Abstract
Oral tolerance (OT) is defined as the specific suppression of cellular and/or humoral immune responses to an antigen by prior administration of the antigen through the oral route. Although the investigation of OT has classically involved antigen feeding, we have found that oral administration of anti-CD3 monoclonal antibody induced tolerance through regulatory T (Treg) cell generation. However, the mechanisms underlying this effect remains unknown. Here we show that conventional, but not plasmocytoid dendritic cells (DCs), are required for anti-CD3-induced OT. Moreover, oral anti-CD3 promotes XCL1 secretion by small intestine lamina propria (SILP) γδ T cells that in turn induces tolerogenic XCR1+ DCs migration to the mesenteric lymph node, where Treg cells are induced and OT established. Consistent with this, TCRδ−/− mice did not develop OT upon oral administration of anti-CD3. However, XCL1 was not required for OT induced by fed antigens, indicating that a different mechanism underlies this effect. Accordingly, oral administration of anti-CD3 enhanced OT induced by fed MOG35-55 peptide resulting in less severe experimental autoimmune encephalomyelitis, which was associated with decreased inflammatory immune cell infiltration in the central nervous system and increased Treg cells in the spleen. Thus, Treg cell induction by oral anti-CD3 is a consequence of the cross talk between γδ T cells and tolerogenic DCs in the gut. Furthermore, anti-CD3 may serve as an adjuvant to enhance OT to fed antigens.
INTRODUCTION
The gastrointestinal immune system has the unique capacity to discriminate between potentially dangerous and harmless material, promoting an inflammatory immune response against pathogenic microbes and toxins while inducing tolerance to food antigens and commensal microbes. Dysfunction of this balance can lead to pathologies such as food allergy, autoimmune diseases and infections. In this context, oral administration of foreign antigen induces local and systemic hyporesponsiveness to a subsequent challenge with the fed antigen and this phenomenon has been named “oral tolerance” (1).
Multiple mechanisms have been proposed to explain the immune hyporesponsiveness to fed antigens: low doses of orally administered antigen favor active suppression with the generation of regulatory T (Treg) cells, whereas high doses favor clonal anergy/deletion (2). However, induction of Treg cells expressing the transcription factor Foxp3 and the latency-associated peptide (LAP; a membrane-bound TGF-β) stands out as the major players in oral tolerance (3, 4). Although oral tolerance has classically involved oral administration of antigens, we have previously shown that oral administration of anti-CD3 monoclonal antibody induced tolerance in several animal models of autoimmune and inflammatory diseases, including experimental autoimmune encephalomyelitis (EAE) (4), streptozotocin-induced and NOD autoimmune diabetes (5-7), type 2 diabetes in the Ob/Ob mouse (8), lupus prone SNF1 mice (9) and atherosclerosis (10). Moreover, oral anti-CD3 has also been tested in a single-blind randomized placebo-controlled phase 2a study in patients with nonalcoholic steatohepatitis (NASH) and altered glucose metabolism that included subjects with type-2 diabetes. Positive results including a reduction in liver enzymes and reduced blood levels of glucose and insulin were found (11). Importantly, oral tolerance induced by anti-CD3 involved Treg cell expansion in both animal models (4, 12) and humans (11), but the mechanism underlying this effect is not known. The fact that the Fc portion of anti-CD3 was not required for oral tolerance induction, as anti-CD3 Fab′2 fragment is active orally and induces Treg cells (13, 14), suggests that the tolerogenic effects of anti-CD3 depends on T cell activation rather than an indirect effect through a putative Fc receptor activation on antigen-presenting cells (APCs) in the gut. However, because of the indispensable role of dendritic cells (DCs) in promoting Treg cell differentiation (15, 16), tolerogenic DCs are likely to be indirectly involved in anti-CD3-induced oral tolerance.
Generation of Treg cells requires several steps with a critical participation of the innate immune system present in the gut lamina propria called GALT (gut-associated lymphoid tissue). Antigen uptake by DCs underlying regular villus epithelium is critical for the development of oral tolerance (17). After sampling food or microbe antigens, tolerogenic DCs migrate to the mesenteric lymph node (mLN), where they induce Treg cells by releasing TGF-β and retinoic acid (RA) (18). Two major subtypes of tolerogenic DCs responsible for oral tolerance induction have been recently characterized. IRF4-dependent migratory DCs, also called conventional DC type 2 (cDC2) express CD11c, CD11b, CD103 and the signal-regulatory protein alpha (Sirpα, also known as CD172a), which are distinguished from the IRF8/BATF3-dependent migratory DCs (named cDC1) that are CD11c+, CD11b−, CD103+ and express the lymphotactin (XCL1) receptor XCR1. Importantly, cDC1 are the most potent tolerogenic subset because of the expression of high levels of TGF-β and the retinoic acid-catalyzing enzyme RALDH (19). The primary factor responsible for DC migration to the secondary lymphoid organs such as mLN is the chemokine receptor CCR7, which binds to the chemokines CCL19 and CCL21 that are highly expressed in these sites (20). Consistent with this, mice deficient for CCR7 failed to induce oral tolerance (21). Importantly, lymphocytes from both IEL and lamina propria compartments have been shown to secrete XCL1, which binds to its receptor XCR1 expressed on CD103+ DCs from the gut lamina propria, likely resulting in CCR7 upregulation on these DCs and migration to the mLN (22). Once in the mLN, CD11c+CD103+ DCs present antigens to cognate CD4+ T cells and differentiate them into Treg cells (15).
As mentioned above, oral administration of anti-CD3 is known to induce Treg cells, but the mechanism underlying this effect remains elusive. Here we show that orally administered anti-CD3 induces a population of γδ T cells in the small intestine lamina propria to produce the XCL1 that in turn leads to XCR1-expressing tolerogenic cDC1 to upregulate CCR7 and migrate to mLN to induce Treg cells and tolerance. Importantly, XCL1 was not required for oral tolerance induced by fed myelin oligodendrocyte glycoprotein peptide (MOG35-55), indicating that the mechanisms involved in anti-CD3 and fed antigen-induced oral tolerance are different. Accordingly, oral anti-CD3 potentiated oral tolerance induced by MOG35-55, resulting in less severe experimental autoimmune encephalomyelitis (EAE), a rodent model for multiple sclerosis (MS). Thus, we provide strong evidences that the mechanism by which anti-CD3 induces oral tolerance relies on the increased migration of tolerogenic DC from the small intestine lamina propria to the mesenteric lymph node in a XCL1/XCR1 dependent fashion and the subsequent induction of Treg cells. Moreover, anti-CD3 may serve as an adjuvant to enhance oral tolerance to fed antigens for the treatment of autoimmune diseases such as MS.
MATERIALS AND METHODS
Mice.
Male and female, 6–10-week-old on a B6 genetic background mice were used in this study. C57BL/6J wild type, TCRδ−/−, zDC-DTR and BDCA2-DTR mice were purchased from the Jackson Laboratory and housed in a conventional specific pathogen-free facility at the Hale Building for Transformative Medicine according to the animal protocol with the full knowledge and permission of the Standing Committee on Animals at Harvard Medical School and Brigham and Women’s Hospital.
FACS and intracellular cytokine staining.
In the experiments where sorted cells were required, cells from the intraepithelial lymphocyte (IEL) compartment of C57BL/6 were sorted for CD45+CD3+TCRβ+ or CD45+CD3+TCRγδ+ cells using APC-anti-CD45 (30-F11; 1:300; Biolegend), AF700-anti-CD3ε (17A2; 1:300; Biolegend), BV605-anti-TCRβ (H57-597; 1:300; Biolegend) and BV421-anti-TCRγδ (GL3; 1:200; Biolegend). To sort αβ and γδ T cells from the small intestine lamina propria, cells were first enriched using CD45 microbeads (Miltenyi Biotec) and sorted using the fluorescent-labeled antibodies described for IEL cell sorting. Dead cells were excluded based on 7-AAD (BD Biosciences) staining. In some experiments, the fixable viability dye Aqua Zombie (1:1000; Biolegend) was used to exclude dead cells.
For intracellular cytokine staining, cells were first stimulated for 4 h with PMA (phorbol 12-myristate 13-aceate; 50 ng ml−1; Sigma-Aldrich) and ionomycin (1 μM; Sigma-Aldrich) and a protein-transport inhibitor containing monensin (1 μg ml−1 GolgiStop; BD Biosciences) before detection by staining with antibodies. Surface markers were stained for 25 min at 4°C in Mg2+ and Ca2+ free HBSS with 2% FCS, 0.4% EDTA (0.5 M) and 2.5% HEPES (1M) then were fixed in Cytoperm/Cytofix (eBioscience), permeabilized with Perm/Wash Buffer (eBiosciences). Flow-cytometric acquisition was performed on a Fortessa (BD Biosciences) by using DIVA software (BD Biosciences) and data were analyzed with FlowJo software versions 10.1 (TreeStar Inc). Intracellular staining antibodies used: FITC-anti-Foxp3 (FJK-16s; 1:100; ThermoFisher), BV421-anti-IFN-γ (XMG1.2; 1:300; Biolegend), PE-Cy7-anti-IL-17A (eBio17B7; 1:300; eBioscience), PE-anti-IL-10 (JES5.16E3; 1:100; eBioscience). Other antibodies included: AF488-anti-CD45 (30-F11; 1:200; Biolegend), , BV605-anti-CD4 (RM4.5; 1:300; BD Bioscience), APC-anti-TCRγδ (GL3; 1:100; Biolegend), PerCP-Cy5.5-anti-CD11c (N418; 1:300; Biolegend), BV421-anti-CCR7 (4B12; 1:200; Biolegend), APC-anti-XCR1 (ZET; 1:200; Biolegend), PE-Cy7-anti-Sirpα (P84; 1:300; Biolegend), AF700-anti-CD11b (M1/70; 1:300; Biolegend), PE-anti-CD103 (2E7; 1:200; Biolegend).
Oral tolerance induction.
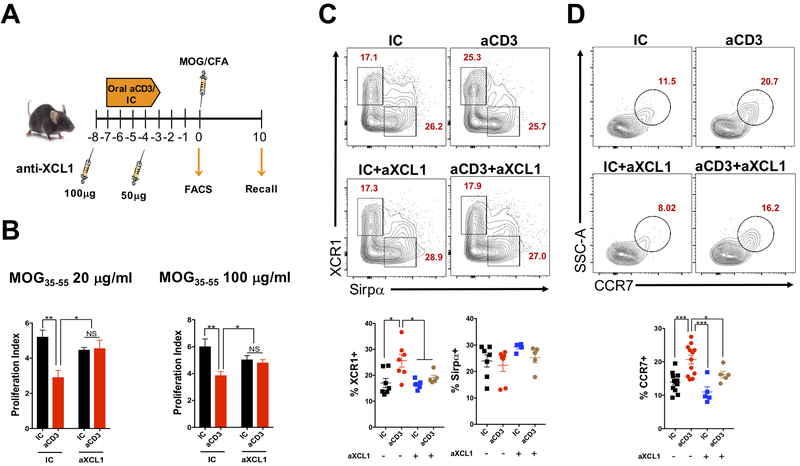
Oral tolerance was induced by gavaging mice with 10 μg/200μl/mouse of anti-CD3 (145-2C11; Biolegend) or isotype control for 5 days. In another experiment, 250 μg/200μl/mouse of MOG35-55 peptide (Genemed Synthesis) was given orally for 5 consecutive days. Control animals received only 200 μl of PBS. Three days after the last feeding, mice were immunized with 100 μg of MOG35-55 in complete Freund’s adjuvant (CFA; BD™ Difco™) in the ventral flanks. In vitro recall responses were measured at day 10 after immunization. For this, splenocytes were stimulated with 4, 20 and 100 μg/ml of MOG35-55 (antigen-specific stimulation) or 0.01, 0.1 and 1 μg/ml of anti-CD3 (antigen non-specific stimulation), and proliferation measured using 3H-thymidine incorporation. Proliferation index was calculated by dividing the counts per minute (cpm) of stimulated cells by the average of the cpm of non-stimulated cells from the same group. To investigate the role of XCL1 in oral tolerance, 100 μg and 50 μg/200 μl/mouse of anti-XCL1 mAb (80222; R&D Systems) was injected intraperitoneally one day before oral administration of anti-CD3 or MOG35-55 and on the 4th day of the oral treatments, respectively.
In vivo depletion of dendritic cell populations.
For transient diphtheria toxin (DT) ablation, BDCA2-DTR mice or WT mice reconstituted with zDC-DTR mouse bone marrow were intraperitoneally injected with 20 ng of DT (Sigma-Aldrich) per gram of bodyweight. Mice were euthanized 48 h after DT injection and mesenteric lymph node and spleen used for controlling depletion. To maintain DT ablation during oral tolerance induction, mice received three doses of DT, on day −1, day +1 and day+3 of oral tolerance induction by feeding anti-CD3 or isotype control for 5 consecutive days, as described above.
EAE induction.
EAE was induced by injecting mice with 80 μg MOG35-55 peptide (Genemed Synthesis) emulsified in complete Freund’s adjuvant (CFA) (BD™ Difco™) per mouse subcutaneously in the flanks, followed by intraperitoneal administration of 150 ng of pertussis toxin (List biological laboratories, Inc.) per mouse on the day of immunization and 48 h later. Clinical signs of EAE were assessed according to the following criteria: 0, no signs of disease; 0.5, partial tail paralysis; 1, tail paralysis or waddling gait; 1.5, partial tail paralysis and waddling gait; 2, tail paralysis and waddling gait; 2.5, partial limb paralysis; 3, paralysis of one limb; 3.5, paralysis of one limb and partial paralysis of another; 4, complete hind-limb paralysis; 4.5, complete hind-limb paralysis and front-limb weakness; 5, moribund.
Real-time PCR.
Small intestine lamina propria and intraepithelial lymphocyte αβ and γδ T cells were sorted from naïve, IC or anti-CD3 treated mice 3 days after the last dose and RNA was extracted with a RNeasy Plus micro kit (Qiagen), then was reverse-transcribed with a high capacity cDNA reverse transcription kit (ThermoFisher Scientific) and analyzed by quantitative RT-PCR with a Vii 7 Real-time PCR system (Applied Biosystems) with the following primers and probes (from ThermoFisher Scientific; identifier in parentheses): Xcl1 (Mm00434772_m1) The comparative threshold cycle method and the internal control Gapdh (Mm99999915-g1) was used for normalization of Xcl1 target gene.
Statistics.
GraphPad Prism 7.0 was used for statistical analysis (unpaired, two-tailed Student’s t-test or one-way ANOVA, followed by Tukey multiple comparisons). Two-way ANOVA was used for EAE experiments. Differences were considered statistically significant with a p value of less than 0.05.
RESULTS
Oral administration of anti-CD3 induces cDC1 migration.
To investigate the mechanisms involved in oral tolerance induced by anti-CD3, we first treated mice with oral anti-CD3 or isotype control (IC) for 5 consecutive days and 3 days later we immunized them with MOG35-55 emulsified in complete Freund’s adjuvant (CFA). Oral tolerance was measured 10 days later by splenocyte proliferation upon MOG35-55 stimulation (Supplementary Fig. 1A). As expected (4), anti-CD3, but not IC, induced oral tolerance as shown by decreased splenocyte proliferation after in vitro stimulation with 4, 20 and 100 μg/ml of MOG35-55 (Supplementary Fig. 1B, C). Consistent with this, anti-CD3-treated mice showed increased frequencies of MOG35-55-specific CD4+ Treg cells (Vβ11+) expressing LAP in the spleen (Th3 cells) as well as Foxp3 and LAP in the inguinal lymph node (iLN; Supplementary Fig. 1D), which drains the inguinal subcutaneous region where immunization was performed.
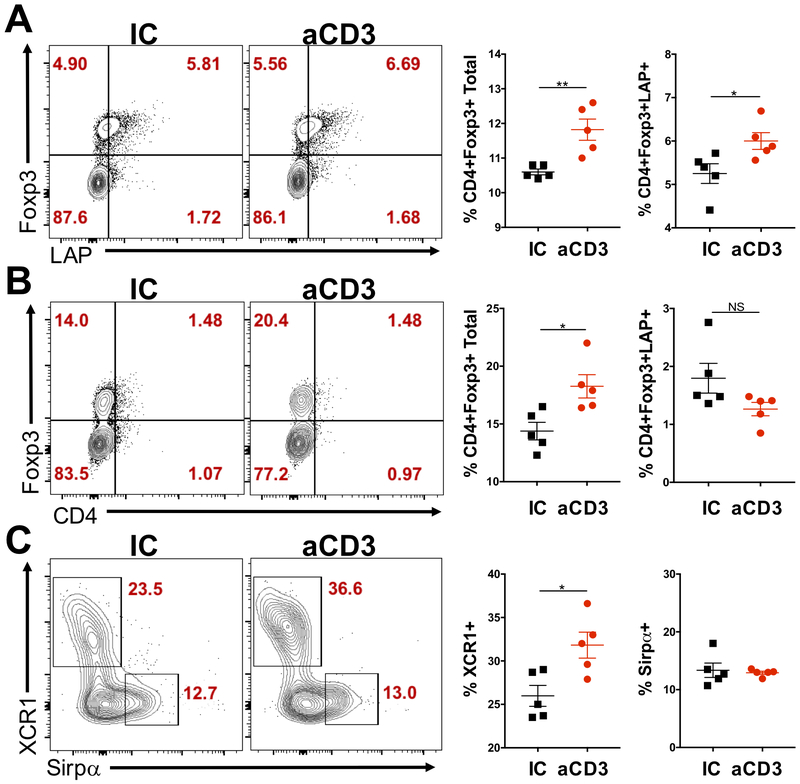
Importantly, flow cytometric analysis of mesenteric lymph node (mLN) T cells 3 days after the last dose of anti-CD3 or IC showed increased frequencies of Foxp3+ Treg cells in mice treated with anti-CD3, but not IC (Fig. 1A). Moreover, these Foxp3+ Treg cells expressed more LAP (Fig. 1A), indicating a higher activation status. Furthermore, Foxp3+ Treg cells were increased in the small intestine lamina propria (SILP) 5 days after the last dose of oral anti-CD3, but not IC (Fig. 1B), suggesting that expanded Treg cells may have migrated from the mLN to the SILP, as previously demonstrated during oral tolerance induced by fed antigens (23). However, no difference was observed in LAP-expressing Treg cells (Fig. 1B). Thus, oral administration of anti-CD3 induces Foxp3+LAP+ Treg cells, which play a pivotal role in the induction of oral tolerance by anti-CD3 (4).
Figure 1. Increased Treg cells and cDC1s in the mLN from mice treated with oral anti-CD3.
(A-C) FACS plots and bar graphs showing Foxp3 and LAP expression in CD4+ cells from the mesenteric lymph node (mLN; A), small intestine lamina propria (SILP; B), as well as XCR1 and Sirpα on CD11c+CD103+ dendritic cells (DCs; C) in the mLN from mice treated for 5 consecutive days with 10 μg of either anti-CD3 (aCD3) or isotype control (IC); n=5 mice/group. mLN and SILP were removed 3 and 5 days, respectively, after the last dose of anti-CD3 or IC; n=5 mice/group. Data are shown as mean ± SEM and are representative of two to three independent experiments. Student’s t test was used. * p<0.05, ** p<0.01.
Because DCs are critical for Treg cell induction (15, 16, 19), we investigated whether DCs migrated to mLN after oral anti-CD3 administration in order to induce Treg cell expansion (Fig. 1A, B), as previously shown for oral tolerance induced by fed antigens (19). We analyzed the two major intestinal migratory DC populations, cDC1 (CD11c+CD11b−CD103+XCR1+) and cDC2 (CD11c+CD11b+CD103+Sirpα+) in the mLN from mice 3 days after the last dose of anti-CD3 or IC. We found a significant increase in the frequency of cDC1s, but not cDC2s in the mLN from mice treated with anti-CD3, but not IC (Fig. 1C), suggesting that oral administration of anti-CD3 may induce intestinal XCR1-expressing DC migration to the mLN and that these cells may play a critical role in the oral tolerance induced by anti-CD3.
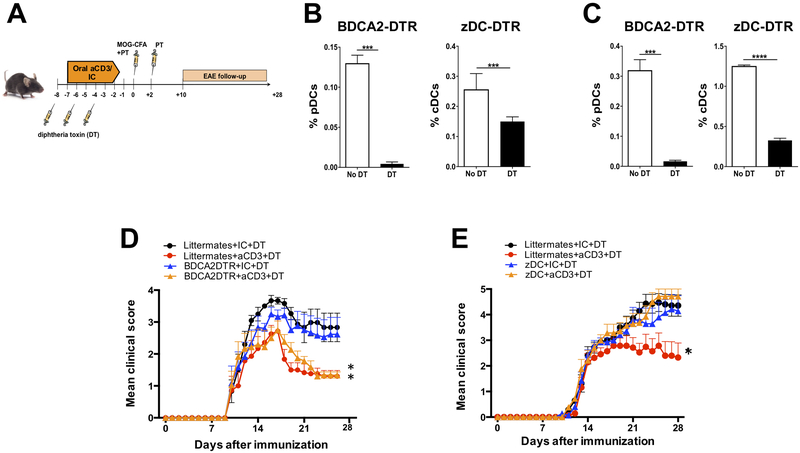
To confirm the involvement of DCs in the oral tolerance induced by anti-CD3, we depleted the two major subsets of DCs: the plasmocytoid DC (pDC) and conventional DCs (cDCs) and tested the ability of anti-CD3 to induce oral tolerance in the EAE mouse model of multiple sclerosis. To specifically deplete pDCs, we used the BDCA2-DTR mice (24) followed by diphtheria toxin (DT) injection; and to deplete cDCs, we used lethally irradiated C57BL/6J WT mice repopulated with bone marrow from the zDC-DTR (Zbtb46-DTR) mice (25) followed by DT injection (Fig. 2A). The use of chimera mice in this procedure is critical, because DT administration in zDC-DTR mice is fatal within 24–48 hours due to a yet unknown essential group of radioresistant cells (26). We used the zDC-DTR animals instead of other transgenic mice for DC depletion because zDC (Zbtb46, Btbd4) is specifically expressed by cDCs and committed cDC precursors but not by monocytes, pDCs, or other immune cell populations. Accordingly, in contrast to the previously characterized CD11c-DTR mice, for example, non-cDCs, including pDCs, monocytes, macrophages, and NK cells, are spared after DT injection in zDC-DTR mice, indicating a selective ablation of cDCs (25). Of note, DT was injected three times during oral anti-CD3 or IC administration to assure depletion of DCs in the critical period of oral tolerance induction, and a significant depletion of pDCs and cDCs were observed in secondary lymphoid organs (mLN and spleen) of BDCA2-DTR and zDC-DTR, respectively (Fig. 2B, C). We found that depletion of cDCs, but not pDCs, completely abrogated the tolerogenic effect of orally-administered anti-CD3 as shown by the reversion of the decreased severity of EAE induced by anti-CD3 (Fig. 2D, E). This is consistent with our findings that cDC1s, which are conventional DCs, are increased in the mLN from mice treated with anti-CD3 (Fig. 1C). Thus, conventional DCs play a crucial role in the anti-CD3-induced oral tolerance.
Figure 2. Anti-CD3-induced oral tolerance is abrogated in the absence of cDC, but not pDC.
(A) Scheme for testing tolerance induction by oral anti-CD3 in the EAE model with or without depletion of conventional dendritic cells (cDCs) or plasmocytoid DCs (pDCs). (B, C) Depletion of plasmocytoid (pDC) and conventional dendritic cells (cDC) in the mLN (B) and spleen (C) from BDCA2-DTR and zDC-DTR mice 48 h after 20 ng of diphtheria toxin (DT) injection. (D, E) EAE clinical score of of DT-treated BDCA2-DTR mice (D), zDC-DTR mice (E) or respective littermate controls with or without previous feeding with anti-CD3; n=7-10 mice/group. Data are shown as mean ± SEM and are representative of two independent experiments. Two-way ANOVA was used. * p<0.05, ** p<0.01. NS= not significant.
Intestinal γδ T cells produce XCL1 upon oral administration of anti-CD3.
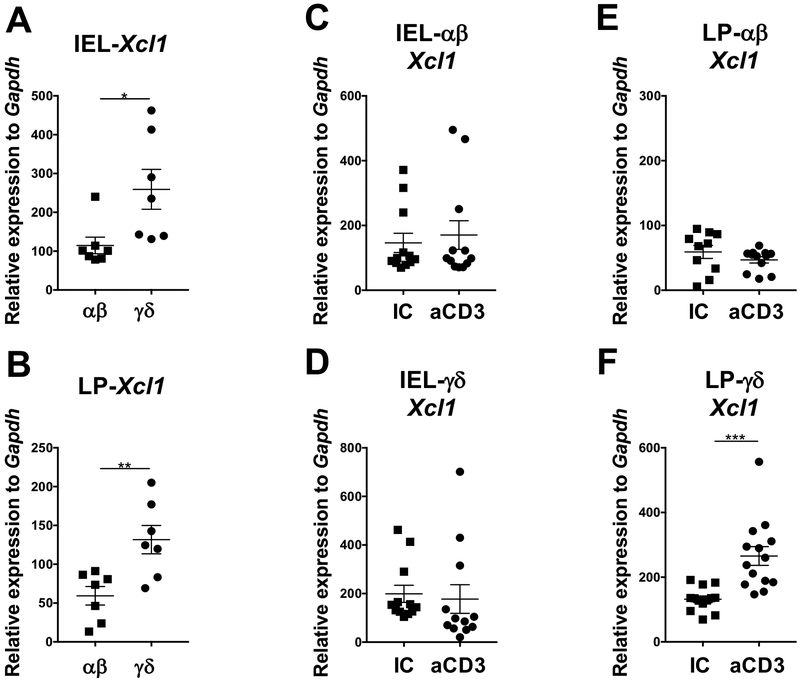
It has been suggested that the chemokine XCL1 upon binding to its receptor XCR1 expressed on intestinal cDC1s induce the upregulation of CCR7, a chemokine receptor critical for DC migration to the mLN (22). Because anti-CD3 must bind to the CD3 molecule, which is solely expressed on T cells, we investigated whether oral administration of anti-CD3 induced XCL1 production by T cells from the small intestine (SI) intraepithelial lymphocyte (IEL) compartment and SI lamina propria (LP). To address this question, we fed mice with anti-CD3 for 5 consecutive days and analyzed αβ and γδ T cells from IEL and SILP 3 days after the last dose of anti-CD3 (Fig. 3A). We found that frequency of αβ and γδ T cells did not change after anti-CD3 administration in both IEL and SILP compartments (not shown). Compared to αβ T cells, IEL and SILP γδ T cells naturally expressed more Xcl1 mRNA, regardless of anti-CD3 administration (Fig. 3A, B). However, only SILP γδ T cells upregulated Xcl1 mRNA after oral administration of anti-CD3 (Fig. 3C-F). Thus, these data suggest that XCL1 produced by γδ T cells may be the link between T and DCs involved in anti-CD3-induced oral tolerance.
Figure 3. Small intestine γδ T cells, but not αβ T cell, express Xcl1 mRNA after oral administration of anti-CD3.
(A, B) Baseline Xcl1 mRNA expression in αβ and γδ T cells from intraepithelial lymphocyte (IEL; A) and small intestine lamina propria (LP; B) compartments. (C-F) Xcl1 mRNA expression in IEL-αβ (C), IEL-γδ (D), LP-αβ (E) and LP-γδ (F) three days after the last dose of five consecutive days of oral administration of anti-CD3 (aCD3) or isotype control (IC) both given at 10 μg/mouse; n=7-14 mice/group. Data are shown as mean ± SEM and are representative of three independent experiments. Student’s t test was used. * p<0.05, ** p<0.01, *** p<0.001.
Anti-CD3-induced oral tolerance is impaired in γδ T cell deficient mice.
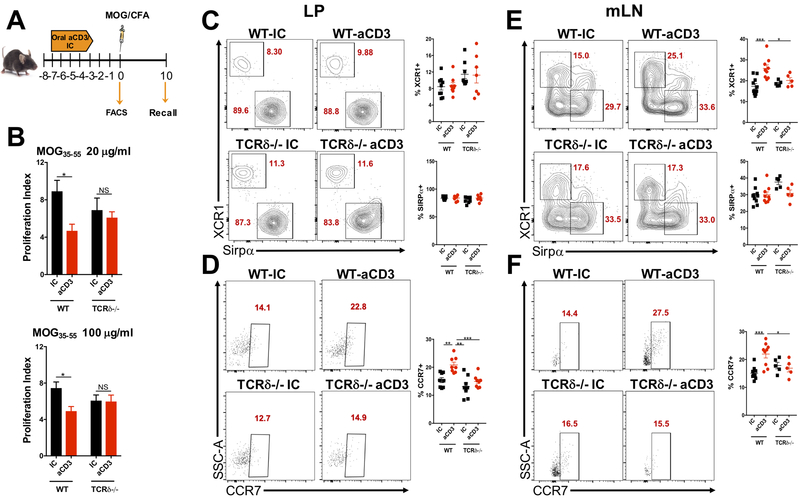
Because we found that γδ, but not αβ T cells upregulated Xcl1 mRNA after oral administration of anti-CD3, we investigated whether γδ T cell deficient (TCRδ−/−) mice had impaired oral tolerance to anti-CD3. To address this, WT and TCRδ−/− mice were fed anti-CD3 or IC for 5 consecutive days, immunized with MOG35-55/CFA 3 days after the last dose of anti-CD3 or IC and sacrificed 10 days after immunization for spleen removal and recall assay. A group of WT and TCRδ−/− mice treated with anti-CD3 or IC was sacrificed before immunization for flow cytometric analyzes of DCs from the SILP and mLN (Fig. 4A). We found that anti-CD3 failed to induce oral tolerance in TCRδ−/− mice as shown by increased splenocyte proliferation upon MOG35-55 stimulation (Fig. 4B). Consistent with the fact that TCRδ−/− mice may have reduced production of XCL1, we observed a trend in cDC1, but not cDC2 accumulation in the SILP of these mice (Fig. 4C). Accordingly, the increased frequency of CCR7-expressing cDC1s in the SILP observed after oral administration of anti-CD3 in WT mice was completely abolished in TCRδ−/− mice (Fig. 4D), suggesting an impaired migration of these cells to the mLN. In fact, anti-CD3 treatment induced increased frequencies of cDC1s in the mLN from WT mice (Fig. 1C) and these cells expressed higher levels of CCR7, which we did not observe in the mLN from TCRδ−/− mice (Fig. 4D, E), confirming that the migration of tolerogenic SILP cDC1s is impaired in γδ T cell deficient mice. Thus, upon oral administration of anti-CD3 in WT mice, cDC1s migrate from the SILP to the mLN. However, in TCRδ−/− mice, production of XCL1 is impaired due to the lack of γδ T cells and XCR1-expressing DCs (cDC1s) tend to accumulate in the lamina propria (and consequently not to migrate to the mLN, where Treg cells are induced and oral tolerance is established) because of the lower expression of CCR7, which is likely induced by the interaction between XCL1 and its receptor XCR1.
Figure 4. TCRδ−/− mice have impaired anti-CD3-induced oral tolerance.
(A) Scheme for oral administration of anti-CD3 (aCD3) or isotype control (IC), MOG35-55/CFA immunization and recall assay in wild type (WT) and TCRδ−/− mice. (B) Ten days after MOG35-55/CFA immunization, mice were sacrificed, and spleens removed for recall assay. Splenocytes were stimulated in the presence of 20 or 100 μg/ml of MOG35-55 for three days and proliferation index analyzed by H3-thymidine incorporation; n=5-10 mice/group. (C, D) FACS plots showing XCR1 and Sirpα expression on CD11c+CD103+ dendritic cells (DC; C) as well as CCR7 expression on CD11c+CD103+XCR1+ cDC1s (D) from small intestine lamina propria (LP) of WT or TCRδ−/− mice treated orally with 10 μg of either anti-CD3 (aCD3) or isotype control (IC) and sacrificed 3 days after the last dose of aCD3 or IC; n=7-9 mice/group. (E, F) FACS plots showing XCR1 and Sirpα expression on CD11c+CD103+ DCs (E) as well as CCR7 expression on CD11c+CD103+XCR1+ cDC1s (F) from mesenteric lymph node (mLN) of WT or TCRδ−/− mice treated orally with 10 μg of either anti-CD3 (aCD3) or isotype control (IC) and sacrificed 3 days after the last dose of aCD3 or IC; n=5-9 mice/group. Data are shown as mean ± SEM and are representative of three independent experiments. One-way ANOVA was used. * p<0.05, *** p<0.001, **** p<0.0001.
Thus, the impaired oral tolerance induced by anti-CD3 in TCRδ−/− mice may be associated with a deficient migration of intestinal cDC1s to the mLN due to an impaired XCL1/XCR1 signaling pathway in DCs from these mice.
XCL1 is critical for anti-CD3-induced oral tolerance.
To investigate the role of XCL1 in the oral tolerance induced by anti-CD3, WT mice were fed either anti-CD3 or IC for 5 consecutive days with or without anti-XCL1 monoclonal antibody (mAb) treatment, immunized with MOG35-55/CFA 3 days after the last dose of anti-CD3 or IC and sacrificed 10 days after immunization for spleen removal and recall assay. A group of WT mice treated with anti-CD3 or IC that received or not anti-XCL1 mAb was sacrificed before immunization for flow cytometric analyzes of DCs from the mLN (Fig. 5A). We found that anti-CD3 failed to induce oral tolerance in mice treated with anti-XCL1 as shown by increased splenocyte proliferation upon MOG35-55 stimulation (Fig. 5B), confirming that XCL1 plays a critical role in the oral tolerance induced by anti-CD3. Importantly, this effect was associated with decreased frequencies of cDC1s, but not cDC2s, in the mLN of oral anti-CD3 fed mice and treated with anti-XCL1 mAb (Fig. 5C). Moreover, mLN cDC1s from these mice showed a reduced expression of CCR7 (Fig. 5D), indicating that XCL1 is indeed crucial for cDC1 migration to the mLN upon oral administration of anti-CD3.
Figure 5. Anti-XCL1 monoclonal antibody administration blocks oral tolerance induced by anti-CD3.
(A) Scheme for anti-XCL1 monoclonal antibody (mAb) administration on oral administration of anti-CD3 (aCD3) or isotype control (IC); MOG35-55/CFA immunization and recall assay. (B) Ten days after MOG35-55/CFA immunization, mice were sacrificed, and spleens removed for recall assay. Splenocytes were stimulated in the presence of 20 or 100 μg/ml of MOG35-55 for three days and proliferation index analyzed by H3-thymidine incorporation; n=5-10 mice/group. (C, D) FACS plots showing XCR1 and Sirpα expression on CD11c+CD103+ dendritic cells (DC; C) as well as CCR7 expression on CD11c+CD103+XCR1+ cDC1s (D) from mesenteric lymph node (mLN) of WT mice treated orally with 10 μg of either anti-CD3 (aCD3) or isotype control (IC) and injected subcutaneously with two doses (100 and 50 μg) of anti-XCL1 (aXCL1) mAb or isotype control. Mice were sacrificed 3 days after the last dose of aCD3 or IC; n=5-12 mice/group. Data are shown as mean ± SEM and are representative of two independent experiments. One-way ANOVA was used. * p<0.05, ** p<0.01, *** p<0.001.
To investigate whether XCL1 also played a role in oral tolerance induced by fed antigens, WT mice were fed MOG35-55 or PBS for 5 consecutive days with or without anti-XCL1 mAb, immunized with MOG35-55/CFA 3 days after the last dose of MOG35-55 or PBS and sacrificed 10 days after immunization for spleen removal and proliferation assay (Supplemental Fig. 2A). We found that oral administration of MOG35-55 induced tolerance as expected, and this effect was not affected by XCL1 blockage (Supplemental Fig. 2B), suggesting that XCL1 is only required for anti-CD3-induced oral tolerance and that a different immune pathway is involved in the oral tolerance induced by antigen feeding.
Anti-CD3 enhances oral tolerance induced by MOG.
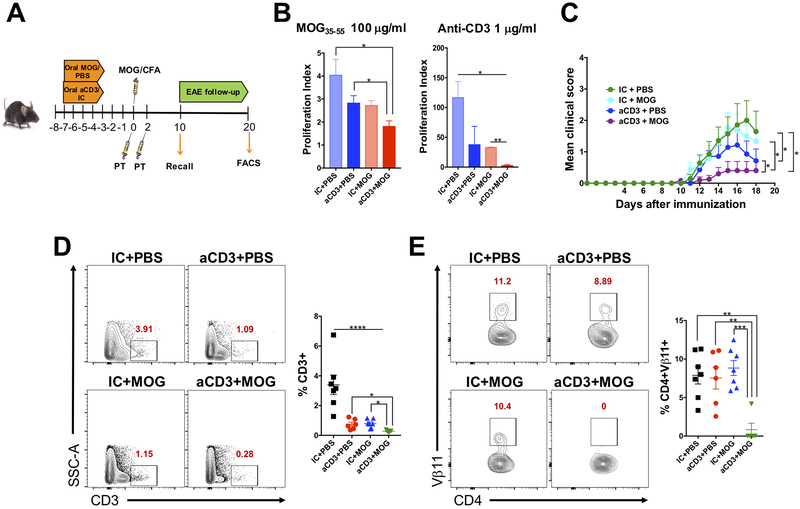
Because we found that oral tolerance induced by antigen feeding and anti-CD3 involves different pathways, we hypothesized that anti-CD3 may enhance MOG35-55-induced oral tolerance and thus function as an adjuvant. To address this, WT mice were fed both anti-CD3 and MOG35-55 for 5 consecutive days, immunized with MOG35-55/CFA 3 days after the last dose of anti-CD3/MOG and sacrificed 10 days later for spleen removal and recall assay as well as flow cytometric analyzes of Treg cells (Fig. 6A). We found that anti-CD3 enhanced oral tolerance induced by MOG35-55, as shown by reduced splenocyte proliferation upon MOG35-55 (specific) and anti-CD3 (unspecific) stimulation (Fig. 6B). Thus, anti-CD3 works as an adjuvant that enhances oral tolerance induced by fed antigens.
Figure 6. Anti-CD3 serves as an adjuvant to enhance oral tolerance induced by a fed antigen.
(A) Scheme for anti-CD3 (aCD3) or isotype control (IC), both given at 10 μg/mouse to enhance oral tolerance induced by MOG35-55, given at 250 μg/mouse. EAE was induced three days after the last dose of anti-CD3 or MOG35-55 and recall assay and FACS analysis performed 10 and 18 days, respectively, after immunization. (B) Ten days after MOG35-55/CFA immunization, mice were sacrificed, and spleens removed for recall assay. Splenocytes were stimulated in the presence of 100 μg/ml of MOG35-55 (specific stimulus) or 1 μg/ml of anti-CD3 (unspecific stimulus) for three days and proliferation index analyzed by H3-thymidine incorporation; n=6 mice/group. (C) EAE clinical score; n=6-7 mice/group. Only statistics for the last day of EAE follow-up (18 days post immunization) are shown. (D, E) FACS plots showing CD3+ T cell infiltration in the spinal cord (D) and Vβ11 expression on infiltrating CD3+CD4+ T cells in the spinal cord (E) 18 days after immunization; n=6-7 mice/group. Data are shown as mean ± SEM and are representative of two independent experiments. One-way ANOVA (B, D, E) or two-way ANOVA (C) were used. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
To further confirm the role of anti-CD3 as an adjuvant for oral tolerance induced by fed antigens, WT mice were fed both anti-CD3 and MOG-35–55 for 5 consecutive days and EAE was induced (Fig. 6A). We found that mice treated with both oral anti-CD3 and MOG35-55 showed less severe EAE (Fig. 6C), which was associated with decreased total CD3+ T cell and CD4+Vβ11+ MOG35-55-specific T cell infiltration into the spinal cord as compared to the other groups (oral vehicle, anti-CD3 or MOG35-55, separately) (Fig. 6D). Among the infiltrating cells, frequency of IFN-γ and IL-17-producing cells were significantly reduced in mice treated with both anti-CD3 and MOG35-55. (Supplemental Fig. 3). This suggests that combination of anti-CD3 and a fed antigen prevents effector T cell infiltration into the spinal cord during an inflammatory process. This effect likely occurred due to an expansion of Treg cells in the periphery, since we found that oral administration of both anti-CD3 and MOG35-55 induced increased Treg cell frequencies in the spleen 10 days after MOG35-55/CFA immunization and thus, before EAE onset (Supplemental Fig. 4). Thus, anti-CD3 functions as an adjuvant for the classical fed antigen oral tolerance induction by decreasing T cell infiltration and the consequent inflammation in the spinal cord.
DISCUSSION
A major goal of immunotherapy of autoimmune diseases is the induction of Treg cells that mediate immunologic tolerance. The intestinal mucosa is a perfect site for this purpose due to its tolerogenic microenvironment. However, for several autoimmune diseases, the self-antigens recognized and attacked by the immune system are not known, precluding the use of a specific oral therapy to promote Treg cell expansion and tolerance in these cases. An ideal approach would be the oral administration of a compound that induces tolerance independent of the antigen involved in the autoimmune disorder but that does not cause immunosuppression. Based on this idea, we have found that oral administration of anti-CD3 monoclonal antibody induced oral tolerance through a mechanism dependent on both CD4+CD25+LAP+ and CD4+CD25-LAP+ Treg cells in mLN and spleen (27). Importantly, oral anti-CD3 ameliorated disease in several mouse models of autoimmunity and inflammation (4-10, 12), indicating that anti-CD3 is a good candidate for oral tolerance induction to treat autoimmune and inflammatory diseases. In fact, human trials using humanized anti-CD3 have shown promising results and the mechanisms underlying the tolerogenic effects in people also appear to involve the generation of Treg cells (11). However, how oral administration of anti-CD3 increases Treg cell population is unknown and the present study aimed to investigate this mechanism.
A critical question that needed to be answered was whether anti-CD3 directly expanded Treg cells by binding to the CD3 complex expressed on these cells or whether it indirectly modulated DCs that in turn induced Treg cell differentiation and expansion as previously shown during oral tolerance induced by oral administration of antigens (19). We showed that mice lacking conventional DCs (cDCs), but not plasmocytoid DCs (pDCs), did not develop tolerance upon oral administration of anti-CD3, providing a strong evidence that the mechanism underlying Treg cell induction by oral administration of anti-CD3 likely relies on its ability to modulate migration of tolerogenic cDCs to the mLN. Consistent with this, we found that both frequencies and numbers of cDC1s, but not cDC2s, increased in the mLN from mice orally treated with anti-CD3 as soon as 3 days after the last dose of the antibody. This correlated with Treg cell expansion at the same time point in the mLN, with the subsequent increase in the frequency of these cells in the SILP 2 days later. cDC1 migration appeared to be dependent on CCR7 expression since CCR7-expressing cDC1 frequencies were increased in the mLN from anti-CD3-treated mice. This suggested that T cells in the gut, upon activation by anti-CD3, modulated DC migration. Consistent with this, it has been recently shown that DCs from mice deficient in either the chemokine XCL1 or its receptor XCR1 have impaired migration to the mLN due to reduced expression of CCR7 on these cells, suggesting that the XCL1/XCR1 axis controls CCR7 expression on DCs (22).
Interestingly, XCR1-expressing DCs are potent tolerogenic cells likely because of their high expression of TGF-β and retinoic acid, which are critical factors for Treg cell induction and migration from the mLN to the gut to promote tolerance (19). Since T cells from the intestinal lamina propria and IEL compartments can secrete XCL1 (22), we hypothesized that oral anti-CD3 induced XCL1 production by intestinal T cells. In fact, γδ T cells from both LP and IEL compartments expressed higher Xcl1 mRNA levels than αβ T cells and only γδ T cells from the SILP upregulated Xcl1 mRNA after oral administration of anti-CD3. This suggests that the CD3 complex from γδ T cells may be more sensitive to stimulation by low doses of anti-CD3 or express more CD3 epsilon chains than αβ T cells (28). However, the reason why anti-CD3 preferentially activates γδ T cells from the SILP is less clear, but it could be related to the fact that anti-CD3 accumulates in the lamina propria upon oral administration (4). Thus, it is possible that anti-CD3 reaches the lamina propria in a way that it does not encounter γδ T cells from the IEL compartment, such as through Microfold (M) cells. Furthermore, the mechanisms by which anti-CD3 induces the expression of XCL1 in γδ T cells are not clear. We believe that CD3 signaling pathway may explain the link between XCL1 production and CD3 engagement. This is because chemokine expression is usually induced by transcription factors (TFs) such as NF-kB and AP-1 (29), which are both activated upon CD3/TCR engagement and the subsequent signaling pathway. Thus, we hypothesize that upon anti-CD3 binding to the CD3/TCR complex, NF-kB and AP-1 may induce XCL1 gene expression.
The role of γδ T cells in the mechanism of DC migration induced by oral anti-CD3 became clearer when we attempted to induce oral tolerance in γδ T cell deficient mice. These animals did not develop tolerance upon oral anti-CD3 administration and this effect correlated with a decreased CCR7 expression on cDC1s from the SILP and the consequent impaired migration of these cells to the mLN. Furthermore, blocking XCL1 by using a monoclonal antibody, we recapitulated the findings observed in TCRδ−/− mice, indicating that XCL1 secreted by γδ T cells is the link connecting DC migration and Treg cell induction by oral administration of anti-CD3.
Strikingly, the XCL1/XCR1 axis was not required for the oral tolerance induced by fed MOG35-55. Because both anti-CD3- and fed antigen-induced oral tolerance depends on Treg cell induction in the mLN (4, 19), this finding suggests that the mechanism involved in DC migration to the mLN may differ in anti-CD3 vs. antigen feeding. In this context, orally administered antigens are sampled by DCs from the SILP, processed and presented to T cells in a MHC-II-dependent manner after migration to the mLN. On the other hand, anti-CD3 may potentiate SILP antigen-loaded DC migration to the mLN by inducing XCL1 production through γδ T cells. Based on this concept, we hypothesized that anti-CD3 could serve as an adjuvant for fed antigen-induced oral tolerance. Consistent with this, we found that oral administration of anti-CD3 enhanced oral tolerance to MOG35-55, as observed by decreased splenocyte proliferation and reduced EAE severity, an effect associated with decreased effector T cell infiltration into the spinal cord and increased Treg frequencies in the spleen of EAE mice. Thus, anti-CD3 may be an important adjuvant that can potentiate oral tolerance induced by fed antigens to treat autoimmune conditions, particularly where the antigen involved in the autoimmune disease is known.
In summary, we have identified the mechanism by which oral tolerance induced by anti-CD3 promotes regulatory T cell expansion and propose that anti-CD3 may be used in combination with fed antigens to potentiate oral tolerance and the consequent immunoregulation for the treatment of autoimmune diseases.
Supplementary Material
Key points.
Oral anti-CD3 induces gut lamina propria γδ T cells to produce XCL1
XCL1-activated CCR7+XCR1+ cDCs migrate to the mesenteric lymph node
In the mesenteric lymph node, Tregs are induced and oral tolerance established
ACKNOWLEDGEMENT
We thank Deneen Kozoriz for her excellent technical support in cell sorting.
This work was supported by the NIH (R01 AI43458 to H.L.W.).
Footnotes
DISCLOSURE
Authors declare no conflict of interest.
REFERENCES
- 1.Rezende RM, and Weiner HL. 2017. History and mechanisms of oral tolerance. Semin Immunol 30: 3–11. [DOI] [PubMed] [Google Scholar]
- 2.Rezende RM, and Weiner HL. 2018. Cellular Components and Mechanisms of Oral Tolerance Induction. Crit Rev Immunol 38: 207–231. [DOI] [PubMed] [Google Scholar]
- 3.Rezende RM, Oliveira RP, Medeiros SR, Gomes-Santos AC, Alves AC, Loli FG, Guimaraes MA, Amaral SS, da Cunha AP, Weiner HL, Azevedo V, Miyoshi A, and Faria AM. 2013. Hsp65-producing Lactococcus lactis prevents experimental autoimmune encephalomyelitis in mice by inducing CD4+LAP+ regulatory T cells. J Autoimmun 40: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, Chen ML, Gandhi R, Miller A, Maron R, and Weiner HL. 2006. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25− LAP+ T cells. Nat Med 12: 627–635. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, and Weiner HL. 2007. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes 56: 2103–2109. [DOI] [PubMed] [Google Scholar]
- 6.Bevier WC, Trujillo AL, Primbs GB, Bradley MK, and Jovanovic L. 2011. Oral anti-CD3 monoclonal antibody delays diabetes in non-obese diabetic (NOD) mice: effects on pregnancy and offspring--a preliminary report. Diabetes Metab Res Rev 27: 480–487. [DOI] [PubMed] [Google Scholar]
- 7.Hu C, Ding H, Zhang X, Wong FS, and Wen L. 2013. Combination treatment with anti-CD20 and oral anti-CD3 prevents and reverses autoimmune diabetes. Diabetes 62: 2849–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, and Weiner HL. 2010. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A 107: 9765–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu HY, Center EM, Tsokos GC, and Weiner HL. 2009. Suppression of murine SLE by oral anti-CD3: inducible CD4+CD25-LAP+ regulatory T cells control the expansion of IL-17+ follicular helper T cells. Lupus 18: 586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki N, Yamashita T, Takeda M, Shinohara M, Nakajima K, Tawa H, Usui T, and Hirata K. 2009. Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation 120: 1996–2005. [DOI] [PubMed] [Google Scholar]
- 11.Ilan Y, Shailubhai K, and Sanyal A. 2018. Immunotherapy with oral administration of humanized anti-CD3 monoclonal antibody: a novel gut-immune system-based therapy for metaflammation and NASH. Clin Exp Immunol 193: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Cunha AP, Wu HY, Rezende RM, Vandeventer T, and Weiner HL. 2015. In vivo anti-LAP mAb enhances IL-17/IFN-gamma responses and abrogates anti-CD3-induced oral tolerance. Int Immunol 27: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatenoud L, Primo J, and Bach JF. 1997. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol 158: 2947–2954. [PubMed] [Google Scholar]
- 14.Kuhn C, and Weiner HL. 2016. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy 8: 889–906. [DOI] [PubMed] [Google Scholar]
- 15.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, and Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, and Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317: 256–260. [DOI] [PubMed] [Google Scholar]
- 17.Chirdo FG, Millington OR, Beacock-Sharp H, and Mowat AM. 2005. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol 35: 1831–1840. [DOI] [PubMed] [Google Scholar]
- 18.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, and Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med 204: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esterhazy D, Loschko J, London M, Jove V, Oliveira TY, and Mucida D. 2016. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nat Immunol 17: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, and Lipp M. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99: 23–33. [DOI] [PubMed] [Google Scholar]
- 21.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Förster R, and Pabst O. 2006. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. Journal of Experimental Medicine 203: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta T, Sugiyama M, Hemmi H, Yamazaki C, Okura S, Sasaki I, Fukuda Y, Orimo T, Ishii KJ, Hoshino K, Ginhoux F, and Kaisho T. 2016. Crucial roles of XCR1-expressing dendritic cells and the XCR1-XCL1 chemokine axis in intestinal immune homeostasis. Sci Rep 6: 23505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassani B, Villablanca EJ, Quintana FJ, Love PE, Lacy-Hulbert A, Blaner WS, Sparwasser T, Snapper SB, Weiner HL, and Mora JR. 2011. Gut-tropic T cells that express integrin alpha4beta7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology 141: 2109–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swiecki M, Gilfillan S, Vermi W, Wang Y, and Colonna M. 2010. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity 33: 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, and Nussenzweig MC. 2012. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med 209: 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaft T, Sapoznikov A, Krauthgamer R, Littman DR, and Jung S. 2005. CD11chigh dendritic cell ablation impairs lymphopenia-driven proliferation of naive and memory CD8+ T cells. J Immunol 175: 6428–6435. [DOI] [PubMed] [Google Scholar]
- 27.Chen ML, Yan BS, Bando Y, Kuchroo VK, and Weiner HL. 2008. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol 180: 7327–7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thibault G, and Bardos P. 1995. Compared TCR and CD3 epsilon expression on alpha beta and gamma delta T cells. Evidence for the association of two TCR heterodimers with three CD3 epsilon chains in the TCR/CD3 complex. J Immunol 154: 3814–3820. [PubMed] [Google Scholar]
- 29.Rahman I, and MacNee W. 1998. Role of transcription factors in inflammatory lung diseases. Thorax 53: 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.