Abstract
Fusarium graminearum is a pervasive plant pathogenic fungal species. Biological control agents employ various strategies to weaken their targets, as shown by Bacillus species, which adopt various mechanisms, including the production of bioactive compounds, to inhibit the growth of F. graminearum. Various efforts to uncover the antagonistic mechanisms of Bacillus against F. graminearum have been undertaken and have yielded a plethora of data available in the current literature. This perspective article attempts to provide a unified record of these interesting findings. The authors provide background knowledge on the use of Bacillus as a biocontrol agent as well as details on techniques and tools for studying the antagonistic mechanism of Bacillus against F. graminearum. Emphasizing its potential as a future biological control agent with extensive use, the authors encourage future studies on Bacillus as a useful antagonist of F. graminearum and other plant pathogens. It is also recommended to take advantage of the newly invented analytical platforms for studying biochemical processes to understand the mechanism of action of Bacillus against plant pathogens in general.
Keywords: Bacillus, Fusarium graminearum, antagonism, mode of action
1. Introduction
Biotic stresses such as plant pests and pathogens are the major factors threatening global crop production. Proliferation in plants of these pathogens can cause devastating epidemics, which can cause severe food shortages especially in countries with limited resources. The current state of crop losses due to pathogenic diseases is alarming, with an estimated 8–40% of crop yield losses caused by plant pathogens worldwide [1,2]. One of such prominent pathogens of health and economic importance is Fusarium graminearum. This fungus is a known causative agent of Fusarium head blight (FHB), which is an economically important cereal crop disease that accounts for worldwide losses estimated between 20 and 100% [3,4,5,6]. According to Dean et al. [7], F. graminearum is ranked the fourth most important plant fungal pathogen, on the basis of its scientific and economic importance. This filamentous ascomycete infects floral tissues of cereal plants and contaminates food grains [7]. Infection is associated with premature bleaching symptoms, which mainly reduce grain quality and, less often, yield [3,7,8,9].
In addition to grain quality reduction, F. graminearum also produces various types of mycotoxins, which if ingested in huge amounts, cause various toxicoses in animals and humans [9]. The major mycotoxin produced by F. graminearum is deoxynivalenol (DON) together with other mycotoxins including the trichothecene nivalenol (NIV) and its derivatives, 3- and 15-acetyldeoxynivalenol (3-ADON and 15-ADON). These mycotoxins are reported to contaminate grain food products, thereby posing a threat to humans and animals by causing neurological disorders and immunosuppression [10,11,12] amongst other dysfunctions. However, these health complications vary from one animal species to the other and according to several factors such as trichothecene type, level, and route of exposure. This assembled body of evidence justifies the need for the biocontrol of F. graminearum in several foodstuffs [12]. In the past three decades, control strategies against this devastating plant pathogen have been based solely on fungicide application, which has resulted in long-term undesirable environmental pollution [13]. Herbicides and insecticides have also been used over the years to suppress the activity of this pathogenic microorganism causing FHB, amongst other diseases, in crops. Coupled with fungicides are various control practices such as sanitation, good agricultural practices, as well as the use of resistant cultivars. With the increase in awareness of the danger of chemical control applications, fungicides are beginning to take a back foot, with the use of biocontrol products being exploited.
With the increased desire for environmental friendliness and sustainability, the biocontrol of pathogens is equally receiving attention. Biocontrol is defined as the use of natural products and living organisms to suppress pathogen populations. The use of biocontrol agents either as an alternative to other forms of plant disease control or as a supplement has attracted worldwide attention to be included in an integrated pathogen management strategy in various food systems. However, to prevent an irrational selection of plant pathogen antagonists to be adopted as commercial products, the modes of the antagonists’ activities and effects need to be fully understood. Bacterial antagonists are commonly used, and many of them belong to the genus Bacillus [14].
In this perspective manuscript, we summarize the current knowledge about the mode of action of Bacillus species against the pervasive plant pathogen F. graminearum. Background information about Bacillus is provided, the antagonism of Bacillus and its mode of action, tools and techniques to uncover the mechanisms of the antagonism are described, and future prospects are presented.
2. Overview of Bacillus Species as a Protective Agent against Pathogens
Bacillus is one of the largest genera of bacteria that produce aerobically dormant endospores under diverse growth conditions [15]. Species belonging to this genus can play a role as human pathogens, whilst others promote plant health and development [16]. Due to their different genetic characteristics, Bacillus species are ideal candidates as biocontrol agents. Bacillus species play a role as bacterial antagonists to pathogens due to their ability to reproduce actively and their resistance to unfavorable environmental conditions [14]. The species’ antagonistic activities are associated with the production of metabolites with antibiotic properties [17]. Particularly, volatile metabolites produced by these microorganisms also play an important role in the activation of plant defense mechanisms by triggering induced systemic resistance (ISR) in plants [18]. In addition, plant host defense responses can also be activated during the production of metabolites by Bacillus species [19]. As documented in the literature, Bacillus spp. also directly antagonize fungal pathogens by competing and depriving them of essential nutrients, by producing fungitoxic compounds, and by inducing systemic acquired resistance in plants [20,21,22,23].
A wide range of pathogenic microorganisms have been controlled using Bacillus-based biocontrol agents [17,24,25,26]. Several disease control products produced from various strains have also been registered and are commercially available. A broad spectrum of resistance mechanisms against plant diseases have been reported to be induced by Bacillus strains in many studies [17,27]. Furthermore, the activity of other Bacillus strains was also investigated in different crops and found to be effective against various fungal plant pathogens and diseases, including Fusarium wilt in tomato [28] as well as FHB in wheat and barley [19,27].
2.1. Biological Activity of Bacillus in General and Against F. graminearum
Bacillus species can produce different antimicrobial substances that confer protection and act as biological agents [29]. Such substances include subtilin [30], bacilysin [31], mycobacillin [32], bacillomycin [33,34], mycosubtilin [35,36], iturins, fengycins, and surfactins [37]. These substances have been reported to exert antibacterial and/or antifungal activities against pathogenic microorganisms [17,19,26,27,28,29,30,31,32,33,34,35,36,37,38]. As noted in the literature, among these antimicrobial substances produced by Bacillus, the most studied with regard to F. graminearum are surfactin, fengycin, and iturin. For this reason, the literature reported herein focuses on these three Bacillus-produced antimicrobial agents.
The antagonism of these antimicrobial substances has been reported against F. graminearum [26,27], Fusarium oxysporum [39,40], Fusarium solani, and Rhizoctonia solani [40], amongst many other plant pathogenic fungi. In a study by Földes and colleagues [29], antagonistic compounds produced by Bacillus subtilis IFS-01 exhibited antimicrobial effects against phytopathogenic, food-borne, and spoilage microorganisms. In an agar diffusion assay, some of the filamentous fungi and yeasts tested showed no visible growth within the inhibition zone (about 10 mm from the colony) due to the antagonistic effect of B. subtilis IFS-01. These findings confirmed the biological control ability of this Bacillus strain against these fungi and yeasts. The iturin family of the lipopeptides produced by Bacillus amyloliquefaciens PPCB044 strain showed antagonism against pathogenic fungi from seven citrus plants during postharvest [41]. All the fungal pathogens were deterred by the B. amyloliquefaciens PPCB004 strain, as the strain produced compounds related to iturin A, fengycin, and surfactin [41]. Similar results were also described by Gong et al. [26], who reported the antagonism of iturin A and plipastatin A from B. amyloliquefaciens S76-3 in wheat inoculated with F. graminearum. The data obtained from both the growth chamber and the field plot assays revealed a strong antagonistic activity of strain S76-3 against the growth and development of F. graminearum. Iturin A killed the conidia at the minimal inhibitory concentration of 50 µg/mL, while plipastatin A exhibited a strong fungal activity at 100 µg/mL.
Zalila-Kolsi et al. [19] studied the FZB42 strain of B. amyloliquefaciens and found that the commercial bacterial strain produces the lipopeptide bacillomycin D, which contributes to its antimicrobial activity. Bacillomycin D showed a strong antagonism against F. graminearum at 30 µg/mL, which is its 50% effective concentration. The plasma membrane morphology and cell wall of F. graminearum were affected by bacillomycin D, while inducing the accumulation of reactive oxygen species (ROS) [19]. Furthermore, this lipopeptide caused cell death of the tested F. graminearum. Lipopeptide-type compounds from the iturin, fengycin, and surfactin families, synthesized by various strains of Bacillus, effectively suppressed the growth of pathogenic microorganisms [26,27,28,39,40,41]. These lipopeptides have different residues at specific positions but consist of variants with the same peptide length. Molecules of the iturin lipopeptide family are linked to a β-amino fatty acid of variable length (C14–C17), those of the surfactin family to a β-hydroxyl fatty acid (C12–C16), while fengycin decapeptides are linked to a β-hydroxyl fatty acid chain (C14–C18) [42]. These nonribosomal peptide synthetase-mediated compounds are surface-active and have emulsifying and foaming properties and haemolytic activity [43,44,45].
Different strains of Bacillus produce different groups of lipopeptides [46], and their role in suppressing/controlling plant pathogens may vary. Similar lipopeptides produced by various Bacillus strains can also suppress and control other pathogens of economic importance. In a study by Guo et al. [47], the antagonistic effect of the B. subtilis strain NCD-2, a fengycin-deficient mutant, was strong against R. solani in vitro and suppressed cotton damping-off disease in vivo. In addition, B. amyloliquefacien CM-2 and T-5 showed their antagonistic activities against the bacterium Ralstonia solanacearum in tomato [28]. The disease incidences were reduced by over 70% by both strains in comparison to the control. On the other hand, crude lipopeptide extracts of B. amyloliquefaciens SS-12.6 successfully suppressed leaf spot disease severity on sugar beet plants [48]. These studies showed significant antagonism of the various Bacillus strains against various pathogens. Many studies have reported the success of Bacillus as a biological control agent against F. graminearum in various crops and diseases. However, the potential of these biocontrol agents has not been fully exploited to control other pathogens. Therefore, different strains of Bacillus species should be studied further as potential biocontrol agents against other pathogenic microorganisms.
2.2. Surfactins, Fengycins, and Iturins in Bacillus Species
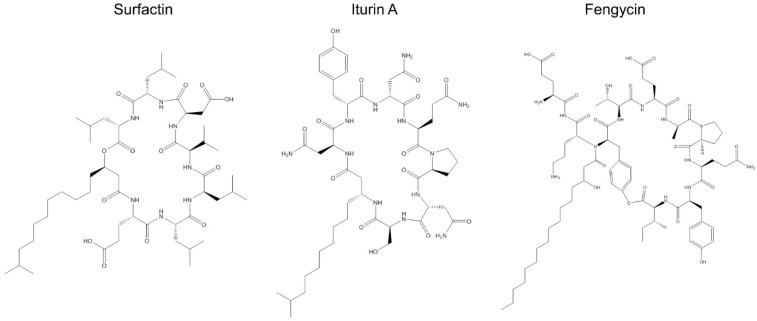
The production of surfactins, fengycins, and iturins by various strains of B. subtilis has been reported by numerous researchers [49,50,51,52,53,54,55,56,57,58], and a crude lipopeptide mixture of the supernatant of B. subtilis was once found to contain these polypeptides [59]. The main congener structures of these cyclic lipopeptide families are shown in Figure 1. Among the most studied in the surfactin family are surfactin linchenysin, pumilacidin WH1, and fungin; for the iturin family, the various iturin isomers—bacillomycins, mycosubtilin—are the best known, while for fengycin, the main compounds are feycin, plipastatin, and agrastatin 1 [60]. An overview of the activity of these three lipopeptides against fungi, with emphasis on F. graminearum, is provided in the following sections of this review.
Figure 1.
Congener structures of the cyclic lipopeptides; surfactin, iturin A, and fengycin. source: [61].
2.2.1. Surfactins
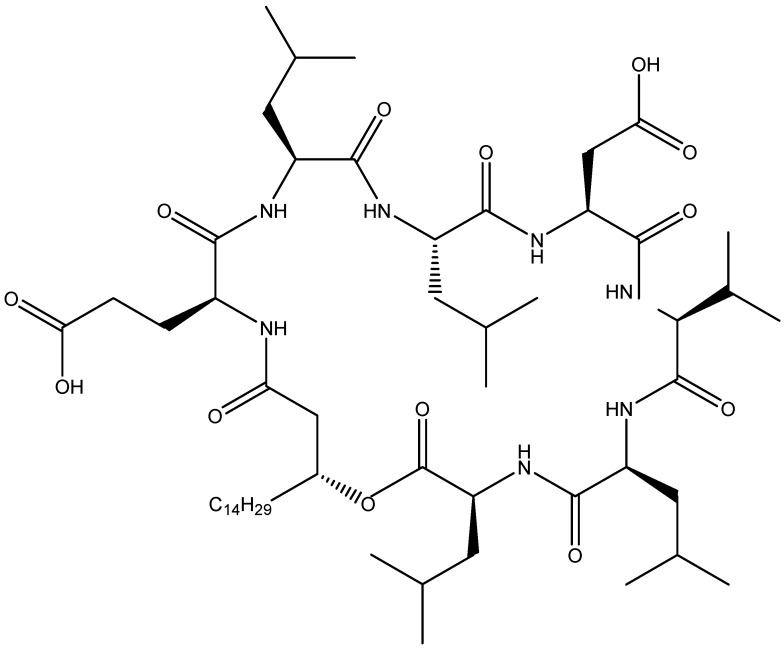
Surfactins are natural lipopeptides that have been reported to possess antifungal activity [42,61]. They include β-hydoxy hepta cyclic depsipeptides with possibile alanine, valine, leucine, or isoleucine amino acid variations at positions 2, 4, and 7 in the cyclic depsipeptide moiety and C13 to C16 variation in the β-hydroxy fatty acid chains [62,63,64]. Surfactin is amphiphilic, with a polar amino acid head and a hydrocarbon chain. This molecular structure makes surfactin a strong biosurfactant, which is at the basis of its antifungal properties. It is assumed that its antibiotic properties are due to its ability to produce selective cationic channels in the membrane phospholipid bilayer [65]. Several studies have been conducted to determine the effect of surfactin on fungi. Qi et al. [66] found a new surfactin, WH1fungin (Figure 2), which induces apoptosis in fungal cells. The same surfactin has also been reported in other studies as an oral immunoadjuvant that could be used for the development of vaccines [67,68]. Surfactin was also found to be effective against the plant pathogenic fungus Colletotrichum gloeosporiodes [57]. Another surfactin, Leu7-surfactin, produced by Bacillus mojavensis, was found to be effective against Fusarium verticillioides [69]. A similar inhibitory activity of surfactin was discovered against F. graminearum [17], F. oxysporum [70], and Fusarium moniliforme (presently F. verticillioides) [71]. This effect on F. graminearum can be culture condition-dependent [17,19], with iron concentration being the most important determinant [19].
Figure 2.
Structure of WH1fungin; source: [72].
2.2.2. Fengycin
The antimicrobial activity of Bacillus-produced lipopeptides is based on their chemistry. This is also the case with fengycin, which is a cyclic lipodecapeptide that contains a β-hydroxy fatty acid with a side chain consisting of 16–19 carbon atoms [73]. Fengycin is particularly active against filamentous fungi and inhibits the functions of the enzymes phospholipase A2 and aromatase [73]. It has various isoforms, which differ in length and branching of the β-hydroxy fatty acid moiety, as well as in the amino-acid composition of the peptide ring [50]. For instance, position 6 d-alanine (as in fengycin A) can be replaced by d-valine (as in fengycin B) [73,74]. Fengycin A presents 1 d-Ala, 1 l-Ile, 1 l-Pro, 1 d-allo-Thr, 3 l-Glx, 1 d-Tyr, 1 l-Tyr, 1 d-Orn, whereas in fengyicn B, d-Ala is replaced by d-Val.
Fengycin affects the integrity of biological membranes in a molar-ratio-dependent manner. The effects of fengycin on biological membranes depend on the concentration, but ultimately high concentrations completely disrupt membranes [75]. Fengycins are elicitors of plant defense [76] and have been found to be effective against many fungi including Magnaporthe grisea [77], Plasmodiophora brassicae [78], Botryosphaeria dothidea [79], C. gloeosporiodes [57], and a number of other fungi [80]. A cluster of fengycin homologues were found to be effective against F. verticillioides [80], F. solani [81], F. solani f. sp. radicicola [80], F. oxysporum [25,39], F. oxysporum f. sp. spinaciae [27], fumonisin production by F. verticillioides [82] and proliferation of F. graminearum [17,27,80,83,84,85]. On F. graminearum, fengycin causes structural deformations of the hyphae and suppresses in planta proliferation and mycotoxin production [27,84], permeabilization of hyphae [85], and in planta arrest of ear rot development of maize [83]. The study of Liu et al. [86] also revealed that fengycin could block the growth of F. graminearum, disrupt cell membrane structure increasing permeability, and create primary lesions in the membrane of fungal cells, thus compromising cell integrity. While the efficacy of fengycin cannot be disputed, its effect on F. graminearum can be concentration-dependent [80,86].
2.2.3. Iturin
Iturins exhibit strong fungitoxic properties by forming ion-conducting pores upon contact with fungal membranes. These amphiphilic compounds possess a heptapeptide backbone connected to a C13-to-C17 β-amino fatty acid chain [56,87]. Iturins vary in structure, their differences consisting in the type of amino acid residues and in the length and branching of the fatty acid chain. Some examples include iturins A, C, D, and E, bacillomycins D, F, and L, bacillopeptin, and mycosubtilin, all of which are arranged in an lddlldl configurational sequence [88]. Length and fatty acid chain branching heterogeneity is clearly demonstrated by iturin A, which has up to 8 isomers with between the 10 to 14 carbons and branching with n-, iso-, or anteiso configurations of the fatty acid chain [89]. Members of the iturin family bacillomycin and bacillopeptin have different amino acids at the third, fourth, and fifth positions. Mycosubtilin, a B. subtilis-produced iturin family member, targets, through its sterol group, ergosterol present in the membranes of sensitive fungi [90]. Bacillomycin L is presumed to act by inducing membrane permeabilization and disruption, as well as by targeting intercellular structrues [91]. Iturins have been found to be effective against a number of plant pathogenic fungi, which include Botrytis, Penicillium, Monilinia [92], R. solani [93], Colletotricum [94], F. oxysporum [95,96,97], and F. graminearum [19,26].
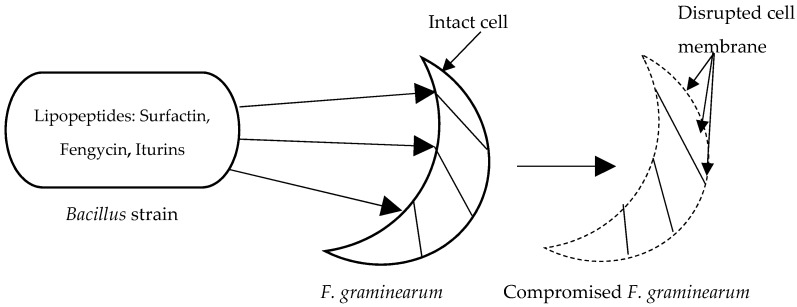
On F. graminearum, iturin causes morphological distortions in conidia and hyphae and severe damage to the plasma membrane, which lead to leakage of the cell contents [26]. Figure 3 illustrates the effects of iturin on F. graminearum conidia. Co-cultured with Bacillus, F. graminearum is not able to decrease the germination ability of wheat seed [19].
Figure 3.
Graphical illustration of Fusarium graminearum cell disruption by Bacillus.
3. Techniques Applied to Establish Potential Modes of Action of Bacillus against Fusarium graminearum
The effect of an organism or a substance against the growth of a target organism is traditionally studied by means of bioassays. In a bioassay, the organism is grown in the presence of the antagonist, and its growth monitored over time in comparison to that of an experimental control. Characteristically, a zone of growth inhibition is formed around the inhibited microbe. Various bioassays have been conducted to assess the effect of B. subtilis on the growth of F. graminearum. Notable is the study of Zhao et al. [27], which clearly demonstrated an antagonism of Bacillus against F. graminearum, whose mechanism still remains not fully elucidated. If a polypeptide is suspected to be a growth deterrent against target microorganisms, genes (their presence or relative expression) which code for the polypeptide can be detected in the growth culture by means of the polymerase chain reaction (PCR) technique. This was the case in the studies of Arrebola et al. [41], Velho et al. [98], and He et al. [99].
The questions needing answers would then be: What are the antagonistic compounds and how do these antagonistic compounds inhibit growth? Studies based on bioassays analyze the growth medium in which the antagonistic microbe and its target are grown. As part of the biochemical analysis, this growth medium is compared with a control growth medium, and inhibitory compounds are detected. Detection is done using techniques such as liquid chromatography–mass spectrometry (LC–MS). Examples of these studies are those which were conducted to detect and/or analyze surfactin, fengycin, and iturin produced by Bacillus against various plant pathogenic fungi [77,100,101,102,103,104,105,106,107]. Two initial scenarios may require this type of testing. The first is when the presence of a specific compound responsible for the antagonistic effect is supposed. This is a targeted analysis, which seeks to confirm the presence of the ‘suspected’ compound. Alternatively, if the presence of a specific molecule is not presumed, an untargeted analysis to assess culture conditions in comparison to the control is performed. A target analysis follows this untargeted analysis. Studying the antagonistic effect of Bacillus against F. graminearum for the protection of durum wheat, Zalisa-Kolsi et al. [19] performed an in vitro bioassay, which was followed by an in planta growth inhibitory test. Similarly, in studying the effect of three Bacillus strains against Fusarium, Dunlap et al. [17] followed a radial diffusion assay with analysis of candidate lipopeptides using high-performance liquid chromatography (HPLC) and a matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) system. Zhao et al. [27] performed a similar experiment and discovered an antagonistic effect of B. subtilis strain SG6 on F. graminearum, as many other similar studies [26,108].
4. Tools for the Detection of Surfactin, Fengycin, and Iturin Genes in Bacillus Strains and Culture with Biological Activity against Fusarium graminearum
Genomic analysis of Bacillus has shown that these bacteria possess genes which code for metabolites associated with biological control [38,109,110,111,112,113]. Genetic information made available by genomic sequencing has led to a better understanding of Bacillus biocontrol features. Chen et al. [112] characterized the genome of the Bacillus velezensis LM2303 strain, known for its strong biocontrol potential against F. graminearum. This strain presented the largest number of biocontrol genes and gene clusters when compared with strains studied earlier. Thirteen biosynthetic gene clusters associated with biocontrol activity were identified using an integrated approach of genome mining and chemical analysis, including the three antifungal metabolites fengycin B, iturin A, and surfactin A [112]. Another strain, B. velezensis LM2303, which has antimicrobial activity against F. graminearum in addition to other plant pathogenic fungi, also presented a plethora of genes encoding antimicrobial compounds. These findings demonstrated the value of genomic analysis in both biocontrol strain characterization and understanding of the basis of biocontrol activity. A plethora of co-culturing studies have utilized PCR to detect genes involved in biological control in culture, to later identify the basis of their biological control activity. However, biological control genes are sometimes detected in pure Bacillus strains undergoing characterization [46]. The study by Adeniji et al. [46] analyzed seven isolates of Bacillus with bio-suppressive effects against F. graminearum and found them to have valuable gene clusters encoding biocontrol agents. The fingerprint of the combination of genes detected by PCR indicates that strain differentiation and selection are important to identify the strain demonstrating the highest antimicrobial activity as a candidate biocontrol agent. Studies to identify surfactin, fengycin, and iturin in culture are routinely carried out and have uncovered a myriad of antimicrobial substances able to act against plant pathogenic fungi, including F. graminearum. These studies make use of combined chromatography and mass spectrometry to identify the compounds which have antagonistic activity. Using reverse-phase high-performance liquid chromatography and electrospray ionization mass spectrometry (RP-HPLC/ESI–MS) analyses, Gong et al. [26] identified iturin and surfactin in a culture of B. amyloliquefaciens isolated from wheat infected with F. graminearum. Further characterization of iturin showed that it causes leakage and/or inactivation of F. graminearum cellular contents. Using thin-layer chromatography–bioautography, Lee et al. [96] identified iturin A in a butanol extract of a culture of B. amyloliquefaciens strain DA12, which was found to be active against F. graminearum. The same study also attributed this activity to volatile heptanones, some of which were detected using gas chromatography–mass spectrometry (GC–MS). A similar study was performed using ultra-high-performance liquid chromatography coupled with mass spectrometry (UHPLC–MS) to confirm the presence of fengycin B, iturin A, and surfactin A in B. velezensis [112]. Also, the study of Adeniji et al. [46] used electrospray ionization–quadrupole mass spectrometry (ESI–Q-ToF-MS) to detect surfactin, fengycin, and iturin in the F. graminearum-supressing B. velezensis strain NWUMFkBS10.5. The power of these analytical techniques lies on their sensitivity and accuracy of detection, and their application is critical for, amongst other things, the detection of toxins in food to ensure compliance with food safety standards based on critical threshold values. Moreover, their application to detect bioactive components of Bacillus against F. graminearum is particularly relevant.
5. Future Prospects and Conclusions
The evidence that Bacillus species can act as biocontrol agents against F. graminearum encourages the exploitation of Bacillus in crop protection and their potential use for organic farming to supplement the despised control measures that pose various environmental hazards and health risks. Ideally, their use may completely replace the current strategies for the control of F. graminearum in wheat and other crops. This is supported by various studies conducted to assess the suitability of Bacillus to control wheat diseases, in particular FHB. The biofungicide, B. subtilis strain QST 713 suspension concentrate (Serenade®ASO) was tested against yellow rust in wheat and showed promising applicability for the control of this fungal infection. However, control tests proved that this biofungicide can be more effective as part of an integrated control strategy than as a standalone remedy [114]. Further work is, therefore, necessary to design an integrated control strategy which utilizes Serenade®ASO together with other organic disease control methods. B. amyloliquefaciens CC09 was also reported to have great potential as a biocontrol agent for wheat powdery mildew [115]. The same CC09 strain was found to be effective against take-all disease caused by Gaeumannomyces graminis and against a myriad of symptoms caused by Bipolaris sorokiniana. This strain effectively colonized the wheat tissue and was found to express genes encoding iturin A synthetase, thereby gaining the name “potential vaccine” [116]. Through its ability to also form spores, Bacillus can be an effective biological control agent against F. graminearum in wheat. With spore formation, Bacillus can overwinter and protect wheat against FHB over several growing seasons. Although the use of biocontrol agents must be extensively tested, ensuring they have a reasonable shelf life, compatibility with other treatments and affordability must be ascertained. Such is not the case with Bacillus, which seems to have passed many of these hurdles to become an effective commercial biocontrol product against F. graminearum. This is evident in available patents registered, such as those for Bacillus species against FHB in cereals [117,118]. The widespread adoption of these patented products to control FHB can benefit organic farming with a healthier and more sustainable wheat product.
Massive screening of various Bacillus strains against a wide array of crop pathogens is still nonetheless necessary to identify new antagonistic species. Furthermore, the application of new tools and techniques for assessing the efficacy of biocontrol agents against crop pathogens can accelerate the discovery of new biocontrol strains of Bacillus. Equally important is the study of the mechanism of action of Bacillus against F. graminearum, which should be analyzed more accurately using the new tools of genome-wide studies and the sensitive and accurate platforms of metabolomics. High-resolution techniques of chromatography and mass spectrometry can make the detection of new antagonistic molecules possible even at traceable levels. Specifically, if explored extensively, Bacillus may replace in the control F. graminearum most of the current widely applied control agents, such as fungicides, and cultural practices which impact negatively on health and the environment.
Author Contributions
Conceptualization, K.N.; writing, K.N., L.K.L., M.E.R., O.A.A., P.B.N.; editing, K.N., L.K.L., M.E.R., O.A.A., P.B.N.; supervision, K.N., P.B.N.
Funding
This study was funded by the National Research Foundation of South Africa under grant number Reference: TTK170413227119.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This article reports the overall mode of action of the bacteria Bacillus against the mycotoxin-producing plant pathogenic fungus Fusarium graminearium and provides a perspective of the techniques used to study antagonist metabolites. The goal is to illustrate research done so far and recommend study directions for the future.
References
- 1.Savary S., Ficke A., Aubertot J.N., Hollier C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012;4:519–537. doi: 10.1007/s12571-012-0200-5. [DOI] [Google Scholar]
- 2.Savary S., Willocquet L., Pethybridge S.J., Esker P., McRoberts N., Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Food Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- 3.McMullen M.P., Jones R., Gallenberg D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997;81:1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- 4.Manning B., Southwell R., Hayman P., Moore K. ‘Fusarium head blight in Northern NSW. NSW Agriculture; Orange, Australia: 2000. [Google Scholar]
- 5.Nganje W.E., Bangsund D.A., Leistritx F.L., Wilson W.W., Tlapo N.M. Regional economic impacts of Fusarium head blight in wheat and barley. Rev. Agric. Econ. 2004;26:332–347. doi: 10.1111/j.1467-9353.2004.00183.x. [DOI] [Google Scholar]
- 6.Dweba C.C., Figlan S., Shimelis H.A., Motaung T.E., Sydenham S., Mwadzingeni L., Tsilo T.J. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017;91:114–122. doi: 10.1016/j.cropro.2016.10.002. [DOI] [Google Scholar]
- 7.Dean R., van Kan J.A.L., Pretorius Z.A., Hammond-Kosack K.E., Di Pietro A., Spanu P.D., Rudd J.J., Dickman M., Kahmann R., Ellis J., et al. The top-10 fungal pathogens in molecular plant pathology. Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pestka J.J., Smolinski A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Heal. B Crit. Rev. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud A.F. Genetic variation and biological control of Fusarium graminearum isolated from wheat in Assiut-Egypt. Plant Pathol. 2015;32:145–156. doi: 10.5423/PPJ.OA.09.2015.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjardins A.E., Hohn T.M., McCormick S.P. Trichothecene biosynthesis in Fusarium species: Chemistry, genetics, and significance. Microbiol. Mol. Biol. Rev. 1993;157:595–604. doi: 10.1128/mr.57.3.595-604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desjardins A.E., Hohn T.M. Mycotoxins in plant pathogenesis. Mol. Plant Microbe Interact. 1997;10:147–152. doi: 10.1094/MPMI.1997.10.2.147. [DOI] [Google Scholar]
- 12.Goswami R.S., Kistler H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004;5:515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y.J., Yu J.J., Zhang Y.N., Zhang X., Cheng C.J., Wang J.X., Hollomon D.W., Fan P.S., Zhou M.G. Effect of carbendazim resistance on trichothecene production and aggressiveness of Fusarium graminearum. Mol. Plant Microbe Interact. 2009;22:1143–1150. doi: 10.1094/MPMI-22-9-1143. [DOI] [PubMed] [Google Scholar]
- 14.Shafi J., Tian H., Ji M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotech. Equip. 2017;31:446–459. doi: 10.1080/13102818.2017.1286950. [DOI] [Google Scholar]
- 15.Zeigler D.R., Perkins J.B. The genus Bacillus. In: Goldman E., Green L.H., editors. Practical Handbook of Microbiology. CRC Press; Boca Raton, FL, USA: 2018. pp. 309–326. [Google Scholar]
- 16.Chitlaru T., Altboum Z., Reuveny S., Shafferman A. Progress and novel strategies in vaccine development and treatment of Anthrax. Immunol. Rev. 2011;239:221–236. doi: 10.1111/j.1600-065X.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- 17.Dunlap C.A., Schisler D.A., Price N.P., Vaughn S.F. Cyclic lipopeptide profile of three Bacillus subtilis strains; antagonists of Fusarium head blight. J. Microbiol. 2011;49:603–609. doi: 10.1007/s12275-011-1044-y. [DOI] [PubMed] [Google Scholar]
- 18.Compant S., Duffy B., Nowak J., Clement C., Barka E.A. Use of plant growth promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ Microbiol. 2005;71:4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zalila-Kolsi I., Mahmoud A.B., Ali H., Sellami S., Nasfi Z., Tounsi S., Jamoussi K. Antagonist effects of Bacillus spp. strains against Fusarium graminearum for protection of durum wheat (Triticum turgidum L. subsp. durum) Microbiol. Res. 2016;192:148–158. doi: 10.1016/j.micres.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Whipps J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001;511:487–511. doi: 10.1093/jxb/52.suppl_1.487. [DOI] [PubMed] [Google Scholar]
- 21.Cawoy H., Debois D., Franzil L., De Pauw E., Thonart P., Ongena M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 2015;8:281–295. doi: 10.1111/1751-7915.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan N., Maymon M., Hirsch A.M. Combating Fusarium infection using Bacillus-based antimicrobials. Microorganisms. 2017;5:75. doi: 10.3390/microorganisms5040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radhakrishnan R., Hashem A., Abd Allah E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017;8:667. doi: 10.3389/fphys.2017.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayed H.B., Hmidet N., Béchet M., Chollet M., Chataigné G., Leclère V., Jacques P., Nasri M. Identification and biochemical characteristics of lipopeptides from Bacillus mojavensis A21. Process Biochem. 2014;49:1699–1707. doi: 10.1016/j.procbio.2014.07.001. [DOI] [Google Scholar]
- 25.Cao Y., Xu Z., Ling N., Yuan Y., Yang X., Chen L., Shen B., Shen Q. Isolation and identification of lipopeptides produced by B. subtilis SQR 9 for suppressing Fusarium wilt of cucumber. Sci. Hortic. 2012;135:32–39. doi: 10.1016/j.scienta.2011.12.002. [DOI] [Google Scholar]
- 26.Gong A.D., Li H.P., Yuan Q.S., Song X.S., Yao W., He W.J., Zhang J.B., Liao Y.C. Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS ONE. 2015;10:e0116871. doi: 10.1371/journal.pone.0116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y., Selvaraj J.N., Xing F., Zhou L., Wang Y., Song H., Tan X., Sun L., Sangare L., Folly Y.M.E., et al. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS ONE. 2014;9:e92486. doi: 10.1371/journal.pone.0092486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan S., Dong Y., Liao H., Huang J., Song S., Xu Y., Shen Q. Antagonistic bacterium Bacillus amyloliquefaciens induces resistance and controls the bacterial wilt of tomato. Pest Manag. Sci. 2013;69:1245–1252. doi: 10.1002/ps.3491. [DOI] [PubMed] [Google Scholar]
- 29.Földes T., Bánhegyi I., Herpai Z., Varga L., Szigeti J. Isolation of Bacillus strains from the rhizosphere of cereals and in vitro screening for antagonism against phytopathogenic, food-borne pathogenic and spoilage micro-organisms. J. Appl. Microbiol. 2000;89:840–846. doi: 10.1046/j.1365-2672.2000.01184.x. [DOI] [PubMed] [Google Scholar]
- 30.Gross E., Kiltz H.H., Nebelin E., Subtilin V.I. Die Struktur des Subtilins. Hoppe-Seyler Z. Physiol. Chem. 1973;354:810–812. [PubMed] [Google Scholar]
- 31.Walker J.E., Abraham E.P. The structure of bacilysin and other products of Bacillus subtilis. Biochem. J. 1970;118:563–570. doi: 10.1042/bj1180563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sengupta S., Banerjee A.B., Bose S.K. γ-Glutamyl and D-or L-peptide linkages in mycobacillin, a cyclic peptide antibiotic. Biochem. J. 1971;121:839–846. doi: 10.1042/bj1210839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besson F., Peypoux F., Michel G., Delcambe L. The structure of bacillomycin L, an antibiotic from Bacillus subtilis. Eur. J. Biochem. 1977;77:61–67. doi: 10.1111/j.1432-1033.1977.tb11641.x. [DOI] [PubMed] [Google Scholar]
- 34.Peypoux F., Marion D., Maget-Dana R. Structure of bacillomycin F, a new peptidolipid antibiotic of the iturin group. Eur. J. Biochem. 1985;153:335–340. doi: 10.1111/j.1432-1033.1985.tb09307.x. [DOI] [PubMed] [Google Scholar]
- 35.Peypoux F., Michel G., Delcambe L. The structure of mycosubtilin, an antibiotic isolated from Bacillus subtilis. Eur. J. Biochem. 1976;63:391–398. doi: 10.1111/j.1432-1033.1976.tb10240.x. [DOI] [PubMed] [Google Scholar]
- 36.Peypoux F., Pommier M.T., Marion D., Ptak M., Das B.C., Michel G. Revised structure of mycosubtilin, a peptidolipid antibiotic from Bacillus subtilis. J. Antibiot. 1986;39:636–641. doi: 10.7164/antibiotics.39.636. [DOI] [PubMed] [Google Scholar]
- 37.Zeriouh H., Romero D., Garcia-Gutierrez L., Cazorla F.M., de Vicente A., Perez-Garcia A. The iturin-like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of cucurbits. Mol. Plant Microbe Interact. 2011;24:1540–1552. doi: 10.1094/MPMI-06-11-0162. [DOI] [PubMed] [Google Scholar]
- 38.Dunlap C.A., Bowman M.J., Schisler D.A. Genomic analysis and secondary metabolite production in Bacillus amyloliquefaciens AS 43.3: A biocontrol antagonist of Fusarium head blight. Biol. Control. 2013;64:166–175. doi: 10.1016/j.biocontrol.2012.11.002. [DOI] [Google Scholar]
- 39.Yuan J., Raza W., Huang Q., Shen Q. The ultrasound-assisted extraction and identification of antifungal substances from B. amyloliquefaciens strain NJN-6 suppressing Fusarium oxysporum. J. Basic Microbiol. 2012;52:721–730. doi: 10.1002/jobm.201100560. [DOI] [PubMed] [Google Scholar]
- 40.Kumar P., Dubey R.C., Maheshwari D.K. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 2012;167:493–499. doi: 10.1016/j.micres.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Arrebola E., Jacobs R., Korsten L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol. 2010;108:386–395. doi: 10.1111/j.1365-2672.2009.04438.x. [DOI] [PubMed] [Google Scholar]
- 42.Stein T. Bacillus subtilis antibiotics: Structure, syntheses and specific functions. Mol. Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 43.Roongsawang N., Washio K., Morikawa M. Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int. J. Mol. Sci. 2011;12:141–172. doi: 10.3390/ijms12010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deleu M., Razafindralambo H., Popineau Y., Jacques P., Thonard P., Paquot M. Interfacial and emulsifying properties of lipopeptides from Bacillus subtilis. Coll. Surf. A Physicochem. Eng. Asp. 1999;152:3–10. doi: 10.1016/S0927-7757(98)00627-X. [DOI] [Google Scholar]
- 45.Peypoux F., Bonmatin J., Wallach J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 1999;51:553–563. doi: 10.1007/s002530051432. [DOI] [PubMed] [Google Scholar]
- 46.Adeniji A.A., Aremu O.S., Babalola O.O. Selecting lipopeptide-producing, Fusarium-suppressing Bacillus spp.: Metabolomic and genomic probing of Bacillus velezensis NWUMFkBS10. 5. MicrobiologyOpen. 2019;8:e00742. doi: 10.1002/mbo3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Q., Dong W., Li S., Lu X., Wang P., Zhang X., Wang Y., Ma P. Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiol. Res. 2014;169:533–540. doi: 10.1016/j.micres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Nikolić I., Berić T., Dimkić I., Popović T., Lozo J., Fira D., Stanković S. Biological control of Pseudomonas syringae pv. aptata on sugar beet with Bacillus pumilus SS-10.7 and Bacillus amyloliquefaciens (SS-12.6 and SS-38.4) strains. J. Appl. Microbiol. 2019;126:165–176. doi: 10.1111/jam.14070. [DOI] [PubMed] [Google Scholar]
- 49.Winkelmann H., Allgaier H., Lu R., Jung G. Iturin AL—A new long chain iturin A possessing an unusual high content of C16 β amino acids. J. Antibiot. 1983;11:1451–1457. doi: 10.7164/antibiotics.36.1451. [DOI] [PubMed] [Google Scholar]
- 50.Vanittanakom N., Loeffler W., Koch U., Jung G. Fengycin—A novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-2. J. Antibiot. 1986;7:888–901. doi: 10.7164/antibiotics.39.888. [DOI] [PubMed] [Google Scholar]
- 51.Vollenbroich D., Ozel M., Vater J., Kamp R.M., Pauli G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals. 1997;25:289–297. doi: 10.1006/biol.1997.0099. [DOI] [PubMed] [Google Scholar]
- 52.Vollenbroich D., Pauli G., Ozel M., Vater J. Antimycoplasma properties and application in cell culture of surfactin, a lipopeptide antibiotic from Bacillus subtilis. Appl. Environ. Microbiol. 1997;63:44–49. doi: 10.1128/aem.63.1.44-49.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kracht M., Rokos H., Ozel M., Kowall M., Pauli G., Vater J. Antiviral and hemolytic activities of surfactin isoforms and their methyl ester derivatives. J. Antibiot. 1999;52:613–619. doi: 10.7164/antibiotics.52.613. [DOI] [PubMed] [Google Scholar]
- 54.Kim S.Y., Kim J.M., Kim S.H., Bae H.J., Yi H., Yoon S.H., Koo B.S., Kwon M., Cho J.Y., Lee C.E., et al. Surfactin from Bacillus subtilis displays anti-proliferative effect via apoptosis induction, cell cycle arrest and survival signalling suppression. FEBS Lett. 2007;581:865–871. doi: 10.1016/j.febslet.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 55.Nagorska K., Bikowski M., Obuchowki M. Multicellular behaviour and production of a wide variety of toxic substance support usage of Bacillus subtilis as powerful biocontrol agent. Acta. Biochim. Pol. 2007;54:495–508. [PubMed] [Google Scholar]
- 56.Ongena M., Jacques P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16:115–124. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Kim P.I., Ryu J., Kim Y.H., Chi Y.T. Production of biosurfactant lipopeptides iturin A, fengycin and surfactin from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporides. J. Microbiol. Biotechnol. 2010;20:138–145. [PubMed] [Google Scholar]
- 58.Geetha L., Manonmani A.M., Paily K.P. Identification and characterization of a mosquito pupicidal metabolite of Bacillus subtilis subsp. subtilis strain. Appl. Microbiol. Biotechnol. 2010;86:1737–1744. doi: 10.1007/s00253-010-2449-y. [DOI] [PubMed] [Google Scholar]
- 59.Pathak K.V., Keharia H. Identification of surfactins and iturins produced by potent fungal antagonist, Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) tree using mass spectrometry. 3 Biotech. 2014;4:283–295. doi: 10.1007/s13205-013-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beltran-Gracia E., Macedo-Raygoza G., Villafaña-Rojas J., Martinez-Rodriguez A., Chavez-Castrillon Y.Y., Espinosa-Escalante F.M., Di Mascio P., Ogura T., Beltran-Garcia M.J. Production of lipopeptides by fermentation processes: Endophytic bacteria, fermentation strategies and easy methods for bacterial selection. In: Menestrina G., Serra M.D., Jozala A.F., editors. Fermentation Processes. 1st ed. Intech Open Science; London, UK: 2017. pp. 260–271. [Google Scholar]
- 61.Geissler M., Oellig C., Moss K., Schwack W., Henkel M., Hausmann R. High-performance thin-layer chromatography (HPTLC) for the simultaneous quantification of the cyclic lipopeptides surfactin, iturin A and fengycin in culture samples of Bacillus species. J. Chromatogr. B. 2017;1044:214–224. doi: 10.1016/j.jchromb.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Peypoux F., Bonmatin J.M., Labbe H., Grangemard I., Das B.C., Ptak M., Wallach J., Michel G. [Ala4] surfactin, a novel isoform from Bacillus subtilis studied by mass and NMR spectroscopies. Eur. J. Biochem. 1994;224:89–96. doi: 10.1111/j.1432-1033.1994.tb19998.x. [DOI] [PubMed] [Google Scholar]
- 63.Kowall M., Vater J., Kluge T., Stein P., Ziessow D. Separation and characterization of surfactin isoforms produced by Bacillus subtilis OKB 105. J. Coll. Interface Sci. 1998;204:1–8. doi: 10.1006/jcis.1998.5558. [DOI] [PubMed] [Google Scholar]
- 64.Hue N., Serani L., Laprevote O. Structural investigation of cyclic peptidolipids from Bacillus subtilis by high energy tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2001;15:203–209. doi: 10.1002/1097-0231(20010215)15:3<203::AID-RCM212>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 65.Sheppard J.D., Jumarie C., Cooper D.G., Laprade R. Ionic channels induced by surfactin in planar lipid bilayer membranes. Biochim. Biophys. Acta. 1991;1064:13–23. doi: 10.1016/0005-2736(91)90406-X. [DOI] [PubMed] [Google Scholar]
- 66.Qi G., Zhu F., Du P., Yang X., Qiu D., Yu Z., Chen J., Zhao X. Lipopeptide induces apoptosis in fungal cells by a mitochondria-dependent pathway. Peptides. 2010;31:1978–1986. doi: 10.1016/j.peptides.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Gao Z., Wang S., Qi G., Pan H., Zhang L., Zhou X., Liu J., Zhao X., Wu J.A. Surfactin cyclopeptide of WH1 fungin used as a novel adjuvant for intramuscular and subcutaneous immunization in mice. Peptides. 2012;38:163–171. doi: 10.1016/j.peptides.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 68.Gao Z., Zhao X., Lee S., Li J., Liao H., Zhou X., Wu J., Qi G. WH1fungin a surfactin cyclic lipopeptide is a novel oral immunoadjuvant. Vaccine. 2013;31:2796–2803. doi: 10.1016/j.vaccine.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 69.Snook M.E., Mitchell T., Hinton D.M., Bacon C.W. Isolation and characterization of Leu7-surfactin from the endophytic bacterium Bacillus mojavensis RRC 101, a biocontrol agent for Fusarium verticillioides. J. Agric. Food Chem. 2009;57:4287–4292. doi: 10.1021/jf900164h. [DOI] [PubMed] [Google Scholar]
- 70.Vitullo D., Di Pietro A., Romano A., Lanzotti V., Lima G. Role of new bacterial surfactins in the antifungal interaction between Bacillus amyloliquefaciens and Fusarium oxysporum. Plant Pathol. 2012;61:689–699. doi: 10.1111/j.1365-3059.2011.02561.x. [DOI] [Google Scholar]
- 71.Jiang J., Gao L., Bie X., Lu Z., Liu H., Zhang C., Lu F., Zhao H. Identification of novel surfactin derivatives from NRPS modification of Bacillus subtilis and its antifungal activity against Fusarium moniliforme. BMC Microbiol. 2016;16:31. doi: 10.1186/s12866-016-0645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nielsen D.S., Shepherd N.E., Xu W., Lucke A.J., Stoermer M.J., Fairlie D.P. Orally absorbed cyclic peptides. Chem. Rev. 2017;117:8094–8128. doi: 10.1021/acs.chemrev.6b00838. [DOI] [PubMed] [Google Scholar]
- 73.Steller S., Vater J. Purification of the fengycin synthetase multienzyme system from Bacillus subtilis b213. J. Chromatogr. B Biomed. Sci. Appl. 2000;737:267–275. doi: 10.1016/S0378-4347(99)00481-8. [DOI] [PubMed] [Google Scholar]
- 74.Vater J., Kablitz B., Wilde C., Franke P., Mehta N., Cameotra S.S. Matrix-assisted laser desorption ionization-time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl. Environ. Microbiol. 2002;68:6210–6219. doi: 10.1128/AEM.68.12.6210-6219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deleu M., Paquot M., Nylander T. Fengycin interaction with lipid monolayers at the air-aqueous interface-implications for the effect of fengycin on biological membranes. J. Coll. Interface Sci. 2005;283:358–365. doi: 10.1016/j.jcis.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 76.Ongena M., Jourdan E., Adam A., Paquot M., Brans A., Joris B., Arpigny J.L., Thonart P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007;9:1084–1090. doi: 10.1111/j.1462-2920.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L., Sun C. Fengycins, cyclic lipopeptides from marine Bacillus subtilis strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl. Environ. Microbiol. 2018;84:e00445-18. doi: 10.1128/AEM.00445-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X.Y., Yang J.J., Mao Z.C., Ho H.H., Wu Y.X., He Y.Q. Enhancement of biocontrol activities and cyclic lipopeptides production by chemical mutagenesis of Bacillus subtilis XF-1, a biocontrol agent of Plasmodiophora brassicae and Fusarium solani. Indian J. Microbiol. 2014;54:476–479. doi: 10.1007/s12088-014-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fan H., Ru J., Zhang Y., Wang Q., Li Y. Fengycin produced by Bacillus subtilis 9407 plays a major role in the biocontrol of apple ring rot disease. Microbiol. Res. 2017;199:89–97. doi: 10.1016/j.micres.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Li L., Ma M., Huang R., Qu Q., Li G., Zhou J., Zhang K., Lu K., Niu X., Luo J. Induction of chlamydospore formation in Fusarium by cyclic lipopeptide antibiotics from Bacillus subtilis C2. J. Chem. Ecol. 2012;38:966–974. doi: 10.1007/s10886-012-0171-1. [DOI] [PubMed] [Google Scholar]
- 81.Li B., Li Q., Xu Z., Zhang N., Shen Q., Zhang R. Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds production. Front. Microbiol. 2014;5:636. doi: 10.3389/fmicb.2014.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu L.B., Zhang T., Yang Z.M., Zhou W., Shi Z.Q. Inhibition of fengycins on the production of fumonisin B1 from Fusarium verticillioides. Lett. Appl. Microbiol. 2009;48:84–89. doi: 10.1111/j.1472-765X.2008.02493.x. [DOI] [PubMed] [Google Scholar]
- 83.Chan Y.K., Savard M.E., Reid L.M., Cyr T., McCormick W.A., Seguin C. Identification of lipopeptide antibiotics ogyjuygthkjf a Bacillus subtilis isolate and their control of Fusarium graminearum diseases in maize and wheat. BioControl. 2009;54:567. doi: 10.1007/s10526-008-9201-x. [DOI] [Google Scholar]
- 84.Hanif A., Zhang F., Li P., Li C., Xu Y., Zubair M., Zhang M., Jia D., Zhao X., Liang J., et al. Fengycin produced by Bacillus amyloliquefaciens FZB42 inhibits Fusarium graminearum growth and mycotoxins biosynthesis. Toxins. 2019;11:295. doi: 10.3390/toxins11050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang J., Liu J., Chen H., Yao J. Characterization of Fusarium graminearum inhibitory lipopeptide from Bacillus subtilis IB. Appl. Microbiol. Biotechnol. 2007;76:889–894. doi: 10.1007/s00253-007-1054-1. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y., Lu J., Sun J., Bie X., Lu Z. Membrane disruption and DNA binding of Fusarium graminearum cell induced by C16-Fengycin A produced by Bacillus amyloliquefaciens. Food Cont. 2019;102:206–213. doi: 10.1016/j.foodcont.2019.03.031. [DOI] [Google Scholar]
- 87.Aranda F.J., Teruel J.A., Ortiz A. Further aspects on the haemolytic activity of the antibiotic lipopeptide iturin A. Biochem. Biophys. Acta. 2005;1713:51–56. doi: 10.1016/j.bbamem.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 88.Bland J.M. The first synthesis of a member of the iturin family, the antifungal cyclic lipopeptide, iturin-A2. J. Org. Chem. 1996;61:5663–5664. doi: 10.1021/jo960452n. [DOI] [Google Scholar]
- 89.Isogai A., Takayama S., Murakoshi S., Suzuki A. Structure of β-amino acids in antibiotics iturin A. Tetrahedron Lett. 1982;23:3065–3068. doi: 10.1016/S0040-4039(00)87534-6. [DOI] [Google Scholar]
- 90.Nasir M.N., Besson F. Interactions of the antifungal mycosubtilin with ergosterol-containing interfacial monolayers. Biochim. Biophys. Acta. 2012;1818:1302–1308. doi: 10.1016/j.bbamem.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 91.Zhang B., Dong C., Shang Q., Han Y., Li P. New insights into membrane-active action in plasma membrane of fungal hyphae by the lipopeptide antibiotic bacillomycin L. Biochim. Biophys. Acta. 2013;1828:2230–2237. doi: 10.1016/j.bbamem.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 92.Calvo H., Mendiara I., Arias E., Blanco D., Venturini M.E. The role of iturin A from B. amyloliquefaciens BUZ-14 in the inhibition of the most common postharvest fruit rots. Food Microbiol. 2019;82:62–69. doi: 10.1016/j.fm.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 93.Zohora U.S., Ano T., Rahman M.S. Biocontrol of Rhizoctonia solani K1 by iturin A producer Bacillus subtilis RB14 seed treatment in tomato plants. Adv. Microbiol. 2016;6:424–431. doi: 10.4236/aim.2016.66042. [DOI] [Google Scholar]
- 94.Arroyave-Toro J.J., Mosquera S., Villegas-Escobar V. Biocontrol activity of Bacillus subtilis EA-CB0015 cells and lipopeptides against postharvest fungal pathogens. Biol. Control. 2017;114:195–200. doi: 10.1016/j.biocontrol.2017.08.014. [DOI] [Google Scholar]
- 95.Fujita S., Yokota K. Disease suppression by the cyclic lipopeptides iturin A and surfactin from Bacillus spp. against Fusarium wilt of lettuce. J. Gen. Plant Pathol. 2019;85:44–48. doi: 10.1007/s10327-018-0816-1. [DOI] [Google Scholar]
- 96.Lee T., Park D., Kim K., Lim S.M., Yu N.H., Kim S., Kim H.Y., Jung K.S., Jang J.Y., Park J.C., et al. Characterization of Bacillus amyloliquefaciens DA12 showing potent antifungal activity against mycotoxigenic Fusarium species. Plant Pathol. J. 2017;33:499–507. doi: 10.5423/PPJ.FT.06.2017.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao Y., Pi H., Chandrangsu P., Li Y., Wang Y., Zhou H., Xiong H., Helmann J.D., Cai Y. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018;8:4360. doi: 10.1038/s41598-018-22782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Velho R.V., Medina L.F.C., Segalin J., Brandelli A. Production of lipopeptides among Bacillus strains showing growth inhibition of phytopathogenic fungi. Folia Microbiol. 2011;56:297–303. doi: 10.1007/s12223-011-0056-7. [DOI] [PubMed] [Google Scholar]
- 99.He Y., Zhu M., Huang J., Hsiang T., Zheng L. Biocontrol potential of a Bacillus subtilis strain BJ-1 against the rice blast fungus Magnaporthe oryzae. Can. J. Plant Pathol. 2019;41:47–59. doi: 10.1080/07060661.2018.1564792. [DOI] [Google Scholar]
- 100.Koumoutsi A., Chen X.H., Henne A., Liesegang H., Hitzero th G., Franke P., Vater J., Borriss R. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 2004;186:1084–1096. doi: 10.1128/JB.186.4.1084-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nanjundan J., Ramasamy R., Uthandi S., Ponnusamy M. Antimicrobial activity and spectroscopic characterization of surfactin class of lipopeptides from Bacillus amyloliquefaciens SR1. Microb. Pathog. 2019;128:374–380. doi: 10.1016/j.micpath.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 102.Ding L., Guo W., Chen X. Exogenous addition of alkanoic acids enhanced production of antifungal lipopeptides in Bacillus amyloliquefaciens Pc3. Appl. Microbiol. Biotechnol. 2019;103:5367–5377. doi: 10.1007/s00253-019-09792-1. [DOI] [PubMed] [Google Scholar]
- 103.Saechow S., Thammasittirong A., Kittakoop P., Prachya S., Thammasittirong S.N.R. Antagonistic activity against dirty panicle rice fungal pathogens and plant growth-promoting activity of Bacillus amyloliquefaciens BAS23. J. Microbiol. Biotechnol. 2018;28:1527–1535. doi: 10.4014/jmb.1804.04025. [DOI] [PubMed] [Google Scholar]
- 104.Sa R.B., An X., Sui J.K., Wang X.H., Ji C., Wang C.Q., Li Q., Hu Y.R., Liu X. Purification and structural characterization of fengycin homologues produced by Bacillus subtilis from poplar wood bark. Australas. Plant Pathol. 2018;47:259–268. doi: 10.1007/s13313-018-0552-1. [DOI] [Google Scholar]
- 105.Zouari I., Jlaiel L., Tounsi S., Trigui M. Biocontrol activity of the endophytic Bacillus amyloliquefaciens strain CEIZ-11 against Pythium aphanidermatum and purification of its bioactive compounds. Biol. Control. 2016;100:54–62. doi: 10.1016/j.biocontrol.2016.05.012. [DOI] [Google Scholar]
- 106.Kaur P.K., Joshi N., Singh I.P., Saini H.S. Identification of cyclic lipopeptides produced by Bacillus vallismortis R2 and their antifungal activity against Alternaria alternata. J. Appl. Microbiol. 2017;122:139–152. doi: 10.1111/jam.13303. [DOI] [PubMed] [Google Scholar]
- 107.Ji S.H., Paul N.C., Deng J.X., Kim Y.S., Yun B.S., Yu S.H. Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology. 2013;41:234–242. doi: 10.5941/MYCO.2013.41.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Palyzová A., Svobodová K., Sokolová L., Novák J., Novotný Č. Metabolic profiling of Fusarium oxysporum f. sp. conglutinans race 2 in dual cultures with biocontrol agents Bacillus amyloliquefaciens, Pseudomonas aeruginosa, and Trichoderma harzianum. Folia Microbiol. 2019 doi: 10.1007/s12223-019-00690-7. [DOI] [PubMed] [Google Scholar]
- 109.Blom J., Rueckert C., Niu B., Wang Q., Borriss R. The complete genome of Bacillus amyloliquefaciens subsp. plantarum CAU B946 contains a gene cluster for non-ribosomal synthesis of Iturin A. J. Bacteriol. 2012;194:1845–1846. doi: 10.1128/JB.06762-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen X.H., Koumoutsi A., Scholz R., Eisenreich A., Schneider K., Heinemeyer I., Morgenstern B., Voss B., Hess W.R., Reva O., et al. Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007;25:1007–1014. doi: 10.1038/nbt1325. [DOI] [PubMed] [Google Scholar]
- 111.Chen L., Heng J., Qin S., Bian K. A comprehensive understanding of the biocontrol potential of Bacillus velezensis LM2303 against Fusarium head blight. PLoS ONE. 2018;13:e0198560. doi: 10.1371/journal.pone.0198560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deng Y., Zhu Y., Wang P., Zhu L., Zheng J., Li R., Ruan L., Peng D., Sun M. Complete genome sequence of Bacillus subtilis BSn5, an endophytic bacterium of Amorphophallus konjac with antimicrobial activity for the plant pathogen Erwinia carotovora subsp. carotovora. J. Bacteriol. 2011;193:2070–2071. doi: 10.1128/JB.00129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deng Q., Wang R., Sun D., Sun L., Wang Y., Pu Y., Fang Z., Xu D., Liu Y., Ye R., et al. Complete genome of Bacillus velezensis CMT-6 and comparative genome analysis reveals lipopeptide diversity. Biochem. Genet. 2019 doi: 10.1007/s10528-019-09927-z. [DOI] [PubMed] [Google Scholar]
- 114.Reiss A., Jørgensen L.N. Biological control of yellow rust of wheat (Puccinia striiformis) with Serenade® ASO (Bacillus subtilis strain QST713) Crop Prot. 2017;93:1–8. doi: 10.1016/j.cropro.2016.11.009. [DOI] [Google Scholar]
- 115.Cai X.C., Liu C.H., Wang B.T., Xue Y.R. Genomic and metabolic traits endow Bacillus velezensis CC09 with a potential biocontrol agent in control of wheat powdery mildew disease. Microbiol. Res. 2017;196:89–94. doi: 10.1016/j.micres.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 116.Kang X., Zhang W., Cai X., Zhu T., Xue Y., Liu C. Bacillus velezensis CC09: A potential ‘vaccine’ for controlling wheat diseases. Mol. Plant Microbe Interact. 2018;31:623–632. doi: 10.1094/MPMI-09-17-0227-R. [DOI] [PubMed] [Google Scholar]
- 117.Schisler D.A., Khan N.I., Boehm M.J. Ohio State University Research Foundation and US Department of Agriculture. Bacillus species NRRL B-30212 for reducing Fusarium head blight in cereals. 7,001,755. U.S. Patent. 2006 Feb 21;
- 118.Da Luz W.C. Empresa Brasileira de Pesquisa Agropecuaria EMBRAPA, Biocontrol of plant diseases caused by Fusarium species with novel isolates of Pantoea agglomerans. 7,118,739. U.S. Patent. 2006 Oct 10;