Abstract
Skeletal vasculature plays a central role in the maintenance of microenvironments for osteogenesis and haematopoiesis. In addition to supplying oxygen and nutrients, vasculature provides a number of inductive factors termed as angiocrine signals. Blood vessels drive recruitment of osteoblast precursors and bone formation during development. Angiogenesis is indispensable for bone repair and regeneration. Dysregulation of the angiocrine crosstalk is a hallmark of ageing and pathobiological conditions in the skeletal system. The skeletal vascular bed is complex, heterogeneous and characterized by distinct capillary subtypes (type H and type L), which exhibit differential expression of angiocrine factors. Furthermore, distinct blood vessel subtypes with differential angiocrine profiles differentially regulate osteogenesis and haematopoiesis, and drive disease states in the skeletal system. This review provides an overview of the role of angiocrine signals in bone during homeostasis and disease.
Keywords: angiogenesis, bone marrow niche, osteogenesis, repair, regeneration
1. Introduction
The vascular system serves as a rapid transport network for delivering oxygen and nutrients. In addition to this traditional role, recent evidence illustrates that endothelial cells (ECs) and perivascular cells engage in signalling with neighbouring cells, and regulate various tissue and organ developments and functions [1–5]. These interactions between the vasculature and tissue cells involve paracrine or juxtacrine signalling, also termed as ‘angiocrine signalling’. The angiocrine signals involve growth factors, extracellular matrix components, secreted signalling molecules such as cytokines and chemokines, and gaseous, physical or cell–cell communication through the cell surface molecules. During such angiocrine crosstalk with neighbouring cell types, blood vessels often form nurturing niche microenvironments required for the maintenance of stem and progenitor cells [6]. In bone, vasculature provides specialized niches for haematopoietic stem cells (HSCs) and osteoprogenitors and regulates haematopoiesis and osteogenesis [6]. This review aims to provide a summary of angiocrine factors and the role of angiogenesis in the skeletal system. It also provides an evaluation of the impact of angiocrine crosstalk on bone physiology and pathophysiology. The angiocrine factors in bone are summarized in table 1.
Table 1.
Angiocrine factors and their crosstalk with tissue cells in bone.
| angiocrine factor | source | target cell | function | reference |
|---|---|---|---|---|
| OPG | endothelial cell | osteoclasts | inhibit osteoclastogenesis | [7] |
| SEMA-III | endothelial cells | osteoclasts | bone remodelling | [8–11] |
| IL-33 | CD105+ endothelial cells | osteoblasts | osteogenesis, haematopoiesis | [12] |
| BMP-2 | endothelial cells | chondrocytes | endochondral bone formation, fracture repair | [13,14] |
| matrix metalloproteinases: Mmp2, Mmp9, Mmp14 | type H endothelial cells | chondrocytes | cartilage resorption, directional bone elongation | [15] |
| Timp1, Timp2, Timp3, Timp4 | type H endothelial cells | chondrocytes | bone resorption and remodelling | [15] |
| SCF | type H, arterial and sinusoidal endothelial cells | HSCs | HSC maintenance | [16] |
| nidogen-1 | sinusoidal and perivascular stromal cells | pro-B cells | pro-B cell maintenance | [17] |
| IL-7 | endothelial cells and perivascular stromal cells | pro-B cells | pro-B cell maintenance | [18,19] |
| CXCL12 | endothelial cells and mesenchymal stem cells | HSCs | HSC maintenance | [20,21] |
| tenascin-C | endothelial cells | HSCs | HSC survival | [17] |
| FGF-2 | endothelial cells | HSPCs | HSPC expansion | [22,23] |
| Jag-1 | endothelial cells | HSCs | HSC regeneration, lymphoma cell proliferation | [24] |
| NOS2 | endothelial cells | osteoblast | negative regulation of osteoblast differentiation | [25] |
| PDGF | endothelial cells | osteoprogenitor | osteoprogenitor proliferation and survival | [26] |
| TGF | endothelial cells | osteoprogenitor | osteoprogenitor survival | [26] |
| FGF1 | endothelial cells | osteoblast and osteoprogenitor | osteoprogenitor survival | [26] |
| Noggin | endothelial cells | osteoblast and osteoprogenitor | bone growth, mineralization and chondrocyte maturation | [27] |
| BMP-4 | endothelial cells | HSPC | expansion of HSPC | [23] |
| angiopotein-1 | endothelial cells | HSPC | protection of HSPC | [22] |
| VCAM-1 | endothelial cells | osteoclasts, leucocytes and fibroblasts | leucocytes trafficking, protection of DTCs | [28–31] |
| E-selectin | endothelial cells | osteoclasts, leucocytes | trafficking leucocytes, cancer metastasis | [28–30,32] |
| von Willebrand factor | endothelial cells | disseminated tumour cells | protection of DTCs | [31] |
| thrombospondin-1 | endothelial cells | disseminated tumour cells | quiescence of DTCs | [33] |
| IGFBP2 | endothelial cells | HSPC | expansion of HSPCs | [23] |
| ICAM-1 | endothelial cells | leucocytes and fibroblasts | leucocytes trafficking | [28–30] |
2. Niche functions of blood vessels during bone formation
The circulatory network in the mammalian skeletal system controls the development of bone through angiocrine signalling. Bone formation starts with the migration and localization of cells to a specific micro-niche followed by condensation of mesenchymal cells [34,35]. This mesenchymal condensate then acts as a template for further differentiation and development [36]. Even though vascular invasion and blood vessel growth is a later event in bone development [37], some blood vessel-derived factors/angiocrine signals from the periphery may play a role as early as during mesenchymal condensation. Transforming growth factor beta 1 (TGFβ1) upregulates the production of connective tissue growth factor (CTGF), and CTGF is a downstream effector of TGFβ1 for surrounding cells, including ECs. CTGF and TGFβ1 are found to be upregulated in mesenchymal condensations [38]. Precursors of ECs termed as ‘angioblasts’ are present early in organ bud formation before vascular development [2]. During limb formation, there is a prominent expression of vascular endothelial growth factor (VEGF) in the mesenchymal condensate [39]. VEGF enhances osteogenesis and helps in the vascular patterning during osteogenesis [40]. After mesenchymal condensation, bone formation occurs by either of the two processes: (i) intramembranous ossification or (ii) endochondral ossification. During intramembranous ossification, mesenchymal cells in the condensate differentiate into osteoblasts, which then differentiate to osteocytes/bone cells to generate flat bones such as the skull and facial bones [41]. Alternatively, in endochondral ossification, long bones develop through an intermediate stage of chondrocyte differentiation and avascular cartilage formation [42].
The central player in blood vessel invasion to the bone tissue after mesenchymal condensation is hypoxia, where hypoxia-inducible factors (HIFs) signal the oxygen level [43]. Under normoxic conditions, the HIF1 subunits are targeted for proteasomal degradation by hydroxylation. While in hypoxia, due to the limiting oxygen levels for hydroxylation, the HIF1-α subunits are stabilized and activate downstream signalling pathways, including VEGF signalling [6,44]. In line with this, HIF1-α loss of function mice shows a decline in bone volume and bone vascularity [37]. VEGF signalling from the avascular regions, which have high levels of VEGF receptors, recruits ECs and drives blood vessel growth. VEGF signalling plays a central role in coupling angiogenesis and osteogenesis [45], through its effect on ECs and also by influencing chondrocytes, osteoblasts and osteoclasts [46].
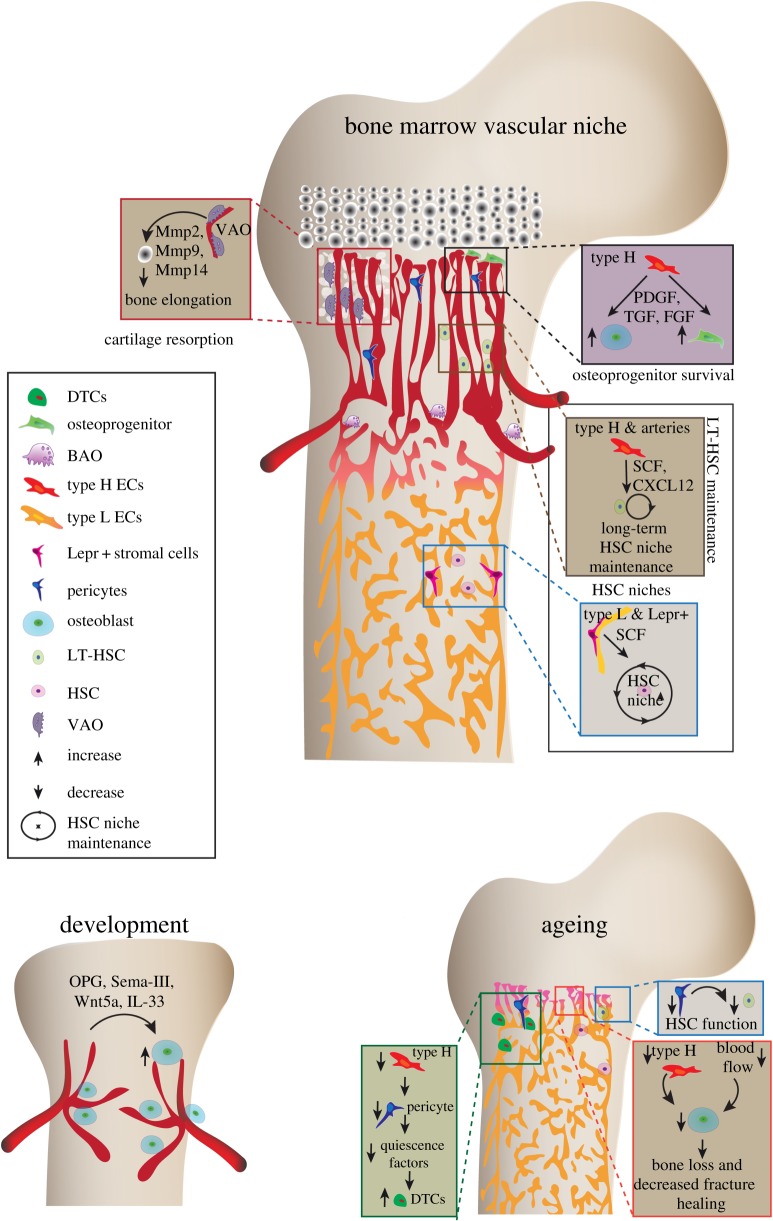
During the postnatal stages, the vasculature of the skeletal system is known to play essential roles in bone growth and bone formation; however, bone vasculature remained vaguely defined as a network of arteries and sinusoidal blood vessels until recent years. Owing to its complex and calcified nature, imaging of the bone tissue remained difficult. Recent improvements with bone imaging techniques provided new insights into the organization of blood vessels and highlighted the heterogeneity of blood vessels in the skeletal system [47]. Notably, in addition to arteries, veins and sinusoidal vessels, a structurally, phenotypically and functionally distinct capillary subtype is present in bone. These capillaries localize in the metaphysis and cortical regions of bones, physically associate with osteoprogenitors and generate an active niche microenvironment for cells of the osteoblast lineage. Due to the high expression of specific markers, they are termed as type H [26]. The abundance of these type H vessels gradually declines in adult and ageing mice, which provides a compelling explanation for the age-associated loss of bone mass seen in rodents and humans. Genetic and pharmacological approaches revealed that the reactivation of type H endothelium in aged mice resulted in increased osteoprogenitor numbers and improved bone mass [48]. Particularly, endothelial Hif1-α maintains these vessels which diminish upon ageing. Treatment with Hif1 stabiliser in aged mice leads to the expansion of type H ECs, accumulation of surrounding osteoprogenitors and increase in bone mass and bone quality [48]. Furthermore, molecular and mechanistic analysis of angiogenesis and type H ECs indicated that these ECs mediate developmental and regenerative angiogenesis in the bone (figure 1).
Figure 1.
Angiocrine crosstalk during bone development, haemostasis and ageing. Displayed are multiple angiocrine factors and their cellular sources that mediate communication between blood vessels, bone cells and haematopoietic cells. Bone development requires blood vessel invasion and osteoprogenitors follow blood vessels. Later, type H blood vessels secrete osteogenic factors and drive the bone formation and bone growth. Further, the proteolytic activity of type H endothelium is required cartilage resorption and directional bone growth. Angiocrine factors derived from different cellular sources maintain HSCs and decline of these cellular sources, particularly, type H and pericytes upon ageing contributes to the declined HSC function. Ageing also leads to enhanced proliferation of DTCs and lowered fracture healing. VAO, vessel-associated osteoclasts; BAO, bone-associated osteoclasts; SCF, stem cell factor; HSC, haematopoietic stem cells; LT, long-term; Mmps, matrix metalloproteinases; PDGF, platelet-derived growth factor; FGF, fibroblast-derived growth factor; TGF, transforming growth factor; CXCL12, C-X-C motif chemokine 12; Lepr+, Leptin receptor; DTC, disseminated tumour cells.
ECs are known to produce Wnt5a [49]. Wnt5a is a secreted glycoprotein that mediates the beta-catenin signalling pathway, which is a central regulator of osteogenesis [50]. VEGF overexpression conditions lead to the stabilization of beta-catenin and excessive bone ossification, indicating the crosstalk between angiogenesis and bone formation via Wnt signalling [50]. Osteoblast-derived Wnt5a is a key player in growth plate ossification and an essential mediator of osteoblastic differentiation through bone morphogenetic protein 2 (BMP-2) [13]. However, the involvement of endothelium-derived Wnts in skeletal system development needs further examination. Another class of extracellular signalling molecules having a strong implication in bone formation and remodelling are semaphorins (Sema) [8,9]. For example, Sema-III is an active member of Sema family with a known role in bone patterning [10,11]. In addition to these factors, ECs secrete proteases like matrix metalloproteinases (Mmps), including Mmp2, Mmp9 and Mmp14 [15]. These Mmps upregulate type H ECs mediate cartilage resorption and bone formation with the help of vessel-associated osteoclasts (VAO), a newly discovered counterpart of bone-associated osteoclasts (BAO). The loss of Mmps in type H ECs leads to misdirected bone growth and abnormal bone elongation [15]. Thus, endothelium-derived factors play a central role in driving osteogenesis and bone growth. The role of angiocrine factors in osteogenesis is summarized and illustrated in figure 1.
3. Therapeutic potential of angiocrine factors in bone repair and regeneration
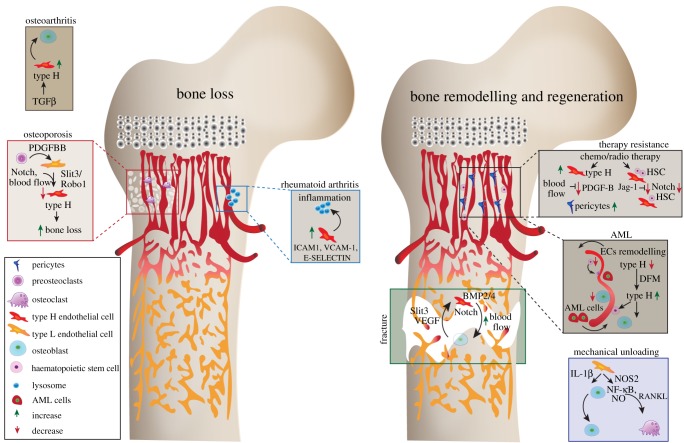
Unlike most other organs in the body, bone possesses a high regenerative potential. Usually, bone repair and regeneration following fracture does not form a fibrotic scar, a common phenomenon occurring during repair of soft tissues. Bone repair occurs in four stages; first, the site of fracture is encapsulated by a haematoma, establishing a hypoxic environment with significant upregulation of HIF-1α and VEGF [51,52]. In response to chronic hypoxia, ECs upregulate the osteogenic factor BMP-2 [14]. Noggin, a secreted BMP agonist, regulated via endothelial Notch signalling reverses both vascular and bone defects [27]. Since Notch signalling is known to play a role in fracture repair [53], there could be a possible angiocrine function via Notch signalling in fracture healing. Second, the fracture site is invaded by new angiogenic blood vessels, laying down a template for osteoclast–fibrocartilaginous callus formation, as blood vessels recruit and guide osteoblast precursors to the site of fracture [54,55]. Third, the soft callus calcifies to generate new bone, which requires early and prolonged exposure to exogenous VEGF to promote vascularization and bone growth. Blocking endogenous VEGF inhibits vascularization and calcification of the callous [56]. Slit homologue 3 protein (SLIT3) is an axon guidance molecule, which has been shown to induce ECs migration via roundabout homologue (ROBO) signalling [57]. Slit3 mutant mice have reduced Type H vessels and impaired fracture repair, whereas Slit3 overexpression creates a mature callus and increased haematopoiesis during fracture repair [58], suggesting a possible role for type H endothelium in fracture repair (figure 2). The final stage involves the reduction of the fracture callus and normalization of the vasculature. Fracture repair requires increased blood to the site of fracture. In line with this, the aged mice with reduced blood flow to bone exhibit impaired ability to regenerate fractures [59].
Figure 2.
Involvement of angiocrine signalling during bone loss, repair and regeneration. Figure illustrating the role of various angiocrine factors, their cellular sources and their influence during radiation and chemotherapy, mechanical loading and also on various pathological conditions like rheumatoid arthritis, OA, inflammation and osteoporosis. The importance of different blood vessel types and associated cells are depicted in the context of bone diseases, repair and regeneration. The angiocrine signalling from the type H ECs plays a crucial role during bone diseases and regeneration. HSC, haematopoietic stem cells; Mmps, matrix metalloproteinases; PDGF, platelet-derived growth factor; FGF, fibroblast-derived growth factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion protein; BMP, bone morphogenetic protein; AML, acute myeloid leukaemia; EC, endothelial cells; NOS, nitric oxide synthase; NO, nitric oxide; IL, interleukin; NF-κB, nuclear factor kappa-light-chain enhancer of activated B cells; RANKL, receptor activator of nuclear factor kappa-B ligand; DFM, deferoxamine mesylate.
The vasculature confers a protective niche for HSCs following chemotherapy, promoting bone and haematopoietic regeneration. Long-term HSCs are associated with arteries and with type H blood vessels which are also referred to as endosteal vessels in some reports [60–62]. The vascular niche is essential to regenerate the HSC population after irradiation [63]. Transplantation of bone marrow ECs following irradiation enhances haematopoiesis and protects radiosensitive tissue [64,65]. Irradiated mice transplanted with bone marrow EC culture conditioned media showed increased survival [64], indicating that angiocrine factors can enhance survival but not compensate for a complete loss of HSCs. Endothelial-specific deletion of the Notch ligand JAG-1 leads to an impairment in HSC regeneration and increase lethality following irradiation [24]. In addition to Notch signalling, ECs upregulate Fgf-2, Bmp4, Igfbp2 and Angiopoetin-1 to expand the haemopoietic stem progenitor cells (HSPCs) [22,23], indicating these factors may be useful to protect HSC following irradiation. Aged bone marrow ECs impair HSCs and promote a myeloid bias, as demonstrated by transplantation of ECs from the bone marrow of the aged mice into the young recipients [66]. The aged bone marrow has a reduction in PDGFR-β expressing pericytes, which correlates with an expansion of disseminated tumour cells (DTCs). Furthermore, the aged bone marrow secretome promotes proliferation of breast cancer cells in bone. Type H ECs expand in response to radiation and chemotherapy and mediate the regenerative angiogenesis in the bone via blood flow-mediated secretion of PDGF-B, which promotes pericyte expansion [67].
4. Dysregulation of angiocrine signalling in bone loss conditions
Osteoporosis is associated with failure to maintain a balance between osteoclasts and osteoblasts, resulting in a loss of bone mass and density. Osteoporosis is predominant in post-menopausal women and linked to a reduction in the Parathyroid hormone. Osteoporosis mouse models demonstrate a decrease in type H blood vessels [68]. Cathepsin K is a protease expressed by osteoclasts and mediates bone resorption. The Cathepsin K inhibitor prevents degradation of the bone matrix by enhancing PDGF-BB in pre-osteoclasts, which in turn, increases type H blood vessels that promote bone formation through the expansion of osterix-associated cells [68]. Schnurri3 (SHN3) acts cell autonomously to regulate bone formation via osteoblasts while also acting non-cell autonomously by enhancing Slit3/Robo1 to increase the type H blood vessels. The increase in type H blood vessels precedes the increase in bone mass seen in Shn3−/− mice [58], demonstrating the angiocrine crosstalk between type H vessels and osteoblasts. Importantly, type H blood vessels also serve as a biomarker for osteoporosis and bone loss in humans [69]. The physical proximity of type H ECs and osteoblasts supports that notion that these blood vessels secrete a localized gradient of factors that works synergistically with osteoblasts to enhance bone formation [26]. Oestrogen-dependent osteoporosis treatment prevents bone reabsorption, whereas increasing type H blood vessels increases osteoprogenitors [68]. Therefore, the absence of type H vessels may serve as a useful biomarker for disease progression. In addition, the expansion of type H blood vessels during osteoporosis may provide a strategy to increase bone formation, thereby improving the bone quality in this condition. However, the impact on oestrogen on type H blood vessels and the crosstalk of type H blood vessels with tissue during osteoporosis treatment is undetermined. Clinical studies indicate a link between reduced blood flow and bone mineral density in osteoporosis [70]. Further studies in mice demonstrate that reduced blood flow results in a significant reduction of osteoprogenitors [71]. These data suggest a potential therapeutic avenue via increased blood flow and angiogenesis in osteoporosis treatment.
Reduction in mechanical loading leads to a decrease in bone mass [72]. Bone mineral density, volume and blood vessel numbers are unchanged in exercised mice treated with an angiogenesis inhibitor [73]. Capillary density increases in swim exercised mice, suggesting that mechanical loading from muscle is sufficient to promote blood vessel increase [74]. Decreased mechanical loading induces IL-1β in ECs and nitric oxide synthase 2 (NOS2) expression, activating the nitric oxide (NO) and nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) signalling pathways in osteoblasts, which inhibits osteoblast proliferation. Further, Lipocalin 2 inhibits osteoblast differentiation and activates receptor activator of nuclear factor kappa-Β ligand (RANKL) to induce osteoclasts, which combined results in an imbalance of bone turnover resulting in bone loss [25] (figure 2).
5. Angiocrine signals during inflammation associated bone loss
Under inflammatory milieu, ECs express BMP-2 [75–77] indicative of their role in bone remodelling. Likewise, ECs produce a glycoprotein–cytokine osteoprotegerin (OPG) in response to a higher concentration of glucose, which inhibits osteoclastogenesis [7]. Production of OPG by ECS under high glucose concentration may minimize bone resorption under diabetic conditions. Interleukin 33 (IL-33), a pro-inflammatory cytokine secreted by Endoglin expressing ECs, is believed to play an essential role in osteogenesis. IL-33 induces the differentiation of bone marrow-derived stromal cells to osteoblasts and increase calcium deposition [12] (figure 2).
Rheumatoid arthritis (RA) is a chronic inflammatory disease leading to bone degradation and joint deformities [78]. Rheumatoid arthritis joints display increased angiogenesis and ECs play a central role in the trafficking of leucocytes into the joint [79]. Additionally, ECs expresses several cytokines and proteases, which enhance inflammation. Intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion protein 1 (VCAM-1) and E-selectin expressed on ECs stimulate leucocyte and fibroblast migration onto the joint [28–30] (figure 2). Osteoarthritis (OA) displays a similar pathology to RA, with the underlying cause due to mechanical wear and tear. Anterior cruciate ligament transection causes OA like phenotypes, with abnormal bone formation and an increase in angiogenesis in the subchondral bone [80,81]. Increased TGFβ initiates a signalling cascade that recruits mesenchymal stem cells (MSCs) and type H vessels, while exogenous blocking of TGFβ results in a reduction in MSC recruitment and type H vessels, attenuating the OA phenotype [80,81] (figure 2).
6. Angiocrine signals in complex and ageing HSC niches
Blood vessels in the skeletal system play crucial roles in blood cell formation by providing nurturing nutrient niche microenvironments for HSCs. Although a common precursor has been suggested for vasculogenesis and primitive haematopoiesis [82], interest to understand the vascular microenvironment in definitive haematopoiesis started with the identification of HSC near blood vessels [61]. Analysing the distribution of HSCs in the whole bone marrow suggest their preferential localization near to the vasculature [83,84]. The recent studies using novel markers such as α-catulin [84] and Hoxb5 [85] also support the existence of blood vessel microenvironment for HSCs. Several blood vessel subtypes and perivascular cell subsets have been reported to interact and regulate HSCs. Interactions of HSCs with different vascular and perivascular cell types in the bone marrow microenvironment is reviewed elsewhere [86–88]. Thus, HSCs reside in specialized, complex niches, which are maintained by a heterogeneous group of cells [20,21,89]. Angiocrine factors secreted by blood vessels regulate HSC self-renewal and quiescence [17,90,91]. Recent improvements with bone imaging methods provide insights into the localization of HSCs within the bone marrow where they frequently localize close to blood vessels [16,84,92]. The stem cell factor (SCF) secreted by type H ECs, sinusoidal ECs and arterial ECs is one of the critical angiocrine factors in HSC maintenance [16]. SCF also plays a role during erythropoiesis and lymphopoiesis [16]. Interleukins (ILs) are a class of cytokines that regulates HSCs and are produced by a wide variety of cells including ECs. In mice, IL-33 alters the HSC fate [12]. IL-33 is known to be secreted during tissue damage; however, its role in HSC niche modification and tissue regeneration is not well studied. Interleukin 7 (IL-7) produced from the perivascular stromal cells maintains a pro-B cell niche associated with HSC niche in the bone marrow [18,19]. IL-7 is necessary for controlling the commitment of lymphoid progenitors to B cells [19]. Perivascular stromal cells, the bone marrow ECs, and osteoblasts produce C-X-C motif chemokine 12 (CXCL12), which is a potent chemokine required for the long-term maintenance and quiescence of the HSC niche [20,89]. The involvement of SCF and CXCL12 in HSC maintenance is depicted in figure 1. Angiocrine factors in HSC regulation and crosstalk between HSCs and endothelium is currently an intense area of study and extensively reviewed [86].
Recent studies highlight the importance of type H capillaries and arteries in maintaining HSCs. The cells forming these vascular structures are strongly positive for SCF. Endothelial Notch activation, which promotes arteriole formation and expansion of type H ECs [27], leads to an increase in platelet-derived growth factor receptor-β (PDGFR-β)/Nestin/Neuron-glial antigen 2 (NG2)-positive perivascular cells, HSCs and augmented SCF levels, suggesting an enhancement of vascular niche function. Remarkably, EC-specific activation of HIF pathway, which enhanced the number of type H capillaries but had no effect on artery formation and perivascular cells, fails to enhance the number of HSCs. Further detailed analyses of endothelial Notch and HIF signalling in bone indicate that both pathways mediate type H EC expansion independently, whereas only Notch signalling enhances the frequency of HSCs by improving the vascular niche function [48]. The number of arterioles, type H capillaries, PDGFR-β/NG2-positive perivascular cells and hence SCF levels decline in the ageing bone. This reduced number of arterioles upon ageing is in line with the reported decrease in blood flow to the bone in ageing. The decline in arterioles upon ageing not only provides compelling evidence for the decreased blood circulation in bone but is also likely to induce metabolic changes in aged bones. The decrease in blood flow to bone reduces angiogenesis and type H vessels that lead to a reduction in osteoprogenitor cells and new bone formation [71] (figure 1). The formation of new blood vessels leads to increased blood flow, and tissue perfusion and thereby may lead to alteration in vascular niches, metabolic microenvironments and their functions. Supporting this notion endothelial activation of Notch signalling in aged mice not only lead to increased blood flow to the bone but also improved the vascular niche function and improved the abundance of HSCs [48]. However, long-term repopulation analysis of HSCs from niche-activated aged mice shows that HSC functionality is not improved, which is a consequence of cell-autonomous aspects of HSC ageing such as the accumulation of DNA damage. The EC-derived Notch ligands are able to enhance proliferation and prevent the depletion of long-term HSCs [93]. The activation status of ECs can have a profound influence on modulating the number of long-term HSCs [23]. Taken together, skeletal and HSC ageing is an outcome of complex multicellular vascular microenvironments in combination with HSC intrinsic factors contributing to the age-dependent alterations and loss of stem cells functionality (figure 1).
7. Angiocrine crosstalk with tissue during malignancies in bone
Vascular niches in bone hold potential to provide a protective microenvironment for cancer cells via secretion of angiocrine factors [94] Technical advances in high-resolution microscopy, coupled with optimization in processing bone tissue, have allowed the investigation of spatio-temporal dynamics in leukaemia and cancer metastasis mouse models [95]. Acute myeloid leukaemia (AML) presents with disorganized bone marrow vasculature, significant remodelling and reduction of the type H endothelium and trans-endothelial migration of HSC [96,97] (figure 2). In addition, ECs support the growth of AML cells in vitro and AML cells localized near ECs show resistance to chemotherapy [98,99]. Inhibition of EC remodelling in AML shows an increase in HSC survival [96]. Lymphoma cells secrete fibroblast growth factor 4 and activate FGFR1 on ECs, upregulating the Notch ligand Jag1 on tumour ECs [100]. This crosstalk establishes the vascular niche as a supportive microenvironment, which in turn supports the proliferation of lymphoma cells in a Notch-dependent manner. ECs in multiple myeloma show an upregulation of genes encoding factors involved in extracellular matrix suggesting that pathological remodelling of the bone marrow microenvironment is dependent on extrinsic factors rather than cell-intrinsic mechanisms to promote angiogenesis and tumour progression [101]. Increased E-selectin expression on bone ECs enhances bone metastasis through an unknown angiocrine signalling pathway that interacts with the Golgi glycoprotein 1 (Glg1) ligand [32]. DTCs remain in a quiescent state in the bone marrow microenvironment and changes to this microenvironment, such as ageing, leads to reactivation and metastasis [102]. Therefore, bone is one of the most common sites for secondary tumour metastasis. Dormant DTCs are closely associated with the endothelium of the bone marrow [33]. Thrombospondin-1 secreted from ECs creates a stabilized vascular niche, in which DTCs become quiescent [33]. The angiocrine signalling via von Willebrand factor and VCAM1 induce an integrin-mediated chemotherapeutic effect on DTCs, with disruptions in this pathway providing potential treatment modalities to eradicate DTCs [31]. Furthermore, a recent study provides the first insights on the impact of the age-related angiocrine signals in regulating the proliferation and quiescence of tumour cells in bones. Specifically, bone EC-derived PDGF-B signalling regulates dormancy and therapy resistance in bone [67]. However, the cell types and niches promoting the proliferation versus the microenvironments supporting the dormancy of DTCs in bones remains elusive. Likewise, the dissection of the mechanisms leading to the reactivation of the dormant tumour cells in the bone marrow needs further investigation.
8. Concluding remarks
It is now becoming increasingly clear that the skeletal vasculature is heterogeneous, and specialized to secrete osteogenesis and haematopoiesis supporting angiocrine factors. Loss of these nurturing angiocrine signals leads to the decline in haematopoietic and mesenchymal stem and progenitor cell function during ageing. Dysregulation of the angiocrine crosstalk drives bone loss diseases and other pathobiological conditions in the skeletal system. Thus, in-depth mechanistic insights into the angiocrine crosstalk within and across heterogeneous bone marrow vascular niches would be of high relevance for designing strategies to manage ageing and pathobiological processes in the skeletal system. Furthermore, the identification of new angiocrine factors and dissecting their role in the bone marrow microenvironment holds the potential to provide new therapeutic targets. Thus, there is an exciting opportunity to unravel a plethora of new players and interactions in complex bone marrow niches, with important implications for basic research and medicine.
Supplementary Material
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
A.P.K is supported by European Research Council (grant no. StG: metaNiche, 805201), Leuka (grant no. 2017/JGF/001), Medical Research Council (grant no. CDA: MR/P02209X/1), The Royal Society (grant no. RG170326), CRUK Development Fund (grant no. CRUKDF 0317-AK), Kennedy Trust for Rheumatology Research (grant no. KENN 15 16 09) and John Fell Fund, University of Oxford (grant no. 161/061).
References
- 1.Ramasamy SK, Kusumbe AP, Adams RH. 2015. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 25, 148–157. ( 10.1016/j.tcb.2014.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. 2001. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294, 559–563. ( 10.1126/science.1063889) [DOI] [PubMed] [Google Scholar]
- 3.Serluca FC, Drummond IA, Fishman MC. 2002. Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr. Biol. 12, 492–497. ( 10.1016/S0960-9822(02)00694-2) [DOI] [PubMed] [Google Scholar]
- 4.Ding B-S, et al. 2010. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468, 310–315. ( 10.1038/nature09493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding B-S, et al. 2011. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147, 539–553. ( 10.1016/j.cell.2011.10.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stegen S, Carmeliet G. 2018. The skeletal vascular system—breathing life into bone tissue. Bone 115, 50–58. ( 10.1016/j.bone.2017.08.022) [DOI] [PubMed] [Google Scholar]
- 7.de Ciriza CP. 2015. OPG expression on endothelial cells and modulation by IL-1B, PDGF, insulin, and glucose . Biochem. Physiol. Open Access 4, 179 ( 10.4172/2168-9652.1000179) [DOI] [Google Scholar]
- 8.Kang S, Kumanogoh A. 2013. Semaphorins in bone development, homeostasis, and disease. Semin. Cell Dev. Biol. 24, 163–171. ( 10.1016/j.semcdb.2012.09.008) [DOI] [PubMed] [Google Scholar]
- 9.Serini G, et al. 2003. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424, 391–397. ( 10.1038/nature01784) [DOI] [PubMed] [Google Scholar]
- 10.Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. 1996. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383, 525–528. ( 10.1038/383525a0) [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. 2012. Osteoprotection by semaphorin 3A. Nature 485, 69–74. ( 10.1038/nature11000) [DOI] [PubMed] [Google Scholar]
- 12.Kenswil KJG, et al. 2018. Characterization of endothelial cells associated with hematopoietic niche formation in humans identifies IL-33 as an anabolic factor. Cell Rep. 22, 666–678. ( 10.1016/j.celrep.2017.12.070) [DOI] [PubMed] [Google Scholar]
- 13.Nemoto E, Ebe Y, Kanaya S, Tsuchiya M, Nakamura T, Tamura M, Shimauchi H. 2012. Wnt5a signaling is a substantial constituent in bone morphogenetic protein-2-mediated osteoblastogenesis. Biochem. Biophys. Res. Commun. 422, 627–632. ( 10.1016/j.bbrc.2012.05.039) [DOI] [PubMed] [Google Scholar]
- 14.Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, Longaker MT. 2002. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast. Reconstr. Surg. 109, 2384–2397. ( 10.1097/00006534-200206000-00033) [DOI] [PubMed] [Google Scholar]
- 15.Romeo SG, Alawi KM, Rodrigues J, Singh A, Kusumbe AP, Ramasamy SK. 2019. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat. Cell Biol. 21, 430–441. ( 10.1038/s41556-019-0304-7) [DOI] [PubMed] [Google Scholar]
- 16.Comazzetto S, Murphy MM, Berto S, Jeffery E, Zhao Z, Morrison SJ. 2019. Restricted hematopoietic progenitors and erythropoiesis require SCF from leptin receptor+ niche cells in the bone marrow. Cell Stem Cell 24, 477–486. ( 10.1016/j.stem.2018.11.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balzano M, et al. 2019. Nidogen-1 contributes to the interaction network involved in pro-B cell retention in the peri-sinusoidal hematopoietic stem cell niche. Cell Rep. 26, 3257–3271. ( 10.1016/j.celrep.2019.02.065) [DOI] [PubMed] [Google Scholar]
- 18.Pillai M, Torok-Storb B, Iwata M. 2004. Expression and function of IL-7 receptors in marrow stromal cells. Leuk. Lymphoma 45, 2403–2408. ( 10.1080/10428190412331283189) [DOI] [PubMed] [Google Scholar]
- 19.Dias S, Silva H, Cumano A, Vieira P. 2005. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J. Exp. Med. 201, 971–979. ( 10.1084/jem.20042393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding L, Saunders TL, Enikolopov G, Morrison SJ. 2012. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462. ( 10.1038/nature10783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. 2010. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387–399. ( 10.1016/j.immuni.2010.08.017) [DOI] [PubMed] [Google Scholar]
- 22.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. 2004. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161. ( 10.1016/j.cell.2004.07.004) [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi H, et al. 2010. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol. 12, 1046–1056. ( 10.1038/ncb2108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poulos MG, et al. 2013. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 4, 1022–1034. ( 10.1016/j.celrep.2013.07.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veeriah V, Zanniti A, Paone R, Chatterjee S, Rucci N, Teti A, Capulli M. 2016. Interleukin-1β, lipocalin 2 and nitric oxide synthase 2 are mechano-responsive mediators of mouse and human endothelial cell-osteoblast crosstalk. Sci. Rep. 6, 29880 ( 10.1038/srep29880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusumbe AP, Ramasamy SK, Adams RH. 2014. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328. ( 10.1038/nature13145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. 2014. Endothelial notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376–380. ( 10.1038/nature13146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann-Geller B, et al. 2019. Interactions between rheumatoid arthritis synovial fibroblast migration and endothelial cells. Immunol. Cell Biol. 97, 178–189. ( 10.1111/imcb.12208) [DOI] [PubMed] [Google Scholar]
- 29.Kriegsmann J, Keyszer GM, Geiler T, Lagoo AS, Lagoo-Deenadayalan S, Gay RE, Gay S. 1995. Expression of E-selectin messenger RNA and protein in rheumatoid arthritis. Arthritis Rheum. 38, 750–754. ( 10.1002/art.1780380606) [DOI] [PubMed] [Google Scholar]
- 30.Klimiuk P, Sierakowski S, Latosiewicz R, Cylwik J, Cylwik B, Skowronski J, Chwiecko J. 2002. Soluble adhesion molecules (ICAM-1, VCAM-1, and E-selectin) and vascular endothelial growth factor (VEGF) in patients with distinct variants of rheumatoid synovitis. Ann. Rheum. Dis. 61, 804–809. ( 10.1136/ard.61.9.804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlson P, et al. 2019. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238 ( 10.1038/s41556-018-0267-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esposito M, et al. 2019. Bone vascular niche E-selectin induces mesenchymal–epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 21, 627–639. ( 10.1038/s41556-019-0309-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghajar CM, et al. 2013. The perivascular niche regulates breast tumor dormancy. Nat. Cell Biol. 15, 807–817. ( 10.1038/ncb2767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jabalee J, Franz-Odendaal T. 2012. Mesenchymal condensation sets the stage for intramembranous bone development. FASEB J. 26, 907.7 ( 10.1096/fasebj.26.1_supplement.907.7)21990375 [DOI] [Google Scholar]
- 35.Long F, Ornitz DM. 2013. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 5, a008334 ( 10.1101/cshperspect.a008334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takebe T, et al. 2015. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16, 556–565. ( 10.1016/j.stem.2015.03.004) [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, et al. 2007. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Invest. 117, 1616–1626. ( 10.1172/JCI31581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song JJ, Aswad R, Kanaan RA, Rico MC, Owen TA, Barbe MF, Safadi FF, Popoff SN. 2007. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-β1 to induce mesenchymal cell condensation. J. Cell. Physiol. 210, 398–410. ( 10.1002/jcp.20850) [DOI] [PubMed] [Google Scholar]
- 39.Eshkar-Oren I, Viukov SV, Salameh S, Krief S, Oh C, Akiyama H, Gerber H-P, Ferrara N, Zelzer E. 2009. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development 136, 1263–1272. ( 10.1242/dev.034199) [DOI] [PubMed] [Google Scholar]
- 40.Zelzer E, McLean W, Ng Y-S, Fukai N, Reginato AM, Lovejoy S, D'Amore PA, Olsen BR. 2002. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Dev. Camb. Engl. 129, 1893–1904. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert SF. 2000. Osteogenesis: the development of bones. In Developmental biology, 6th edn. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 42.Yang Y-Q, Tan Y-Y, Wong R, Wenden A, Zhang L-K, Rabie ABM. 2012. The role of vascular endothelial growth factor in ossification. Int. J. Oral Sci. 4, 64–68. ( 10.1038/ijos.2012.33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang LE, Gu J, Schau M, Bunn HF. 1998. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl Acad. Sci. USA 95, 7987–7992. ( 10.1073/pnas.95.14.7987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semenza GL. 2012. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408. ( 10.1016/j.cell.2012.01.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grosso A, Burger MG, Lunger A, Schaefer DJ, Banfi A, Di Maggio N.. 2017. It takes two to tango: coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 5, 68 ( 10.3389/fbioe.2017.00068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai J, Rabie ABM. 2007. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J. Dent. Res. 86, 937–950. ( 10.1177/154405910708601006) [DOI] [PubMed] [Google Scholar]
- 47.Kusumbe AP, Ramasamy SK, Starsichova A, Adams RH. 2015. Sample preparation for high-resolution 3D confocal imaging of mouse skeletal tissue. Nat. Protoc. 10, 1904–1914. ( 10.1038/nprot.2015.125) [DOI] [PubMed] [Google Scholar]
- 48.Kusumbe AP, Ramasamy SK, Itkin T, Mäe MA, Langen UH, Betsholtz C, Lapidot T, Adams RH. 2016. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384. ( 10.1038/nature17638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan K, et al. 2019. Loss of endothelium-derived Wnt5a Is associated with reduced pericyte recruitment and small vessel loss in pulmonary arterial hypertension. Circulation 139, 1710–1724. ( 10.1161/CIRCULATIONAHA.118.037642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maes C, et al. 2010. Increased skeletal VEGF enhances β-catenin activity and results in excessively ossified bones. EMBO J. 29, 424–441. ( 10.1038/emboj.2009.361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Street J, Winter D, Wang JH, Wakai A, McGuinness A, Redmond HP. 2000. Is human fracture hematoma inherently angiogenic? Clin. Orthop. Relat. Res. 378, 224–237. ( 10.1097/00003086-200009000-00033) [DOI] [PubMed] [Google Scholar]
- 52.Wan C, et al. 2008. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc. Natl Acad. Sci. USA 105, 686–691. ( 10.1073/pnas.0708474105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Inzana JA, Mirando AJ, Ren Y, Liu Z, Shen J, O'Keefe RJ, Awad HA, Hilton MJ. 2016. NOTCH signaling in skeletal progenitors is critical for fracture repair. J. Clin. Invest. 126, 1471–1481. ( 10.1172/JCI80672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. 2010. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344. ( 10.1016/j.devcel.2010.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerber H-P, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. 1999. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 5, 623 ( 10.1038/9467) [DOI] [PubMed] [Google Scholar]
- 56.Street J, et al. 2002. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl Acad. Sci. USA 99, 9656–9661. ( 10.1073/pnas.152324099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang B, Dietrich UM, Geng J-G, Bicknell R, Esko JD, Wang L. 2009. Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood 114, 4300–4309. ( 10.1182/blood-2008-12-193326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu R, et al. 2018. Targeting skeletal endothelium to ameliorate bone loss. Nat. Med. 24, 823–833. ( 10.1038/s41591-018-0020-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu C, Hansen E, Sapozhnikova A, Hu D, Miclau T, Marcucio RS. 2008. Effect of age on vascularization during fracture repair. J. Orthop. Res. 26, 1384–1389. ( 10.1002/jor.20667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heissig B, et al. 2002. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109, 625–637. ( 10.1016/S0092-8674(02)00754-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiel MJ, Yilmaz ÖH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. 2005. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121. ( 10.1016/j.cell.2005.05.026) [DOI] [PubMed] [Google Scholar]
- 62.Kunisaki Y, et al. 2013. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643. ( 10.1038/nature12612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hooper AT, et al. 2009. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2 mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263–274. ( 10.1016/j.stem.2009.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poulos MG, Crowley MJP, Gutkin MC, Ramalingam P, Schachterle W, Thomas J-L, Elemento O, Butler JM. 2015. Vascular platform to define hematopoietic stem cell factors and enhance regenerative hematopoiesis. Stem Cell Rep. 5, 881–894. ( 10.1016/j.stemcr.2015.08.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chute JP, Muramoto GG, Salter AB, Meadows SK, Rickman DW, Chen B, Himburg HA, Chao NJ. 2007. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood 109, 2365–2372. ( 10.1182/blood-2006-05-022640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poulos MG, Ramalingam P, Gutkin MC, Llanos P, Gilleran K, Rabbany SY, Butler JM. 2017. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J. Clin. Invest. 127, 4163–4178. ( 10.1172/jci93940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh A, Veeriah V, Xi P, Labella R, Chen J, Romeo S, Ramasamy SK, Kusumbe AP. 2019. Angiocrine signals regulate quiescence and therapy resistance in bone metastasis. JCI Insight 4, 125679 ( 10.1172/jci.insight.125679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie H, et al. 2014. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 20, 1270–1278. ( 10.1038/nm.3668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L, et al. 2017. Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis. 8, e2760 ( 10.1038/cddis.2017.36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogt MT, Cauley JA, Kuller LH, Nevitt MC. 1997. Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J. Bone Miner. Res. 12, 283–289. ( 10.1359/jbmr.1997.12.2.283) [DOI] [PubMed] [Google Scholar]
- 71.Ramasamy SK, et al. 2016. Blood flow controls bone vascular function and osteogenesis. Nat. Commun. 7, 13601 ( 10.1038/ncomms13601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suva LJ, Gaddy D, Perrien DS, Thomas RL, Findlay DM. 2005. Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr. Osteoporos. Rep. 3, 46–51. ( 10.1007/s11914-005-0003-0) [DOI] [PubMed] [Google Scholar]
- 73.Yao Z, Lafage-Proust M-H, Plouët J, Bloomfield S, Alexandre C, Vico L. 2004. Increase of both angiogenesis and bone mass in response to exercise depends on VEGF. J. Bone Miner. Res. 19, 1471–1480. ( 10.1359/jbmr.040517) [DOI] [PubMed] [Google Scholar]
- 74.Viboolvorakul S, Niimi H, Wongeak-in N, Eksakulkla S, Patumraj S. 2009. Increased capillary vascularity in the femur of aged rats by exercise training. Microvasc. Res. 78, 459–463. ( 10.1016/j.mvr.2009.07.003) [DOI] [PubMed] [Google Scholar]
- 75.Willette RN, Gu JL, Lysko PG, Anderson KM, Minehart H, Yue T-L. 1999. BMP-2 gene expression and effects on human vascular smooth muscle cells. J. Vasc. Res. 36, 120–125. ( 10.1159/000025634) [DOI] [PubMed] [Google Scholar]
- 76.Dhore CR, et al. 2001. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 21, 1998–2003. ( 10.1161/hq1201.100229) [DOI] [PubMed] [Google Scholar]
- 77.Basic-Jukic N, et al. 2016. Expression of BMP-2 in vascular endothelial cells of recipient may predict delayed graft function after renal transplantation. Kidney Blood Press. Res. 41, 781–793. ( 10.1159/000450568) [DOI] [PubMed] [Google Scholar]
- 78.Aletaha D, Smolen JS. 2018. Diagnosis and management of rheumatoid arthritis: a review. JAMA 320, 1360–1372. ( 10.1001/jama.2018.13103) [DOI] [PubMed] [Google Scholar]
- 79.Walsh DA, McWilliams DF, Turley MJ, Dixon MR, Franses RE, Mapp PI, Wilson D. 2010. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology 49, 1852–1861. ( 10.1093/rheumatology/keq188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhen G, et al. 2013. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 19, 704–712. ( 10.1038/nm.3143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cui Z, et al. 2016. Halofuginone attenuates osteoarthritis by inhibition of TGF-β activity and H-type vessel formation in subchondral bone. Ann. Rheum. Dis. 75, 1714–1721. ( 10.1136/annrheumdis-2015-207923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. 1998. A common precursor for hematopoietic and endothelial cells. Development 125, 725–732. [DOI] [PubMed] [Google Scholar]
- 83.Nombela-Arrieta C, et al. 2013. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat. Cell Biol. 15, 533–543. ( 10.1038/ncb2730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Acar M, et al. 2015. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130. ( 10.1038/nature15250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen JY, et al. 2016. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature 530, 223–227. ( 10.1038/nature16943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szade K, et al. 2018. Where hematopoietic stem cells live: the bone marrow niche. Antioxid. Redox Signal. 29, 191–204. ( 10.1089/ars.2017.7419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He N, Zhang L, Cui J, Li Z. 2014. Bone marrow vascular niche: home for hematopoietic stem cells. Bone Marrow Res. 2014, 128436 ( 10.1155/2014/128436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morrison SJ, Scadden DT. 2014. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334. ( 10.1038/nature12984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greenbaum A, Hsu Y-MS, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. 2013. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230. ( 10.1038/nature11926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rafii S, Butler JM, Ding B-S. 2016. Angiocrine functions of organ-specific endothelial cells. Nature 529, 316–325. ( 10.1038/nature17040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakamura-Ishizu A, Okuno Y, Omatsu Y, Okabe K, Morimoto J, Uede T, Nagasawa T, Suda T, Kubota Y. 2012. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood 119, 5429–5437. ( 10.1182/blood-2011-11-393645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spencer JA, et al. 2014. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273. ( 10.1038/nature13034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Butler JM, et al. 2010. Endothelial cells are essential for the self-renewal and repopulation of notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251–264. ( 10.1016/j.stem.2010.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Butler JM, Kobayashi H, Rafii S. 2010. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer 10, 138–146. ( 10.1038/nrc2791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allocca G, Kusumbe AP, Ramasamy SK, Wang N. 2016. Confocal/two-photon microscopy in studying colonisation of cancer cells in bone using xenograft mouse models. BoneKEy Rep. 5, 851 ( 10.1038/bonekey.2016.84) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duarte D, et al. 2018. Inhibition of endosteal vascular niche remodeling rescues hematopoietic stem cell loss in AML. Cell Stem Cell 22, 64–77. ( 10.1016/j.stem.2017.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Passaro D, et al. 2017. Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell 32, 324–341. ( 10.1016/j.ccell.2017.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bosse RC, et al. 2016. Chemosensitizing AML cells by targeting bone marrow endothelial cells. Exp. Hematol. 44, 363–377. ( 10.1016/j.exphem.2016.02.003) [DOI] [PubMed] [Google Scholar]
- 99.Meacham A, Wise E, Scott EW, Cogle CR. 2013. Bone marrow endothelial cells protect acute myeloid leukemia from chemotherapy by direct contact: the BCAM/Laminin/VLA5 axis as a potential therapeutic target. Blood 122, 2546. [Google Scholar]
- 100.Cao Z, et al. 2014. Angiocrine factors deployed by tumor vascular niche induce B-cell lymphoma invasiveness and chemoresistance. Cancer Cell 25, 350–365. ( 10.1016/j.ccr.2014.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ria R, et al. 2009. Gene expression profiling of bone marrow endothelial cells in patients with multiple myeloma. Clin. Cancer Res. 15, 5369–5378. ( 10.1158/1078-0432.CCR-09-0040) [DOI] [PubMed] [Google Scholar]
- 102.Kusumbe AP. 2016. Vascular niches for disseminated tumour cells in bone. J. Bone Oncol. 5, 112–116. ( 10.1016/j.jbo.2016.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.