Abstract
Background
Bovine babesiosis is caused by protozoan parasites of the genus Babesia and presents a wide spectrum of clinical manifestations. Disease severity depends on the type of Babesia species infection. Generally, B. bovis and B. bigemina are considered as the causative agents of bovine babesiosis; in addition, Babesia ovata and B. major are a group of benign bovine piroplasms. Therefore, species identification is important for diagnosis, epidemiological investigations and follow-up management.
Methods
Real-time PCR combined with high resolution melting (RT-PCR-HRM) analysis was used to detect and discriminate four Babesia species infective to cattle, including Babesia bovis, B. bigemina, B. major and B. ovata. The melting profiles and melting temperatures (Tm) of the amplicon targeting 18S rRNA revealed differences that can discriminate the four Babesia spp. Sensitivity and specificity of the analytical method were evaluated using 50 blood samples collected from experimentally infected cattle and 240 blood samples from areas where bovine babesiosis is an issue.
Results
RT-PCR-HRM analysis allowed to detect and discriminate four Babesia spp. (B. bovis, B. bigemina, B. major and B. ovata), which were responsible for bovine babesiosis in China. The protocol was validated with DNA samples from experimentally infected cattle and field infection in cattle.
Conclusions
Our results indicate that RT-PCR-HRM is a fast and robust tool for the simultaneous detection and discrimination of four Babesia species that are responsible for bovine babesiosis in China. This approach is applicable for both field and experimental samples, thus it could be useful in epidemiological investigations and diagnoses of bovine babesiosis.
Keywords: Real-time PCR, High resolution melting analysis, Diagnosis, Bovine babesiosis, 18S rRNA
Background
The protozoan parasites of the genus Babesia (phylum Apicomplexa, order Piroplasmida), cause a large spectrum of clinical manifestations known as babesiosis. This disease is a public health burden to humans and domesticated and wild animals in tropical and subtropical regions of the world. To date, more than 100 Babesia species have been identified in wild and domestic animals across the world [1]. However, only a few Babesia species have been identified in bovines and cause bovine babesiosis, namely Babesia bovis, B. bigemina, B. major, B. divergens, B. ovata, B. orientalis, B. occultans and B. jakimivi.
In China, bovine babesiosis was first reported as early as 1948 [2]. Shortly thereafter, B. bigemina and B. bovis were identified in 22 and 16 provinces, respectively [3–8]. Six Babesia species strains infective to bovines have been isolated by the Vectors and Vector-Borne Diseases (VVBD) Laboratory, Lanzhou Veterinary Research Institute (LVRI). These Babesia spp. were identified as four distinct species, B. bovis, B. bigemina, B. major and B. ovata, which were isolated from cattle in bovine babesiosis domestic areas in China [9–12].
Disease severity depends on the host’s immune status and the species of Babesia. Generally, B. bovis and B. bigemina are considered the causative agents of bovine babesiosis [13]. While the clinical signs caused by these parasites are similar, characterized by fever, anemia and even death, more negative effects are observed in cattle infected with B. bovis than B. bigemina [14]. Babesia ovata and B. major are a group of benign bovine piroplasms [15]. Thus, species identification is essential for diagnosis, follow-up management and epidemiological studies.
Traditionally, microscopy, considered to be the gold standard for babesiosis confirmation, is used to detect Babesia species in peripheral blood smears [16]. However, this approach requires skilled personnel and has several disadvantages, which include low sensitivity and unreliability in species identification [17, 18]. Recently, polymerase chain reaction (PCR) based methods (e.g. restriction fragment length polymorphism, reverse line blot, nested-PCR and multiplex PCR) are predominately used to detect and distinguish Babesia species with high sensitivity, specificity and repeatability [8, 19–21]. However, these assays are labor-intensive and require multiple step manipulation of the PCR product in order to determine the specific species [22]. Thus, a rapid, efficient and reliable diagnostic approach is essential for species identification.
The high-resolution melting (HRM) assay is a novel and powerful molecular method applied to mutation detection, genotype analysis and species identification [23–26]. During double strand DNA (dsDNA) dissociation to single stranded DNA with increasing temperature, melting curve and melting temperature (Tm) are generated by monitoring the fluorescence of the binding dye present in dsDNA [27]. The changes in melting curve shape and Tm peaks are generated due to differences in amplicon composition. Currently, there are no reports on HRM assays for discrimination of Babesia species infective to bovines. However, assays have been performed to identify Babesia caballi and Theileria equi, discriminate four Babeisa sp. infective to humans and diagnose five Plasmodium spp. [27–29]. In the present study, we developed a reliable, rapid and powerful RT-PCR-HRM assay targeting the 18S rRNA gene to discriminate between four Babesia spp. in cattle.
Methods
Plasmid DNA preparation
Positive blood samples for each of B. bovis, B. bigemina, B. major, B. ovata, Theileria annulata, Theileria orientalis, Theileria sinensis and Anaplasma marginale were provided by the Vectors and Vector-Borne Diseases (VVBD) Laboratory, Lanzhou Veterinary Research Institute (LVRI). Total DNA was extracted from 200 μl of blood sample using a commercial DNA extraction kit (QIAamp DNA Blood Mini Kit; Qiagen, Hilden, Germany) according to the manufacturerʼs instructions. Negative control DNA was isolated from the whole blood of piroplasm-free cattle, which was examined by blood smear microscopy and nested PCR [30]. The extracted DNA was stored at −20 °C until required.
18S rRNA gene was amplified from DNA samples positive for B. bovis, B. bigemina, B. major and B. ovata using the primer pair (Piro1-S: 5′-CTT GAC GGT AGG GTA TTG GC-3′ and Piro3-AS: 5′-CCT TCC TTT AAG TGA TAA GGT TCA C-3′) [30]. A 25 μl PCR reaction mixture was prepared containing 2.5 μl of 10× PCR buffer (Mg2+ plus), 2.0 μl of dNTPs (2.5 mM each), 1.25 U of Taq DNA polymerase (TaKaRa, Dalian, China), 2.0 μl of template DNA, 1.0 μl of each primer (10 pM) and 16.25 μl of double-distilled water. PCR reactions parameters were as follows: initial denaturation at 95 °C for 3 min; 35 cycles of denaturation at 95 °C for 30 s, annealing 55 °C for 30 s and extension at 72 °C for 90 s; with a final extension step at 72 °C for 5 min.
PCR amplicons from each Babesia species were purified and cloned into a pGEM-T vector using the Easy Vector System (Promega, Madison, WI, USA). Briefly, PCR amplicons were purified with a Zymoclean™ Gel DNA Recovery Kit (Zymo, Los Angeles, USA), cloned into a PGEM-T Easy vector (Promega) and then transformed into Escherichia coli DH5α competent cells. Three clones of each sample were selected, prepared and sequenced using BigDye Terminator Mix (Genscript, Nanjing, China). Sequences of 18S rRNA gene from B. bovis, B. bigemina, B. major and B. ovata were subjected to Blast analysis on the NCBI website using the BLASTn program (http://www.ncbi.nlm.nih.gov).
Experimentally infected and field-collected samples
Six cattle were purchased from Wuwei county of Gansu Province and were confirmed to be free of piroplasm infection by the microscopy, nested PCR and ELISA assay [30–33]. Three cattle were inoculated intravenously using 10 ml of cryopreserved blood infected with B. bovis. The rest were injected with B. bigemina-infected blood. After inoculation, weekly blood samples were collected for DNA extraction.
From August 2008 to July 2014, 240 blood samples were randomly collected into EDTA coated tubes from cattle distributed across ten counties, across seven provinces in China [34]. All blood samples were transported to the VVBD, LVRI, Chinese Academy of Agricultural Sciences (CAAS) in iceboxes and stored at −20 °C. DNA extractions were performed according to the manufacturers’ instructions above and the concentration evaluated using a Nano Drop 2000 spectrophotometer (Thermo Fisher Scientific, MA, USA). All samples were initially detected using a nested PCR assay [30, 31]. From these results, the field samples were composed of 71 cases of Babesia spp. infections, 33 cases of B. bovis, 20 cases of B. bigemina, five cases of B. ovata, four cases of B. major, 7 cases of co-infection with two species (3 cases of B. bovis + B. bigemina; 2 cases of B. bovis + B. ovata; 1 case of B. bigemina + B. ovata; 1 case of B. bovis + B. major and 2 cases of triple co-infection (B. bovis + B. bigemina + B. ovata and B. bovis + B. bigemina + B. major).
Real-time PCR high resolution melting analysis (RT-PCR-HRM)
HRM assays were performed using a Rotor-Gene Q6000 Real-Time PCR system (Qiagen, Sydney, Australia). Each reaction was performed in a total volume of 20 μl containing 10 μl of Forget-Me-Not™ qPCR Master Mix (Biotium, Fremont, USA), 2 pmol of each primer (BovisB-7F: 5′-CCT GAC ACA GGG AGG TAG TGA CAAG-3′ and BovisB-7R: 5′-GGC TGC TGG CAC CAG ACT TGC CCT CC-3′), 1 μl (10 ng) plasmid DNA bearing the 18S rRNA gene sequence. The reaction parameters were as follows: initial denaturation at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 5 s and annealing at 60 °C for 30 s. After real-time PCR, HRM was performed from 75 °C to 90 °C rising by 0.2 °C with a 2 s hold time at each acquisition step. Finally, melting curves were normalized using the High Resolution Melt software v.2.3.1 (Qiagen).
Evaluation of sensitivity and specificity of the RT-PCR-HRM
To determine the analytical sensitivity and detection limits of the HRM assay, 10-fold serial dilutions of positive control plasmid DNA were used as template, ranging from 107 to 1 copy number/μl. Each plasmid dilution was duplicated in three independent experiments to ensure reproducibility of the threshold cycle number (Cq). Standard curves were plotted using the software v.2.3.1 (Qiagen) to evaluate the amplified efficiency percentages and linear correlations of each Babesia species.
The specificity of the HRM analysis was also determined using genomic DNA extracted from bovines and parasites infective for bovines, including T. annulata, T. orientalis, T. sinensis and Anaplasma marginale.
Results
Polymorphic regions on the 18S rRNA gene and primer design
Sequences for the 18S rRNA gene of piroplasms infective for bovines were retrieved from GenBank (accession numbers JQ723013 for B. bovis, JX495402 for B. bigemina, AY603399 for B. major and AY603401 for B. ovata). After sequence alignment using DNAMAN v.2.0 software, conserved and variable regions were identified for each Babesia species. A primer pair was designed for the conserved regions to amplify variable gene fragment using the Primer v.5.0 software (Fig. 1). A 122-bp amplicon was amplified using the primer pair Bovis-B-7F and Bovis-B-7R; the reactions were performed to discriminate four Babesia spp.
Fig. 1.
Nucleotide sequences of the 18S rRNA amplicon and the primer location. Sequence alignment of the nucleotide sequence of the Babesia spp. amplicon used in the HRM analysis. The underlined sequences indicate the position of the primer pair used for the real-time PCR assay
Specificity and sensitivity of the assay
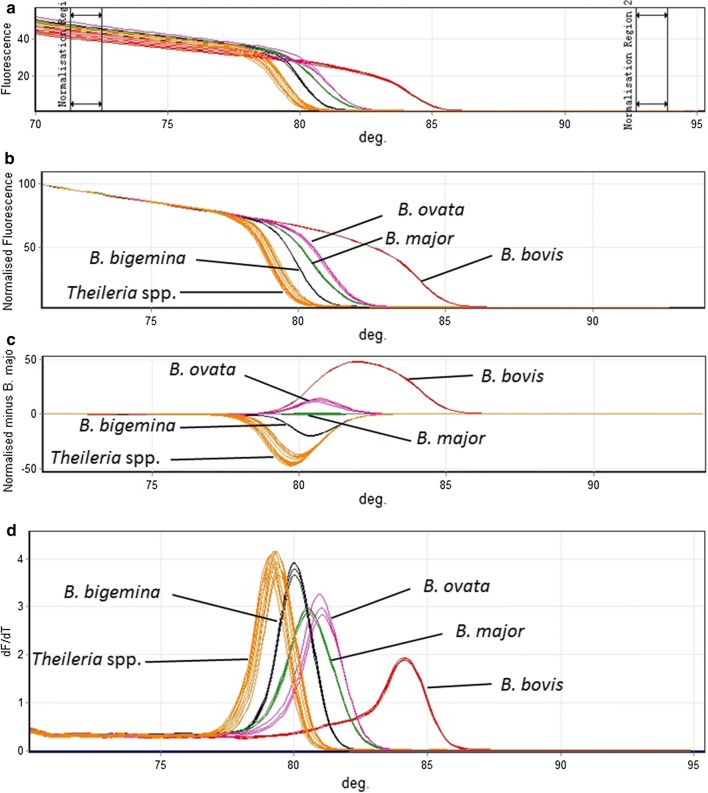
A primer pair (Bovis-B-7F and Bovis-B-7R) was designed to amplify DNA of all four Babesia spp. using the RT-PCR-HRM. However, there were cross-reactions with T. annulata, T. orientalis and T. sinensis. According to melting profiles and Tm values, the generated amplicon distinguished these species into five groups: Theileria spp., B. bovis, B. bigemina, B. ovata and B. major. The approach was not suitable for discriminating between species of the genus Theileria because no differences in melting profiles and Tm values were observed between T. annulata, T. orientalis and T. sinensis (Fig. 2). There was no amplification plot using genomic DNA isolated from bovine free-piroplasm infection and A. marginale.
Fig. 2.
Detection and discrimination of the four Babesia spp. and Theileria spp. a Raw data from melt curve analysis. b Normalized HRM plots for 18S rRNA amplicon; each sample was performed in duplicates in three independent experiments. c Normalized difference curves. d Derivative melting curve peaks
To evaluate the detection limit of the RT-PCR-HRM assay, 10 times serial dilutions from 107 to 1 copy number/μl plasmid DNA containing target sequences of each Babesia spp. were performed in duplicate. The detection limit was one copy per reaction for each Babesia species. Standard curves for quantification assays, as confirmed by duplicates showed good correlations and efficiencies varying from 93.4 to 100.4% for all Babesia spp., with ranges of 101 to 107 copies (Additional file 1: Figure S1). The standard deviation and average of the melting temperature (Tm) of the amplicon were determined using variable DNA concentrations as template, isolated from each Babesia spp. infective to bovines. The average Tm values and their standard deviations for the 18S rRNA amplicon of each Babesia spp. are presented in Table 1 and Fig. 3.
Table 1.
Tm values of average melting curve peak for each Babesia spp.
| Species | Tm value range (mean Tm ± SD) (°C) | |
|---|---|---|
| DNAa | Variable amount of DNAb | |
| B. bovis | 84.15 ± 0.04 | 84.16 ± 0.19 |
| B. bigemina | 80.02 ± 0.02 | 80.04 ± 0.12 |
| B. major | 80.54 ± 0.03 | 80.62 ± 0.15 |
| B. ovata | 81.02 ± 0.05 | 81.24 ± 0.25 |
| Theileria spp. | 79.20 ± 0.19 | 79.24 ± 0.24 |
aThe Tm values obtained from HRM using 20 ng of genomic DNA in 3 repeat reactions in 3 independent experiments
bThe Tm values obtained from HRM using a variable amount of genomic DNA ranging from 5 to 50 ng in 3 repeat reactions in 3 independent experiments
Fig. 3.
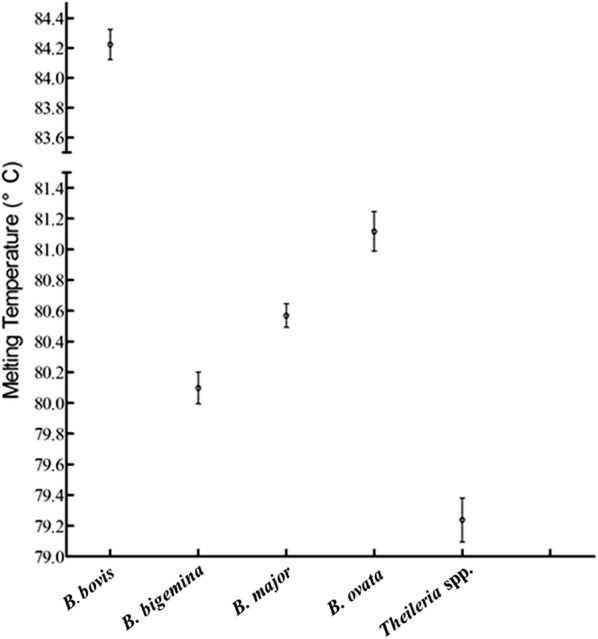
Tm values from the HRM analysis targeting the 18S rRNA amplicon using a variable amount of initial DNA as a template. Each point represents the average and standard deviation of the Tm values, measured in duplicate
Evaluation of the RT-PCR-HRM assay using experimental and clinical samples
The RT-PCR-HRM assay for 18S rRNA gene amplicon was also evaluated using genomic DNA extracted from cattle experimentally infected with B. bovis and B. bigemina, respectively. On the basis of melting curves and Tm values, species identified herein using the HRM analysis were 100% consistent with previous results (Table 2).
Table 2.
Comparison of detection and discrimination results for Babesia spp. in experimentally infected animal samples and field samples
| Sample source | Diagnostic method | |
|---|---|---|
| HRM assay | nPCR assay and 18S rRNA gene sequencing | |
| Experimentally infected samples | B. bovis (n = 25) | B. bovis (n = 25) |
| B. bigemina (n = 25) | B. bigemina (n = 25) | |
| Field samples | B. bovis (n = 31) | B. bovis (n = 33) |
| B. bigemina (n = 20) | B. bigemina (n = 20) | |
| B. major (n = 4) | B. major (n = 4) | |
| B. ovata (n = 5) | B. ovata (n = 5) | |
| B. bovis + B. bigemina (n = 3) | B. bovis + B. bigemina (n = 3) | |
| B. bovis + B. ovata (n = 2) | B. bovis + B. ovata (n = 2) | |
| B. bigemina + B. ovata (n = 1) | B. bigemina + B. ovata (n = 1) | |
| B. bovis + B. major (n = 1) | B. bovis + B. major (n = 1) | |
| B. bovis + B. bigemina + B. ovata (n = 1) | B. bovis + B. bigemina + B. ovata (n = 1) | |
| B. bovis, B. bigemina + B. major (n = 0) | B. bovis + B. bigemina + B. major (n = 1) | |
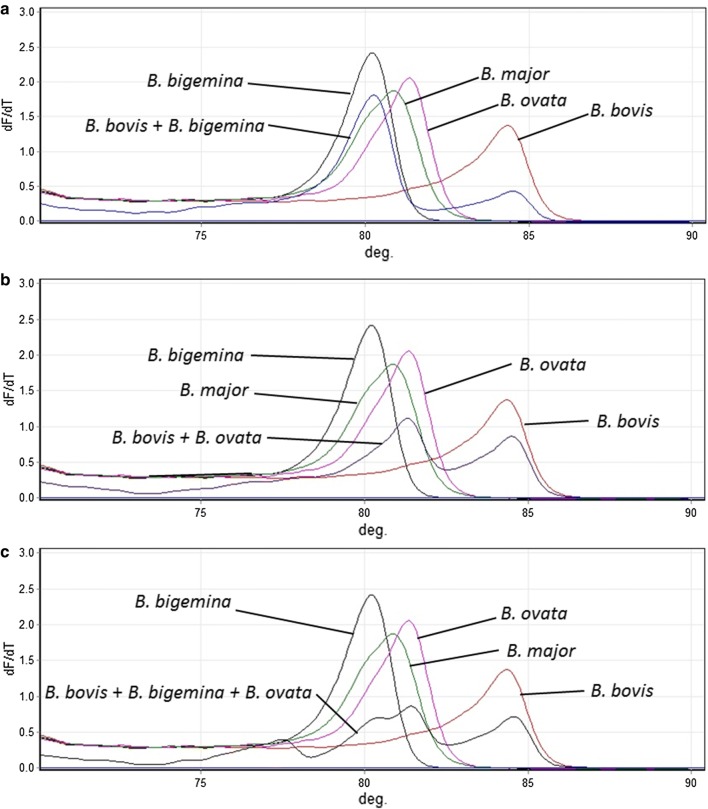
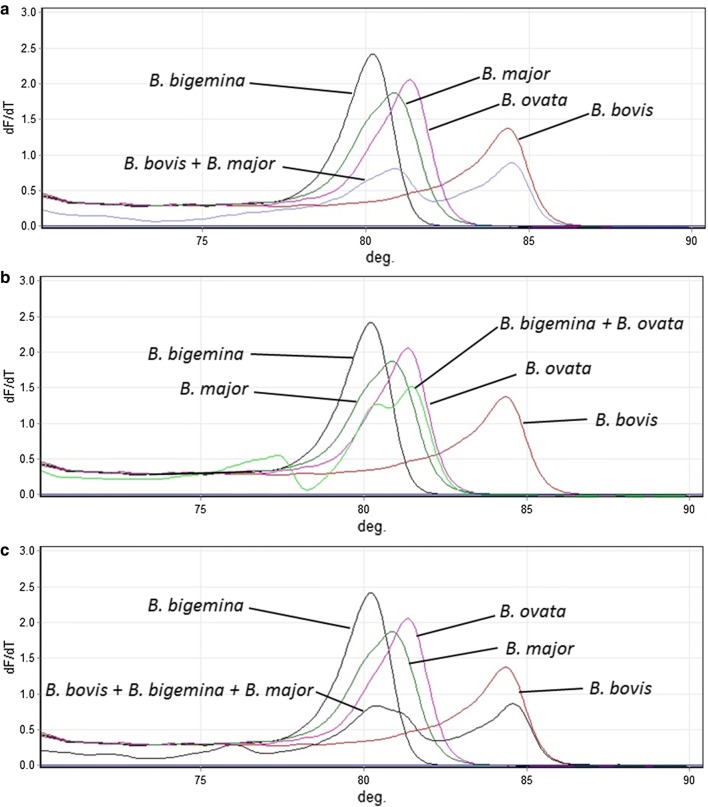
From the screening of clinical samples, 33 were identified as B. bovis infections. However, there were no amplifications for two clinical samples using the RT-PCR HRM assays. For the remaining samples, the HRM assays results were in agreement with the results, determined by nested PCR analysis and gene sequencing (Table 2). This approach was also suitable for the detection and discrimination of double and triple infections, except for mixed infections for B. bovis, B. bigemina and B. major, which only generated two peaks, observed on the melting curve plot (Figs. 4, 5). Moreover, the Tm values of the peaks were ambiguous.
Fig. 4.
Detection and discrimination of mixed infectious clinical samples. a Babesia bovis + B. bigemina co-infection. b Babesia bovis + B. ovata co-infection. c Babesia bovis + B. bigemina + B. ovata triple infection
Fig. 5.
Detection and discrimination of mixed infectious clinical samples. a Babesia bovis + B. major co-infection. b Babesia bigemina + B. ovata co-infection. c Babesia bovis + B. bigemina + B. major triple infection
Discussion
Each Babesia spp. infective to bovines has different contribution to economic losses of livestock industry. For example, B. bovis and B. bigemina are the major causative agents of bovine babesiosis in China but B. ovata and B. major are considered as benign Babesia spp. which cause mild anemia or subclinical signs [3, 35]. Therefore, rapid detection and accurate discrimination of Babesia spp. are critical to clinical diagnosis and epidemiological study. Microscopy is considered to be the gold standard for babesiosis confirmation [16]. However, this approach has several limitations: it is time consuming, has a low sensitivity and is unable to distinguish species, which ultimately requires a combined approach with other diagnostic methods [17, 18]. Due to its high sensitivity, specificity, time efficiency and reproducibility, PCR based methods are predominately used to identify and distinguish Babesia species [8, 19–21]. However, these assays require the subsequent manipulation of the PCR product, which may introduce amplicon contamination [36]. HRM analysis is an alternative method for the simultaneous detection and discrimination of species. Based on slight differences in nucleotide composition, specific double-stranded DNA dissociation curve profiles and Tm values can be generated to discriminate Babesia spp. The HRM methodology has been employed to identify Theileria equi and Babesia caballi and to differentiate Babesia spp. infecting humans and dogs [28, 29]. Recently, Chua et al. [27] explored HRM analysis for the simultaneous detection of all five human Plasmodium spp. These available results indicated that HRM treating the 18S rRNA gene is an attractive approach for the detection and differentiation of species.
Here, we successfully developed a HRM assay for the rapid detection and differentiation of four Babesia spp. circulating in Chinese bovine herds. Targeting the Babesia spp. 18S rRNA gene, a primer pair was designed to amplify an amplicon containing polymorphic regions in different species and conserved intra-species. The HRM approach generated melting profiles and Tm values, which can be used to discriminate B. bovis, B. bigemina, B. major and B. ovata.
Initial DNA template concentrations were found to impact Tm fluctuations, which had previously been reported [37, 38]. Once Tm values and melting curves shape are shifted, it can lead to a misidentification of specific species. In our analysis, DNA concentration slightly influenced Tm values for all four Babesia spp., only exceeding 0.2 °C for B. ovata. For reliable differentiation, only Tm values exceeding 0.25 °C were considered as different species. The four species that did not present an overlapping Tm range and melting curve profiles were B. bovis, B. bigemina, B. major and B. ovata, which could be accurately discriminated by the HRM analysis. In addition, all available 18S rRNA sequences from the NCBI database were aligned and screened for interspecific and intraspecific polymorphisms. The theoretical Tm values and sequence similarities of these four Babesia spp. isolated from different geographical regions were predicted by the in silico analyses using Oligo Calculator v.3.27 [39]. Of the isolates, two strains of B. ovata and one of B. bovis presented theoretical Tm values whose differences exceeded 0.25 °C, which could be the result of real sequence polymorphisms, sequencing errors or reflect different taxa (Additional file 2: Table S1).
Blood samples comprising both field-collected samples and samples from experimentally infected cattle were used to evaluate and compare HRM with the identification results using nested PCR and 18S rRNA gene sequencing. Not all amplicons were successfully amplified for all the samples using the RT-PCR HRM approach; this could be due to differences in sensitivity of the diagnostic assay and the low parasitemia may have led to lack of amplification for these field-collected samples. The double or triple species co-infection was also accurately distinguished by HRM in this study. Multiple melting curve peaks, generated by combinational species, could be presented on the derivative melting curve profiles of samples infected with Babeisa spp. The melting curve shapes from field samples could be accurately distinguished Babesia spp. infective to cattle, when standard positive controls were included in each independent analysis.
Conclusions
The RT-PCR-HRM analysis developed in this study is a promising tool for the detection and discrimination of Babesia spp., the causative agents of bovine babesiosis in China. There are no requirements for further manipulation of PCR products, such as gel fractionation, restriction fragment length polymorphism analysis and gene sequencing, thereby avoiding PCR cross-contamination in the laboratory.
Supplementary information
Additional file 1: Figure S1. Efficiency and correlation of 18S rRNA amplicon real-time PCR for DNA isolated from four Babesia spp. Standard curves were derived from a plasmid bearing the 18S rRNA gene from B. bovis (a), B. bigemina (b), B. major (c) and B. ovata (d). A 10-fold dilution of the plasmid, ranging from 107 to 101 copies per reaction, was used as a template and performed in duplicate.
Additional file 2: Table S1. Sequence analysis of 18S rRNA amplicons from four Babesia spp. The nucleotide sequences containing the 18S rRNA amplicon were retrieved from the GenBank database.
Acknowledgements
Not applicable.
Abbreviations
- RT-PCR-HRM
Real-time PCR high resolution melting analysis
- VVBD
Vectors and Vector-Borne Diseases Laboratory
- LVRI
Lanzhou Veterinary Research Institute
- Tm
melting temperature
- Cq
threshold cycle number
- PCR
polymerase chain reaction
- DNA
deoxyribonucleic acid
- dsDNA
double stranded DNA
Authors’ contributions
Designed the study: HY, JLu and GG. Performed the experiments, analyzed the results and wrote the manuscript: JW and SZ. Contributed reagents/materials/equipment: JLi, ZLiu, QM, XW, ZLi, AL, YL and MR. All authors read and approved the final manuscript.
Funding
This study was financially supported by the National Key R&D Programme of China (2017YFD0501200; 2018YFD0501804; 2018YFD0502305; 2016YFD0501503), ASTIP (CAAS-ASTIP-2016-LVRI), NBCIS (CARS-37), 973 Programme (2015CB150300) and Jiangsu Co-innovation Center Programme for Prevention and Control of Important Animal Infectious Disease and Zoonosis.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
The collection and manipulation of blood samples were approved by the Animal Ethics Committee of the Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. All sampling procedures were handled in accordance with the Animal Ethics Procedures and Guidelines of the People’s Republic of China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jinming Wang, Email: wjm0403@caas.cn.
Aihong Liu, Email: liuaihong@caas.cn.
Shangdi Zhang, Email: 251358058@qq.com.
Shandian Gao, Email: gaoshandian@caas.cn.
Muhammad Rashid, Email: rashidkhan.laghari@gmail.com.
Youquan Li, Email: liyouquan@caas.cn.
Junlong Liu, Email: liujunlong@caas.cn.
Quanying Ma, Email: 405261056@qq.com.
Zhi Li, Email: 136545267@qq.com.
Zhijie Liu, Email: liuzhijie@caas.cn.
Jianxun Luo, Email: luojianxun@caas.cn.
Guiquan Guan, Email: guanguiquan@caas.cn.
Hong Yin, Email: yinhong@caas.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-019-3781-4.
References
- 1.Levine ND. Progress in taxonomy of the Apicomplexan protozoa. J Protozool. 1988;35:518–520. doi: 10.1111/j.1550-7408.1988.tb04141.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang CJ. Bovine piroplasmosis. In: Division of Veterinary and Animal Husbandry, Minstry of Agriculture, editor. The diseases of domestic animals in China. Beijing: Academic Press; 1993. pp. 381–390. [Google Scholar]
- 3.Yin H, Lu WS, Luo JX. Babesiosis in China. Trop Anim Health Prod. 1997;29(Suppl.):11S–15S. doi: 10.1007/BF02632908. [DOI] [PubMed] [Google Scholar]
- 4.Liu JL, Guan GQ, Liu AH, Li YQ, Yin H, Luo J. A PCR method targeting internal transcribed spacers: the simultaneous detection of Babesia bigemina and Babesia bovis in cattle. Acta Parasitol. 2014;59:132–138. doi: 10.2478/s11686-014-0222-6. [DOI] [PubMed] [Google Scholar]
- 5.Niu QL, Liu ZJ, Yu PF, Yang JF, Abdallah MO, Guan GQ, et al. Genetic characterization and molecular survey of Babesia bovis, Babesia bigemina and Babesia ovata in cattle, dairy cattle and yaks in China. Parasit Vectors. 2015;8:518. doi: 10.1186/s13071-015-1110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun M, Wang J, Liu A, Liu Z, Nie Y, Wei H, et al. Development of a nested PCR assay for detection of Babesia bovis. Chin Vet Sci. 2019;49:183–189. [Google Scholar]
- 7.Luo JX, Yin H, Guan GQ, Zhang QC, Lu WS. Description of a new Babesia sp. infective for cattle in China. Parasitol Res. 2002;88(Suppl. 1):S13–S15. doi: 10.1007/s00436-001-0562-7. [DOI] [PubMed] [Google Scholar]
- 8.Liu JL, Yin H, Liu GY, Guan GQ, Ma ML, Liu AH, et al. Discrimination of Babesia major and Babesia ovata based on ITS1-5.8S-ITS2 region sequences of rRNA gene. Parasitol Res. 2008;102:709–713. doi: 10.1007/s00436-007-0818-y. [DOI] [PubMed] [Google Scholar]
- 9.Luo JX, Yin H, Liu GY, Guan GQ, Liu ZJ, Liu AH, et al. Collection and identification of piroplasma infected to cattle and sheep in China. Chin J Parasitol Parasit Dis. 2006;24:48–53. [Google Scholar]
- 10.Bai Q, Yin SX, Chen ZH, Liu GY, Zhou JY. Isolation of single species of Babesia bigemina. Chin J Vet Sci Technol. 1987;9:25–27. [Google Scholar]
- 11.Bai Q, Liu GY, Zhang L, Zhou J. Isolation of single species of Babesia bovis. Chin J Vet Sci Technol. 1991;1:20–22. [Google Scholar]
- 12.Bai Q, Liu GY, Zhang L, Zhou J. Discovey and isolation of Babesia ovata in China. Chin J Vet Sci. 1990;16:2–4. [Google Scholar]
- 13.Kim CM, Blanco LB, Alhassan A, Iseki H, Yokoyama N, Xuan X, et al. Development of a rapid immunochromatographic test for simultaneous serodiagnosis of bovine babesioses caused by Babesia bovis and Babesia bigemina. Am J Trop Med Hyg. 2008;78:117–121. doi: 10.4269/ajtmh.2008.78.117. [DOI] [PubMed] [Google Scholar]
- 14.AbouLaila M, Yokoyama N, Igarashi I. Development and evaluation of a nested PCR based on spherical body protein 2 gene for the diagnosis of Babesia bovis infection. Vet Parasitol. 2010;169:45–50. doi: 10.1016/j.vetpar.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Sivakumar T, Tagawa M, Yoshinari T, Ybanez AP, Igarashi I, Ikehara Y, et al. PCR Detection of Babesia ovata from cattle reared in Japan and clinical significance of coinfection with Theileria orientalis. J Clin Microbiol. 2012;50:2111–2113. doi: 10.1128/JCM.00220-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bishop JP, Adams LG. Combination thin and thick blood films for the detection of Babesia parasitemia. Am J Vet Res. 1973;34:1213–1214. [PubMed] [Google Scholar]
- 17.Bose R, Jorgensen WK, Dalgliesh RJ, Friedhoff KT, de Vos AJ. Current state and future trends in the diagnosis of babesiosis. Vet Parasitol. 1995;57:61–74. doi: 10.1016/0304-4017(94)03111-9. [DOI] [PubMed] [Google Scholar]
- 18.Almeria S, Castella J, Ferrer D, Ortuno A, Estrada-Pena A, Gutierrez JF. Bovine piroplasms in Minorca (Balearic Islands, Spain): a comparison of PCR-based and light microscopy detection. Vet Parasitol. 2001;99:249–259. doi: 10.1016/S0304-4017(01)00464-2. [DOI] [PubMed] [Google Scholar]
- 19.Liu A, Guan G, Du P, Gou H, Liu Z, Liu J, et al. Loop-mediated isothermal amplification (LAMP) method based on two species-specific primer sets for the rapid identification of Chinese Babesia bovis and B. bigemina. Parasitol Int. 2012;61:658–663. doi: 10.1016/j.parint.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Gubbels JM, de Vos AP, van der Weide M, Viseras J, Schouls LM, de Vries E, et al. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J Clin Microbiol. 1999;37:1782–1789. doi: 10.1128/jcm.37.6.1782-1789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JL, Guan GQ, Liu AH, Li YQ, Yin H, Luo JX. A PCR method targeting internal transcribed spacers: the simultaneous detection of Babesia bigemina and Babesia bovis in cattle. Acta Parasitologica. 2014;59:132–138. doi: 10.2478/s11686-014-0222-6. [DOI] [PubMed] [Google Scholar]
- 22.Annoscia G, Latrofa MS, Cantacessi C, Olivieri E, Manfredi MT, Dantas-Torres F, et al. A new PCR assay for the detection and differentiation of Babesia canis and Babesia vogeli. Ticks Tick Borne Dis. 2017;8:862–865. doi: 10.1016/j.ttbdis.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Tong SY, Giffard PM. Microbiological applications of high-resolution melting analysis. J Clin Microbiol. 2012;50:3418–3421. doi: 10.1128/JCM.01709-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchant-Patel S, Blackall PJ, Templeton J, Price EP, Tong SY, Huygens F, et al. Campylobacter jejuni and Campylobacter coli genotyping by high-resolution melting analysis of a flaA fragment. Appl Environ Microbiol. 2010;76:493–499. doi: 10.1128/AEM.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller KE, Zampieri RA, Aoki JI, Muxel SM, Nerland AH, Floeter-Winter LM. Amino acid permease 3 (aap3) coding sequence as a target for Leishmania identification and diagnosis of leishmaniases using high resolution melting analysis. Parasit Vectors. 2018;11:421. doi: 10.1186/s13071-018-2989-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palais RA, Liew MA, Wittwer CT. Quantitative heteroduplex analysis for single nucleotide polymorphism genotyping. Anal Biochem. 2005;346:167–175. doi: 10.1016/j.ab.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Chua KH, Lim SC, Ng CC, Lee PC, Lim YAL, Lau TP, et al. Development of high resolution melting analysis for the diagnosis of human malaria. Sci Rep. 2015;5:15671. doi: 10.1038/srep15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salim B, Bakheit MA, Sugimoto C. Rapid detection and identification of Theileria equi and Babesia caballi by high-resolution melting (HRM) analysis. Parasitol Res. 2013;112:3883–3886. doi: 10.1007/s00436-013-3581-2. [DOI] [PubMed] [Google Scholar]
- 29.Rozej-Bielicka W, Masny A, Golab E. High-resolution melting PCR assay, applicable for diagnostics and screening studies, allowing detection and differentiation of several Babesia spp infecting humans and animals. Parasitol Res. 2017;116:2671–2681. doi: 10.1007/s00436-017-5576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JF, Li YQ, Liu ZJ, Liu JL, Guan GQ, Chen Z, et al. Molecular evidence for piroplasms in wild Reevesʼ muntjac (Muntiacus reevesi) in China. Parasitol Int. 2014;63:713–716. doi: 10.1016/j.parint.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Olmeda AS, Armstrong PM, Rosenthal BM, Valladares B, delCastillo A, deArmas F, et al. A subtropical case of human babesiosis. Acta Trop. 1997;67:229–234. doi: 10.1016/S0001-706X(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 32.Li AY, Liu AH, Wang JM, Zhu H, Ma ML, Ren QY, et al. Development of an ELISA for diagnosis of babesiosis in cattle. Chin Vet Sci. 2014;44:62–67. [Google Scholar]
- 33.Yuan LQ, Guan GQ, Liu JL, Zhao SY, Yang CS, Lu HY, et al. Cloning and expression of RAP-1C gene from Babesia bigemina and reactivity of the expressed recombinant protein. Chin Vet Sci. 2014;44:823–828. [Google Scholar]
- 34.Wang JM, Yang JF, Liu JL, Wang XX, Xu JL, Liu AH, et al. Molecular detection and genetic diversity of Theileria orientalis in cattle in China. Parasitol Res. 2018;117:3689–3694. doi: 10.1007/s00436-018-6023-3. [DOI] [PubMed] [Google Scholar]
- 35.Hakimi H, Yamagishi J, Kegawa Y, Kaneko O, Kawazu S, Asada M. Establishment of transient and stable transfection systems for Babesia ovata. Parasit Vectors. 2016;9:171. doi: 10.1186/s13071-016-1439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zampieri RR, Laranjeira-Silva MF, Muxel SM, de Lima ACS, Shaw JJ, Floeter-Winter LM. High resolution melting analysis targeting Hsp70 as a fast and efficient method for the discrimination of Leishmania species. PLoS Negl Trop Dis. 2016;10:e0004485. doi: 10.1371/journal.pntd.0004485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernandez C, Alvarez C, Gonzalez C, Ayala MS, Leon CM, Ramirez JD. Identification of six New World Leishmania species through the implementation of a high-resolution melting (HRM) genotyping assay. Parasit Vectors. 2014;7:501. doi: 10.1186/s13071-014-0501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuang ZW, Zhang CY, Pang HS, Ma Y. A rapid high-resolution melting method for differentiation of Leishmania species targeting lack gene. Acta Trop. 2018;178:103–106. doi: 10.1016/j.actatropica.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Kibbe WA. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res. 2007;35:W43–W46. doi: 10.1093/nar/gkm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Efficiency and correlation of 18S rRNA amplicon real-time PCR for DNA isolated from four Babesia spp. Standard curves were derived from a plasmid bearing the 18S rRNA gene from B. bovis (a), B. bigemina (b), B. major (c) and B. ovata (d). A 10-fold dilution of the plasmid, ranging from 107 to 101 copies per reaction, was used as a template and performed in duplicate.
Additional file 2: Table S1. Sequence analysis of 18S rRNA amplicons from four Babesia spp. The nucleotide sequences containing the 18S rRNA amplicon were retrieved from the GenBank database.
Data Availability Statement
Not applicable.