonitoring
Graphical Abstract
The picture was taken by Nathan Dodder.
INTRODUCTION
Measurements of the human exposome aim to assess environmental exposure and associated risks in their entirety1. Within this context, the uptake of exogenous lipophilic chemicals by lactating women will result in contaminant elimination via the fatty portion of milk, and since the 1950s breast milk has been used as a biomonitoring matrix to assess human contaminant exposure, the mother’s internal dose, pre-natal exposure, and transfer to the infant through breast feeding2–4. Although breast-feeding has well-established benefits (for both mothers and infants) and is recommended5,6, breast milk contaminants, including biotransformation products, are concerns for infant and children’s health7–9. Contaminant detection in breast milk also serves as an indicator of general human exposure10. Prior breast milk contaminant surveys have detected multiple classes of compounds, including persistent organic pollutants (POPs) such as polychlorinated biphenyls (PCBs) and organochlorine pesticides11,12; components of personal care products4 including cosmetic UV filters, phthalates, fragrances and parabens; bisphenol A13; and polycyclic aromatic hydrocarbons (PAHs)11.
Historically, the analysis of breast milk has been key to the investigation of unexpected environmental contaminants. In 1973–1974, cattle feed in Michigan was erroneously mixed with the flame retardant polybrominated biphenyl (PBB), severely affecting the health of cattle and subsequently contaminating meat and dairy products from exposed farms14. The farms were quarantined, and it was initially thought human exposure was limited to individuals living on the farms or directly receiving their products. However, during a pesticide screening of breast milk in 1976, it was discovered that mothers from the general population of Michigan were exposed to PBB15, and subsequent work confirmed PBB had entered the food supply16,17. In another case, temporal trend surveys of Swedish breast milk collected between 1972–1997 were among the first studies to indicate widespread and increasing human exposure to another brominated flame retardant, polybrominated diphenyl ether (PBDE)18,19. These PBDE breast milk studies were among the initial investigations that lead to a multitude of occurrence and toxicological studies, and the eventual reductions of PBDE production and use approximately 10 years later20.
Breast milk contaminant analyses are typically targeted (the compounds of interest are pre-determined)2, and will miss unexpected compounds regardless of their abundance21. Unexpected contaminants in this and other matrices are usually identified in an ad hoc manner, which may result in the widespread environmental occurrence and increasing environmental concentration of compounds missed by routine targeted contaminant screening. In contrast, non-targeted analysis is a relatively new class of full-scan mass spectrometry based methods that aim to systematically identify both known and unknown compounds. For example, non-targeted analysis has been used to identify contaminants in inland waters22,23 and marine mammal blubber24–28. It has also been integrated with toxicological investigations to identify and prioritize chemicals of interest29–31. Mass spectrometry based non-targeted analysis of environmental contaminants has been developed using different types of instruments23,29. In the present study, we apply non-targeted analysis based on comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS) to determine the number and identity of unexpected contaminants in human breast milk. GC×GC provides improved chromatographic separation compared to one-dimensional GC, and is advantageous for the analysis of complex matricies32. The current study is a proof-of-concept test of non-targeted mass spectrometry for contaminant biomonitoring.
METHODS
Breast Milk Collection.
Breast milk was collected from three individuals who gave birth at the University of California San Diego hospital in November 2011. Upon discharge, the breast milk was donated for scientific research by the mother and stored at −20 °C. The milk was received frozen with no identifying information and was stored at −20 °C until sample preparation.
Sample Preparation.
The extraction and cleanup procedure is summarized in Figure S1, and details are provided in supporting information-1 (SI-1). Each milk sample was 16 mL, and was first separated into lipid and water portions. Each portion underwent a specific cleanup procedure, resulting in two lipid fractions with differing extraction polarity (via silica solid phase extraction with hexane/dichloromethane, and dichloromethane) and one water extract. Each of the three final fractions per sample were individually analyzed. In total, three breast milk samples were processed to give 9 fractions. Each breast milk sample was coupled with a procedural blank.
Instrumental Analysis and Initial Compound Identifications.
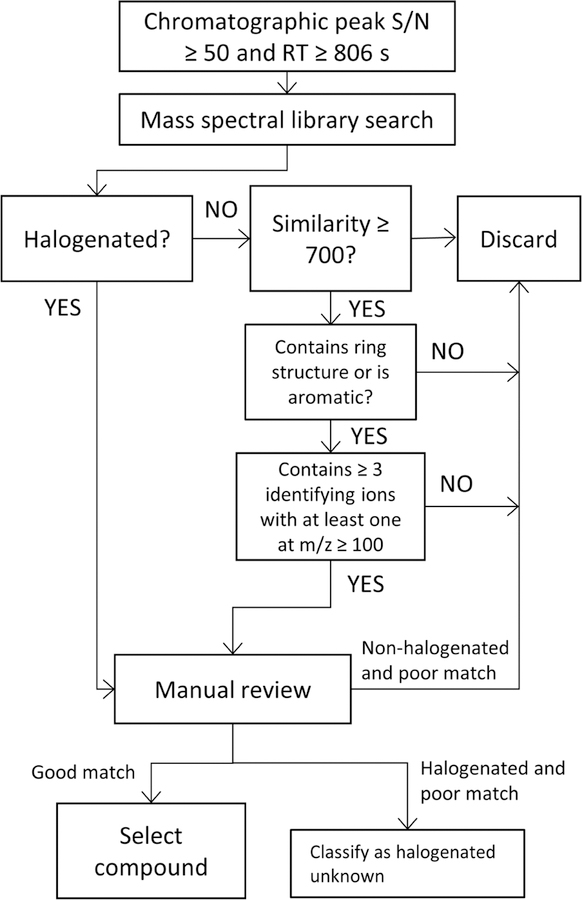
Non-targeted analysis was performed using a Pegasus® 4D GC×GC/TOF-MS (LECO, St. Joseph, MI, USA) with the instrumental parameters described in Table S1. Compounds were tentatively identified using LECO® ChromaTOF® software (version 4.43.3.0) through comparison of the experimental spectra against the 2011 NIST Mass Spectral Library and a series of screening criteria, as outlined in Figure 1. First, peaks with a signal to noise ratio (S/N) ≥ 50 and a 1st dimension retention time ≥ that of naphthalene (806 s) were selected and searched against the mass spectral library. Compounds identified as halogenated were manually reviewed for spectral similarity to the library match; in particular, the presence of halogenated isotopic patterns and fragments due to halogen loss. This chemical information is not incorporated in the similarity score33, and for this reason a strict similarity score threshold was not used for halogenated compounds. Spectra matching a non-halogenated compound with an aromatic or cyclic structure were also selected for further evaluation using two criteria: 1) the spectral similarity or reverse similarity was ≥ 700 out of 999; and 2) the spectrum contained at least three identifying ions of relatively high intensity that matched the mass spectral library hit, with at least one ≥ 100 m/z. The average similarity and reverse similarly (the match factor when excluding experimental spectrum peaks that were not in the library spectrum) scores for all halogenated and non-halogenated compounds meeting these criteria were 849 and 871, respectively. Spectra initially identified as halogenated that did not have a matching library record were classified as halogenated unknowns. Finally, the intensity of the identified compounds (including unknowns) had to be at least three times greater than that found in the corresponding procedural blank.
Figure 1.
Initial contaminant identification criteria. RT = retention time. Identities of selected compounds were then confirmed with authentic standards. Good match = 1) For halogenated compounds, the halogenated isotopic clusters should match those in the corresponding library mass spectrum, and loss of halogen must be observed in the mass spectrum. For non-halogenated compounds, the pattern (m/z values and relative abundances) of the 3 most prominent ions should match that of the corresponding library mass spectrum.
Identification Uncertainty and Confirmation with Authentic Standards.
Tentatively identified compounds were prioritized for confirmation by authentic standards. Using system previously described in Hoh et al. (2012)24, we categorized identifications within one of four categories, with the category names and number of compounds in brackets: 1) The experimental mass spectrum and retention times (within a modulation time of 3.5 s in in the 1st GC dimension and ±0.05 s in the 2nd) were matched to those of a reference standard analyzed under the same conditions [authentic MS RT]. 2) The experimental spectrum, but not the retention times, was matched to a reference standard, indicating the experimental spectrum is that of an isomer [authentic MS]. 3) The experimental spectrum was matched to one within the NIST Electron Ionization (EI) Mass Spectral Library [reference database MS] (the 2011 NIST EI Mass Spectral Library contains 220,460 spectra of 192,108 unique compounds). 4) The experimental spectrum was identified as belonging to a halogenated compound, but the chemical structure could not be further identified [unknown].
Detection limits were estimated by analyzing standard solutions at 0.1, 1, 10, 100, and 1000 ng/mL. The nine standard compounds represented three classes. 1) Benzophenone, 2-methylindole, and ethyl 4-ethoxybenzoate were non-halogenated aromatic compounds at relatively high concentrations in all three mothers’ samples. 2) BDE-47, p,p’-DDE, and PCB-153 were legacy halogenated persistent organic pollutants (DDE = dichlorodiphenyldichloroethylene and PCB =polychlorinated biphenyl). 3) 4,4′-Dichlorodiphenylsulphide, N-(4-chlorophenyl)formamide, and 4-chloro-N-methylaniline were non-legacy halogenated organic compounds at relatively high concentration in the mothers’ samples. The lowest concentration at which the compounds gave a sufficient mass spectrum for identification was 10 ng/mL, except for 2-methylindole and N-(4-chlorophenyl)formamide, for which the lowest concentration was 100 ng/mL. These values correspond to original sample concentrations of approximately 0.2 ng/mL whole milk and 2 ng/mL whole milk, respectively.
The log KOW and water solubility for each compound was determined using the Estimations Programs Interface for Windows (EPI Suite) software34. Regulatory information was obtained using SciFinder’s >347,000 compound Regulated Chemicals Listing35, a database of international lists such as high production volume chemicals, priority chemicals, and pollutant release inventories. Detailed description can be found in SI-1.
RESULTS & DISCUSSION
We used a non-targeted analytical method to identify 1) halogenated compounds and 2) non-halogenated cyclic and aromatic compounds in human breast milk collected in San Diego, California. Halogenated compounds are typically the most common targets in breast milk contamination surveys due to their production volumes, environmental persistence, lipophilic nature, and concerns regarding health impacts36. However, it has been proposed that current targeted methods may be missing uninvestigated anthropogenic halogenated contaminants37. Non-halogenated cyclic and aromatic contaminants in breast milk are currently less frequently investigated, however the targeted detection of non-halogenated UV-filters, parabens, and musks in breast milk4 suggested that non-targeted analysis may identify other non-halogenated contaminants. Our data analysis procedure excluded non-halogenated aliphatic compounds because they are likely endogenous, and their electron impact fragmentation frequently results in non-specific mass spectra.
In total, 172 presumed anthropogenic contaminants were preliminarily identified among the three breast milk samples. The identifications across all samples and fractions are listed in SI-2. Twenty-four compounds were halogenated organic compounds, 141 were non-halogenated cyclic or aromatic organic compounds, and 7 were unknown halogenated compounds (the mass spectrum indicated the compound was halogenated, but a match was not found in the mass spectral library and the structure could not be determined manually). Sixteen of the tentatively identified compounds had a total of 37 isomers. The experimental and matching reference library spectra for all tentative identifications is provided in SI-3. Eight tentatively identified phthalic anhydrides were excluded from the count of 172 because they may rapidly hydrolyze in water38, and are therefore unlikely to exist in breast milk. Unknown reagents may have transformed to the anhydrides in the GC injection port.
The 172 tentatively identified compounds (that were matched only to a reference library spectrum) were prioritized for confirmation by authentic standards using two criteria. 1) All tentatively identified halogenated compounds were selected. 2) Non-halogenated cyclic or aromatic compounds that were present in all three breast milk samples were selected. In total, 22 halogenated and 18 non-halogenated cyclic or aromatic compounds met the prioritization criteria. Table 1 shows all prioritized compounds with the column definitions are as follows: CAS is the Chemical Abstracts Service Registry Number; Regulatory Status indicates if the compound is on a regulatory list catalogued by SciFinder; Additional Isomers indicates the additional number of compounds that share the same mass spectrum as the listed chemical, but have different retention times; ID Category is the chemical identification category specified in the Methods Section; Breast Milk Sample is the number of detects among the three milk samples; Lipid Fraction is the combined number of detects in the two lipid fractions (3 mothers * 2 lipid fractions = 6 samples total); and Water Fraction is the number of detects in the water fraction (3 samples total). 1,7-Dimethylnaphthalene and 1,4,5-trimethylnaphthalene were prioritized because one of their isomers were detected in all three breast milk samples (see below).
Table 1.
Contaminants identified in human breast milk by non-targeted analysis. Det. Freq. = Detection Frequency.
| Name | CAS | Regulatory Status | Additional Isomers | ID Category | Breast Milk Sample Det. Freq. (Out of 3) | Lipid Fraction 1 Det. Freq. (Out of 3 | Lipid Fraction 2 Det. Freq. (Out of 3 | Water Fraction Det. Freq. (Out of 3) |
|---|---|---|---|---|---|---|---|---|
| Halogenated Compounds | ||||||||
| 1-Chloro-3-dimethylaminobenzene | 6848-13-1 | Regulated | 1 [authentic MS RT] | 3 | 0 | 2 | 1 | |
| 4-Chloro-N-methylaniline | 932-96-7 | Regulated | 1 | 1 [authentic MS RT] | 3 | 3 | 2 | 2 |
| N-(4-Chlorophenyl)formamide | 2617-79-0 | Not regulated | 1 [authentic MS RT] | 3 | 2 | 1 | 3 | |
| p-Chlorophenyl methyl sulfoxide | 934-73-6 | Regulated | 3 [reference database MS] | 3 | 0 | 3 | 0 | |
| 1-(4-Chlorophenyl)pyrrole | 5044-38-2 | Not regulated | 1 [authentic MS RT] | 2 | 1 | 1 | 1 | |
| 4,4′-Dichlorodiphenylether | 2444-89-5 | Regulated | 1 [authentic MS RT] | 2 | 1 | 1 | 0 | |
| 4,4′-Dichlorodiphenylsulphide | 5181-10-2 | Regulated | 1 [authentic MS RT] | 2 | 1 | 2 | 1 | |
| 4-Chlorodiphenyl ether | 7005-72-3 | Regulated | 1 [authentic MS RT] | 2 | 1 | 1 | 1 | |
| 4-Chlorothioanisole | 123-09-1 | Regulated | 1 [authentic MS RT] | 2 | 1 | 2 | 1 | |
| HCB | 118-74-1 | Regulated | 1 [authentic MS RT] | 2 | 2 | 0 | 0 | |
| beta-HCH | 319-85-7 | Regulated | 1 [authentic MS RT] | 1 | 1 | 0 | 1 | |
| BDE-47 | 5436-43-1 | Regulated | 1 [authentic MS RT] | 1 | 1 | 0 | 0 | |
| PCB-153 | 35065-27-1 | Regulated | 1 [authentic MS RT] | 1 | 1 | 0 | 0 | |
| 3’-Chloroacetanilide | 588-07-8 | Regulated | 1 [authentic MS RT] | 1 | 1 | 0 | 0 | |
| 4-Chlorobutyrophenone | 939-52-6 | Regulated | 1 [authentic MS RT] | 1 | 0 | 1 | 0 | |
| 7-Chloroquinaldine | 4965-33-7 | Regulated | 1 [authentic MS RT] | 1 | 0 | 1 | 0 | |
| p,p′-DDE | 72-55-9 | Regulated | 1 [authentic MS RT] | 1 | 1 | 0 | 0 | |
| 2-Chloro-N,N-dimethylaniline | 698-01-1 | Not regulated | 2 [authentic MS] | 1 | 1 | 0 | 0 | |
| p,p′-DDT | 50-29-3 | Regulated | 1 [authentic MS RT] | 1 | 1 | 0 | 0 | |
| 3-Chloro-2,6-dimethylpyridine | 2405-06-3 | Not regulated | 1 | 3 [reference database MS] | 1 | 1 | 0 | 0 |
| 4-Formylphenyl 3-chloropropanoate | NA | Not regulated | 3 [reference database MS] | 1 | 0 | 0 | 1 | |
| Thiophene-2-carbonitrile, 5-tert-butyl-3-(4-chlorobenzylidenamino)- | NA | Not regulated | 3 [reference database MS] | 1 | 0 | 1 | 0 | |
| Non-Halogenated Aromatic or Cyclic Compounds | ||||||||
| 1,3-Diacetylbenzene | 6781-42-6 | Regulated | 1 | 1 [authentic MS RT] | 3 | 3 | 2 | 1 |
| 2,4-Di-tert-butylphenol | 96-76-4 | Regulated | 1 | 1 [authentic MS RT] | 3 | 1 | 1 | 1 |
| 2-Cyanobenzoic acid | 3839-22-3 | Not regulated | 1 [authentic MS RT] | 3 | 1 | 2 | 0 | |
| 2-Hydroxymethylbenzoic acid | 612-20-4 | Regulated | 1 [authentic MS RT] | 3 | 2 | 2 | 1 | |
| 2-Methylindole | 95-20-5 | Regulated | 1 [authentic MS RT] | 3 | 2 | 0 | 3 | |
| 4-Methylbiphenyl | 644-08-6 | Regulated | 2 | 1 [authentic MS RT] | 3 | 3 | 2 | 0 |
| Benzophenone | 119-61-9 | Regulated | 1 [authentic MS RT] | 3 | 2 | 2 | 3 | |
| Benzyl butyl phthalate | 85-68-7 | Regulated | 1 [authentic MS RT] | 3 | 3 | 0 | 1 | |
| Ethyl 4-ethoxybenzoate | 23676-09-7 | Regulated | 1 [authentic MS RT] | 3 | 2 | 3 | 2 | |
| Isatin | 91-56-5 | Regulated | 1 [authentic MS RT] | 3 | 0 | 0 | 3 | |
| 2,4-Dimethylpropiophenone | 35031-55-1 | Regulated | 2 [authentic MS] | 3 | 1 | 1 | 1 | |
| 1,1,6-Trimethyltetralin | 475-03-6 | Regulated | 3 [reference database MS] | 3 | 3 | 0 | 0 | |
| 5H-1-Pyrindine | 270-91-7 | Not regulated | 3 [reference database MS] | 3 | 3 | 0 | 3 | |
| 5-Methyltetralin | 2809-64-5 | Not regulated | 1 | 3 [reference database MS] | 3 | 2 | 3 | 0 |
| N-(2-acetylphenyl)formamide | 5257-06-7 | Regulated | 3 [reference database MS] | 3 | 0 | 0 | 3 | |
| Phenylamide | 55-21-0 | Regulated | 1 [authentic MS RT] | 3 | 0 | 0 | 3 | |
| 1,7-Dimethylnaphthalene | 575-37-1 | Regulated | 2 | 1 [authentic MS RT] | 2 | 2 | 2 | 0 |
| 1,4,5-Trimethylnaphthalene | 2131-41-1 | Regulated | 3 | 1 [authentic MS RT] | 1 | 0 | 1 | 0 |
Thirty-two authentic standards were used to verify the identities of 30 of the 40 prioritized compounds (Table 1 ID Category = 1 [authentic MS RT]); a verification success rate of 94%. The remaining 2 authentic standards matched the mass spectra but not the retention time of the corresponding breast milk contaminants, indicating they were isomers or a different chemical structure with the same mass spectrum. (Table 1 ID Category = 2 [authentic MS]). Authentic standards could not be obtained for 8 of the prioritized compounds (Table 1 ID Category = 3 [reference database MS]). Seven additional mass spectra that were halogenated based on the presence of characteristic bromine and/or chlorine isotopic patterns, but did not have a matching spectrum in the NIST EI Library, were classified as unknown halogenated compounds. Their mass spectra are provided in SI-3. Two of the unknown halogenated compounds were found in two of three mothers’ samples (Unknown # 2 and Unknown #5), the other unknown halogenated compounds were found in one of three mothers’ samples.
We detected the common breast milk targets p,p′-DDT, p,p′-DDE, PCB-153, BDE-47, HCB, and beta-HCH (DDT = dichlorodiphenyltrichloroethane, HCB = hexachlorobenzene, and HCH = hexachlorocyclohexane). We did not, however, detect other legacy or pharmaceutical and personal care product contaminants that have been measured in targeted studies, such as multiple PCB and PBDE congeners, and other organochlorine pesticides4,11,12,39. This is likely due to the detection limit of the non-targeted method. Assuming a whole milk density of 1.031 g/mL40, and an average lipid content of 3.5%41, our estimated range of detection limits was approximately 5 to 50 ng/g lipid. This range is higher than the concentration of many individual compounds typically quantified in breast milk surveys4,11,12,39. It also indicates the concentrations of compounds detected by the non-targeted analysis may be higher than those of the typically monitored contaminants.
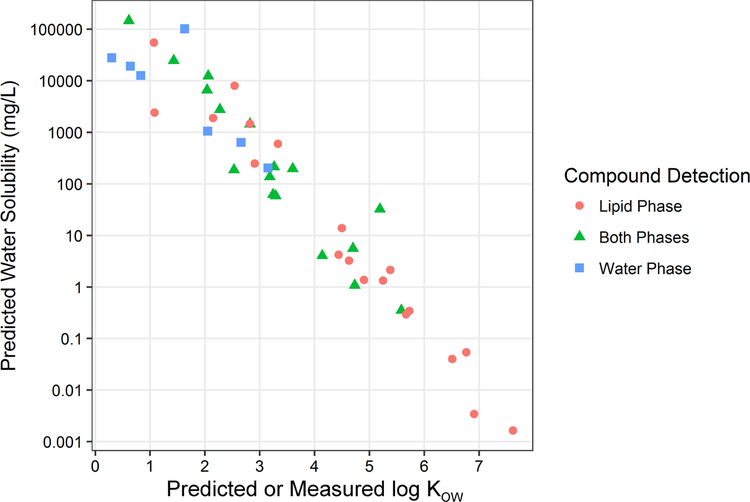
We applied the non-targeted GC×GC-TOF/MS method to the analysis of contaminants in the lipid and water fractions of breast milk, where lipid fraction refers to the combined hexane/DCM and DCM silica SPE extracts of the lipid portion of whole milk. The frequency of detection in each fraction is shown in Table 1. A majority of the 40 prioritized detections were in the lipid phase only (n = 19), perhaps due a greater proportion of lipophilic vs. water soluble compounds in whole milk, and/or instrumental detection bias towards non-polar compounds. The other compounds were detected in the both the lipid and water phases (n = 17) and water phase only (n = 4). Of the 172 total contaminants detected (prioritized plus non-prioritized), 44 were detected in the water phase only (26%). This indicates the water phase may be a significant matrix for breast milk contaminant measurements, perhaps with the addition of derivatization methods for GC based analysis, or liquid chromatography/tandem mass spectrometry. This finding was reinforced by comparison among the predicted or measured log KOW of the contaminants identified in each fraction (Figure 2), where smaller log KOW values indicates greater water solubility (Figure 3). The maximum log KOW among contaminants detected in the lipid fraction only was 7.6, in both the lipid and water fraction was 5.6, and in the water fraction only was 3.2.
Figure 2.
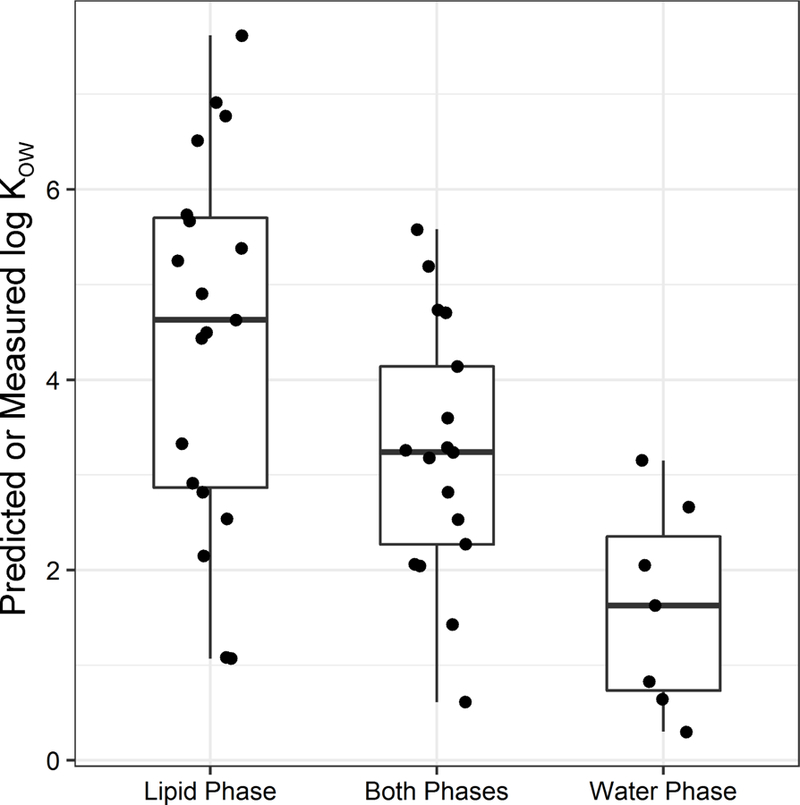
Logarithmic octanol-water partition coefficients (log KOW) for all prioritized breast milk contaminants listed in Table 1, plus the three additional water phase compounds identified in 2 of 3 samples (to increase from n = 3 to n = 6 in this category).
Figure 3.
Logarithmic octanol-water partition coefficient (log Kow) vs. water solubility (mg/L) at 25 °C for the contaminants listed in Table 1, plus three additional water phase compounds as discussed in the text. The point shape and color indicates the phase in which the compound was detected (see legend).
The six persistent organic pollutants p,p′-DDT, p,p′-DDE, PCB-153, BDE-47, HCB, and beta-HCH were the only contaminants regularly measured in breast milk surveys. The other 34 prioritized contaminants (85%) are not typically monitored. Thirty-one of the 40 prioritized chemicals (77%) are regulated in at least one market worldwide. Presence on a regulatory listing indicates the compound is produced in significant quantities and has risk-associated properties. However, the exact source of the chemicals to the subjects in this study is unknown, and we cannot exclude the possibility that a chemical may have an alternate natural source or is a transformation product. Since chemical concentrations were not quantified by the non-targeted identification method, we did not further assess the risk of these compounds.
Two PAH identifications were verified with authentic standards: 1,7-dimethylnaphthalene and 1,4,5-trimethylnaphthalene (Table 1). Apparent isomers of both compounds (with identical mass spectra but differing retention times) were also observed. In total, dimethylnaphthalene had three isomers and trimethylnaphthalene had four isomers. Exposure to PAHs is usually determined through measurement of urinary phase 1 hydroxy-PAH metabolites42; however, targeted measurements have found unmetabolized PAHs in human blood34 and breast milk11,43–45. The two PAHs we identified were not reported in these prior targeted analyses.
In conclusion, results of this study indicate GC×GC-TOF/MS non-targeted analysis of breast milk is capable of comprehensively identifying unexpected exogenous chemical exposure to the mother and infant. Non-targeted analysis is an initial step in assessing a broad range of contaminant exposure. Future related work is to 1) further increase the sensitivity of detection ; 2) evaluate the risk of exposure through expanded occurrence measurements and toxicological assessment; and 3) determine if the unknown spectra frequently occur in larger sample sets, and if so, identify and further investigate these compounds as emerging contaminants.
Supplementary Material
SI-1 contains detailed descriptions of the materials, sample preparation method, GC×GC/TOF-MS parameters (Table S1), and Figure S1.
SI-2 is a comma separated value (CSV) file describing the identification of all 172 compounds across all samples and fractions.
SI-3 shows the GC×GC/TOF-MS electron impact mass spectra and the matching library spectrum for the identified compounds and unknown spectra in all the fractions of the three samples (in total 9 fractions).
Highlights:
breast milk as a useful biomonitoring matrix for non-targeted analysis
successful screening, prioritization, and identification via nontargeted analysis
A total of 172 anthropogenic halogenated and non-halogenated cyclic and aromatic compounds identified in breast milk by non-targeted analysis
85% of 40 prioritized contaminants are not typically monitored in breast milk surveys
Thirty compounds out of 32 prioritized compounds were matched with their corresponding authentic standards (at 94% verification success rate achieved)
Acknowledgements
We acknowledge Renee Bridge for assisting in obtaining the samples at the University of California San Diego Medical Center and Kayo Watanabe for assisting with the analysis.
FUNDING SOURCES
This study was supported by National Children’s Study Formative Research Contract (HHSN267200700021C), and San Diego State University’s University Grants Program. This publication was made possible in part by the National Institute of Environmental Health Sciences (NIEHS: P01-ES021921).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interest.
References
- (1).Wild CP The exposome: from concept to utility. Int. J. Epidemiol 2012, 41 (1), 24–32. [DOI] [PubMed] [Google Scholar]
- (2).Needham LL; Wang RY Analytic considerations for measuring environmental chemicals in breast milk. Environ. Health Perspect 2002, 110 (6), A317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wigle DT; Arbuckle TE; Turner MC; Bérubé A; Yang Q; Liu S; Krewski D Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J. Toxicol. Environ. Health Part B 2008, 11 (5–6), 373–517. [DOI] [PubMed] [Google Scholar]
- (4).Schlumpf M; Kypke K; Wittassek M; Angerer J; Mascher H; Mascher D; Vökt C; Birchler M; Lichtensteiger W Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere 2010, 81 (10), 1171–1183. [DOI] [PubMed] [Google Scholar]
- (5).Promotion & Support | Breastfeeding | CDC; https://www.cdc.gov/breastfeeding/promotion/index.htm (accessed Feb 9, 2017). [Google Scholar]
- (6).WHO | 10 facts on breastfeeding http://www.who.int/features/factfiles/breastfeeding/en/ (accessed Feb 9, 2017).
- (7).Somogyi A; Beck H Nurturing and breast-feeding: exposure to chemicals in breast milk. Environ. Health Perspect 1993, 101 (Suppl 2), 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Landrigan PJ; Sonawane B; Mattison D; McCally M; Garg A Chemical contaminants in breast milk and their impacts on children’s health: an overview. Environ. Health Perspect 2002, 110 (6), A313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Mead MN Contaminants in human milk: weighing the risks against the benefits of breastfeeding. Env. Health Perspect 2008, 116 (10), A427–A434. [PMC free article] [PubMed] [Google Scholar]
- (10).Solomon GM; Weiss PM Chemical contaminants in breast milk: time trends and regional variability. Environ. Health Perspect 2002, 110 (6), A339–A347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Çok I; Mazmanci B; Mazmanci MA; Turgut C; Henkelmann B; Schramm K-W Analysis of human milk to assess exposure to PAHs, PCBs and organochlorine pesticides in the vicinity Mediterranean city Mersin, Turkey. Environ. Int 2012, 40, 63–69. [DOI] [PubMed] [Google Scholar]
- (12).Lee S; Kim S; Lee H-K; Lee I-S; Park J; Kim H-J; Lee JJ; Choi G; Choi S; Kim S; et al. Contamination of polychlorinated biphenyls and organochlorine pesticides in breast milk in Korea: Time-course variation, influencing factors, and exposure assessment. Chemosphere 2013, 93 (8), 1578–1585. [DOI] [PubMed] [Google Scholar]
- (13).Zimmers SM; Browne EP; O’Keefe PW; Anderton DL; Kramer L; Reckhow DA; Arcaro KF Determination of free Bisphenol A (BPA) concentrations in breast milk of U.S. women using a sensitive LC/MS/MS method. Chemosphere 2014, 104, 237–243. [DOI] [PubMed] [Google Scholar]
- (14).Kay K Polybrominated biphenyls (PBB) environmental contamination in Michigan, 1973–1976. Environ. Res 1977, 13 (1), 74–93. [DOI] [PubMed] [Google Scholar]
- (15).Brilliant L; Van Amburg G; Isbister J; Humphrey H; Wilcox K; Eyster J; Bloomer A; Price H Breast-milk monitoring to measure Michigan’s contamination with polybrominated biphenyls. The Lancet 1978, 312 (8091), 643–646. [DOI] [PubMed] [Google Scholar]
- (16).Miller FD; Brilliant LB; Copeland R Polybrominated biphenyls in lactating Michigan women: persistence in the population. Bull. Environ. Contam. Toxicol 1984, 32 (2), 125–133. [DOI] [PubMed] [Google Scholar]
- (17).Wolff MS; Anderson HA; Selikoff IJ Human Tissue Burdens of Halogenated Aromatic Chemicals in Michigan. JAMA 1982, 247 (15), 2112–2116. [PubMed] [Google Scholar]
- (18).Meironyté D; Norén K; Bergman A Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J. Toxicol. Environ. Health A 1999, 58 (6), 329–341. [DOI] [PubMed] [Google Scholar]
- (19).Norén K; Meironyté D Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere 2000, 40 (9–11), 1111–1123. [DOI] [PubMed] [Google Scholar]
- (20).Dodder NG; Maruya KA; Lauenstein GG; Ramirez J; Ritter KJ; Schiff KC Distribution and sources of polybrominated diphenyl ethers in the Southern California Bight. Environ. Toxicol. Chem. SETAC 2012, 31 (10), 2239–2245. [DOI] [PubMed] [Google Scholar]
- (21).Lebedev AT Environmental Mass Spectrometry. Annu. Rev. Anal. Chem 2013, 6 (1), 163–189. [DOI] [PubMed] [Google Scholar]
- (22).Moschet C; Lew BM; Hasenbein S; Anumol T; Young TM LC- and GC-QTOF-MS as Complementary Tools for a Comprehensive Micropollutant Analysis in Aquatic Systems. Environ. Sci. Technol 2017, 51 (3), 1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Albergamo V; Schollee JE; Schymanski EL; Helmus R; Timmer H; Hollender J; de Voogt P Nontarget screening reveals time trends of polar micropollutants in a riverbank filtration system. Environ. Sci. Technol 2019, 53 (13), 7584–7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Hoh E; Dodder NG; Lehotay SJ; Pangallo KC; Reddy CM; Maruya KA Nontargeted Comprehensive Two-Dimensional Gas Chromatography/Time-of-Flight Mass Spectrometry Method and Software for Inventorying Persistent and Bioaccumulative Contaminants in Marine Environments. Environ. Sci. Technol 2012, 46 (15), 8001–8008. [DOI] [PubMed] [Google Scholar]
- (25).Shaul NJ; Dodder NG; Aluwihare LI; Mackintosh SA; Maruya KA; Chivers SJ; Danil K; Weller DW; Hoh E Nontargeted Biomonitoring of Halogenated Organic Compounds in Two Ecotypes of Bottlenose Dolphins (Tursiops truncates) from the Southern California Bight. Environ. Sci. Technol 2015, 49 (3), 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Alonso MB; Maruya KA; Dodder NG; Brito JL; Azevedo A; Santos-Neto E; Torres JPM; Malm O; Hoh E A Comprehensive Non-Targeted Screening of Halogenated Organic Compounds in Bottlenose Dolphins from Brazil. Environ. Sci. Technol 2017, 51 (3), 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Mackintosh SA; Dodder NG; Shaul NJ; Aluwihare LI; Maruya KA; Chivers SJ; Danil KD; Weller DW; Hoh E Newly Identified DDT-Related Compounds Accumulating in Southern California Bottlenose Dolphins. Environ. Sci. Technol 2016, 50 (22), 12129–12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Cossaboon JM; Hoh E; Chivers SJ; Weller DW; Danil K; Maruya KA; Dodder NG Apex Marine Predators and Ocean Health: Proactive Screening of Halogenated Organic Contaminants reveals Ecosystem Indicator Species. Chemosphere 2019, 221, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Chibwe L; Titaley IA; Hoh E; Simonich SLM Integrated Framework for Identifying Toxic Transformation Products in Complex Environmental Mixtures. Environ. Sci. Technol. Lett 2017, 4 (2), 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Chibwe L; Davie-Martin CL; Aitken MD; Hoh E; Massey Simonich SL Identification of polar transformation products and high molecular weight polycyclic aromatic hydrocarbons (PAHs) in contaminated soil following bioremediation. Sci. Total. Environ 2017, 599–600, 1099–1107. [DOI] [PubMed] [Google Scholar]
- (31).Xu EG; Richardot WH; Li S; Buruaem L; Wei H; Dodder NG; Schick SF; Novotny T; Schlenk D; Gerberg RM; Hoh E Assessing toxicity and in vitro bioactivity of smoked cigarette leachate using cell-based assays and chemical analysis. Chem. Res. Toxicol 2019. 10.1021/acs.chemrestox.9b00201 [DOI] [PubMed]
- (32).Dälluge J,; Vreuls RJJ; Beens J Optimization and characterization of comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection (GC x GC-TOF MS). J. Sep. Sci 2002, 25, 201–214. [Google Scholar]
- (33).Stein SE; Scott DR Optimization and testing of mass spectral library search algorithms for compound identification. J. Am. Soc. Mass Spectrom 1994, 5 (9), 859–866. [DOI] [PubMed] [Google Scholar]
- (34).United States Environmental Protection Agency. EPI Suite; United States Environmental Protection Agency, 2017. [Google Scholar]
- (35).American Chemical Society. SciFinder; 2017.
- (36).Nickerson K Environmental Contaminants in Breast Milk. J. Midwifery Womens Health 2006, 51 (1), 26–34. [DOI] [PubMed] [Google Scholar]
- (37).Howard P; Muir D Identifying New Persistent and Bioaccumulative Organics Among Chemicals in Commerce. Environ. Sci. Technol 2010, 44 (7), 2277–2285. [DOI] [PubMed] [Google Scholar]
- (38).Muir D; Howard P Are there other persistent organic pollutants? A challenge for environmental chemists. Environ. Sci. Technol 2006, 40 (23), 7157–7166. [DOI] [PubMed] [Google Scholar]
- (39).Lee S; Kim S; Kim E; Lee I-S; Choi G; Kim H-J; Park J; Jae Lee J; Choi S; Young Kim S; et al. Polybrominated diphenyl ethers (PBDEs) in breast milk of Korea in 2011: Current contamination, time course variation, influencing factors and health risks. Environ. Res 2013, 126, 76–83. [DOI] [PubMed] [Google Scholar]
- (40).How much expressed milk will my baby need? • KellyMom.com. KellyMom.com, 2011.
- (41).Campoy C; Jimenez M; Olea-Serrano MF; Frias MM; Canabate F; Olea N; Bayés R; Molina-Font JA Analysis of organochlorine pesticides in human milk: preliminary results. Early Hum. Dev 2001, 65, S183–S190. [DOI] [PubMed] [Google Scholar]
- (42).Pleil JD; Stiegel MA; Sobus JR; Tabucchi S; Ghio AJ; Madden MC Cumulative exposure assessment for trace-level polycyclic aromatic hydrocarbons (PAHs) using human blood and plasma analysis. J. Chromatogr. B 2010, 878 (21), 1753–1760. [DOI] [PubMed] [Google Scholar]
- (43).Pulkrabova J; Stupak M; Svarcova A; Rossner P; Rossnerova A; Ambroz A; Sram R; Hajslova J Relationship between atmospheric pollution in the residential area and concentrations of polycyclic aromatic hydrocarbons (PAHs) in human breast milk. Sci. Total Environ 2016, 562, 640–647. [DOI] [PubMed] [Google Scholar]
- (44).Tsang HL; Wu S; Leung CKM; Tao S; Wong MH Body burden of POPs of Hong Kong residents, based on human milk, maternal and cord serum. Environ. Int 2011, 37 (1), 142–151. [DOI] [PubMed] [Google Scholar]
- (45).Santonicola S; De Felice A; Cobellis L; Passariello N; Peluso A; Murru N; Ferrante MC; Mercogliano R Comparative study on the occurrence of polycyclic aromatic hydrocarbons in breast milk and infant formula and risk assessment. Chemosphere 2017, 175, 383–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SI-1 contains detailed descriptions of the materials, sample preparation method, GC×GC/TOF-MS parameters (Table S1), and Figure S1.
SI-2 is a comma separated value (CSV) file describing the identification of all 172 compounds across all samples and fractions.
SI-3 shows the GC×GC/TOF-MS electron impact mass spectra and the matching library spectrum for the identified compounds and unknown spectra in all the fractions of the three samples (in total 9 fractions).