Abstract
Systematic glucocorticosteroids (GCS) are used to treat chronic obstructive pulmonary disease (COPD) and can cause leukocytosis. Distinguishing the effect of GCS on leukocyte level from infection-induced leukocytosis is important. We sought to quantify the effect of chronic GCS treatment on leukocytosis level in patients with COPD exacerbation. We reviewed the records of patients with COPD exacerbation and fever hospitalized in a tertiary medical center in 2003–2014. Patients were classified according to the GCS treatment they received: chronic GCS treatment (CST), acute GCS treatment (AST), and no prior GCS treatment (NGCS). We used the eosinophil absolute count as a marker of compliance and efficacy of steroid treatment. The primary outcome was the maximal white blood cell (WBC) count within the first 24 h of admission. Of 834 patients, 161 were categorized as CST, 116 AST, and 557 NGCS. The overall maximal leukocyte count was higher and the eosinophil count lower in the two GCS therapy groups. In patients with COPD exacerbation and fever, acutely treated with GCS, the mean increase in the WBC count was more evident when the eosinophils were undetectable (absolute count of zero). This supports leukocytosis level as a marker of disease course in COPD and fever.
Keywords: glucocorticosteroids (GCS), chronic obstructive pulmonary disease (COPD), leukocytosis, eosinophils
1. Introduction
Treatment with systematic glucocorticoids (GCS) is occasionally a component of the therapeutic regimen of chronic obstructive pulmonary disease (COPD) [1]. Due to the wide range of side effects of GCS, the therapeutic strategy is usually conservative, and treatment is reserved for periods of exacerbation. Nevertheless, for some patients, chronic use of steroids is the only solution that affords disease stabilization [2].
GCS are known to increase the number of white blood cells (WBC), a phenomenon called “glucocorticoid induced leukocytosis”. The increase is mainly in the neutrophilic cells, while the count of the other circulating cells, especially the eosinophils, decreases [3]. Indeed, previous studies used blood eosinophil count as a marker for GCS treatment, for patient compliance and for titration of GCS therapy [4].
The level of leukocytes is a key parameter in the clinical assessment of patients with COPD exacerbation, with and without fever. The presentation of high WBC count makes it important to distinguish the effect of the GCS from the effect of the possible infection. Other studies of steroid-induced leukocytosis in patients with acute infections [5,6] did not focus specifically on patients with fever and acute exacerbation of COPD. Therefore, the main purpose of this study was to quantify the effect of steroid treatment on the level of leukocytosis in patients hospitalized with fever and acute exacerbation of COPD.
2. Methods
2.1. Study Population
In this retrospective cohort study, we assessed all COPD patients admitted to Soroka University Medical Center with exacerbation of their disease during the years 2003–2014. We included patients who were over 18 years old, with a core body temperature of 38 °C or above, with COPD exacerbation as their main diagnosis. We excluded from the analysis all patients with oncologic or hematologic diseases.
2.2. Steroid Treatment
Three groups of patients were compared in this study, according to treatment with GCS: acute, chronic, and no GCS therapy (NGCS). Chronic GCS treatment (CST) was defined as a daily oral dose or an alternate-day oral dose of 5 mg or more of prednisone, for a period of at least 28 days. Acute GCS treatment (AST) was defined as a daily oral dose or an alternate-day oral dose of 5 mg or more of prednisone, for less than 28 days.
The primary outcome was the maximal WBC count, and the secondary outcome was the maximal neutrophil (PMN) count. Both outcomes were measured within the first 24 h from hospital admission and were compared to the minimal eosinophil count and maximal fever (°C).
2.3. Statistical Analysis
Normally distributed variables were described by means ± standard deviations; and other distributions by medians and interquartile range (IQR). Categorical variables were summarized by frequencies of available cases. For between covariate associations, the t-test or ANOVA test were used for the comparison of normally distributed continuous variables and the Mann–Whitney or Kruskal–Wallis tests for those not normally distributed. Chi-Square or Fisher’s exact test were used for the comparison of dichotomous variables. Data were summarized using frequency tables, summary statistics, confidence intervals, and p-values. Associations of WBC and PMN counts with the eosinophil count and body temperature were analyzed by fitting linear curves.
Multivariate analyses for WBC and PMN counts were conducted using a linear regression model. The models were first fitted in the NGCS group. The variables were introduced to the model based on clinical and statistical significance (p-value < 0.05 in univariate analysis). The final models included the following variables: age, gender, and degree of fever.
Using the linear regression equation, the expected WBC count and expected PMN count were calculated for each patient in both the AST and CST groups. For the steroid groups, differences between the observed and expected values of WBC and PMN counts (calculated based on the NGCS group regression model) were shown using linear curves as a function of the minimal eosinophil count and body temperature.
All statistical analyses were conducted with a two-sided significance level of α = 0.05 and 95% confidence interval. The statistical analysis was performed by the Statistical Package for the Social Sciences (IMB SPSS STATISTICS, version 20, IBM, Armonk, NY, USA)
Ethics: The work was conducted with the formal approval of the local human subjects committees at Soroka University Medical Center, Israel, Ethics approval number 0304-15(SUMC).
3. Results
3.1. Patient Population
The study comprised 834 patients diagnosed with COPD exacerbation and fever: 161 in the CST group, 116 in the AST group, and 557 who were not treated with GCS (NGCS). Table 1 summarizes characteristics of the study population: The oldest mean age was noted in the CST group (71.7 ± 11.0 years). The majority of patients in all three groups were current or former smokers: 77.0% in the CST, 74.1% in the AST, and 69.8% in the NGCS. The majority of the patients were males. The proportion of patients with type 2 diabetes was significantly higher in the CST group (57.8%) than in the other two groups (40.5% in the AST group, 39.0% in the NGCS group, p < 0.001). The most prevalent diagnosis, in addition to COPD, was pneumonia (33.5% of CST, 37.1% of AST, and 37.5% of the NGCS group). The proportion of patients with bacteremia was higher in the NGCS group (4.7%) than in the AST (2.6%) and CST groups (1.9%). The maximal body temperature measured during hospitalization was similar in all three groups: the NGCS group (38.6 ± 0.6 °C), the AST group (38.5 ± 0.4 °C), and the CST group (38.5 ± 0.5 °C).
Table 1.
Population characteristics.
| Acute Steroid Use (Under 28 Days) (N = 116) |
Chronic Steroid Use (More than 28 Days) (N = 161) |
No Steroid Use (N = 557) |
p Value | |
|---|---|---|---|---|
| Age | 0.124 | |||
|
Mean ± SD
min, max |
69.40 ± 12.03 45.00, 102.00 |
71.74 ± 11.00 31.00, 95.00 |
69.48 ± 13.26 22.00, 108.00 |
|
| Gender | 0.932 | |||
| Male % (N) | 61.2% (71) | 63.4% (102) | 62.1% (346) | |
| BMI | 0.183 | |||
|
Mean ± SD
min, max |
25.20 ± 5.71 15.62, 42.97 |
24.82 ± 4.80 15.43, 40.25 |
25.88 ± 6.24 13.28, 49.95 |
|
| Weight | 0.268 | |||
|
Mean ± SD
min, max |
70.63 ± 17.27 35.00, 115.00 |
70.31 ± 14.98 40.00, 120.00 |
72.60 ± 17.97 34.00, 150.00 |
|
| Obesity (BMI > 30 kg/m2) % (N) | 29.6% (32) | 20.4% (29) | 31.3% (160) | 0.040 |
| Smoker (current or former) % (N) | 74.1% (86) | 77.0% (124) | 69.8% (389) | 0.170 |
| Type 2 diabetes % (N) | 40.5% (47) | 57.8% (93) | 39.0% (217) | <0.001 |
| HTN % (N) | 45.7% (53) | 44.1% (71) | 44.3% (247) | 0.960 |
| Ischemic heart disease % (N) | 19.8% (23) | 16.8% (27) | 24.4% (136) | 0.096 |
|
Charlson’s comorbidity index,
Median, (IQR) |
2, (1,3) | 2, (1,2) | 2 (1, 2) | 0.301 |
| Bacteremia, % (N) | 2.6% (3) | 1.9% (3) | 4.7% (26) | 0.199 |
| Pneumonia, % (N) | 37.1% (43) | 33.5% (54) | 37.5% (209) | 0.650 |
| Sepsis, % (N) | 3.4% (4) | 4.3% (7) | 4.1% (23) | 0.927 |
|
Maximal fever during hospitalization,
Mean ± SD min, max |
38.5 ± 0.4 38.00, 39.69 |
38.5 ± 0.5 38.00, 40.20 |
38.6 ± 0.6 38.00, 42.00 |
0.302 |
| Transfer to ICU % (N) | 0.9% (1) | 1.2% (2) | 2.3% (13) | 0.452 |
| SLE % (N) | 0.9% (1) | 0.0% (0) | 0.0% (0) | 0.045 |
| RA % (N) | 3.4% (4) | 1.9% (3) | 1.1% (6) | 0.162 |
BMI: body mass index; HTN: hypertension; ICU: intensive care unit; SLE: systemic lupus erythematosus; RA: rheumatoid arthritis.
3.2. White Blood Cell Count
Baseline laboratory tests of the patients prior to the current illness (Table 2) showed, as expected, that the mean WBC count was significantly higher in the two GCS treatment groups (10.0 ± 5.5 × 109 /L in the AST and 10.2 ± 2.9 × 109/L in the CST) compared to the NGCS group (8.5 × 109/L ± 2.6). During hospitalization, the mean maximal leukocyte count was also higher in the GCS treatment groups than in the NGCS group: 16.1 ± 9.8 × 109/L in the AST group, 14.2 ± 6.9 × 109 /L in the CST group, and 12.1 ± 6.2 × 109 /L in the NGCS group (p < 0.001). The eosinophil count was suppressed more in the GCS groups than in the NGCS group: mean eosinophil count of 0.08 ± 0.09 × 109 /L in the AST group, 0.05 ± 0.07.5 × 109 /L in the chronic GCS treatment and 0.11 ± 0.14 × 109/L in the NGST group (p < 0.001).
Table 2.
Comparison of laboratory tests between the groups.
| Acute Steroid Use (Under 28 Days) (N = 116) |
Chronic Steroid Use (More than 28 Days) (N = 161) |
No Steroid Use (N = 557) |
p Value | ||
|---|---|---|---|---|---|
|
Lab Tests During the First 24 h of Hospitalization
Mean ± SD Min, Max |
Initial WBC * | 15.36 ± 9.32 2.58, 75.48 |
13.90 ± 5.67 1.28, 37.40 |
12.34 ± 5.86 2.00, 42.10 |
<0.001 |
| WBC (mean) ** | 14.87 ± 8.87 2.58, 72.97 |
13.53 ± 5.47 1.57, 37.40 |
12.15 ± 5.73 2.43, 44.70 |
<0.001 | |
| WBC (max) ** | 15.70 ± 9.51 2.58, 75.48 |
14.14 ± 5.70 1.85, 37.40 |
12.74 ± 6.07 2.43, 47.30 |
<0.001 | |
| Initial Neutrophil | 12.60 ± 6.49 2.13, 36.90 |
11.90 ± 5.40 1.20, 39.50 |
10.39 ± 5.61 0.97, 39.90 |
<0.001 | |
| Neutrophils (mean) | 12.32 ± 6.15 2.13, 31.01 |
11.70 ± 5.29 1.32, 35.50 |
10.30 ± 5.50 0.97, 42.35 |
<0.001 | |
| Neutrophils (max) | 13.12 ± 6.79 2,13, 36.90 |
12.18 ± 5.44 1.44, 35.50 |
10.82 ± 5.83 0.97, 44.80 |
<0.001 | |
| Initial Eosinophils * | 0.07 ± 0.11 0.00, 0.75 |
0.07 ± 0.14 0.00, 1.00 |
0.06 ± 0.10 0.00, 1.19 |
0.469 | |
| Eosinophils (mean) ** | 0.06 ± 0.13 0.00, 1.08 |
0.07 ± 0.13 0.00, 1.00 |
0.06 ± 0.09 0.00, 1.19 |
0.517 | |
| Eosinophils (min) ** | 0.05 ± 0.10 0.00, 0.75 |
0.05 ± 0.13 0.00, 1.00 |
0.04 ± 0.08 0.00, 1.19 |
0.508 | |
|
Lab Tests During Hospitalization, from 24 h Following Admission until Discharge
Mean ± SD Min, Max |
WBC (mean) | 13.93 ± 6.88 6.06, 47.53 |
12.51 ± 5.01 3.40, 29.69 |
10.77 ± 4.46 3.38, 37.97 |
<0.001 |
| WBC (max) | 16.14 ± 9.84 6.32, 67.09 |
14.17 ± 6.92 3.40, 44.32 |
12.12 ± 6.22 3.39, 51.88 |
<0.001 | |
| Neutrophils (mean) | 11.28 ± 5.28 5.07, 31.51 |
10.56 ± 5.02 2.70, 28.69 |
8.50 ± 4.38 1.02, 35.79 |
0.001 | |
| Neutrophils (max) | 13.49 ± 7.86 5.07, 41.22 |
12.17 ± 6.85 2.70, 43.28 |
9.84 ± 6.13 1.02, 39.97 |
0.001 | |
| Eosinophils (mean) | 0.08 ± 0.09 0.00, 0.40 |
0.05 ± 0.07 0.00, 0.50 |
0.11 ± 0.14 0.00, 1.13 |
<0.001 | |
| Eosinophils (min) | 0.04 ± 0.05 0.00, 0.20 |
0.05 ± 0.07 0.00, 0.50 |
0.88 ± 0.14 0.00, 0.96 |
<0.001 | |
|
Baseline Lab Tests: Data from within 30 Days Prior to Admissions
Mean ± SD Min, Max |
WBC (mean) | 10.00 ± 5.47 5.62, 25.46 |
10.15 ± 2.85 5.59, 17.13 |
8.50 ± 2.55 3.77, 15,64 |
0.027 |
| WBC (max) | 10.05 ± 5.62 5.62, 26.10 |
10.17 ± 2.83 5.59, 17.13 |
8.51 ± 2.57 3.77, 15.64 |
0.027 | |
| Neutrophils (mean) | 6.94 ± 4.79 2.70, 18.62 |
7.47 ± 2.84 3.38, 14.45 |
5.54 ± 2.47 1.93, 12.44 |
0.007 | |
| Neutrophils (max) | 7.07 ± 5.14 2.70, 20.30 |
7.50, 2.82 3.38, 14.45 |
5.57 ± 2.47 1.93, 12.44 |
0.008 |
* Refers to the first blood test at admissions. ** Refers to all tests during the first 24 h of admission.
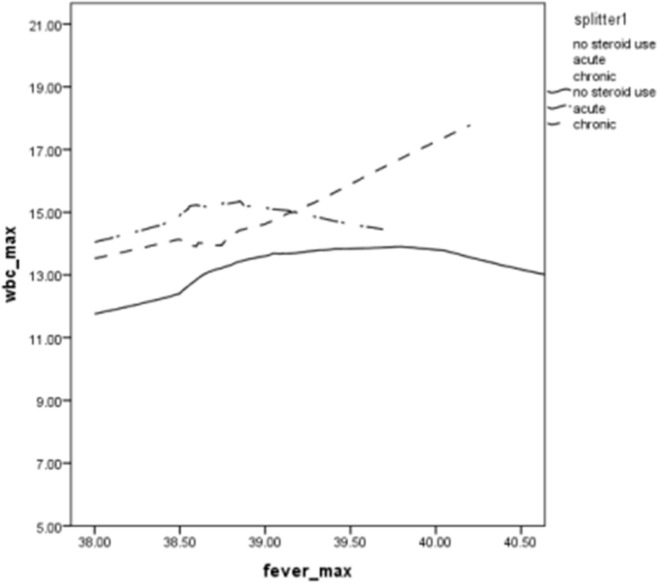
The mean neutrophil count was also higher in both GCS treatment groups than in the NGCS group: 13.1 ± 6.8 × 109/L in the AST group, 12.2 ± 5.4 × 109/L in the CST group, and 10.8 ± 5.8 × 109/L in the NGCS group (p < 0.001). WBC count was higher for the two treatment groups than for the NGCS group at all body temperatures; values increased with temperature, particularly for the CST group (Figure 1). The trends for maximum neutrophil values as a function of body temperature were similar to those of maximum WBC count as a function of body temperature (data not shown). The difference increases with temperature and reaches 6 × 109/L at 40.2 °C. A multivariable linear regression analysis showed significantly elevated WBC counts after adjustment for age, smoking, maximal fever, pneumonia, and sepsis, which were similar in the two GCS treatment groups (Table 3). The R square value was 0.76, and the adjusted R square value 0.68.
Figure 1.
Maximum WBC count as a function of maximum body temperature for COPD patients receiving acute steroid treatment, chronic steroid treatment or no steroid treatment.
Table 3.
Linear regression model for the prediction of maximal white blood cell (WBC) count within 24 h from admissions.
| Variable | Unstandardized B | Standardized B | 95% CI | p Value | |
|---|---|---|---|---|---|
| Min | Max | ||||
| No steroids (reference group) | 1 | ||||
| Chronic Steroids | 1.776 | 0.099 | 0.526 | 3.026 | 0.005 |
| Acute Steroids | 2.945 | 0.144 | 1.523 | 4.368 | <0.001 |
| Age | 0.016 | 0.028 | −0.024 | 0.056 | 0.433 |
| Smoker | 0.820 | 0.052 | −0.300 | 1.939 | 0.151 |
| Maximal Fever | 1.113 | 0.085 | 0.207 | 2.020 | 0.016 |
| Pneumonia | 2.342 | 0.159 | 1.311 | 3.372 | <0.001 |
| Sepsis | 4.061 | 0.104 | 1.384 | 6.737 | 0.003 |
3.3. Eosinophil Count as a Marker of Glucocorticoid Effect
Among patients treated with steroids and with undetectable absolute eosinophil count (undetectable eosinophils), the mean difference between the expected and the observed WBC count was 5.1 ± 12.7.5 × 109/L for the AST and 2.8 ± 7.4 × 109/L for the CST groups. By contrast, for patients who were treated with GCS and had a detectable eosinophil count (absolute count higher than zero), the mean differences between the expected and observed WBC counts were 1.5 ± 5.7 × 109/L for the AST and 0.8 ± 5.7 × 109 /L for the CST (Table 4 and Table 5).
Table 4.
Linear regression model for prediction of expected WBC.
| Eosinophils = 0 | Eosinophils > 0 | |||
|---|---|---|---|---|
| Acute Steroids (46) |
Chronic Steroids (72) |
Acute Steroids (63) |
Chronic Steroids (82) |
|
|
Difference between observed and expected
(WBC) |
||||
|
Mean ± SD,
Min, Max Median, IQR |
5.06 ± 12.70 −10.71, 61.19 1.76 −3.25, 9.44 |
2.82 ± 7.43 −7.75, 29.02 0.51 −1.60, 5.60 |
1.49 ± 5.66 −10.29, 15.64 0.86 −2.93, 3.57 |
0.83 ± 5.65 −8.28, 19.02 0.05 −3.36, 4.20 |
|
Difference between observed and expected
(NEUT) |
||||
|
Mean ± SD
Min, Max Median IQR |
4.65 ± 9.47 −9.60, 31.11 2.37 −2.37, 9.61 |
3.03 ± 7.20 −7.29, 29.84 0.99 −1.30, 5.97 |
0.97 ± 4.99 −0.853, 12.92 0.33 −2.55, 2.41 |
0.86 ± 5.54 −9.88, 17.15 0.47 −3.37, 3.33 |
Table 5.
Adjusted maximal WBC count.
| Variable | B | β | 95% CI | p Value | |
|---|---|---|---|---|---|
| Min | Max | ||||
| Age | 0.034 | 0.069 | −0.009 | 0.077 | 0.118 |
| Gender (Female) | −0.508 | −0.038 | −1.682 | 0.666 | 0.396 |
| Maximal Fever | 0.994 | 0.086 | −0.005 | 1.992 | 0.051 |
Linear regression model 1 in the “no-steroid group”. Adjusted R square = 0.008.
4. Discussion
The main finding of this study is that in patients with COPD exacerbation, both chronic and acute GCS therapy was associated with an independent increase in the WBC count to the level of 3–5 × 109/L. This finding was more evident in patients with undetectable levels of eosinophils.
According to a systemic review published in 2015 [7], the majority of the clinical guidelines recommend leukocytosis as a standalone criterion for administering antibiotic treatment in patients with COPD exacerbation. Since the level of leukocytosis is influenced by the infection process [8] and the physiologic stress response [9], as well as the steroid effect, distinguishing the degree of effects of these factors is crucial. Therefore, based on our results, in patients with acute or chronic treatment with GCS, we recommend subtracting 3–5 × 109/L from the actual WBC count to obtain an unbiased, nonsteroid-induced estimate. We emphasize that physicians should consider all clinically available data in deciding whether to administer antibiotic treatment and not rely solely on the level of leukocytosis.
Similarly, the ultimate duration of antibiotic therapy in COPD patients is unclear [10] and usually ranges from 5 to 10 days in most clinical guidelines. Since it is reasonable to assume that the degree of leukocytosis serves as only one of the parameters that guides clinicians in the decision to cease antibiotics therapy, the same approach of subtracting 3–5 × 109/L from the actual WBC count seems appropriate also here.
Blood eosinophil has been proposed as a personalized biomarker-guide to reduce systemic corticosteroid exposure [11]. The issue investigated in the current study is different. Since peripheral blood eosinophils are known to be suppressed by systemic GCS [3], we considered if blood eosinophil level can serve as a direct marker of the effect of GCS. Indeed, we found that the increase of WBC in patients with COPD exacerbation treated with chronic or acute GCS was more evident in patients with undetectable levels of eosinophils. Thus, we assume that mild elevation of WBC in patients with COPD exacerbation treated with GCS, together with normal levels of eosinophils, can be a clue for patient noncompliance or a clue for an inadequate effect of GCS due to other reasons, such as malabsorption, patient error in medical consumption, and vomiting. Moreover, such an eosinophil level can represent a coexisting disease, for example, atopic dermatitis, inflammatory bowel disease, lymphoma, leukemia or drug allergy.
In this cohort study, more than 60% of our COPD patients were males, and the mean age was 69 years. These characteristics are similar to those of COPD patients in a general population in the Netherlands: about 57% in that study were males, and the mean age was 67.8 + −12.1 years [12].
The novelty of this study is its focus on patients with fever and acute exacerbations of COPD. The current study has a number of limitations. First, it included only 834 patients with COPD exacerbation and fever, which is considered a relatively small retrospective study, from a single center. Second, GCS are known to have an antipyretic effect [13]. This would have excluded from the analysis normothermic patients with COPD exacerbation who were treated with GCS therapy. Third, the current study does not distinguish between steroid doses or route of administration, while these factors could have specific effects on the results.
We believe that our findings, combined with bedside inspection of eosinophil and leukocytosis levels, may contribute to clinicians’ understanding of patient compliance to steroid therapy, as well as the understanding of the specific, distinct effect of steroids on WBC count.
Author Contributions
Writing—Review and Editing, A.F. and E.K.; Methodology and Formal Analysis, V.N.; Conceptualization M.K.; Validation E.B.; Writing–Original Draft Preparation L.K.; Supervision N.M. All authors approved the submitted version. All authors agree to be personally accountable for their own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Niewoehner D.E., Erbland M.L., Deupree R.H., Collins D., Gross N.J., Light R.W., Anderson P., Morgan N.A. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N. Engl. J. Med. 1999;340:1941–1947. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 2.Walters J.A., Walters E.H., Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2005;3:005374. doi: 10.1002/14651858.CD005374. [DOI] [PubMed] [Google Scholar]
- 3.Altman L.C., Hill J.S., Hairfield W.M., Mullarkey M.F. Effects of Corticosteroids on Eosinophil Chemotaxis and Adherence. J. Clin. Investig. 1981;67:28–36. doi: 10.1172/JCI110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng S.L. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:2775–2784. doi: 10.2147/COPD.S175017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frenkel A., Kachko E., Cohen K., Novack V., Maimon N. Estimations of a degree of steroid induced leukocytosis in patients with acute infections. Am. J. Emerg. Med. 2018;36:749–753. doi: 10.1016/j.ajem.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Bhattarai B., Ghosh M., Ray A.S., Azad M.R., Sivasambu B., Wan S.K., Saha S., Kandel S., Kharel P., Pokharel S., et al. Leukocytosis in Patients With COPD/BA Exacerbation: Steroid-Induced or Sign of an Infection. Chest. 2014;146:28A. doi: 10.1378/chest.1993849. [DOI] [Google Scholar]
- 7.Laue J., Reierth E., Melbye H. When should acute exacerbation of COPD be treated with systemic corticosteroids and antibiotics in primary care: A systemic review of the current COPD guidelines. NPJ Prim Care Respir Med. 2015;25:15002. doi: 10.1038/npjpcrm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zubler M.A. Leukocytosis in Infection. Arch. Intern. Med. 1987 doi: 10.1001/archinte.1987.00370020017008. [DOI] [PubMed] [Google Scholar]
- 9.Abramson N., Melton B. Leukocytosis: Basics of clinical assessment. Am. Fam. Physician. 2000;62:2053–2060. [PubMed] [Google Scholar]
- 10.Stolbrink M., Amiry J., Blakey J.D. Does antibiotic treatment duration affect the outcomes of exacerbations of asthma and COPD? A systematic review. Chronic Respir. Dis. 2017;15:225–240. doi: 10.1177/1479972317745734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivapalan P., Lapperre T.S., Janner J., Laub R.R., Moberg M., Bech C.S., Eklöf J., Holm F.S., Armbruster K., Sivapalan P., et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): A multicentre, randomised, controlled, open-label, non-inferiority trial. Lancet Respir. Med. 2019;7:699–709. doi: 10.1016/S2213-2600(19)30176-6. [DOI] [PubMed] [Google Scholar]
- 12.Afonso A.S., Verhamme K.M., Sturkenboom M.C., Brusselle G.G. COPD in the general population: Prevalence, incidence and survival. Respir. Med. 2011;105:1872–1884. doi: 10.1016/j.rmed.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Coelho M.M., Luheshi G., Hopkins S.J., Pela I.R., Rothwell N.J. Multiple mechanisms mediate antipyretic action of glucocorticoids. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1995;269:R527–R535. doi: 10.1152/ajpregu.1995.269.3.R527. [DOI] [PubMed] [Google Scholar]