Graphical abstract
Keywords: Infective endocarditis, Culture-negative endocarditis, Prosthetic valve endocarditis, Aortic valve, Dehiscence
Highlights
-
•
The authors describe culture-negative endocarditis causing mechanical AV dehiscence.
-
•
Early diagnosis and prompt surgical treatment are necessary.
-
•
TEE is useful in identifying the etiology of AR in this instance.
-
•
Pseudoaneurysm involving aortic homograft is rare and can occur without reinfection.
Introduction
Culture-negative endocarditis constitutes up to 16% to 18% of prosthetic valve endocarditis (PVE)1, 2 and can rarely be complicated by prosthetic aortic valve (AV) dehiscence. We report the case of a patient with culture-negative endocarditis affecting a mechanical bileaflet AV resulting in valve dehiscence, severe paravalvular aortic regurgitation (AR) and acute left ventricular (LV) failure, complicated by aortic root homograft pseudoaneurysm. A detailed case report and brief overview of dehiscence of prosthetic valves are presented, and the utility of three-dimensional echocardiography and multimodality imaging highlighted.
Case Presentation
A 66-year-old man presented to the emergency department with sudden-onset dyspnea and was found to be in heart failure. The patient had undergone mechanical AV replacement (25-mm On-X bileaflet; CryoLife, Kennesaw, GA) 1 year prior for severe aortic stenosis with preserved LV ejection fraction. There was only minor coronary artery disease on computed tomographic coronary angiography before valve replacement. Other background medical history included permanent pacemaker with single right ventricular lead for complete heart block in the context of permanent atrial fibrillation and sick sinus syndrome and chronic obstructive pulmonary disease. His predominant symptom was exertional dyspnea, elicited on mobilizing very short distances. This was associated with atypical nonpleuritic right-sided chest pain. However, the patient reported no fevers or cough. The patient was independent in activities of daily living before presentation and had a 30-pack-year history of smoking. Medications at the time of admission were metoprolol 100 mg twice daily, digoxin 250 μg daily, warfarin 3 mg daily at admission (variable dose on the basis of international normalized ratio), tiotropium/olodaterol two puffs in the morning, and salbutamol metered dose inhaler as needed. On cardiovascular examination, heart rate was 60 beats/min in atrial fibrillation, blood pressure was 120/40 mm Hg, and respiratory rate was 28 breaths/min with oxygen saturation of 96% on low-flow oxygen delivered via nasal prongs. The patient was afebrile. There was a metallic second heart sound, and an ejection systolic murmur was audible in the aortic area, radiating toward the carotid arteries. There were bibasal coarse crepitations. No diastolic murmur could be appreciated. There were no signs of right heart failure (jugular venous pressure was not elevated, and there was no pitting lower limb edema or ascites). Twelve-lead electrocardiography showed rate-controlled atrial fibrillation (not paced) with a left bundle branch block pattern. Chest radiography showed interstitial pulmonary edema and no cardiomegaly (Figure 1). Transthoracic echocardiography (TTE) revealed mild global LV systolic impairment (biplane LV ejection fraction 42%; Video 1). The prosthetic valve appeared well seated with a mild paravalvular leak (Figure 2, Video 2), there was no other significant valvular dysfunction, and mild pulmonary hypertension was demonstrated, with an estimated right ventricular systolic pressure of 46 mm Hg.
Figure 1.
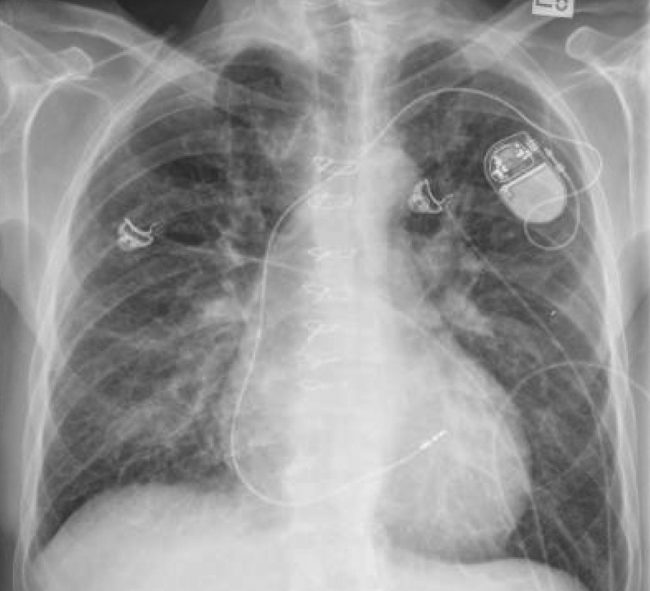
Plain posterior-anterior chest radiograph obtained in the emergency department on the day of presentation. There is interstitial pulmonary edema, without evident cardiomegaly. A permanent pacemaker is in situ with a single right ventricular lead.
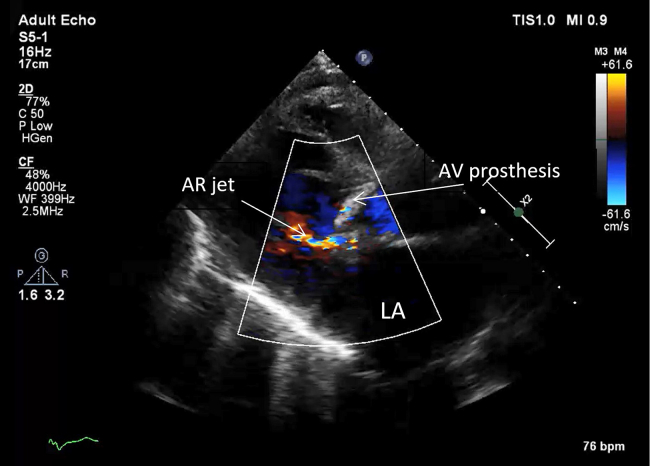
Figure 2.
Initial transthoracic echocardiogram. The mechanical AV prosthesis in parasternal long axis view. Early diastole. There is mild AR. LA, Left atrium.
The patient's condition deteriorated gradually over 4 days, and he had a cardiac arrest with pulseless electrical activity and downtime of 30 min before return of spontaneous circulation. There was no pulmonary embolus on computed tomographic pulmonary angiography. The patient developed cardiorespiratory failure necessitating intubation and inotropes (dobutamine and noradrenaline), complicated by ischemic hepatitis with coagulopathy (international normalized ratio 8.9), and acute renal failure (creatinine 150 μmol/L, 90 μmol/L at baseline) with lactic acidosis (lactate 16 mmol/L, pH 7.19). The cause for acute deterioration was unclear. Inflammatory markers were raised (white cell count 22.0 × 109/L, C-reactive protein 150 mg/L), but the patient was never febrile, and detailed septic screen was negative. The patient was treated for presumed sepsis with meropenem after consultation with the infectious diseases team.
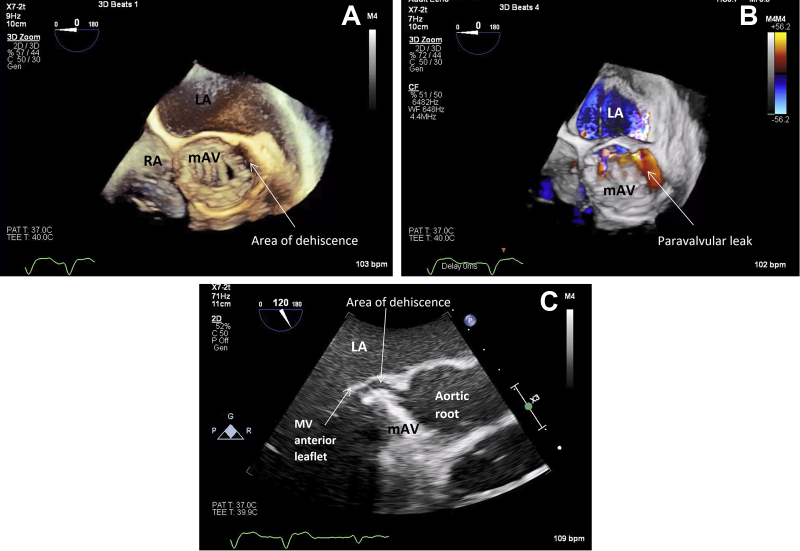
The coagulopathy was corrected, and the patient stabilized with a new AR murmur heard. Thus, transesophageal echocardiography (TEE) was performed, which demonstrated rocking of the prosthetic AV (Figure 3C, Video 3C) with severe eccentric paravalvular AR (diastolic flow reversal in the descending aorta). Three-dimensional acquisition demonstrated dehiscence of the prosthetic valve annulus (or sewing ring) in the region of the native left coronary cusp (∼3 o'clock to 6 o'clock using the interatrial septum as 12 o'clock) involving 25% of the circumference (Figures 3A and 3B, Videos 3A and 3B). There were no obvious vegetations or root abscess. The aortic root was dilated (42 × 44 mm at the sinuses of Valsalva), the left ventricle was dilated, and there was moderate to severe biventricular dysfunction despite inotropes. Antimicrobial treatment was changed to ceftriaxone and vancomycin for broad-spectrum coverage and moxifloxacin, azithromycin, and rifampicin for Mycobacterium spp. The patient proceeded to undergo an urgent redo sternotomy with AV, root, and ascending aorta replacement with a 24-mm cadaveric homograft. The surgeon noted that the mechanical prosthesis was held only by two sutures, and the annulus was largely “liquified” along the suture line. There was no overt abscess formation. The cause of dehiscence appeared infectious. Blood and tissue fungal cultures were negative. Blood and tissue mycobacterial cultures, polymerase chain reaction, and acid-fast bacilli cultures were negative. Blood and tissue Legionella nucleic acid test results were similarly negative. Blood polymerase chain reaction for Bartonella henselae and Tropheryma whipplei were negative. We performed 16S recombinant ribonucleic acid gene sequencing using polymerase chain reaction on AV tissue samples, and similarly this did not identify any pathogen, including Coxiella burnetii.
Figure 3.
Transesophageal echocardiogram performed day 14 of admission. Short-axis three-dimensional image in end-systole showing dehiscence of aortic prosthesis in 3 o'clock to 6 o'clock position (A, B) and resultant paravalvular leak (B). (C) Long-axis view demonstrating separation of the aortic prosthesis from the aortomitral curtain. The valve is seen rocking (see Video 3C). LA, Left atrium; mAV, mechanical AV; MV, mitral valve; RA, right atrium.
Repeat TTE 3 days postoperatively demonstrated marked improvement in LV systolic function and normal estimated pulmonary artery pressures (35 mm Hg), and the homograft appeared to function normally with trivial central AR (Figure 4, Video 4). The patient was discharged 1 month later on a 6-week course of ceftriaxone 2 g daily intravenously.
Figure 4.
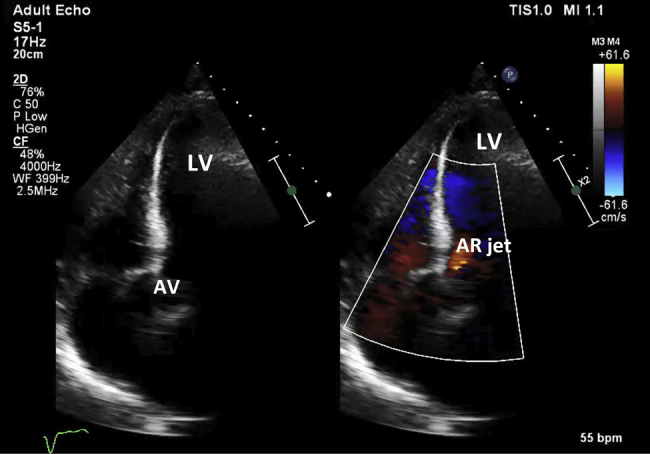
Day 3 after excision of mechanical aortic prosthesis and replacement with an AV homograft. The valve is functioning normally, with only trivial AR (see Video 4).
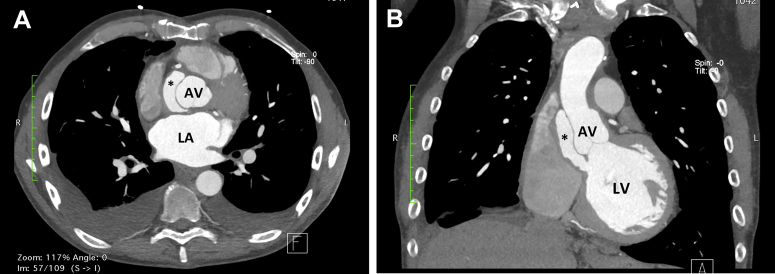
The patient underwent cardiac computed tomography 1 month after discharge. This showed a false aneurysm anterior to the noncoronary cusp of the homograft extending from the LV outflow tract (Figure 5). TEE confirmed this and demonstrated flow during systole into the false aneurysm with systolic collapse of the noncoronary cusp (Figure 6, Video 5). There was no evidence of reinfection or homograft AV failure. The patient proceeded to undergo bovine patch repair of the false aneurysm (Figure 7) and had an uncomplicated postoperative recovery.
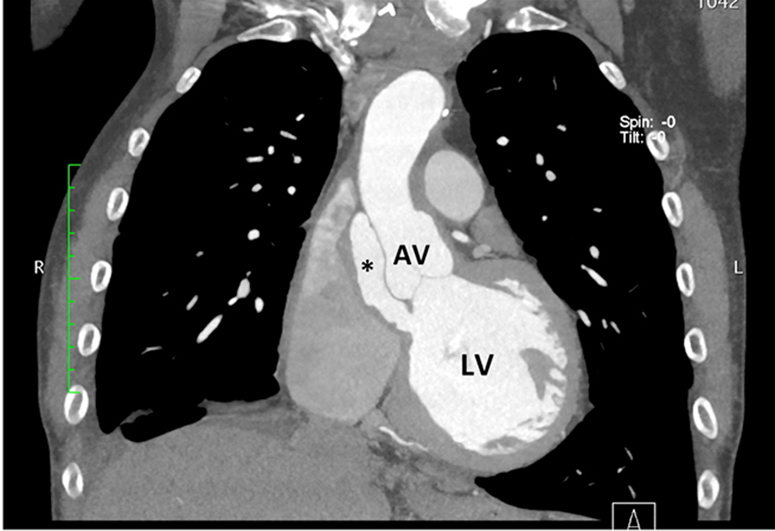
Figure 5.
Computed tomographic chest axial plane at the level of the AV (A) and coronal plane (B) demonstrating presence of pseudoaneurysm (asterisk) and connection to the LV outflow tract (B). LA, Left atrium.
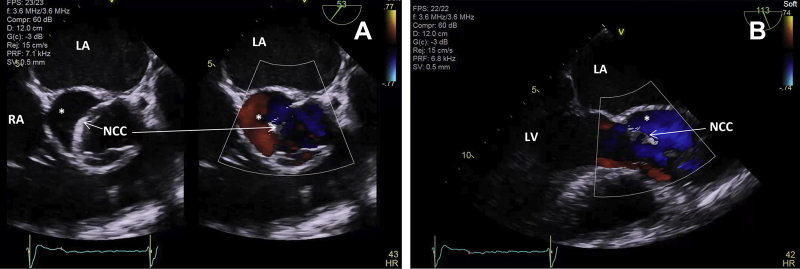
Figure 6.
Transesophageal echocardiogram before repair of homograft pseudoaneurysm shown in end-systole in short axis (A) and long axis (B). Collapse of noncoronary cusp (NCC) of AV homograft by systolic flow through the pseudoaneurysm (asterisk). LA, Left atrium; RA, right atrium.
Figure 7.
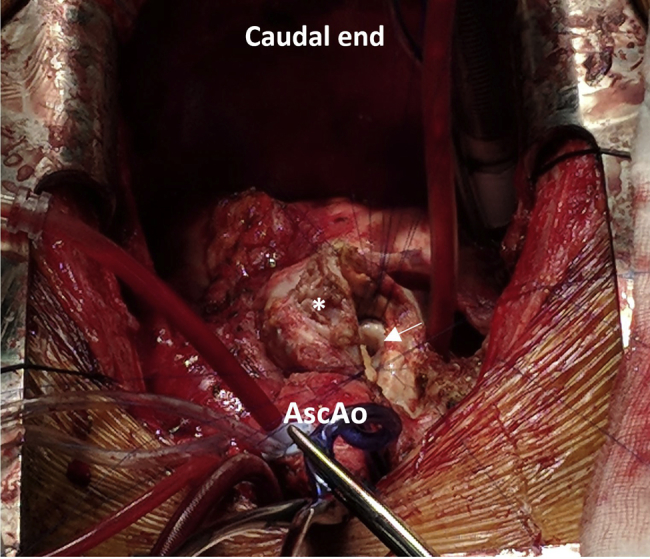
Bovine patch repair of pseudoaneurysm with access via the pseudoaneurysmal sac (asterisk). The mouth of the pseudoaneurysm is depicted by the arrow. AscAo, Ascending aorta.
Discussion
This case highlights the occurrence of early-onset (within 12 months) culture-negative endocarditis in the setting of a mechanical AV and the importance of three-dimensional TEE in both diagnosis and guidance of surgical treatment. In our case, absence of fevers, negative blood cultures, and early TTE, which did not demonstrate vegetations with what was considered mild paravalvular leak (severity likely underestimated because of the extremely eccentric AR jet, with shadowing and artifact from the mechanical prosthesis), delayed the diagnosis and urgent surgical treatment. TEE using three-dimensional echocardiography identified severe paravalvular leak and dehiscence of the mechanical prosthesis, including the point of disjunction along the annulus, which was able to direct surgical treatment. Microbiologic test results of blood and tissue samples were negative for the usual bacterial pathogens, Mycobacterium spp, HACEK organisms, C burnetii, Bartonella spp, and T whipplei. The reason for culture-negative endocarditis may be because of antibiotic treatment before adequate microbiologic investigation or, much less likely, noninfective endocarditis. This case also documents the rare occurrence of a noninfective pseudoaneurysm complicating homograft treatment of early PVE.
PVE has an incidence of 0.3% to 1.2% per patient-year,3 and the risk is similar for both bioprosthetic and mechanical valve replacements.4 It accounts for up to one-fifth (20%) of all cases of infective endocarditis3 and is a serious complication of surgical valve replacement, with combined risk for mortality and morbidity ranging from 20% up to 80% for AV endocarditis.1, 2, 5 Most patients die of heart failure and cardiogenic shock.2 The most common organism in PVE is Staphylococcus aureus (23%), followed by coagulase-negative Staphylococcus (17%), and health care–associated PVE has been seen to occur in 37% of patients.5 The clinical presentation of PVE can be atypical and more difficult to diagnose than native valve endocarditis.6 Positive blood cultures and vegetations are less frequently seen in PVE compared with native valve endocarditis, whereas abscess formation is more frequent.7, 8 Negative echocardiographic findings may occur because of small or absent (as in our case) vegetations or because of difficulty with detecting them in the context of a prosthesis with associated shadowing and artifact.6 Unlike native valve endocarditis, the Duke criteria cannot be applied to PVE, because of lower sensitivity.9 The infection in PVE is usually localized to the prosthesis-tissue junction at the sewing ring, accompanied by tissue destruction leading to dehiscence and paravalvular leak.3, 10 TEE is crucial in the diagnosis of infective endocarditis, especially PVE. However, both TTE and TEE have lower sensitivity and specificity for PVE compared with native valve endocarditis.3
Complications of PVE include paravalvular abscess formation, prosthesis regurgitation, heart failure, dehiscence or prosthesis malfunction, systemic embolization of vegetations, complete heart block, and multiorgan failure.1, 2, 5 In a prospective, observational multicenter cohort study involving 556 patients with definite PVE, 71% of health care–associated PVE occurred between 60 days and 1 years of implantation. Surgery was performed in 49% during the index hospitalization, and in-hospital death occurred in 23%. In-hospital death was predicted by older age, health care–associated infection, S aureus infection, and complications of PVE, including heart failure (33%), stroke (34%), intracardiac abscess (33%), and persistent bacteremia (55%).5 Severe heart failure, prosthetic valve dehiscence, and Staphylococcus infection are independent predictors of long-term mortality.11
Blood culture–negative endocarditis may be due to highly fastidious microorganisms (e.g., HACEK bacteria) or intracellular bacteria that cannot be routinely cultured in blood (e.g., C burnetii, Bartonella spp, T whipplei) and inadequate microbiological techniques.12, 13 Culture-negative endocarditis has a slow clinical progression and history of treatment with antimicrobials before blood cultures. Noninfectious endocarditis is rare and usually due to marantic endocarditis and endocarditis secondary to autoimmune diseases.13 The predominant etiologies of culture-negative endocarditis after PVE have been shown in a study to be predominately fungal infections (16%), compared with community-acquired culture-negative endocarditis, which is due predominately to C burnetii (36.5%) and Bartonella spp (14%).12
Surgery is the recommended treatment strategy in PVE complicated by prosthesis dysfunction or heart failure (as in our case) and is associated with a better prognosis compared with medical therapy. However, there is no difference in mortality in uncomplicated PVE treated with a medical versus surgical approach.3 Our case was additionally complicated by pseudoaneurysm formation involving the homograft requiring early reoperation. Reinfection following homograft replacement for PVE has been reported to occur in 4.9%, and most occurred early (i.e., <60 days after surgery).14 However, in our case, the cause of the pseudoaneurysm did not appear to be reinfection from macroscopic appearance of tissue during the surgery. A review of the literature regarding aortic root pseudoaneurysms (outside the context of reinfection) following homograft for PVE yielded no similar reports. However, there was a similar case involving a pseudoaneurysm following porcine AV replacement treated with a homograft.15
Conclusion
We report a case of severe paravalvular AR causing acute LV failure secondary to culture-negative endocarditis with mechanical AV dehiscence. Absence of fever, negative blood cultures, and lack of vegetations on TTE may delay correct diagnosis and appropriate treatment, hence causing progressive tissue damage. Early diagnosis and prompt surgical treatment of complicated PVE is required. Three-dimensional TEE is useful in identifying the etiology of the severity of AR in this instance. In the absence of typical features of infective endocarditis (fever, positive blood cultures, and vegetations), acute valvular insufficiency (especially an eccentric jet) and LV failure should still raise the suspicion of culture negative endocarditis. Pseudoaneurysm involving aortic homograft is rare and can occur without reinfection.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2019.04.008.
Supplementary Data
TTE on day 1 demonstrating mild global LV systolic impairment (LV ejection fraction 42%) in the apical four-chamber view.
TTE on day 1 demonstrating a well-seated prosthetic valve with mild paravalvular leak.
(A) TEE with three-dimensional acquisition demonstrating dehiscence of the aortic annulus in the region of the left coronary cusp (∼3 o’clock to 6 o’clock using the interatrial septum as 12 o’clock) involving 25% of the circumference. (B) The resulting paravalvular leak. (C) The rocking prosthesis. The aortic root was dilated, measuring 42 × 44 mm at the sinuses of Valsalva.
TTE 3 days postoperatively demonstrated marked improvement in LV systolic function, and the homograft appeared to function normally with trivial central AR.
TEE before repair of homograft pseudoaneurysm demonstrating flow during systole into the false aneurysm with systolic collapse of the noncoronary cusp of the AV.
References
- 1.Lopez J., Revilla A., Vilacosta I., Villacorta E., Gonzalez-Juanatey C., Gomez I. Definition, clinical profile, microbiological spectrum, and prognostic factors of early-onset prosthetic valve endocarditis. Eur Heart J. 2007;28:760–765. doi: 10.1093/eurheartj/ehl486. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Valle H., Farinas-Alvarez C., Garcia-Palomo J.D., Bernal J.M., Martin-Duran R., Gutierrez Diez J.F. Clinical course and predictors of death in prosthetic valve endocarditis over a 20-year period. J Thorac Cardiovasc Surg. 2010;139:887–893. doi: 10.1016/j.jtcvs.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Habib G., Thuny F., Avierinos J.F. Prosthetic valve endocarditis: current approach and therapeutic options. Prog Cardiovasc Dis. 2008;50:274–281. doi: 10.1016/j.pcad.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Foster E. Clinical practice. Mitral regurgitation due to degenerative mitral-valve disease. N Engl J Med. 2010;363:156–165. doi: 10.1056/NEJMcp0906782. [DOI] [PubMed] [Google Scholar]
- 5.Wang A., Athan E., Pappas P.A., Fowler V.G., Jr., Olaison L., Pare C. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007;297:1354–1361. doi: 10.1001/jama.297.12.1354. [DOI] [PubMed] [Google Scholar]
- 6.Matsukuma S., Eishi K., Tanigawa K., Miura T., Matsumaru I., Hisatomi K. Afebrile pannus-induced blood culture-negative mechanical valve endocarditis. Ann Thorac Surg. 2016;102:e511–e513. doi: 10.1016/j.athoracsur.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Habib G., Derumeaux G., Avierinos J.F., Casalta J.P., Jamal F., Volot F. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol. 1999;33:2023–2029. doi: 10.1016/s0735-1097(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 8.Habib G. Management of infective endocarditis. Heart. 2006;92:124–130. doi: 10.1136/hrt.2005.063719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamas C.C., Eykyn S.J. Suggested modifications to the Duke criteria for the clinical diagnosis of native valve and prosthetic valve endocarditis: analysis of 118 pathologically proven cases. Clin Infect Dis. 1997;25:713–719. doi: 10.1086/513765. [DOI] [PubMed] [Google Scholar]
- 10.Mahesh B., Angelini G., Caputo M., Jin X.Y., Bryan A. Prosthetic valve endocarditis. Ann Thorac Surg. 2005;80:1151–1158. doi: 10.1016/j.athoracsur.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Habib G., Tribouilloy C., Thuny F., Giorgi R., Brahim A., Amazouz M. Prosthetic valve endocarditis: who needs surgery? A multicentre study of 104 cases. Heart. 2005;91:954–959. doi: 10.1136/hrt.2004.046177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thuny F., Fournier P.E., Casalta J.P., Gouriet F., Lepidi H., Riberi A. Investigation of blood culture-negative early prosthetic valve endocarditis reveals high prevalence of fungi. Heart. 2010;96:743–747. doi: 10.1136/hrt.2009.181594. [DOI] [PubMed] [Google Scholar]
- 13.Tattevin P., Watt G., Revest M., Arvieux C., Fournier P.E. Update on blood culture-negative endocarditis. Med Mal Infect. 2015;45:1–8. doi: 10.1016/j.medmal.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Musci M., Weng Y., Hubler M., Amiri A., Pasic M., Kosky S. Homograft aortic root replacement in native or prosthetic active infective endocarditis: twenty-year single-center experience. J Thorac Cardiovasc Surg. 2010;139:665–673. doi: 10.1016/j.jtcvs.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Tsai K.T., Cheng N.J., Chu J.J., Lin P.J. Aortic root pseudoaneurysm following surgery for aortic valve endocarditis. Chang Gung Med J. 2002;25:133–138. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TTE on day 1 demonstrating mild global LV systolic impairment (LV ejection fraction 42%) in the apical four-chamber view.
TTE on day 1 demonstrating a well-seated prosthetic valve with mild paravalvular leak.
(A) TEE with three-dimensional acquisition demonstrating dehiscence of the aortic annulus in the region of the left coronary cusp (∼3 o’clock to 6 o’clock using the interatrial septum as 12 o’clock) involving 25% of the circumference. (B) The resulting paravalvular leak. (C) The rocking prosthesis. The aortic root was dilated, measuring 42 × 44 mm at the sinuses of Valsalva.
TTE 3 days postoperatively demonstrated marked improvement in LV systolic function, and the homograft appeared to function normally with trivial central AR.
TEE before repair of homograft pseudoaneurysm demonstrating flow during systole into the false aneurysm with systolic collapse of the noncoronary cusp of the AV.