Abstract
The state-of-the-art in wearable flexible sensors (WFSs) for sweat analyte detection was investigated. Recent advances show the development of integrated, mechanically flexible and multiplexed sensor systems with on-site circuitry for signal processing and wireless data transmission. When compared with single-analyte sensors, such devices provide an opportunity to more accurately analyse analytes that are dependent on other parameters (such as sweat rate and pH) by improving calibration from in situ real-time analysis, while maintaining a lightweight and wearable design. Important health conditions can be monitored and on-demand regulating drugs can be delivered using integrated wearable systems but require correlation verification between sweat and blood measurements using in vivo validation tests before any clinical application can be considered. Improvements are necessary for device sensitivity, accuracy and repeatability to provide more reliable and personalized continuous measurements. With rapid recent development, it can be concluded that non-invasive WFSs for sweat analysis have only skimmed the surface of their health monitoring potential and further significant advancement is sure to be made in the medical field.
Keywords: wearable flexible sensor, healthcare, sweat sensor, biosensor, electrolyte
1. Introduction
Sweating, or perspiration, is a form of thermoregulation where between 500 and 700 ml of hypotonic fluid is secreted by the sweat glands of the average adult human, under most climate conditions, per day [1]. Sweat is easily accessible from the skin surface of the human body, secreted by the eccrine glands and is rich in physiological data, containing electrolytes (such as sodium and potassium ions) and metabolites (such as lactate and glucose) [2,3]. This straightforward access renders sweat a particularly useful biofluid as it can be retrieved using non-invasive methods as opposed to other biofluids, such as blood [4].
Using biomarkers in the composition of sweat, diagnostic information can be used to discover genetic conditions, such as cystic fibrosis in infants by testing for elevated chloride levels [5,6], and to detect diseases related to cystic fibrosis due to the loss of sodium [4]. Furthermore, an understanding has been established about glucose-level correlation between sweat and blood leading to potential use in continuous monitoring for diabetes [7]; lactate can be measured to detect ischaemia [8]; and temperature of the skin surface can be used to provide information for various skin injuries and diseases [9].
Analysis of sweat biomarkers has primarily been achieved by a method of electrochemical sensing using biosensors. A biosensor is an analytical device used to provide real-time data (such as concentration) of one or more chemical constituents (analytes) in a sample [10]. One of the earliest appearances of this concept was in 1962, introduced by Dr Leland C. Clark, whose aim was to analyse levels of glucose in the blood using an ‘enzyme electrode’ [11]. In the present day, basic biosensors still follow the same framework as Dr Clark's early example, using a recognition stage (comprising the analyte and a sensing element) and a transduction stage [10,12].
Although sweat can offer a large amount of physiological information for disease detection, drawbacks in previous studies using biosensors have included poor sweat collection methods; separate collection and analysis stages; and an inability to monitor multiple analytes simultaneously [13–15]. Furthermore, there is a lack of correlation verification between sweat and blood measurements using in vivo validation tests, with ethanol the only analyte confirmed thus far with this procedure [16]. With recent advances in integrated sensor arrays in wearable electronics, these shortcomings are beginning to be addressed [3,17]. This review will investigate the state-of-the-art in wearable electronics for disease detection in sweat with a critical discussion on the application and fabrication of such devices as well as quantitative comparison of sensor performance parameters including sensitivity, limit of detection (LoD), linear range and accuracy compared to conventional metrics.
2. Background
2.1. Sweat as a biofluid
Eccrine sweat sensing has been an underdeveloped area of research for wearable sensing until recent years. With the development of sensors with integrated sweat stimulation for continuous sweat access [18,19], and with multiplexed sensing arrays for in situ calibration of analyte measurements [3,20], sweat sensing is emerging as a technology capable of providing continuous analyte access and monitoring, using a non-invasive platform. With these advancements, sweat sensing has undergone an approximately 10-fold increase in academic publishing over the last 5 years [16].
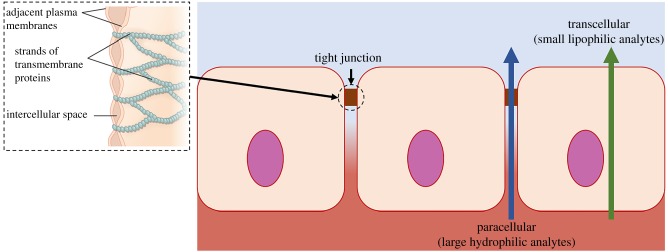
The biomarkers available to be measured in sweat are already well documented; however, the clinical worth of many of these analytes for health condition monitoring is still unproven. Small lipophilic (hydrophobic) analytes, such as steroid hormones (cortisol [13], testosterone [21], etc.) and drugs (methylxanthine [22], levodopa [23], ethanol [16], etc.), exhibit strong correlation between sweat and blood concentrations. While these biomarkers are known to partition transcellularly through the lipophilic cell membranes, larger and/or more hydrophilic analytes are speculated to enter the sweat through a paracellular route, active channels or vesicular/exosomes which will confound attempts at sweat–blood correlation (figure 1) [21].
Figure 1.
Analyte partitioning pathway from interstitial fluid and blood to sweat through lipophilic cell membranes. Adapted from [24]. (Online version in colour.)
Due to the greater number of cellular barriers, the level of filtering in the tight junctions is increased, leading to higher dilution of larger biomarkers. An example of this is sweat glucose, which is transported through a paracellular pathway and is approximately 100 times more diluted than interstitial fluid or blood plasma glucose [7]. This considerably lower concentration provides a big challenge in wearable sweat sensing and underlines the necessity for ultra-sensitive and highly selective devices with carefully designed sweat sampling methods.
Some of the most commonly measured analytes in sweat are electrolytes, such as sodium [15,17,25], potassium [3,26] and chloride [27,28]. Although sodium has been shown to be a useful marker for electrolyte imbalance [15], there is no evidence of any correlation between blood and sweat sodium [13]. Despite this, sweat sodium has recently been shown to be valuable for correlating regional sweat levels with whole-body fluid and electrolyte loss, using individualized wearable monitoring to demonstrate a near 1 : 1 relationship between measured and predicted whole-body fluid losses [29]. Blood and sweat potassium correlation is still yet to be demonstrated as the very small changes in blood potassium result in the corresponding sweat potassium measurements to be dominated by interference sources [21]. Sweat chloride has been shown to have clinical application in point-of-care cystic fibrosis testing [5].
Other analytes that are readily available in eccrine sweat but also lack blood–sweat correlation are lactate [3,14,26] and urea [30]. Sweat lactate correlation with blood concentrations is difficult to establish due to additional local generation at the secretory coil of the sweat gland during perspiration generation [31]. While this currently means that sweat lactate cannot be directly related to whole-body conditions, it can at least be related to sweat gland exertion in response to whole-body conditions. Meanwhile, urea levels in sweat have been linked to kidney failure monitoring with the effects of the condition visible as a white crust on the skin of inflicted patients [13].
While advancement in sweat collection methods and sensing is vital for further development of non-invasive wearables, the clinical validation of sweat analytes is also critical for the overall progression of sweat sensing, particularly for any future commercial applications. Blood–sweat correlation must be established through in vivo trials and biomarker partitioning pathways must be fully understood before the full potential of wearable sweat sensing can be realized.
2.2. Sweat biosensor mechanism
Chemical sensors are devices that use a molecular chemical receptor and a physico-chemical detector element (transducer) to extract useful information such as the concentration of a single entity in a sample. Where the recognition system uses a biological component (e.g. antibody, DNA, enzyme, ionophores), the device can be called a biosensor [32]. The bioreceptor chosen for the chemical recognition system is dependent on the desired analyte and must be able to output the analyte concentration as a physical or chemical signal with an identifiable defined sensitivity. Similarly, the transducer will need to be selected depending on the bioreceptor and required measurement technique. The type of transducer is commonly used as the classification for the biosensor (e.g. optical, impedance-based and piezoelectric sensors). Figure 2 displays a schematic of the different components in a biosensor.
Figure 2.
Schematic of the main components of a biosensor. (a) The desired analyte from a sample interacts with the analyte-specific bioreceptor; (b) bioreceptor outputs a signal with defined sensitivity and (c) transducer transforms bioreceptor output into a readable signal for amplification and data processing. (Online version in colour.)
Several different sweat-based biosensors have been used to investigate physiologically important analytes and other data of interest. Electrochemical sensing has been the primary method used for health monitoring using sweat due to its low cost, high performance and device portability [33]. Examples of these types of sensors are listed in table 1, displaying what analytes can be detected using different techniques.
Table 1.
Types of biosensor and the analytes they can detect in sweat.
| sensor | analyte/data of interest | reference |
|---|---|---|
| optical | sweat rate | |
| pH level OH−, H+, Cu+, Fe2+ |
[34] | |
| impedance-based | sweat rate | [35] |
| sweat conductivity | ||
| ion-selective electrodes (ISEs) | pH level Na+, H+, K+, NH4+, Cl−, Mg2+, Zn2+, Ca2+ |
[3,15,28,36–40] |
| enzymatic amperometric | metabolites (glucose, lactate, ethanol, uric acid) | [3,14,18] |
| stripping-based | heavy metals (Cu, Zn, Pb, Cd, Hg) | [20,41] |
Currently, for disease and health condition detection, the analytes of greatest interest are metabolites and electrolytes [42]. The most common methods of detection are enzymatic amperometric and potentiometric ion-selective electrode (ISE) sensors (table 1).
2.3. Enzymatic amperometric sensors
Electrochemical sweat sensors typically use three or four electrodes manufactured on a flexible substrate. These electrodes are the working electrode, counter electrode, reference electrode and cathode [43]. The reference electrode has a known and stable electropotential and therefore can be used as a half-cell to determine the electropotential of the other half of the cell (the working electrode). The most commonly used reference electrode is made of Ag/AgCl with defined potentials ranging from +0.20 to +0.25 V [43].
For metabolite sensing, biosensors with enzyme recognition elements are used where the enzyme is tethered to the working electrode by a process of enzyme immobilization, including entrapment [44] and covalent cross-linking or bonding [45,46]. This is achieved using an insoluble material, typically containing supports made of natural polymers such as chitosan [3,14,18], synthetic polymers [46] or inorganic materials, such as glass [47]. Enzymes used in previous studies have been glucose oxidase (GOx) for glucose sensing [48], lactate oxidase (LOx) for lactate sensing [14,17] and alcohol oxidase (AOx) for ethanol sensing [18]. The enzyme works in the system by catalysing a reduction–oxidation (redox) reaction to initiate an electron transfer process between the redox centre of the enzyme and the working electrode. The product concentration increase caused by the enzymatic reaction can be read using amperometry if the substances are electroactive [49]. Hydrogen peroxide (H2O2) concentration is typically measured for enzymatic amperometric readings as it is a product of the oxidase reaction. In recent working electrode systems, a redox mediator is included to enable more efficient electron transfer from the difficult-to-access redox centre in the enzyme to the electrode [48]. Ferric materials such as ferrocene and Prussian blue are commonly used as mediators [3,48–50]. A schematic of an example glucose sensor using enzymatic amperometric sensing is shown in figure 3b.
Figure 3.
(a) Schematic of enzymatic amperometric biosensor with mediator and (b) schematic applied to example using glucose oxidase to detect glucose. (Online version in colour.)
2.4. Ion-selective electrode sensors
ISEs are transducers capable of converting specific ion activity (dissolved in a solution) into a readable signal. By the Nernst equation, the logarithm of the target ion activity can be related to the voltage allowing selectivity to be achieved by direct potentiometry. For ISE sensors, the ISE is the working electrode and a reference electrode is required as well in the same way as amperometric sensors. ISEs are available in three different classifications: solid-state membranes (fixed ion exchange: e.g. glass, crystal), liquid membranes (mobile ion exchange) and membranes in special electrodes (e.g. gas-sensing) [51].
To prepare an ISE, a base electrode is drop-casted with an ion-selective membrane (ISM) solution and left to dry [3,15,25]. This solution will contain an ionophore, a lipid-soluble chemical species capable of binding to and carrying a specific ion along the membrane. The ionophore will induce ionic activity generating a specific electrical potential. Examples of ionophores used for ISE sensors are monensin [15,25] (for sodium) and valinomycin [3,26] (for potassium).
2.5. Wearable sensors for sweat
There are two broad classifications for sensors: flexible [52] and non-flexible [53]. For the application of collecting physiological data from sweat, the sensor must be in close contact to the skin for optimal in situ sweat collection and analysis, while also being capable of enduring dynamic use and yet still remaining convenient to wear. Hence, for this purpose, wearable flexible sensors (WFSs) are preferred to match the non-planarity of human skin [54]. Recent advances in WFSs have seen embedded and integrated signal processing circuitry introduced for real-time data analysis and wireless transmission to a computer or smart device [3,55]. Typically, Bluetooth has been used for wireless data transmission due to its lower installation costs, good compatibility and fewer hardware requirements than other network protocols such as Wi-Fi or ZigBee [56]. Figure 4 displays a schematic of WFS integrated signal processing and wireless transmission.
Figure 4.
Schematic of transducer output signal processing and transmission to external monitoring device. Adapted from [55]. (Online version in colour.)
Materials and manufacturing methods used to fabricate such flexible devices are discussed in §3.
3. Fabrication
3.1. Sensor manufacturing methods
When considering the fabrication of WFSs, the materials and manufacturing methods must be capable of producing highly sensitive, selective, flexible, biocompatible sensors and circuitry on the micro- and nano-scale. Existing WFSs for sweat sensing include wearable patches [3,50,57,58], epidermal tattoos [14,15,18,25] and wearable articles of clothing such as sweat bands [3] and eyeglasses [26]. For each of these, the manufacturing process must include the assembly of electrodes on a flexible substrate. Methods for this have been screen printing [15,22,29,41], stamp transferring [59], epidermal elastomeric stamping [33], photolithography [3] and ink or aerosol jet printing [60].
While photolithography can provide excellent resolution on the nanoscale (approx. 50 nm) by using electron beam patterning technology, the ease of manufacture is limited by the high cost of equipment and required clean room environment. Screen printing is the most suitable for mass production of electrodes on a variety of flexible substrates due to its low cost, with roll-to-roll screen printing offering a very high throughput of 60 devices per minute [29]. However, screen printing does not yield as high spatial resolution as photolithography and is also hindered by substrate properties and a limited range of printable ink materials [43]. Novel methods such as stamp transferring and ink jet printing (combined with electroplating) have shown to have good resolution (approx. 2 µm) while providing manufacturing capability on non-planar substrates with complex surface morphologies, hence rendering these methods ideal for epidermal sensor fabrication [59,61].
Once the electrode array has been manufactured, the respective recognition element for the analyte of interest must be added. This involves forming a layer or membrane on the working electrode by coating the surface with an analyte-targeting solution. Typically, a solution is prepared, containing the biorecognition element, and drop cast onto the electrode surface [3,62]. The thicknesses of layers can vary due to the poor uniformity of the drop casting technique; however, protection of the recognition element has been achieved using monolayer thickness [63]. Modifications can also be made to electrodes before introducing the recognition element such as adding layers of material to increase selectivity or conductivity as well as attaching porous materials to expand the reaction site surface area [43]. Fabricating highly porous nanofibres using electrospinning and combining them with a bioreceptor is an example of this method for enhancing sensor sensitivity, sensing range and bioreceptor immobilization [64]. Likewise, conductive metal nanoparticles can be used to enhance the performance of non-conductive but highly selective materials [65,66].
3.2. Materials
While the fabrication method is a significant factor in the performance of a biosensor, the chosen material's properties (such as morphology, conductivity, porosity, surface area, selectivity and mechanical properties and surface wetting behaviour) are imperative for optimal sensitivity and range of detection. Furthermore, for WFSs, the materials used in fabrication must be flexible and robust for dynamic use on human skin.
Materials for the flexible substrate used for the foundation of WFSs have included many different polymers such as polydimethylsiloxane (PDMS) [58,67], polyethylene naphthalate (PEN) [68], polyethylene terephthalate (PET) [3,14,15,26], polyimide (PI) [25], poly(methyl methacrylate) (PMMA) [17], poly(vinylidenefluoride-co-trifluoroethylene) (PVDF-TrFE)) [69], Parylene [70] and polypyrrole [71]. The use of paper for substrates has also been successfully demonstrated for wearable disposable sensors, showing an improvement in sensitivity over sensors fabricated on non-porous substrates such as PI [60]. However, PI has still been preferred over PDMS due to the latter's tendency to undergo mild swelling due to ISM solution absorption [25].
For the working electrode fabrication, typical base materials are silver, gold, platinum and carbon (including graphene, graphite ink, CNTs and glassy carbon) [55]. By modifying electrodes with different materials, for example, attaching nanofibres or nanoparticles, enhanced sensor performance can be achieved by increasing sensitivity, robustness and selectivity. A thin redox mediator layer of Prussian blue deposited on a base electrode has been shown to provide greater sensitivity in glucose biosensors, helping to measure the low glucose levels in sweat [3].
An increase in enzyme surface concentrations, enzyme immobilization, surface area and porosity (and hence adsorption sites) have been achieved by use of metal nanoparticles such as those made of silver [65,72], gold [73] and nickel [74]. Similarly, electrospun metal oxide nanofibres, such as zinc oxide, have been used showing a fast sensor response time (4 s using zinc oxide for glucose measurement) and LoD (1 µM for glucose detection) [75]. Tang et al. [76] also quantitatively displayed how titanium oxide nanofibre structuring can be used to yield an electrode response 4.6 times higher than without titanium oxide nanofibres for 100 µM of glucose. Bujes-Garrido et al. [77] displayed an emerging use of electrochemically deposited silver nanoparticles on a low-cost, screen-printed carbon electrode for chloride and other halide ions in sweat samples. This approach yielded excellent reproducibility (with a relative standard deviation of 1.61%) and was shown to have high selectivity towards chloride ions comparable to traditional electrochemical detection techniques using ion selective electrodes, with an LoD of 3.0 µM. Such results have greatly influenced the widespread use of nanoparticles and nanofibres in electrode fabrication.
Additionally, colorimetric sensing has been used for both quantitative and qualitative analysis for wearable optical sensors, capable of inducing a colour change, visible to the naked eye, in the material that can be correlated to the presence of a certain analyte [78]. This has been achieved by functionalizing a polymer matrix by doping, such as by dye-doping [79] or nanoparticle doping [80]. This can be used for basic warning threshold indicators using only the human eye to inspect a change in colour or can make use of spectrophotometry for quantitative measurements of analyte concentrations. Colorimetric sensing has been used for measuring analytes present in sweat using nanofibrous sensors based on doping techniques such as for glucose [81], ammonia [82] and pH level [83]. Koh et al. [84] used chromogenic bioreceptor reagents to produce a colorimetric adhesive patch with a soft microfluidic device, based on PDMS, for sweat collection and analysis of lactate, chloride, glucose and pH. This was used in tandem with image processing software on a smartphone for concentration correlation of analytes with levels of red, green and blue output by the colorimetric sensor (figure 5). A resolution of 0.5 units for the pH sensor and concentration resolutions of 0.2, 0.3 and 0.1 mM for chloride, lactate and glucose were found, respectively.
Figure 5.
(a) Epidermal microfluidic colorimetric sweat sensor with and without artificial sweat applied and (b) process using smartphone software for image capture and analysis of colorimetric sensor [84]. (Online version in colour.)
A major advantage of colorimetric sensing is the ability to detect without the need for electrical contacts, enabling greater flexibility in design and easier personal and portable use [78]. However, issues must still be taken into consideration such as lack of continuous monitoring (single-use patches) and reduced measurement accuracy compared to electrochemical sensing [84]. Such devices may be more suited to threshold measurements and warning indicators as opposed to precise concentration measurements due to relatively poor correlation between laboratory and sensor results.
Further to using metallic materials for electrode modification, non-metallic conductive substances, such as carbon and polymers, have also been shown to be effective for electrode fabrication. Gao et al. and Nyein et al. have shown the use of poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT : PSS), a polymer consisting of a mixture of two ionomers, as an effective material for the detection of electrolytes such as calcium, potassium and sodium due to the high conductivity (up to 5400 S cm−1) [85], flexibility and ductility [3,40] of its thin film. Yang et al. [86] also used PEDOT : PSS to form a nanofiber structure for glucose sensing, enabling increased immobilization of GOx enzyme and decreased impedance of the Pt working electrode compared to flat electrode sensing. Carbon and carbon variants, such as graphite [26], graphene [50] and reduced graphene oxide [87], are widely used materials in sweat biosensor fabrication due to their excellent electronic and mechanical properties as well as high chemical resistance. Carbon has been used for both base electrodes and as an electrode modification device, using both carbon nanofibres (CNFs) [88] and carbon nanotubes (CNTs) [25,89]. While CNTs and CNFs have been used extensively in glucose biosensing [90], Roy et al. [25] showed that CNTs used as the basis of a working electrode for sodium detection can provide stronger attachment to ISM solution and increased selectivity over other working electrode materials such as platinum or gold.
Non-conductive materials with desirable properties such as high selectivity, mechanical strength and flexibility, biocompatibility and large surface areas can still be used in electrode fabrication by doping with conductive materials. Anderson et al. [65] presented this as a useful method in non-enzymatic amperometric glucose sensing, where an intrinsically non-conductive material, molybdenum disulphide (MoS2), was nucleated with silver nanoparticles and used to modify a glassy carbon electrode. The LoD was found to be 0.03 µM with a sensitivity of 9044.6 µA mM−1 cm2 and a linear range of 0.1–1.0 mM of glucose concentration, with high selectivity towards glucose detection. These results were superior to previously recorded results from both enzymatic and non-enzymatic glucose biosensors.
Similarly, Zaryanov et al. recently used a non-enzymatic poly(3-APBA)-based lactate sensor, imprinted with lactate, to replace typical LOx enzyme-based sensors. Results were successful with a comparable linear range (3–100 mM) and LoD (1.5 mM) to enzymatic lactate sensors but with the ability for measurement accuracy to be unchanged after six months. This is unachievable for enzymatic devices due to poor LOx stability [91].
4. Applications
4.1. Detection of diseases and conditions
The most common application for wearable biosensors in current science is for point-of-care medical or physiological monitoring, helping to detect diseases and health conditions in a more convenient manner and with a less invasive approach than conventional monitoring methods. WFSs have been used extensively for studies in recent years for detecting analyte concentrations relevant to diseases and health conditions. However, these non-invasive methods have typically lacked simultaneous multi-analyte detection and on-site circuitry for in-situ analysis and calibration.
Gao et al. [3] have revolutionized the sweat biosensing field by introducing a wearable integrated sensor array where multiple analytes can be simultaneously measured, using a flexible integrated sensing array (FISA), and analysed and transmitted using a flexible printed circuit board (FPCB). The integrated FISA and FPCB fabricated on a flexible PET substrate are displayed on a subject's wrist in figure 6a, with the FISA approximately the same size as a US quarter dollar coin (25 mm2 area) [3]. The analytes measured in this study were metabolites (glucose and lactate) and electrolytes (sodium and potassium). It is described that, by using this device, physiological monitoring during exercise will give real-time data on the subject's condition, outputted visually on a smart device. These data can be used as a marker for conditions such as hypoglycaemia due to diabetes mellitus from glucose, pressure ischaemia from lactate, hypokalaemia from potassium and hyponatraemia from sodium.
Figure 6.
(a) FISA and FPCB worn on the wrist; (b) sensor array worn on the forehead, arm and wrist during cycling exercise and (c) on-body analysis yielded very similar results to ex situ analysis for sodium and glucose [3]. (Online version in colour.)
Measurements were taken using the device while it was worn on the forehead, arm and wrist during cycling exercise (figure 6b) and the on-body data retrieved were compared to ex situ data analysed later, yielding very similar trends (figure 6c). However, glucose concentration was found to decrease with continued exercise due to dilution of glucose in sweat over time and different glucose concentrations were measured from the devices at different locations in the body. It was proposed that increasing temperature by exercising impacted the glucose oxidase (GOx) enzyme activity. Hence, careful monitoring of sweat rate and composition as well as environmental variables are required to ensure accurate monitoring for sweat/blood glucose level relationship. Furthermore, although the sweat rate and relevant biomarkers were correlated, there was no correlation established between sweat glucose and blood glucose concentrations.
In the case of Gao et al., sweat was collected by increasing the sweat rate during exercise. This can be risky due to potential-induced hypoglycaemia in diabetic patients. An alternative method of collection is by on-demand sweat simulation using iontophoresis (figure 7). Emaminejad et al. [42] used iontophoresis by delivering sweat-inducing agonists (e.g. acetylcholine and pilocarpine) to the sweat glands using electrical current. The aim of this study was to non-invasively monitor sodium and chloride levels for cystic fibrosis diagnosis but was also extended to glucose monitoring.
Figure 7.
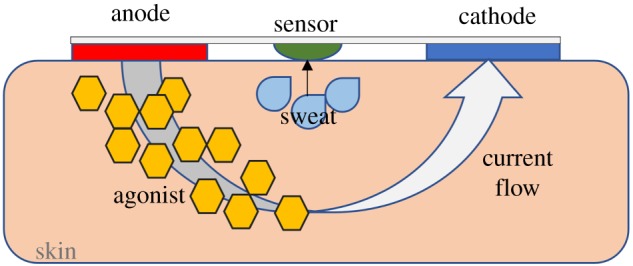
Schematic of iontophoresis with agonist delivered to skin aided by electrical current. Adapted with permission from [18]. Copyright © 2016 American Chemical Society. (Online version in colour.)
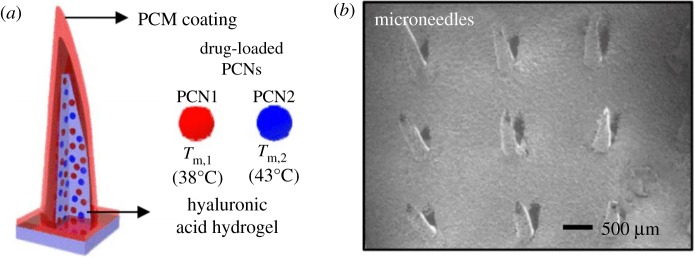
Lee et al. [50,58] investigated the use of a graphene-based glucose sensor integrated with a transdermal drug delivery module. This device was fabricated as both a wearable patch and a disposable strip to provide flexibility for different situations. Metformin delivery was achieved by loading the drug into phase change nanoparticles (PCNs) and containing them inside hyaluronic acid hydrogel microneedles (figure 8). The glucose biosensing coupled with subsequent drug delivery was successfully tested on diabetic mice with results yielding decreased blood glucose concentration to normoglycemic levels (less than 11 mM) after application of metformin. The study concluded that improvements had to be made for long-term stability of the system and that suppression of blood glucose levels could be regulated faster by use of chlorpropamide instead of metformin.
Figure 8.
(a) Schematic of hyaluronic acid hydrogel microneedles, with phase change material (PCM) coatings, containing metformin-loaded PCNs and (b) SEM image of the microneedles [58]. (Online version in colour.)
A recent advancement in sweat biosensors has seen calcium [40] and ethanol [18] concentrations measured using WFSs. Nyein et al. [40] used the multiplexed sensor array framework innovated by Gao et al. to establish a WFS capable of non-invasively measuring both sweat pH and ionized calcium (Ca2+) simultaneously. The multiplexed system was essential for calcium measurements due to its dependence on pH level. Five human subjects were monitored during constant-load cycling with the device attached to their foreheads. The sensitivity of the Ca2+ sensor was found to be 32.7 mV/decade and hence close to the ideal sensitivity of 29.6 mV/decade calculated from the Nernst equation [40]. The sensor array was continuously tested for an hour finding that average sensitivity differentiated by only an 8% error, showing high stability. Furthermore, sensor reading accuracy was confirmed by adding different amounts of Ca2+ and H+ into sweat samples and taking measurements with both the sensor array and by using inductively coupled plasma-mass spectrometry (ICP-MS). Results were similar with only around a 4% average error in concentration measurements. With these findings, this multiplexed sensor array for Ca2+ is a particularly useful advancement in wearable biosensing for disease detection due to a large number of possible detectable conditions such as myeloma, cirrhosis, renal failure, kidney stones, acid–base balance disorder and primary hyperparathyroidism. However, in vivo blood–sweat correlation validation trials are still required to verify any possible clinical application.
Kim et al. [18] integrated an enzymatic amperometric sweat ethanol sensing patch with a pilocarpine iontophoresis drug delivery system and wireless Bluetooth transmission to a smart device (figure 9). The applied electrical current (0.6 mA) for iontophoresis was optimized for efficient drug delivery and subject comfort (high currents tended to cause skin irritation). Test subjects had ethanol concentrations measured before and after alcohol consumption with results yielding expected raised ethanol levels.
Figure 9.
Schematic for the measurement and display of ethanol levels in sweat. Adapted with permission from [18]. Copyright © 2016 American Chemical Society. (Online version in colour.)
The device was found to be more reliable than commonly used breathalysers due to avoiding potential inaccuracies caused by temperature, humidity, environmental factors (such as alcoholic fumes) or consumer products (such as mouthwash). It was also established to be a faster method of blood alcohol concentration measurement than other transdermal devices (SCRAM and Giner WrisTAS), which could take between 0.5 and 2.0 h for a useful output compared to 10 min for this device. Hence, it was suggested that the ethanol sensor could be used for detecting illegal levels of alcohol consumption in automobile drivers.
The study concluded that although a stable iontophoresis-biosensing ethanol monitoring system had been established, further development needed to be made in personalizing the device to meet individual factors such as skin permeability and sweat composition.
Hauke et al. [16] further confirmed the efficacy of wearable enzymatic sweat ethanol sensing by correlating in vitro data, carefully characterized to account for interference sources, with in vivo blood ethanol measurements. The study displayed a highly reproducible and novel device design with a hex wick/sensor interface comprising hydrophilic porous fumed silica which ensured complete wetting of the sensor surface with sweat (figure 10). Although the device had a relatively low ethanol concentration linear range (0.014–3.67 mM), sensor performance was not the primary focus and was deemed good enough for a proof-of-concept study, which showed a linear Pearson correlation of 0.9474–0.9996 between blood and sweat ethanol measurements. With these promising results, there is a potential for this device to provide correlation validation for other small lipophilic analytes in sweat, which exhibit strong correlation between blood and sweat concentrations [13].
Figure 10.
Integrated wearable sweat ethanol sensor with (a) system assembly and (b,c) photos of assembled devices [16]. (Online version in colour.)
In addition to ethanol, there have also been two more small lipophilic drug sensing studies recently established, measuring caffeine [22] and levodopa [23] concentrations in sweat. A wearable sweat band with a CNT/Nafion working electrode was used to detect caffeine by oxidation. A strong correlation was found between caffeine intake and measured sweat caffeine levels with a 0.98 Pearson's correlation coefficient and sensitivity of 45 µM g−1. This study demonstrated an alternative method to urine testing for illicit substance use in athletics competitions [22]. For levodopa sensing, a wearable sweat band sensor, similar to the caffeine sensor design, was employed but with gold nanodendrite modified Au/Cr electrodes with immobilized tyrosinase for enzymatic sensing. From this study, it is believed that sweat sensing can be used to optimize the levodopa dosage required for patients with Parkinson's disease, as opposed to the traditional and limited method of evaluating the subject's motor function [23].
Sempionatto et al. [26], meanwhile, used typical sweat metabolite and electrolyte biosensors for a new style of WFS by incorporating the sensors into the nose pads of eyeglasses with a wireless circuit board attached to the glasses arms. This multiplexed device with wireless transmission provides an interesting alternative to the similar system proposed originally by Gao et al. [3] as it allows spatially separated sensor sites reducing potential interference between sensors both electrically and chemically. The sensors trialled in this study were glucose, lactate and potassium and proved proficient for continuous, simultaneous monitoring. However, necessary control experiments were not conducted to verify the results.
The main WFSs discussed in §§3 and 4 are summarized in table 2 in §4.3 for comparative reference. This table gives a comparison between type of sensor, materials used, quantitative sensor details and relevant diseases to each of the analytes examined.
Table 2.
Summary of wearable sweat sensor findings. AOx, alcohol oxidase; GOx, glucose oxidase; ISE, ion-selective electrode; Lox, lactate oxidase.
| analyte | sensor | sensor array type (substrate material) | recognition element | electrode material(s) | linear range | sensitivity | relevant diseases/conditions | ref. |
|---|---|---|---|---|---|---|---|---|
| calcium | potentiometric ISE | wearable multiplexed sensing system (PET) | ETH 129 | Au/PEDOT-PSS | 125–2000 µM | 32.7 mV/decade | —myeloma —cirrhosis —acid–base balance disorder —normocalcaemic hyperparathyroidism —kidney stones |
[40] |
| chloride | potentiometric ISE | wearable patch (PET) |
AgCl | AgCl | 1000–30 00000 µM | 56 mV/decade | —cystic fibrosis —hyperchloraemia —acid–base disorders |
[28] |
| wearable patch (PET) | Ag/AgCl | Ag/AgCl | 10 000–15 0000 µM | 52.8 mV/decade |
[57] | |||
| ethanol | enzymatic amperometric | temporary tattoo | AOx | Prussian blue | up to 36 000 µM | 0.362 nA mM−1 | —alcohol consumption | [18] |
| wearable patch (PET) | AOx | Pt |
14–3670 µM | 7.25 µA mM−1 | [16] | |||
| glucose | enzymatic amperometric | flexible array patch (PET) | GOx | Prussian blue/Au | up to 200 µM | 2.35 µA mM−1 | —diabetes mellitus | [3]` |
| multi-analyte glasses (PET) | GOx | Au | up to 2000 µM | [26] | ||||
| graphene-based stretchable patch (PDMS) | GOx | Prussian blue/graphene-Au | 10–700 µM | 1 µA mM−1 | [50] | |||
| non-enzymatic voltammetry |
flexible sensor | Ag/MoS2 | glassy carbon | 0.1–1000 µM | 9044.6 µA mM cm−2 | [65] | ||
| lactate | enzymatic amperometric | flexible array patch (PET) | LOx | Prussian blue/Au | up to 30 000 µM | 220 nA mM−1 | —pressure ischaemia —peripheral arterial occlusive disease —panic disorder —Frey's syndrome —hypoxia |
[3] |
| temporary tattoo (PET) | LOx | carbon ink | up to 20 000 µM | 644.2 nA mM−1 | [14] | |||
| multi-analyte glasses (PET) | LOx | Prussian blue/graphite ink | up to 8000 µM | [26] | ||||
| non-enzymatic impedimetric |
wearable patch | lactate-imprinted 3-APBA |
glassy carbon |
3000–10 0000 µM | 23 M−1 | [91] | ||
| levodopa | enzymatic amperometric | flexible sweat band (PET) | tyrosinase | Au nanodendrites on Au/Cr |
1–1000 µM | 1.7 nA µM−1 | —Parkinson's disease | [23] |
| potassium | potentiometric ISE | flexible array patch (PET) |
valinomycin | PEDOT : PSS | 1000–32 000 M | 61.3 mV/decade | —hypertension —hypokalaemia |
[3] |
| multi-analyte glasses (PET) | valinomycin | carbon ink | 100–10 0000 µM | 60.6 mV/log [K+] [M] |
[26] | |||
| sodium | potentiometric ISE | flexible array patch (PET) |
Na ionophore | PEDOT:PSS | 10 000–16 0000 µM | 64.2 mV/decade | —cystic fibrosis —hyponatraemia |
[3] |
| temporary tattoo (PET) | Na ionophore | carbon ink | 100–10 0000 µM | 63.75 mV/log10[Na+] | [15] | |||
| wearable multisensing patch (PMMA) | Na ionophore | Pt/PEDOT | up to 42 000 µM | 56 mV/unit | [17] | |||
| wearable tattoo (PI) | Na ionophore | carbon nanotube | 0.7–1000 µM | 56 mV/decade | [25] |
4.2. Limitations and future scope
In an ever-advancing field, WFSs are evolving with growing microelectromechanical (MEMS) and nanoelectromechanical system (NEMS) technologies leading to lower cost of fabrication in the future [55]. Furthermore, advances must be made in material science for sensor manufacture to continue to improve sensitivity, selectivity, detection range and LoD by incorporating different combinations of nanofibres, nanoparticles, highly selective materials and highly conductive materials. This is essential to establish as near to an ideal Nernstian response as possible from sensors and to achieve a low LoD for low sweat concentration analytes, such as glucose compared to blood glucose. However, careful consideration must still be made to ensure highly sensitive sensors do not generate false positives.
There have been many studies undertaken for comparing sweat sensor results to reference results such as laboratory techniques or conventional measurements with good accuracy and correlation results for electrochemical sensors (as shown in table 3). Choi et al. [27] displayed an excellent correlation between sweat measurement results for laboratory analysis and a potentiometric ISE, using this for investigating chloride concentrations for subjects with and without cystic fibrosis. Further research should be conducted for comparing sweat sensor results to laboratory analysis (or other reference methods), for investigating concentrations for health and physiological conditions specifically, to verify use of these devices in medical monitoring.
Table 3.
Summary of sweat sensor results compared to laboratory analysis/reference methods. ρ, Spearman's rank correlation coefficient; r, Pearson's correlation coefficient; R,2 coefficient of determination.
| sweat sensor analyte | type of sensor | correlation/percentage difference to ex situ laboratory or reference tests | ref. |
|---|---|---|---|
| Ca2+ | potentiometric ISE | 7.0% | [40] |
| Cl− | colorimetric | 0.13363 (ρ) | [84] |
| Cl− | potentiometric ISE | 0.97 (r) | [27] |
| ethanol | enzymatic amperometric | 0.993 (R2) | [18] |
| glucose | enzymatic amperometric | 0.89 (assay R2), 0.83 (meter R2) | [50] |
| glucose | enzymatic amperometric | 3.3–7.6% | [87] |
| lactate | colorimetric | 0.14286 (ρ) | [84] |
| lactate | enzymatic amperometric | 7.0% | [17] |
| lactate | non-enzymatic impedimetric | 0.90 (r) | [91] |
| Na | potentiometric ISE | 6.0% | [17] |
| pH | potentiometric ISE | 9.0% | [17] |
| pH | colorimetric | −0.29554 (ρ) | [84] |
Despite the number of studies conducted on the correlation of WFS results with ex situ laboratory sweat results, for complete validation of results, the concentrations of analytes in sweat and blood must also be correlated. Unfortunately, there has been a lack of literature regarding the validation of results using a wearable sweat sensing device and in vivo blood measurements. Hauke et al. [16] established a strong correlation (0.9474–0.9996 Pearson correlation coefficient) between sweat and blood ethanol using a wearable enzymatic biosensor and in vivo validation trials during a greater than 3 h testing period. Validation trials like this must be conducted for other analytes to verify any useful application in wearable health monitoring, particularly in electrolytic sensing where the only major verified application is in single-use, point-of-care cystic fibrosis testing measuring sweat chloride [21].
Although the correlation between glucose concentration in sweat and in blood has been well established (approx. 100 times diluted in sweat), proper collection of sweat to achieve this correlation has proven challenging and the glucose pathway from blood to sweat is still yet to be corroborated [3,26,48,50,58]. Iontophoretic sweat glucose levels were found to not strongly correlate with corresponding real-time blood glucose measurements, displaying a critical limitation in sweat glucose-based threshold measurements [29]. However, it is believed that by uploading large pools of sweat glucose data from wirelessly transmitting WFS devices to a central online ‘cloud’ database and with further investigation into more complex, individual-specific correlations between sweat and blood glucose, a greater understanding of diabetes biomarkers can be realized [3,29].
Further analysis is required for using multiple analytes (such as pH, skin temperature and humidity) for greater accuracy and continuous calibration of devices. Autonomous systems must be advanced to enable efficient sweat collection and calibration-free sensing for continuous monitoring with as little human interaction as possible. Further research should focus on incorporating efficient iontophoresis systems into every sweat sensor to nullify the requirement of exercise to accurately monitor analyte concentrations. As temperature has been shown to affect enzyme activity [3] and enzymes are known to be unstable with a poor shelf-life [91], non-enzymatic sensors should be developed further to reduce cost and increase available continuous monitoring time.
5. Conclusion
This review investigated the state-of-the-art in WFSs for detecting analytes in human sweat relevant to disease and health condition monitoring. In recent years, extensive research has been conducted on providing non-invasive, in situ, real-time analysis of sweat analyte concentrations. Advancements in fabrication techniques and material science have allowed multiple sensors to be integrated into a single mechanically flexible multiplexed system with on-site circuitry for signal processing and wireless data transmission. Such devices provide an opportunity to better calibrate analytes that are dependent on other parameters (such as the sweat rate dependence of glucose).
Combining tailored materials has proven beneficial for enhancing sensor capabilities with high sensitivity, low LoD and large linear range all shown to improve with the increased surface area and porosity provided by, for example, nanofibre- and nanoparticle-modified electrodes. Doping non-conductive but highly selective materials with conductive nanoparticles has proven useful for fabricating non-enzymatic electrochemical sensors with superior LoD, sensitivity and stability than enzymatic sensors due to higher specificity.
Metabolites and electrolytes available to be non-invasively measured in sweat may provide essential information required for important health condition monitoring but require correlation verification between sweat and blood measurements using in vivo validation tests for useful application in the medical field. Integrated wearable systems are now able to induce sweating by iontophoresis, removing the need to exercise for sweat collection, and are also capable of delivering blood glucose-regulating drugs on-demand in response to hypoglycaemia.
Further development in the field is still required, however, such as a need to establish a clear correlation between glucose concentration in blood and sweat by verifying the partitioning pathway. Sensitivity still needs to be improved to achieve as near an ideal Nernstian response as possible. Furthermore, integrated multiplexed systems need to introduce additional calibration-free, disease-related sensors to establish widespread use of devices. With the current limitations, wearable sweat devices have not yet been introduced for clinical application. Nevertheless, from recent rapid development, it can be concluded that non-invasive WFSs for sweat analysis have only skimmed the surface of their health monitoring potential and further significant advancement is sure to be made in the medical field.
Data accessibility
This article has no additional data.
Authors' contributions
M.C. wrote the first draft of the manuscript and addressed comments for subsequent drafts. G.F. and N.R. provided feedback and supervision.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the EPSRC CDT in Intelligent Sensing and Measurement, grant no. EP /L016753/1.
References
- 1.McArdle WD, Katch FI, Katch VL. 2006. Essentials of exercise physiology, 3rd edn Baltimore, MD: Lippincott Williams & Wilkins. [Google Scholar]
- 2.Montain SJ, Cheuvront SN, Lusaski HC. 2007. Sweat mineral-element responses during 7 h of exercise-heat stress. Int. J. Sport Nutr. Exerc. Metab. 17, 574–582. ( 10.1123/ijsnem.17.6.574) [DOI] [PubMed] [Google Scholar]
- 3.Gao W, et al. 2016. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514. ( 10.1038/nature16521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mena-Bravo A, Luque de Castro MD. 2014. Sweat: a sample with limited present applications and promising future in metabolomics. J. Pharm. Biomed. Anal. 90, 139–147. ( 10.1016/j.jpba.2013.10.048) [DOI] [PubMed] [Google Scholar]
- 5.Rock MJ, Makholm L, Eickhoff J. 2014. A new method of sweat testing: the CF Quantum® sweat test. J. Cyst. Fibros. 13, 520–527. ( 10.1016/j.jcf.2014.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desax M, Ammann RA. 2008. Nanoduct® sweat testing for rapid diagnosis in newborns, infants and children with cystic fibrosis. Eur. J. Pediatr. 167, 299–304. ( 10.1007/s00431-007-0485-0) [DOI] [PubMed] [Google Scholar]
- 7.Moyer J, Wilson D, Finkelshtein I, Wong B, Potts R. 2012. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 14, 398–402. ( 10.1089/dia.2011.0262) [DOI] [PubMed] [Google Scholar]
- 8.Derbyshire PJ, Barr H, Davis F, Higson SPJ. 2012. Lactate in human sweat: a critical review of research to the present day. J. Physiol. Sci. 62, 429–440. ( 10.1007/s12576-012-0213-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb RC, et al. 2013. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 12, 938–944. ( 10.1038/nmat3755.Ultrathin) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banica FG. 2012. Chemical sensors and biosensors: fundamentals and applications, 1st edn New York, NY: Wiley. [Google Scholar]
- 11.Clark LC, Lyons C. 1962. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 102, 29–45. ( 10.1111/j.1749-6632.1962.tb13623.x) [DOI] [PubMed] [Google Scholar]
- 12.Rasooly A, Herold KE. 2009. Preface. In Biosensors and biodetection. Methods and protocols: electrochemical and mechanical detectors, lateral flow ligands for biosensors, vol. 504, pp. 6–7. Totowa, NJ: Humana Press. [PubMed] [Google Scholar]
- 13.Sonner Z, et al. 2015. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 9, 031301 ( 10.1063/1.4921039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia W, Bandodkar AJ, Valdés-Ramírez G, Windmiller JR, Yang Z, Ramírez J, Chan G, Wang J. 2013. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 85, 6553–6560. ( 10.1021/ac401573r) [DOI] [PubMed] [Google Scholar]
- 15.Bandodkar AJ, Molinnus D, Mirza O, Guinovart T, Windmiller JR, Valdés-Ramírez G, Andrade FJ, Schöning MJ, Wang J. 2014. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 54, 603–609. ( 10.1016/j.bios.2013.11.039) [DOI] [PubMed] [Google Scholar]
- 16.Hauke A, et al. 2018. Complete validation of a continuous and blood-correlated sweat biosensing device with integrated sweat stimulation. Lab. Chip 18, 3750–3759. ( 10.1039/c8lc01082j) [DOI] [PubMed] [Google Scholar]
- 17.Anastasova S, Crewther B, Bembnowicz P, Curto V, Ip HM, Rosa B, Zhong-Yang G. 2017. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 93, 139–145. ( 10.1016/j.bios.2016.09.038) [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Jeerapan I, Imani S, Cho TN, Bandodkar A, Cinti S, Mercier PP, Wang J. 2016. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sensors 1, 1011–1019. ( 10.1021/acssensors.6b00356) [DOI] [Google Scholar]
- 19.Heikenfeld J. 2016. Non-invasive analyte access and sensing through eccrine sweat: challenges and outlook circa 2016. Electroanalysis 28, 1242–1249. ( 10.1002/elan.201600018) [DOI] [Google Scholar]
- 20.Gao W, et al. 2016. Wearable microsensor array for multiplexed heavy metal monitoring of body fluids. ACS Sensors 1, 866–874. ( 10.1021/acssensors.6b00287) [DOI] [Google Scholar]
- 21.Heikenfeld J, Jajack A, Feldman B, Granger SW, Gaitonde S, Begtrup G, Katchman BA. 2019. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419. ( 10.1038/s41587-019-0040-3) [DOI] [PubMed] [Google Scholar]
- 22.Tai L, et al. 2018. Methylxanthine drug monitoring with wearable sweat sensors. Adv. Mater. 30, 1–8. ( 10.1002/adma.201707442) [DOI] [PubMed] [Google Scholar]
- 23.Tai L, et al. 2019. Wearable sweat band for noninvasive levodopa monitoring. Nano Lett. 19, 6346–6351. ( 10.1021/acs.nanolett.9b02478) [DOI] [PubMed] [Google Scholar]
- 24.OpenStax. 2016. Epithelial tissue. See https://cnx.org/contents/oWqVExrJ@3/Epithelial-Tissue#fig-ch04_02_01.
- 25.Roy S, David-Pur M, Hanein Y. 2017. Carbon nanotube-based ion selective sensors for wearable applications. ACS Appl. Mater. Interfaces 9, 35 169–35 177. ( 10.1021/acsami.7b07346) [DOI] [PubMed] [Google Scholar]
- 26.Sempionatto JR, Nakagawa T, Pavinatto A, Mensah ST, Imani S, Mercier P, Wang J. 2017. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab. Chip 17, 1834–1842. ( 10.1039/c7lc00192d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi DH, Thaxton A, Jeong I, Kim K, Sosnay PR, Cutting GR, Searson PC. 2018. Sweat test for cystic fibrosis: wearable sweat sensor vs. standard laboratory test. J. Cyst. Fibros. 17, e35–e38. ( 10.1016/j.jcf.2018.03.005) [DOI] [PubMed] [Google Scholar]
- 28.Dam VAT, Zevenbergen MAG, Van Schaijk R.. 2015. Flexible chloride sensor for sweat analysis. Procedia Eng. 120, 237–240. ( 10.1016/j.proeng.2015.08.588) [DOI] [Google Scholar]
- 29.Nyein HYY, et al. 2019. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 5, eaaw9906 ( 10.1126/sciadv.aaw9906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Gao W. 2019. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 48, 1465–1491. ( 10.1039/C7CS00730B) [DOI] [PubMed] [Google Scholar]
- 31.Ament W, Huizenga JR, Mook GA, Gips CH, Verkerke CJ. 1997. Lactate and ammonia concentration in blood and sweat during incremental cycle ergometer exercise. Int. J. Sports Med. 18, 35–39. [DOI] [PubMed] [Google Scholar]
- 32.Thévenot DR, Toth K, Durst RA, Wilson GS. 2001. Electrochemical biosensors: recommended definitions and classification. Biosens. Bioelectron. 16, 121–131. ( 10.1016/S0956-5663(01)00115-4) [DOI] [PubMed] [Google Scholar]
- 33.Bandodkar AJ, Wang J. 2014. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 32, 363–371. ( 10.1016/j.tibtech.2014.04.005) [DOI] [PubMed] [Google Scholar]
- 34.Huang X, et al. 2014. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small 10, 3083–3090. ( 10.1002/smll.201400483) [DOI] [PubMed] [Google Scholar]
- 35.Coyle S, et al. 2010. BIOTEX: biosensing textiles for personalised healthcare management. IEEE Trans. Inf. Technol. Biomed. 14, 364–370. ( 10.1109/TITB.2009.2038484) [DOI] [PubMed] [Google Scholar]
- 36.Bandodkar AJ, et al. 2013. Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring. Analyst 138, 123–128. ( 10.1039/c2an36422k) [DOI] [PubMed] [Google Scholar]
- 37.Rose DP, Ratterman ME, Griffin DK, Hou L, Kelley-Loughnane N, Naik RR, Hagen JA, Papautsky I, Heikenfeld JC. 2015. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 62, 1457–1465. ( 10.1109/TBME.2014.2369991) [DOI] [PubMed] [Google Scholar]
- 38.Glennon T, et al. 2016. ‘SWEATCH’: a wearable platform for harvesting and analysing sweat sodium content. Electroanalysis 28, 1283–1289. ( 10.1002/elan.201600106) [DOI] [Google Scholar]
- 39.Guinovart T, Bandodkar AJ, Windmiller JR, Andrade FJ, Wang J. 2013. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 138, 7031–7038. ( 10.1039/c3an01672b) [DOI] [PubMed] [Google Scholar]
- 40.Nyein HYY, et al. 2016. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano 10, 7216–7224. ( 10.1021/acsnano.6b04005) [DOI] [PubMed] [Google Scholar]
- 41.Kim J, De Araujo WR, Samek IA, Bandodkar AJ, Jia W, Brunetti B, Paixão TRLC, Wang J. 2015. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 51, 41–45. ( 10.1016/j.elecom.2014.11.024) [DOI] [Google Scholar]
- 42.Emaminejad S, et al. 2017. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114, 4625–4630. ( 10.1073/pnas.1701740114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin H, Abu-Raya YS, Haick H. 2017. Advanced materials for health monitoring with skin-based wearable devices. Adv. Healthc. Mater. 6, 1700024 ( 10.1002/adhm.201700024) [DOI] [PubMed] [Google Scholar]
- 44.Singh BD. 2009. a-Amylase immobilization on the silica nanoparticles for cleaning performance towards starch soils in laundry detergents. J. Mol. Catal. B Enzym. 74, 1.5. [Google Scholar]
- 45.Zhao Q, Hou Y, Gong GH, Yu MA, Jiang L, Liao F. 2010. Characterization of alcohol dehydrogenase from permeabilized brewer's yeast cells immobilized on the derived attapulgite nanofibers. Appl. Biochem. Biotechnol. 160, 2287–2299. ( 10.1007/s12010-009-8692-y) [DOI] [PubMed] [Google Scholar]
- 46.Datta S, Christena LR, Rajaram YRS. 2013. Enzyme immobilization: an overview on techniques and support materials. 3 Biotech. 3, 1–9. ( 10.1007/s13205-012-0071-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahney R, Puri BK, Anand S. 2005. Enzyme coated glass pH-electrode: its fabrication and applications in the determination of urea in blood samples. Anal. Chim. Acta 542, 157–161. ( 10.1016/j.aca.2005.03.069) [DOI] [Google Scholar]
- 48.Kim J, Campbell AS, Wang J. 2018. Wearable non-invasive epidermal glucose sensors: a review. Talanta 177, 163–170. ( 10.1016/j.talanta.2017.08.077) [DOI] [PubMed] [Google Scholar]
- 49.Sadeghi SJ. 2013. Amperometric biosensors. In Encyclopedia of biophysics, pp. 61–67. Berlin, Germany: Springer. [Google Scholar]
- 50.Lee H, et al. 2016. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572. ( 10.1038/nnano.2016.38) [DOI] [PubMed] [Google Scholar]
- 51.Ammann D. 1986. Classification of ion-selective electrodes. In Ion-selective microelectrodes: principles, design and application, pp. 3–8. Berlin, Germany: Springer. [Google Scholar]
- 52.Segev-Bar M, Haick H. 2013. Flexible sensors based on nanoparticles. ACS Nano 7, 8366–8378. ( 10.1021/nn402728g) [DOI] [PubMed] [Google Scholar]
- 53.Unno Y, et al. 2011. Development of n-on-p silicon sensors for very high radiation environments. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip. 636, 24–30. ( 10.1016/j.nima.2010.04.080) [DOI] [Google Scholar]
- 54.Keum H, Mccormick M, Liu P, Zhang Y, Omenetto FG. 2011. Epidermal electronics. Science 333, 838–844. ( 10.1126/science.1206157) [DOI] [PubMed] [Google Scholar]
- 55.Nag A, Mukhopadhyay SC, Kosel J. 2017. Wearable flexible sensors: a review wearable flexible sensors: a review. IEEE Sens. J. 17, 3949–3960. ( 10.1109/JSEN.2017.2705700) [DOI] [Google Scholar]
- 56.Song EY, Lee KB.. 2010. 1451.5 Standard-based wireless sensor networks. In Advancement in wireless sensors and sensor networks (eds Mukhopadhyay SC, Leung H), pp. 243–271. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 57.Choi DH, Li Y, Cutting GR, Searson PC. 2017. A wearable potentiometric sensor with integrated salt bridge for sweat chloride measurement. Sens. Actuators, B Chem. 250, 673–678. ( 10.1016/j.snb.2017.04.129) [DOI] [Google Scholar]
- 58.Lee H, et al. 2017. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Windmiller JR, Wang J. 2013. Wearable electrochemical sensors and biosensors: a review. Electroanalysis 25, 29–46. ( 10.1002/elan.201200349) [DOI] [Google Scholar]
- 60.Lombardi J, et al. 2017. Nanoparticle based printed sensors on paper for detecting chemical species. In Proc. Electronic Components and Technology Conf., Orlando, FL, 30 May to 2 June, pp. 764–771. Piscataway, NJ: IEEE. [Google Scholar]
- 61.Zhang X, Guo S, Han Y, Li J, Wang E. 2017. Beyond conventional patterns: new electrochemical lithography with high precision for patterned film materials and wearable sensors. Anal. Chem. 89, 2569–2574. ( 10.1021/acs.analchem.6b04816) [DOI] [PubMed] [Google Scholar]
- 62.Bariya M, Yin H, Nyein Y, Javey A. 2018. Wearable sweat sensors. Nat. Electron. 1, 160 ( 10.1038/s41928-018-0043-y) [DOI] [Google Scholar]
- 63.Xu J, Jia F, Li F, An Q, Gan S, Zhang Q, Ivaska A. 2016. Simple and efficient synthesis of gold nanoclusters and their performance as solid contact of ion selective electrode. Electrochim. Acta 222, 1007–1012. ( 10.1016/j.electacta.2016.11.069) [DOI] [Google Scholar]
- 64.Sapountzi E, Braiek M, Chateaux JF, Jaffrezic-Renault N, Lagarde F. 2017. Recent advances in electrospun nanofiber interfaces for biosensing devices. Sensors (Switzerland) 17, 1887 ( 10.3390/s17081887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson K, Poulter B, Dudgeon J, Li S-E, Ma X. 2017. A highly sensitive nonenzymatic glucose biosensor based on the regulatory effect of glucose on electrochemical behaviors of colloidal silver nanoparticles on MoS2. Sensors 17, 1807 ( 10.3390/s17081807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Senthamizhan A, Balusamy B, Uyar T. 2016. Glucose sensors based on electrospun nanofibers: a review fiber-based platforms for bioanalytics. Anal. Bioanal. Chem. 408, 1285–1306. ( 10.1007/s00216-015-9152-x) [DOI] [PubMed] [Google Scholar]
- 67.Chen CY, Chang CL, Chien TF, Luo CH. 2013. Flexible PDMS electrode for one-point wearable wireless bio-potential acquisition. Sens. Actuators, A Phys. 203, 20–28. ( 10.1016/j.sna.2013.08.010) [DOI] [Google Scholar]
- 68.Someya T. 2014. Bionic skins using flexible organic devices. In 44th European Solid State Device Research Conf. (ESSDERC), Venice, Italy, 22–26 September, pp. 68–71. Piscataway, NJ: IEEE. [Google Scholar]
- 69.Fujita T, Shiono S, Kanda K, Maenaka K, Hamada H, Higuchi K. 2012. Flexible sensor for human monitoring system by using P(VDF/TrFE) thin film. In Int. Conf. Emerging Trends in Engineering and Technology, ICETET, Himeji, Japan, 5–7 November, pp. 75–79. Piscataway, NJ: IEEE. [Google Scholar]
- 70.Ha D, De Vries WN, John SWM, Irazoqui PP, Chappell WJ.. 2012. Polymer-based miniature flexible capacitive pressure sensor for intraocular pressure (IOP) monitoring inside a mouse eye. Biomed. Microdevices 14, 207–215. ( 10.1007/s10544-011-9598-3) [DOI] [PubMed] [Google Scholar]
- 71.Tjahyono AP, Aw KC, Devaraj H, Surendra W, Haemmerle E, Travas-Sejdic J. 2013. A five-fingered hand exoskeleton driven by pneumatic artificial muscles with novel polypyrrole sensors. Ind. Rob. 40, 251–260. ( 10.1108/01439911311309951) [DOI] [Google Scholar]
- 72.Amjadi M, Pichitpajongkit A, Lee S, Ryu S, Park I. 2014. Highly stretchable and sensitive strain sensor based on silver nanowire–elastomer nanocomposite. ACS Nano 8, 5154–5163. ( 10.1021/nn501204t) [DOI] [PubMed] [Google Scholar]
- 73.Hasegawa Y, Shikida M, Ogura D, Suzuki Y, Sato K. 2008. Fabrication of a wearable fabric tactile sensor produced by artificial hollow fiber. J. Micromech. Microeng. 18, 085014 ( 10.1088/0960-1317/18/8/085014) [DOI] [Google Scholar]
- 74.Tang SLP. 2007. Recent developments in flexible wearable electronics for monitoring applications. Trans. Inst. Meas. Control 29, 283–300. ( 10.1177/0142331207070389) [DOI] [Google Scholar]
- 75.Ahmad M, Pan C, Luo Z, Zhu J. 2010. A single ZnO nanofiber-based highly sensitive amperometric glucose biosensor. J. Phys. Chem. C 114, 9308–9313. ( 10.1021/jp102505g) [DOI] [Google Scholar]
- 76.Tang H, Yan F, Tai Q, Chan HLW. 2010. The improvement of glucose bioelectrocatalytic properties of platinum electrodes modified with electrospun TiO2 nanofibers. Biosens. Bioelectron. 25, 1646–1651. ( 10.1016/j.bios.2009.11.027) [DOI] [PubMed] [Google Scholar]
- 77.Bujes-Garrido J, Izquierdo-Bote D, Heras A, Colina A, Arcos-Martínez MJ. 2018. Determination of halides using Ag nanoparticles-modified disposable electrodes. A first approach to a wearable sensor for quantification of chloride ions. Anal. Chim. Acta 1012, 42–48. ( 10.1016/j.aca.2018.01.063) [DOI] [PubMed] [Google Scholar]
- 78.Schoolaert E, Hoogenboom R, De Clerck K.. 2017. Colorimetric nanofibers as optical sensors. Adv. Funct. Mater. 27, 1–26. ( 10.1002/adfm.201702646) [DOI] [Google Scholar]
- 79.Fantini D, Costa L. 2009. Dye, fluorophores and pigment coloration of nanofibers produced by electrospinning. Polym. Adv. Technol. 20, 111–121. ( 10.1002/pat.1283) [DOI] [Google Scholar]
- 80.Mudabuka B, Ondigo D, Degni S, Vilakazi S, Torto N. 2014. A colorimetric probe for ascorbic acid based on copper–gold nanoparticles in electrospun nylon. Microchim. Acta 181, 395–401. ( 10.1007/s00604-013-1114-4) [DOI] [Google Scholar]
- 81.Ji X, Su Z, Wang P, Ma G, Zhang S. 2014. ‘Ready-to-use’ hollow nanofiber membrane-based glucose testing strips. Analyst 139, 6467–6473. ( 10.1039/c4an01354a) [DOI] [PubMed] [Google Scholar]
- 82.Khattab TA, Abdelmoez S, Klapötke TM. 2016. Electrospun nanofibers from a tricyanofuran-based molecular switch for colorimetric recognition of ammonia gas. Chem. Eur. J. 22, 4157–4163. ( 10.1002/chem.201504448) [DOI] [PubMed] [Google Scholar]
- 83.Van Der Schueren L, De Meyer T, Steyaert I, Ceylan Ö, Hemelsoet K, Van Speybroeck V, De Clerck K.. 2013. Polycaprolactone and polycaprolactone/chitosan nanofibres functionalised with the pH-sensitive dye Nitrazine Yellow. Carbohydr. Polym. 91, 284–293. ( 10.1016/j.carbpol.2012.08.003) [DOI] [PubMed] [Google Scholar]
- 84.Koh A, et al. 2016. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 ( 10.1126/scitranslmed.aaf2593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gueye MN, et al. 2016. Structure and dopant engineering in PEDOT thin films: practical tools for a dramatic conductivity enhancement. Chem. Mater. 28, 3462–3468. ( 10.1021/acs.chemmater.6b01035) [DOI] [Google Scholar]
- 86.Yang G, Kampstra KL, Abidian MR. 2014. High-performance conducting polymer nanofiber biosensors for detection of biomolecules. Adv. Mater. 26, 4954–4960. ( 10.1002/adma.201400753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xuan X, Yoon HS, Park JY. 2018. A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens. Bioelectron. 109, 75–82. ( 10.1016/j.bios.2018.02.054) [DOI] [PubMed] [Google Scholar]
- 88.Wang J, Lin Y. 2008. Functionalized carbon nanotubes and nanofibers for biosensing applications. Trends Anal. Chem. 27, 619–626. ( 10.1016/j.trac.2008.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shim BS, Chen W, Doty C, Xu C, Kotov NA. 2008. Smart electronic yarns and wearable fabrics for human biomonitoring made by carbon nanotube coating with polyelectrolytes. Nano Lett. 8, 4151–4157. ( 10.1021/nl801495p) [DOI] [PubMed] [Google Scholar]
- 90.You T. 2017. Carbon nanofibers for electroanalysis. In Nanocarbons for electroanalysis, pp. 27–47. New York, NY: Wiley. [Google Scholar]
- 91.Zaryanov NV, Nikitina VN, Karpova EV, Karyakina EE, Karyakin AA. 2017. Nonenzymatic sensor for lactate detection in human sweat. Anal. Chem. 89, 11 198–11 202. ( 10.1021/acs.analchem.7b03662) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.